Inhibition of Human Cathepsins B and L by Caffeic Acid and Its Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kinetic Measurements
2.3. Kinetic Data Analyses
3. Results
3.1. Evaluation of Cinnamic Acid Derivatives as Cathepsin Inhibitors
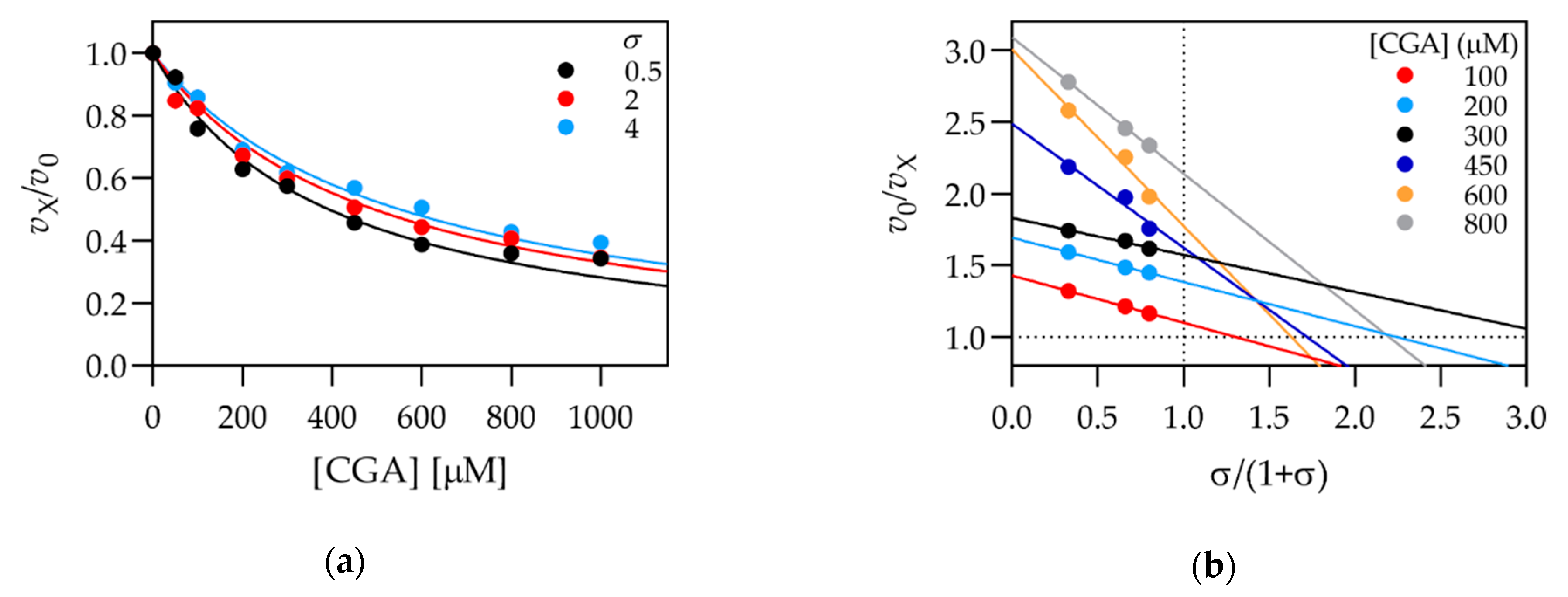
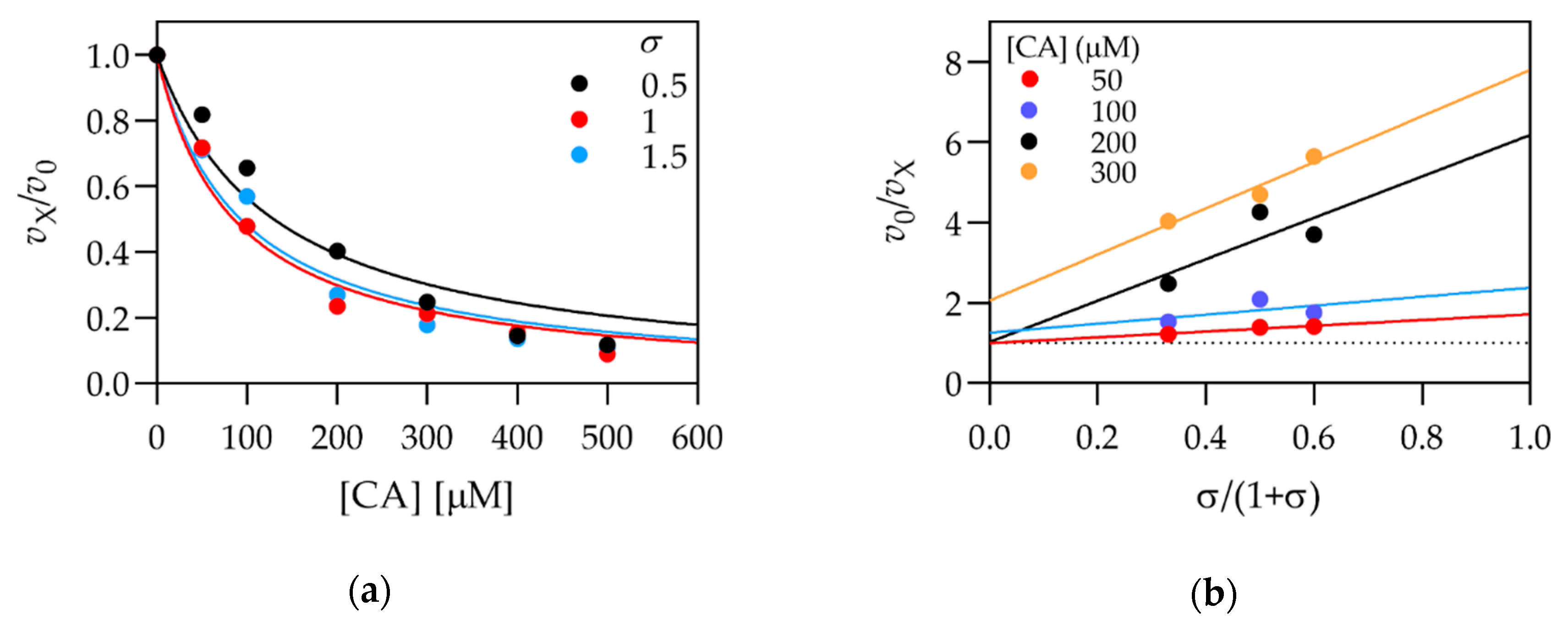
3.2. Kinetic Characterization of Selected Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abz | aminobenzoyl |
| AMC | 7-amino-4-methylcoumarin |
| CA | caffeic acid |
| CAPE | caffeic acid phenethyl ester |
| CGA | chlorogenic acid |
| Dnp | 2,4-dinitrophenylamino |
| DPPI | dipeptidyl-peptidase I |
| DTT | dithiothreitol |
| Z | benzyloxycarbonyl |
References
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Pittalá, V.; Salerno, L.; Romeo, G.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V. Therapeutic Potential of Caffeic Acid Phenethyl Ester (CAPE) in Diabetes. Curr. Med. Chem. 2018, 25, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 87, pp. 71–88. [Google Scholar]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin-a review on their antioxidant activity in vitro (O/W emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Leonardi, F.; Scaccini, C.; Virgili, F. Modulation of ceramide-induced NF-κB binding activity and apoptotic response by caffeic acid in U937 cells: Comparison with other antioxidants. Free Radic. Biol. Med. 2001, 30, 722–733. [Google Scholar] [CrossRef]
- Chung, T.; Moon, S.; Chang, Y.; Ko, J.; Lee, Y.; Cho, G.; Kim, S.; Kim, J.; Kim, C. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004, 18, 1670–1681. [Google Scholar] [CrossRef]
- Yasuko, K.; Tomohiro, N.; Sei-Itsu, M.; Ai-Na, L.; Yasuo, F.; Takashi, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Sud’ina, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V.; Varfolomeev, S.D.; Belozersky, A.N. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Yaqoob, P.; Knox, K.A.; Calder, P.C. Inhibition of ICE-family cysteine proteases rescues murine lymphocytes from lipoxygenase inhibitor-induced apoptosis. FEBS Lett. 1996, 396, 266–270. [Google Scholar] [CrossRef][Green Version]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar] [PubMed]
- Chan, W.S.; Wen, P.C.; Chiang, H.C. Structure-activity relationship of caffeic acid analogues on xanthine oxidase inhibition. Anticancer Res. 1995, 15, 703–707. [Google Scholar] [PubMed]
- Ploemen, J.H.T.M.; van Ommen, B.; de Haan, A.; Schefferlie, J.G.; van Bladeren, P.J. In vitro and in vivo reversible and irreversible inhibition of rat glutathione S-transferase isoenzymes by caffeic acid and its 2-S-glutathionyl conjugate. Food Chem. Toxicol. 1993, 31, 475–482. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Pongsuwan, J.; Wungcharoen, C.; Yibchok-Anun, S. In vitro effects of cinnamic acid derivatives on protein tyrosine phosphatase 1B. J. Enzyme Inhib. Med. Chem. 2013. [Google Scholar] [CrossRef]
- Rebernik, M.; Snoj, T.; Klemenčič, M.; Novinec, M. Interplay between tetrameric structure, enzymatic activity and allosteric regulation of human dipeptidyl-peptidase I. Arch. Biochem. Biophys. 2019, 675, 108121. [Google Scholar] [CrossRef]
- Musil, D.; Zucic, D.; Turk, D.; Engh, R.A.; Mayr, I.; Huber, R.; Popovic, T.; Turk, V.; Towatari, T.; Katunuma, N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: The structural basis for its specificity. EMBO J. 1991, 10, 2321–2330. [Google Scholar] [CrossRef]
- Novinec, M.; Lenarcic, B. Papain-like peptidases: Structure, function, and evolution. Biomol. Concepts 2013, 4, 287–308. [Google Scholar] [CrossRef]
- Michallet, M.-C.; Saltel, F.; Preville, X.; Flacher, M.; Revillard, J.-P.; Genestier, L. Cathepsin-B-dependent apoptosis triggered by antithymocyte globulins: A novel mechanism of T-cell depletion. Blood 2003, 102, 3719–3726. [Google Scholar] [CrossRef]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Baici, A.; Lang, A.; Zwicky, R.; Müntener, K. Cathepsin B in osteoarthritis: Uncontrolled proteolysis in the wrong place. Semin. Arthritis Rheum. 2004, 34, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.J.; Kellett, K.A.B.; Thinakaran, G.; Hooper, N.M. A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis. J. Biol. Chem. 2016, 291, 19235–19244. [Google Scholar] [CrossRef] [PubMed]
- Cavallo-Medved, D.; Mai, J.; Dosescu, J.; Sameni, M.; Sloane, B.F. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J. Cell Sci. 2005, 118, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Abboud-Jarrous, G.; Atzmon, R.; Peretz, T.; Palermo, C.; Gadea, B.B.; Joyce, J.A.; Vlodavsky, I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J. Biol. Chem. 2008, 283, 18167–18176. [Google Scholar] [CrossRef]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 2015, 155, 105–116. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Pan, J.; Sun, J.; Liu, J.; Libby, P.; Sukhova, G.K.; Doria, A.; Katunuma, N.; Peroni, O.D.; et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat. Cell Biol. 2007, 9, 970–977. [Google Scholar] [CrossRef]
- Ebert, D.H.; Deussing, J.; Peters, C.; Dermody, T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002, 277, 24609–24617. [Google Scholar] [CrossRef]
- Gnirß, K.; Kühl, A.; Karsten, C.; Glowacka, I.; Bertram, S.; Kaup, F.; Hofmann, H.; Pöhlmann, S. Cathepsins B and L activate Ebola but not Marburg virus glycoproteins for efficient entry into cell lines and macrophages independent of TMPRSS2 expression. Virology 2012, 424, 3–10. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Mason, R.W.; Johnson, D.A.; Barrett, A.J.; Chapman, H.A. Elastinolytic activity of human cathepsin L. Biochem. J. 1986, 233, 925–927. [Google Scholar] [CrossRef]
- Novinec, M.; Grass, R.N.; Stark, W.J.; Turk, V.; Baici, A.; Lenarčič, B. Interaction between human cathepsins K, L, and S and elastins: Mechanism of elastinolysis and inhibition by macromolecular inhibitors. J. Biol. Chem. 2007, 282, 7893–7902. [Google Scholar] [CrossRef] [PubMed]
- Korenč, M.; Lenarčič, B.; Novinec, M. Human cathepsin L, a papain-like collagenase without proline specificity. FEBS J. 2015, 282, 4328–4340. [Google Scholar] [CrossRef] [PubMed]
- Ishidoh, K.; Kominami, E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem. Biophys. Res. Commun. 1995, 217, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Pavšič, M.; Lenarčič, B. A simple and efficient protocol for the production of recombinant cathepsin v and other cysteine cathepsins in soluble form in Escherichia coli. Protein Expr. Purif. 2012, 82, 1–5. [Google Scholar] [CrossRef]
- Palmier, M.O.; Van Doren, S.R. Rapid determination of enzyme kinetics from fluorescence: Overcoming the inner filter effect. Anal. Biochem. 2007, 371, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Schenker, P.; Baici, A. Paradoxical interactions between modifiers and elastase-2. FEBS J. 2010, 277, 2486–2495. [Google Scholar] [CrossRef]
- Baici, A. The Specific Velocity Plot: A Graphical Method for Determining Inhibition Parameters for Both Linear and Hyperbolic Enzyme Inhibitors. Eur. J. Biochem. 1981, 119, 9–14. [Google Scholar] [CrossRef]
- Wang, S.H.; Chen, C.S.; Huang, S.H.; Yu, S.H.; Lai, Z.Y.; Huang, S.T.; Lin, C.M. Hydrophilic ester-bearing chlorogenic acid binds to a novel domain to inhibit xanthine oxidase. Planta Med. 2009, 75, 1237–1240. [Google Scholar] [CrossRef]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Pagliani, T.; Poggi, A. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Schenker, P.; Alfarano, P.; Kolb, P.; Caflisch, A.; Baici, A. A double-headed cathepsin B inhibitor devoid of warhead. Protein Sci. 2008, 17, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Mirković, B.; Renko, M.; Turk, S.; Sosič, I.; Jevnikar, Z.; Obermajer, N.; Turk, D.; Gobec, S.; Kos, J. Novel Mechanism of CathepsinB Inhibition by Antibiotic Nitroxoline and Related Compounds. ChemMedChem 2011, 6, 1351–1356. [Google Scholar] [CrossRef]
- Sosič, I.; Mitrović, A.; Ćurić, H.; Knez, D.; Brodnik Žugelj, H.; Štefane, B.; Kos, J.; Gobec, S. Cathepsin B inhibitors: Further exploration of the nitroxoline core. Bioorganic Med. Chem. Lett. 2018, 28, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Sosič, I.; Mirković, B.; Arenz, K.; Štefane, B.; Kos, J.; Gobec, S. Development of new cathepsin b inhibitors: Combining bioisosteric replacements and structure-based design to explore the structure-activity relationships of nitroxoline derivatives. J. Med. Chem. 2013, 56, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Renko, M.; Završnik, J.; Turk, D.; Seeger, M.A.; Vasiljeva, O.; Grütter, M.G.; Turk, V.; Turk, B. Non-invasive in vivo imaging of tumour-associated cathepsin B by a highly selective inhibitory DARPin. Theranostics 2017, 7, 2806–2821. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic Acid, A Versatile Pharmacophore: An Overview. Mini Rev. Med. Chem. 2012, 11, 695–713. [Google Scholar] [CrossRef]
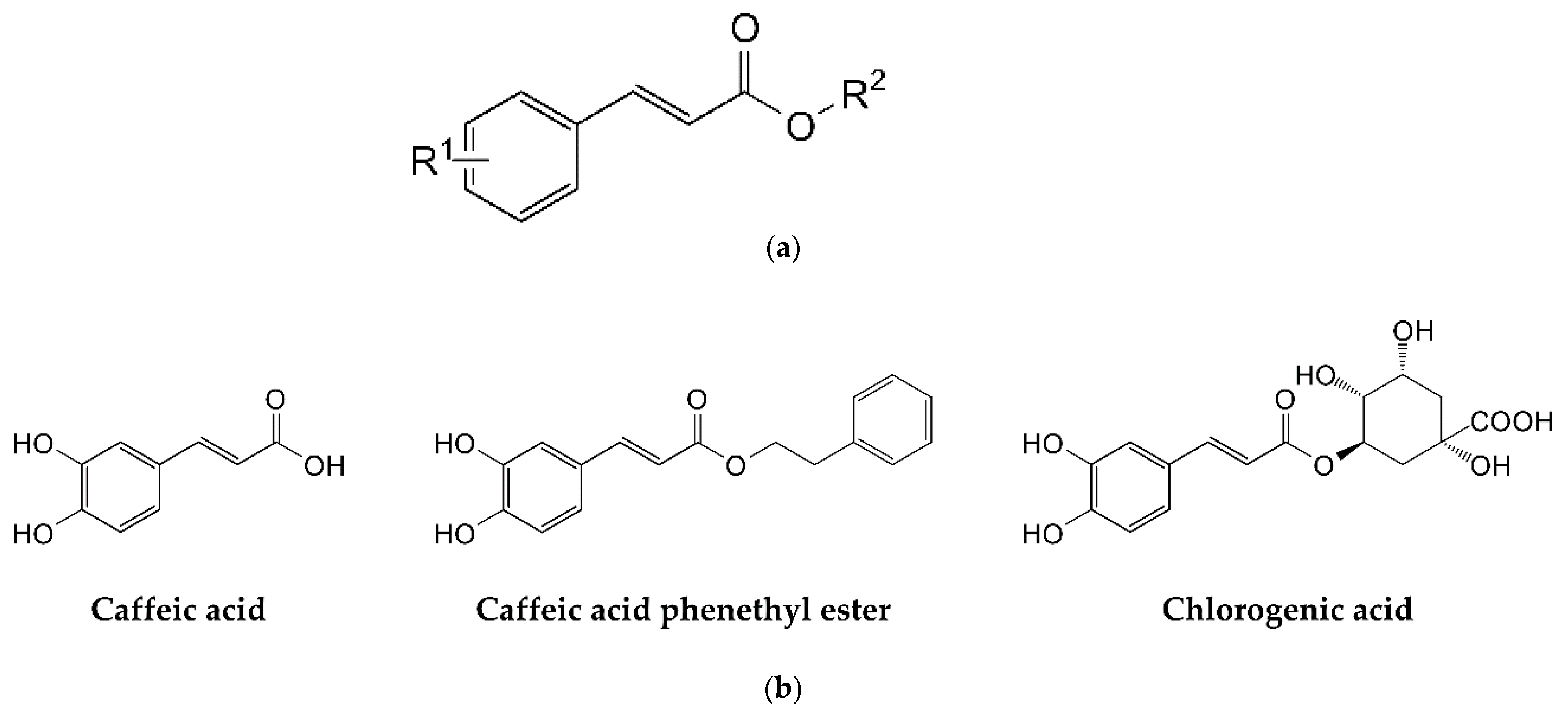
| Common Name | R 1 | R 2 | Cathepsin B IC50 (µM) | Cathepsin L IC50 (µM) |
|---|---|---|---|---|
| Cinnamic acid | None | H- | >1000 | >1000 |
| o-Coumaric acid | 2-hydroxy- | H- | >1000 | >1000 |
| m-Coumaric acid | 3-hydroxy- | H- | >1000 | >1000 |
| p-Coumaric acid | 4-hydroxy- | H- | >1000 | >1000 |
| 2,4-Dihydroxy- cinnamic acid | 2,4-dihydroxy- | H- | 910 ± 30 | 930 ± 150 |
| Caffeic acid 2 | 3,4-dihydroxy- | H- | 110 ± 10 | >1000 |
| Hydrocaffeic acid 1 | 3,4-dihydroxy- | H- | >1000 | >1000 |
| Ferulic acid | 3-methoxy-, 4-hydroxy- | H- | >1000 | >1000 |
| Sinapinic acid | 3,3′-dimethoxy-, 4-hydroxy- | H- | >1000 | >1000 |
| Caffeic acid phenethyl ester 2 | 3,4-dihydroxy- | 2-phenylethyl- | 470 ± 30 | 190 ± 30 |
| Chlorogenic acid 2 | 3,4-dihydroxy- | 3-O-quinic acid | 340 ± 20 | 480 ± 20 |
| Enzyme/Inhibitor | pH | Mechanism | KSp (µM) | KCa (µM) |
|---|---|---|---|---|
| Cathepsin L | ||||
| CA | 5.5 | Mixed | 740 ± 70 | 1270 ± 120 |
| CAPE | 5.5 | Mixed | 120 ± 70 | 170 ± 30 |
| CGA | 5.5 | Mixed | 330 ± 20 | 670 ± 60 |
| Cathepsin B | ||||
| CA | 5.5 | Mixed | 270 ± 140 | 82 ± 16 |
| 7.4 | Catalytic | n.a.1 | 46 ± 4 | |
| CAPE | 5.5 | Catalytic | n.a.1 | 140 ± 10 |
| 7.4 | Mixed | 107 ± 38 | 70 ± 19 | |
| CGA | 5.5 | Mixed | 340 ± 80 | 1200 ± 1100 |
| 7.4 | Mixed balanced | 92 ± 15 | 92 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulčakar, L.; Novinec, M. Inhibition of Human Cathepsins B and L by Caffeic Acid and Its Derivatives. Biomolecules 2021, 11, 31. https://doi.org/10.3390/biom11010031
Ulčakar L, Novinec M. Inhibition of Human Cathepsins B and L by Caffeic Acid and Its Derivatives. Biomolecules. 2021; 11(1):31. https://doi.org/10.3390/biom11010031
Chicago/Turabian StyleUlčakar, Liza, and Marko Novinec. 2021. "Inhibition of Human Cathepsins B and L by Caffeic Acid and Its Derivatives" Biomolecules 11, no. 1: 31. https://doi.org/10.3390/biom11010031
APA StyleUlčakar, L., & Novinec, M. (2021). Inhibition of Human Cathepsins B and L by Caffeic Acid and Its Derivatives. Biomolecules, 11(1), 31. https://doi.org/10.3390/biom11010031





