Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. 2D Culture
2.2. 3D Culture Technique in the Self-Assembling Peptide Scaffold RAD16-I
2.3. Construct Morphology Evaluation
2.4. Cell Viability Assessment
2.4.1. Live/Dead Staining
2.4.2. MTT Assay
2.5. Osteogenic and Adipogenic Differentiation
2.6. Staining for Phenotype Assessment
2.6.1. Alkaline Phosphatase Staining
2.6.2. Toluidine Blue Staining
2.6.3. Immunocytochemistry
2.6.4. Immunofluorescence
2.6.5. Von Kossa Staining
2.6.6. Nile Red Staining
2.7. RNA Extraction, cDNA Synthesis and PCR
2.8. Western Blot
3. Results
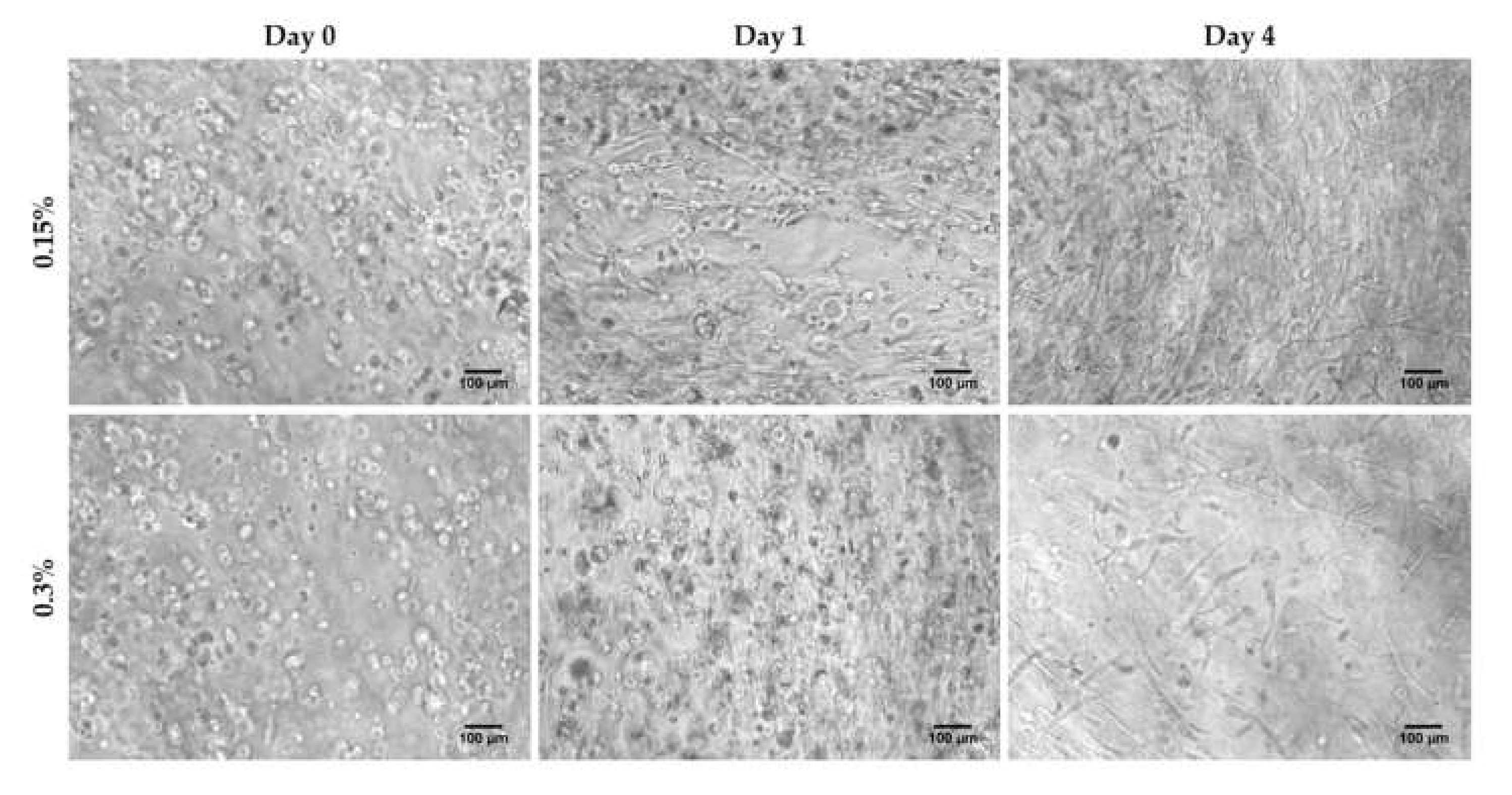
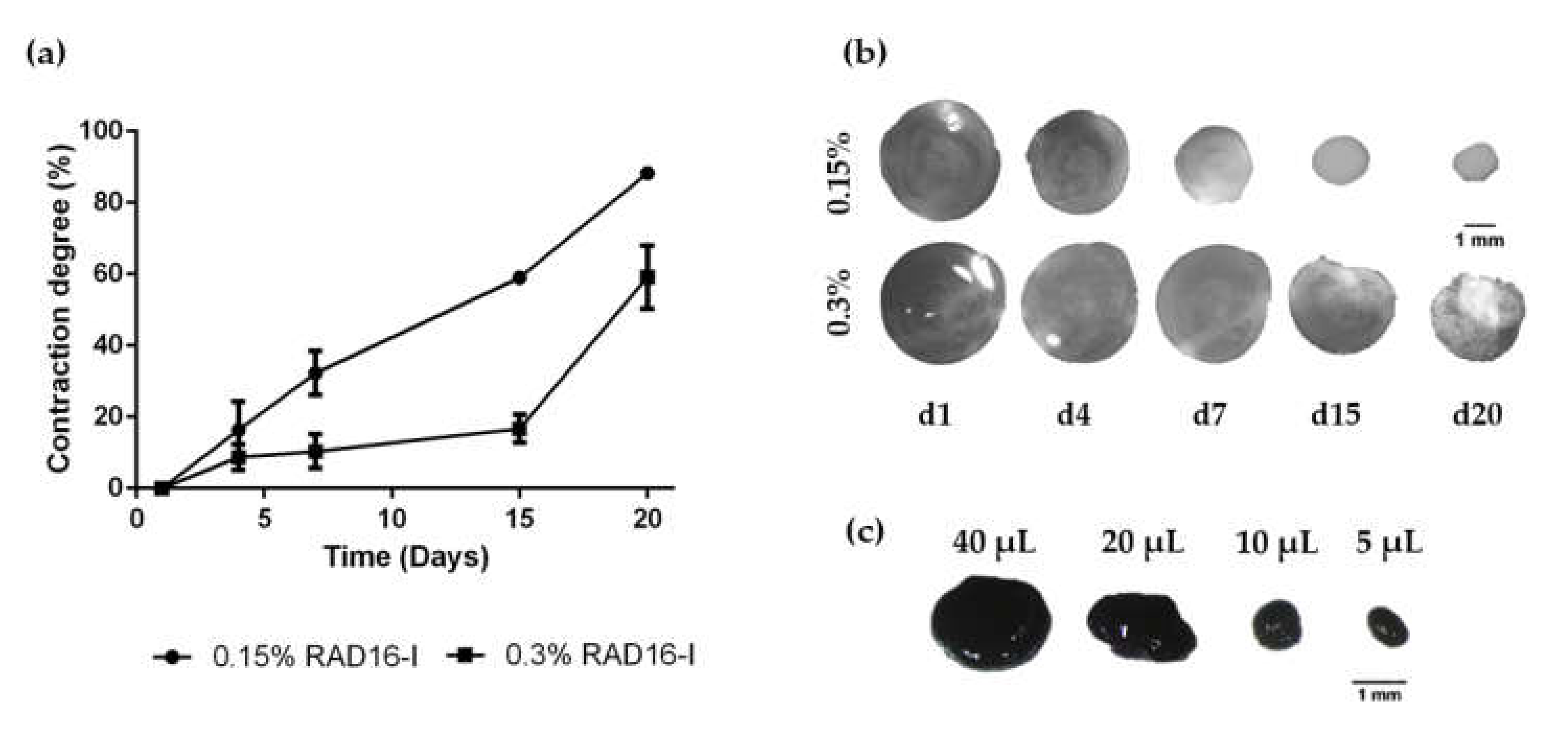
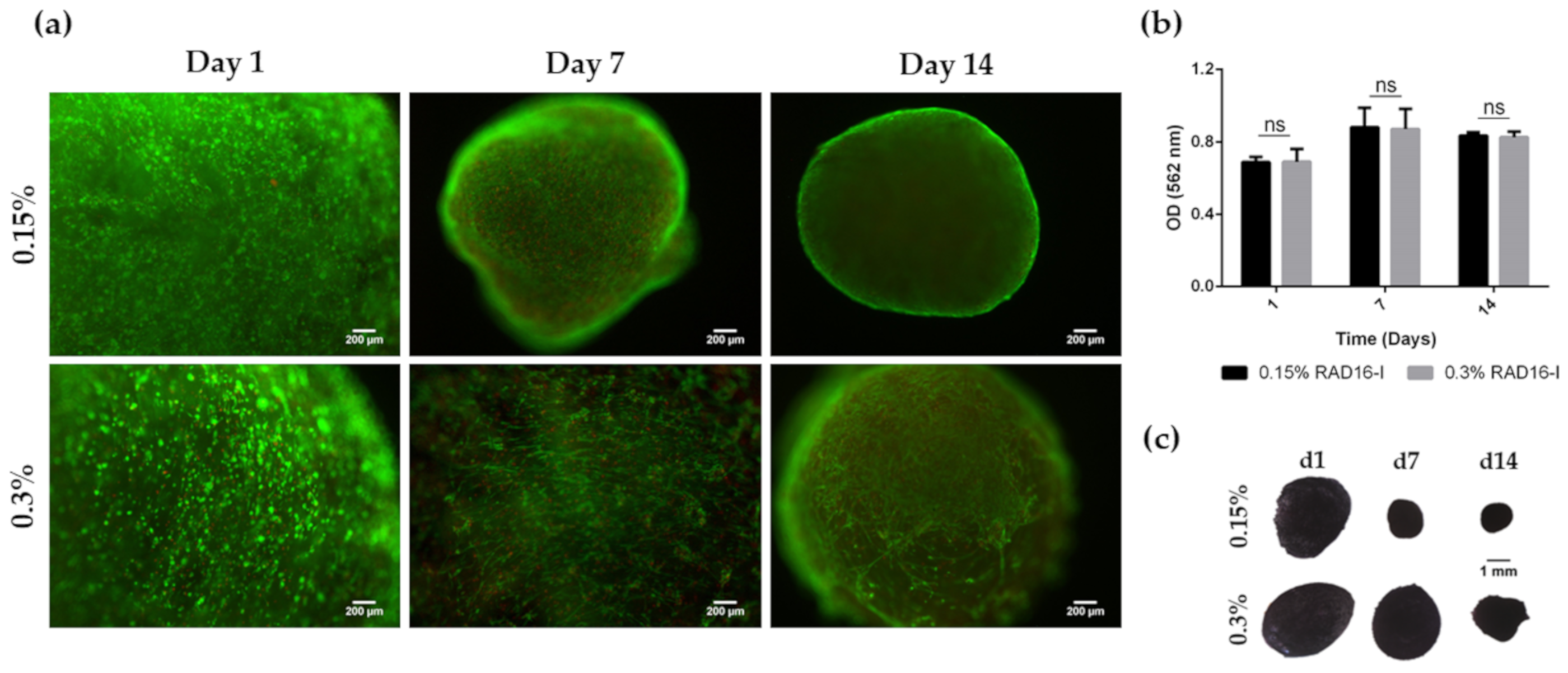
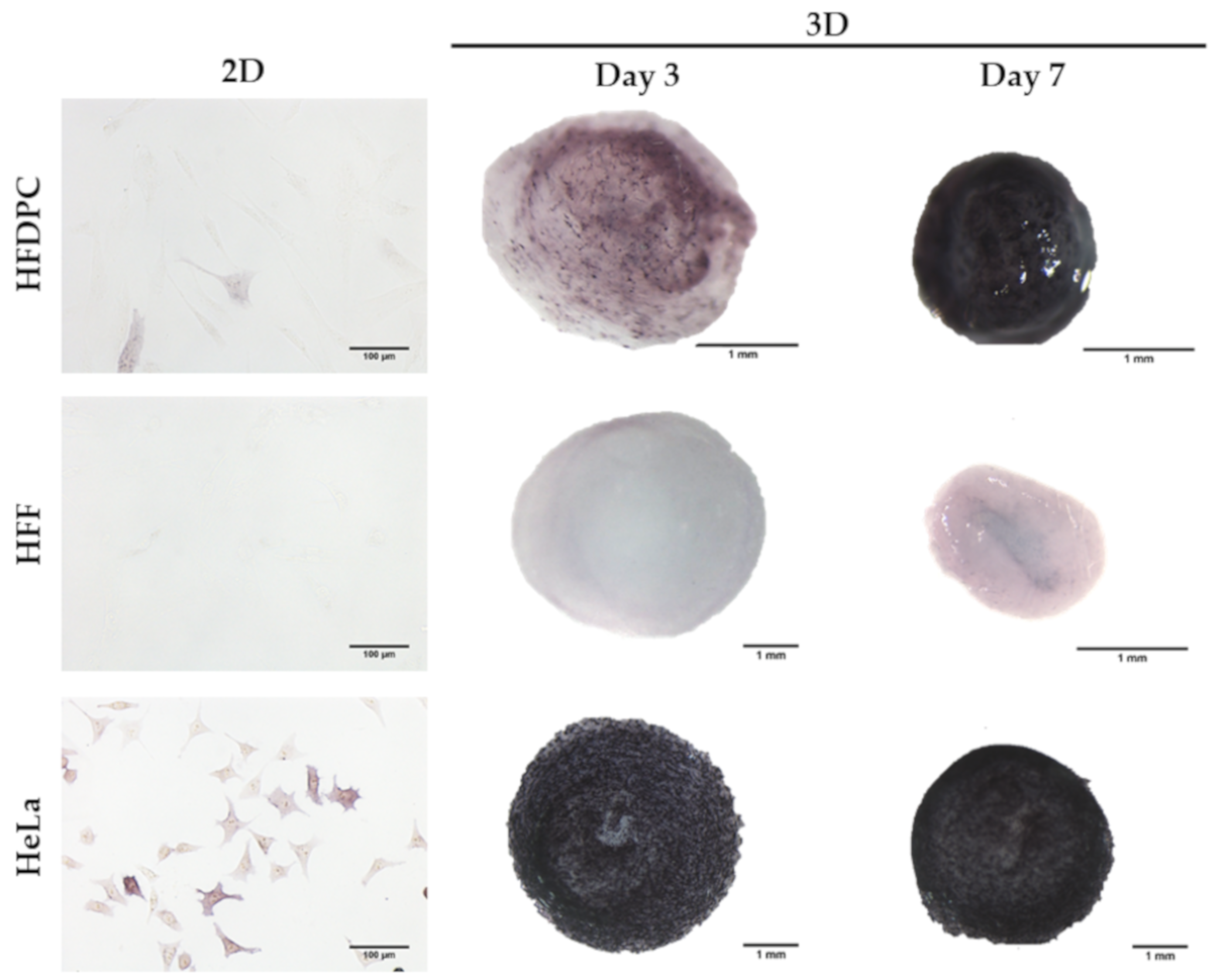
3.1. HFDPC Culture in the 3D Self-Assembling Peptide Scaffold RAD16-I
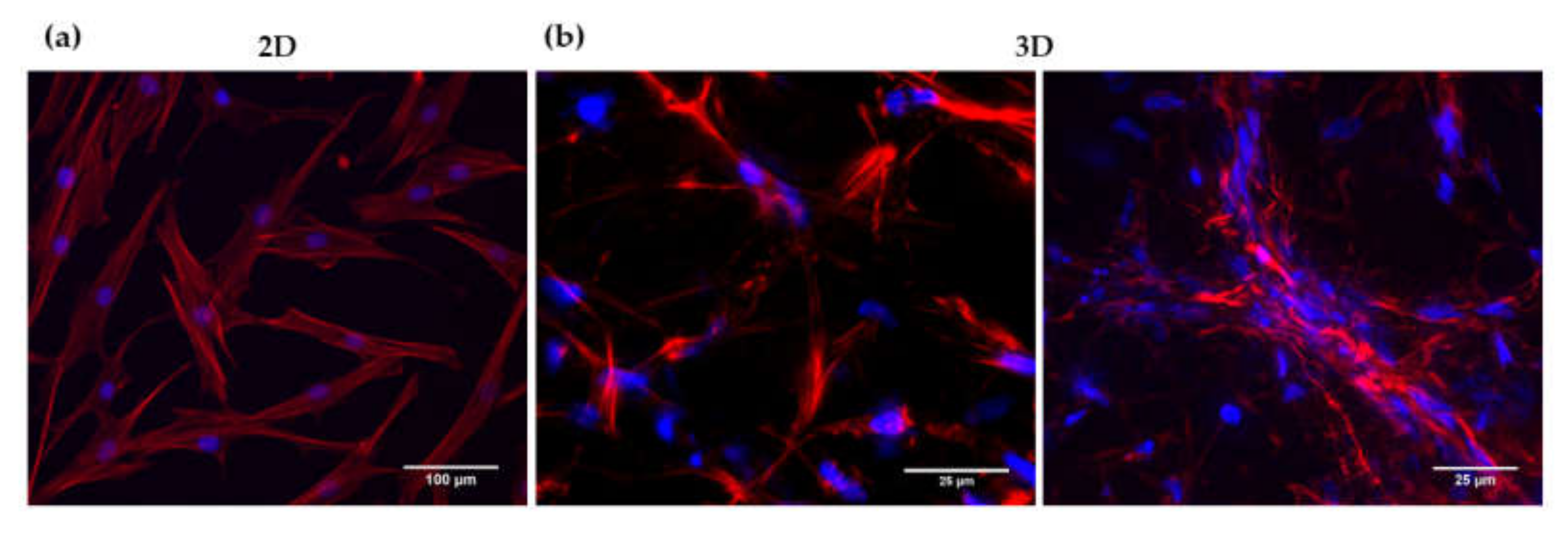
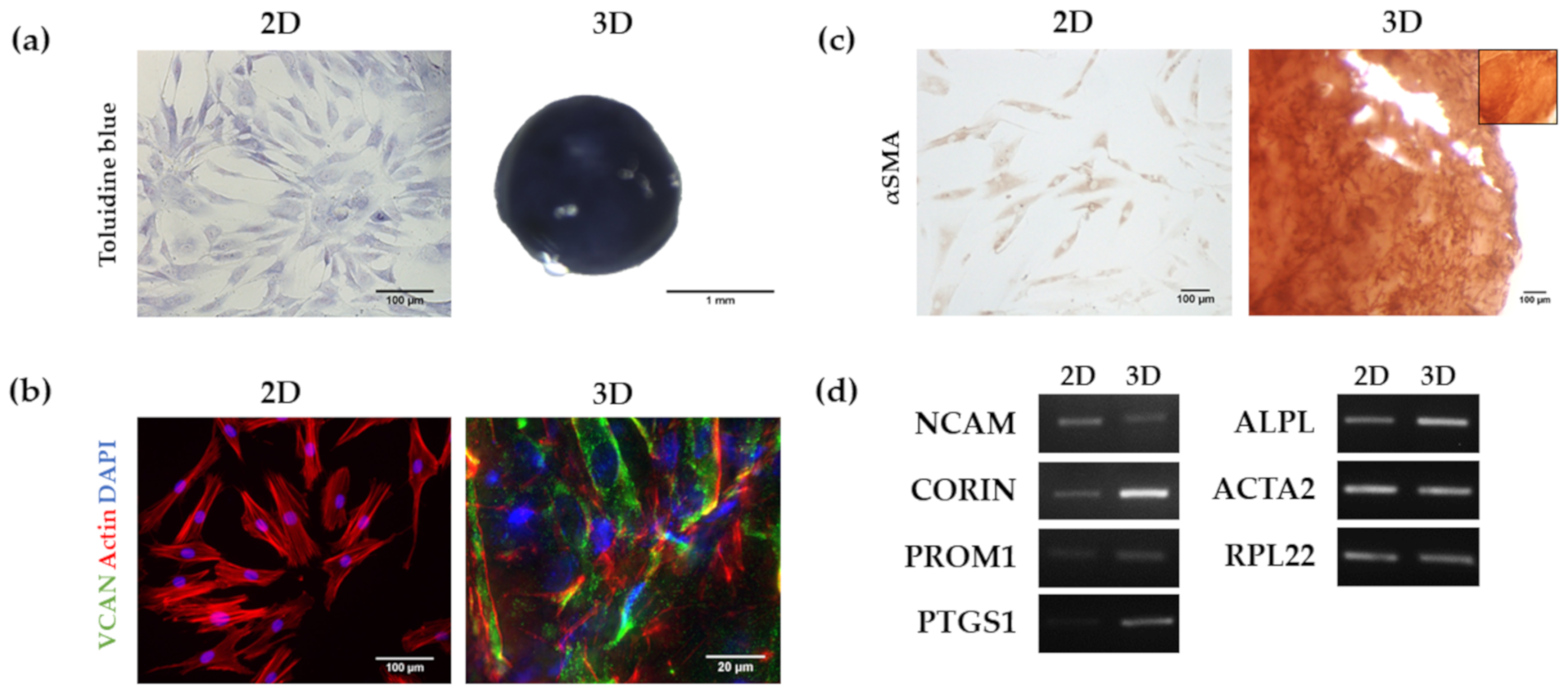
3.2. Analysis of HFDPC Signature Markers
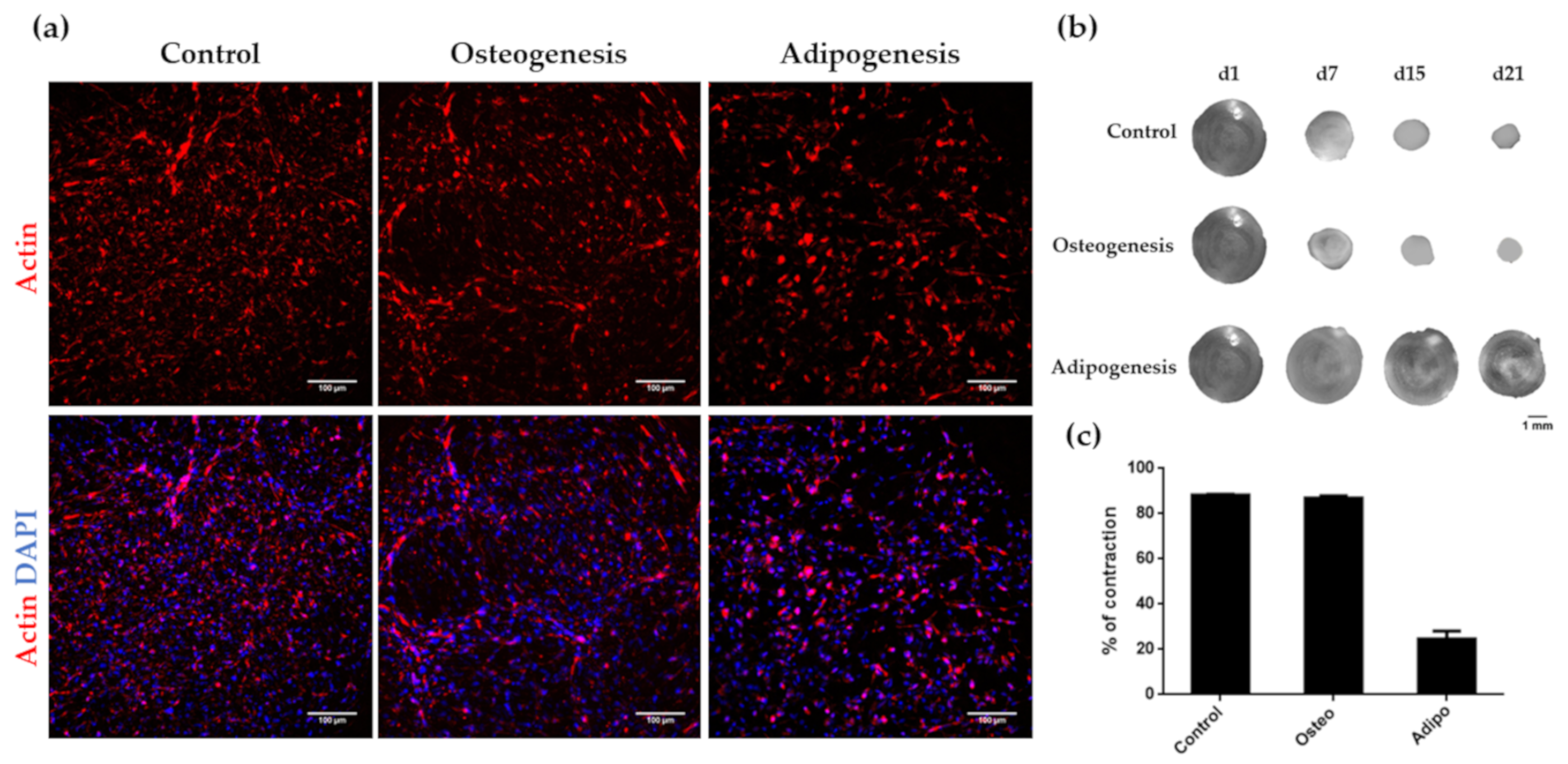
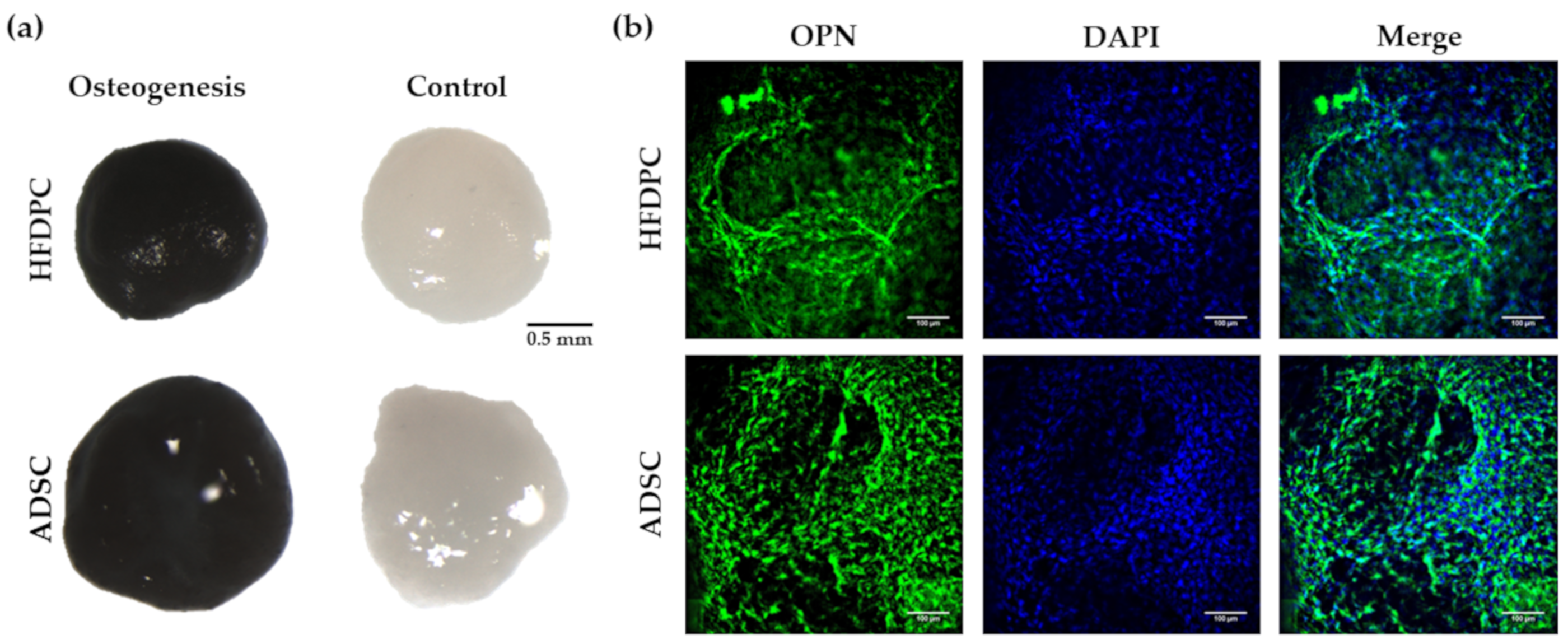
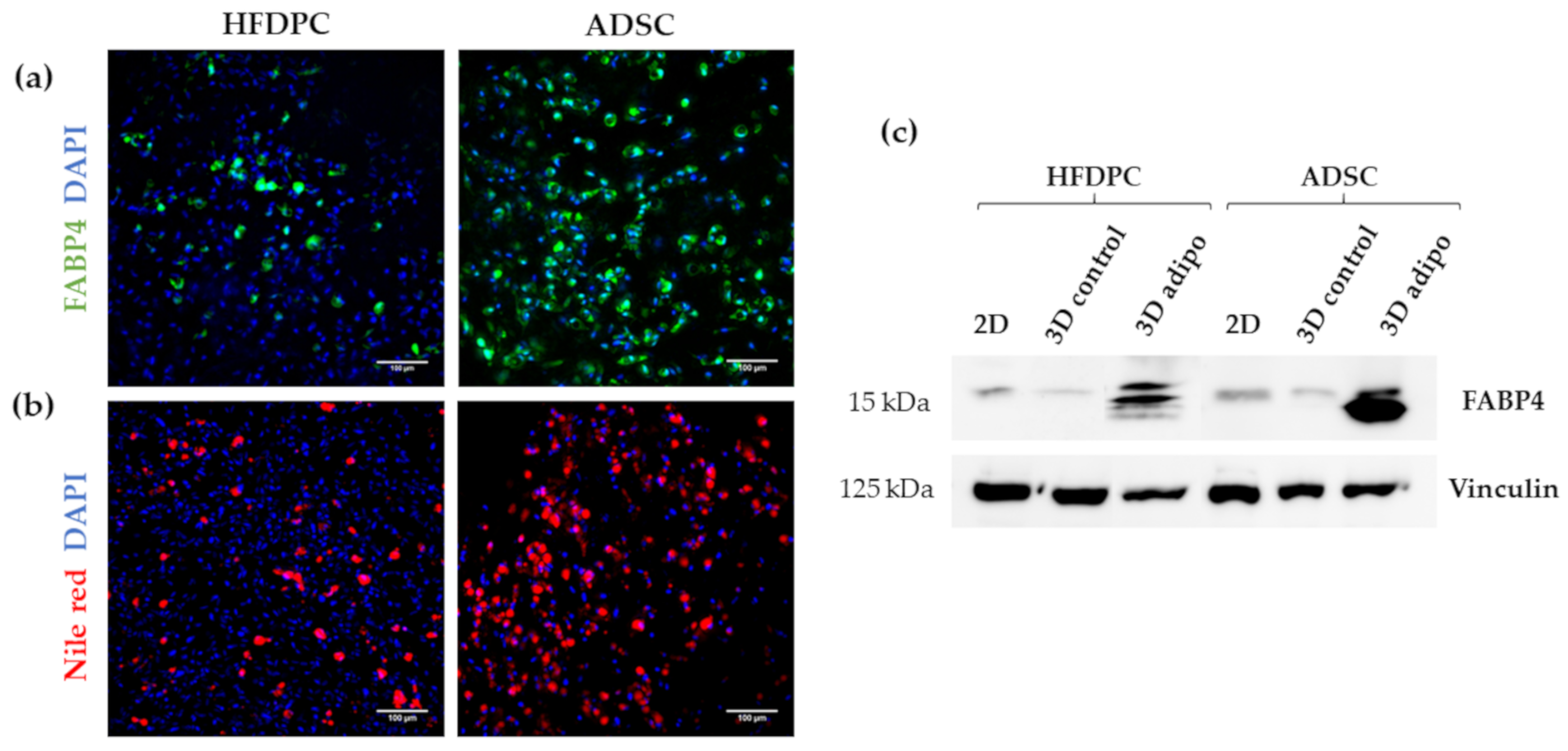
3.3. Osteogenic and Adipogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teumer, J.; Cooley, J. Follicular cell implantation: An emerging cell therapy for hair loss. Semin. Plast. Surg. 2005, 19, 193–200. [Google Scholar] [CrossRef]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef]
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef]
- Jahoda, C.A.B.; Reynolds, A.J.; Chaponnier, C.; Forester, J.C.; Gabbiani, G. Smooth muscle a-actin is a marker for hair follicle dermis In Vivo and In Vitro. J. Cell Sci. 1991, 99, 627–636. [Google Scholar]
- Qiao, J.; Zawadzka, A.; Philips, E.; Turetsky, A.; Batchelor, S.; Peacock, J.; Durrant, S.; Garlick, D.; Kemp, P.; Teumer, J. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen. Med. 2009, 4, 667–676. [Google Scholar] [CrossRef]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef]
- Higgins, C.A.; Richardson, G.D.; Ferdinando, D.; Westgate, G.E.; Jahoda, C.A.B. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp. Dermatol. 2010, 19, 546–548. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.B.; Christiano, A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef]
- Lin, B.; Miao, Y.; Wang, J.; Fan, Z.; Du, L.; Su, Y.; Liu, B.; Hu, Z.; Xing, M. Surface tension guided hanging-drop: Producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5906–5916. [Google Scholar] [CrossRef]
- Driskell, R.R.; Juneja, V.R.; Connelly, J.T.; Kretzschmar, K.; Tan, D.W.M.; Watt, F.M. Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -Negative cells in vitro and in Vivo. J. Investig. Dermatol. 2012, 132, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Sun, Y.B.; Liu, B.C.; Jiang, J.D.; Hu, Z.Q. Controllable production of transplantable adult human high-passage dermal papilla spheroids using 3D matrigel culture. Tissue Eng. Part A 2014, 20, 2329–2338. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.M.; Kim, D.; Heo, W. Characterization of human dermal papilla cells in alginate spheres. Appl. Sci. 2018, 8, 1–16. [Google Scholar]
- Richardson, G.D.; Arnott, E.C.; Whitehouse, C.J.; Lawrence, C.M.; Hole, N.; Jahoda, C.A.B. Cultured cells from the adult human hair follicle dermis can be directed toward adipogenic and osteogenic differentiation. J. Investig. Dermatol. 2005, 124, 1090–1091. [Google Scholar] [CrossRef]
- Richardson, G.D.; Arnott, Ã.E.C.; Whitehouse, Ã.C.J.; Lawrence, Ã.C.M.; Reynolds, A.J.; Hole, Ã.N.; Jahoda, C.A. Plasticity of rodent and human hair follicle dermal cells: Implications for cell therapy and tissue engineering. J. Investig. Dermatol. Symp. Proc. 2005, 10, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Gorjup, E.; Genever, P.G. Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 2006, 60, 49–60. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Mistriotis, P.; Andreadis, S.T. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012, 8, 74–84. [Google Scholar] [CrossRef]
- Liu, J.Y.; Peng, H.F.; Gopinath, S.; Tian, J.; Andreadis, S.T. Derivation of functional smooth muscle cells. Tissue Eng. Part A 2010, 16, 2553–2564. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Lawrence, C.; Cserhalmi-Friedman, P.B.; Christiano, A.M.; Jahoda, C.A.B. Trans-gender induction of hair follicles. Nature 1999, 402, 33–34. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef]
- Schneider, J.P.; Pochan, D.J.; Ozbas, B.; Rajagopal, K.; Pakstis, L.; Kretsinger, J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2002, 124, 15030–15037. [Google Scholar] [CrossRef]
- Zhang, S.; Gelain, F.; Zhao, X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 2005, 15, 413–420. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.; Lockshin, C.; Rich, A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.N.; Knowles, P.F.; McLeish, T.C.B.; Pitkeathly, M.; Radford, S.E. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes. Lett. Nat. 1997, 386, 259–262. [Google Scholar] [CrossRef]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Semino, C.E. Can we build artificial stem cell compartments? J. Biomed. Biotechnol. 2003, 3, 164–169. [Google Scholar] [CrossRef]
- Semino, C.E. Self-assembling peptides: From bio-inspired materials to bone regeneration. J. Dent. Res. 2008, 87, 606–616. [Google Scholar] [CrossRef]
- Genové, E.; Shen, C.; Zhang, S.; Semino, C.E. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 2005, 26, 3341–3351. [Google Scholar] [CrossRef]
- Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Castells-Sala, C.; Mata, A.; Semino, C.E. Bimolecular based heparin and self-assembling hydrogel for tissue engineering applications. Acta Biomater. 2015, 16, 35–48. [Google Scholar] [CrossRef]
- Dégano, I.R.; Quintana, L.; Vilalta, M.; Horna, D.; Rubio, N.; Borrós, S.; Semino, C.; Blanco, J. The effect of self-assembling peptide nanofiber scaffolds on mouse embryonic fibroblast implantation and proliferation. Biomaterials 2009, 30, 1156–1165. [Google Scholar] [CrossRef]
- Wu, J.; Marí-Buyé, N.; Muiños, T.F.; Borrós, S.; Favia, P.; Semino, C.E. Nanometric self-assembling peptide layers maintain adult hepatocyte phenotype in sandwich cultures. J. Nanobiotechnol. 2010, 8, 29. [Google Scholar] [CrossRef]
- Genové, E.; Schmitmeier, S.; Sala, A.; Borrós, S.; Bader, A.; Griffith, L.G.; Semino, C.E. Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity In Vitro. J. Cell Mol. Med. 2009, 13, 3387–3397. [Google Scholar] [CrossRef]
- Castells-Sala, C.; Recha-Sancho, L.; Llucià-Valldeperas, A.; Soler-Botija, C.; Bayes-Genis, A.; Semino, C.E. Three-dimensional cultures of human subcutaneous adipose tissue-derived progenitor cells based on RAD16-I self-assembling peptide. Tissue Eng. Part C Methods 2016, 22, 113–124. [Google Scholar] [CrossRef]
- Garreta, E.; Genove, E.; Borrós, S.; Semino, C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006, 12, 1–14. [Google Scholar] [CrossRef]
- Marí-Buyé, N.; Fernández-Muiños, T.; Semino, C.E. Three-dimensional cultures in soft self-assembling nanofibers núria. J. Hosp. Libr. 2012, 12, 317–326. [Google Scholar]
- Fernández-Muiños, T.; Suárez-Muñoz, M.; Sanmartí-Espinal, M.; Semino, C.E. Matrix dimensions, stiffness, and structural properties modulate spontaneous chondrogenic commitment of mouse embryonic fibroblasts. Tissue Eng. Part A 2014, 20, 1145–1155. [Google Scholar] [CrossRef]
- Recha-Sancho, L.; Semino, C.E. Heparin-based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. J. Biomed. Mater. Res. Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef]
- Recha-Sancho, L.; Semino, C.E. Chondroitin sulfate- and decorin-based self-Assembling scaffolds for cartilage tissue engineering. PLoS ONE 2016, 11, e0157603. [Google Scholar] [CrossRef]
- Betriu, N.; Recha-sancho, L.; Semino, C.E. Culturing mammalian cells in three-dimensional peptide scaffolds. J. Vis. Exp. 2018, 136, 1–6. [Google Scholar] [CrossRef]
- Sarang-Sieminski, A.; Was, A.; Kim, G.; Gong, H.; Kamm, R. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem. Biophys. 2007, 49, 73–83. [Google Scholar] [CrossRef]
- Soma, T.; Tajima, M.; Kishimoto, J. Hair cycle-specific expression of versican in human hair follicles. J. Dermatol. Sci. 2005, 39, 147–154. [Google Scholar] [CrossRef]
- Malgouries, S.; Thibaut, S.; Bernard, B.A. Proteoglycan expression patterns in human hair follicle. Br. J. Dermatol. 2008, 158, 234–242. [Google Scholar] [CrossRef]
- Enshell-seijffers, D.; Lindon, C.; Morgan, B.A. The serine protease Corin is a novel modifier of the agouti pathway. Development 2008, 135, 217–225. [Google Scholar] [CrossRef]
- Ito, Y.; Hamazaki, T.S.; Ohnuma, K.; Tamaki, K.; Asashima, M.; Okochi, H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Investig. Dermatol. 2007, 127, 1052–1060. [Google Scholar] [CrossRef]
- Michelet, J.F.; Commo, S.; Billoni, N.; Mahé, Y.F.; Bernard, B.A. Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J. Investig. Dermatol. 1997, 108, 205–209. [Google Scholar] [CrossRef]
- Combates, N.J.; Chuong, C.M.; Stenn, K.S.; Prouty, S.M. Expression of two ig family adhesion molecules in the murine hair cycle: Dcc in the bulge epithelia and NCAM in the follicular papilla. J. Investig. Dermatol. 1997, 109, 672–678. [Google Scholar] [CrossRef][Green Version]
- Higgins, C.; Roger, M.; Hill, R.; Ali, S.; Garlick, J.; Christiano, M.; Jahoda, C.A.B. Multifaceted role of hair follicle dermal cells in bioengineered skins. Br. J. Dermatol. 2017, 176, 1259–1269. [Google Scholar] [CrossRef]
- Lenoir, M.C.; Bernard, B.A.; Pautrat, G.; Darmon, M.; Shroot, B. Outer root sheath cells of human hair follicle are able to regenerate a fully differentiated epidermis In Vitro. Dev. Biol. 1988, 130, 610–620. [Google Scholar] [CrossRef]
- Jahoda, C.A.B.; Reynolds, A.J. Hair follicle dermal sheath cells: Unsung participants in wound healing. Lancet 2001, 358, 1445–1448. [Google Scholar] [CrossRef]
- Gharzi, A.; Reynolds, A.J.; Jahoda, C.A.B. Plasticity of hair follicle dermal cells in wound healing and induction. Exp. Dermatol. 2003, 12, 126–136. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin enhances the bioperformance of collagen-based matrices preseeded with human-adipose derived stem cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef]
- Wang, X.F.; Song, Y.; Liu, Y.S.; Sun, Y.C.; Wang, Y.G.; Wang, Y.; Lyu, P.-J. Osteogenic differentiation of three-dimensional bioprinted constructs consisting of human adipose-derived stem cells In Vitro and In Vivo. PLoS ONE 2016, 11, e0157214. [Google Scholar] [CrossRef]
- Biggs, L.C.; Mäkelä, O.J.; Myllymäki, S.M.; Roy, R.D.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. Elife 2018, 7, 1–33. [Google Scholar] [CrossRef]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells “feel” outside and in? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef]
- Kishimoto, J.; Ehama, R.; Wu, L.; Jiang, S.; Jiang, N.; Burgeson, R.E. Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc. Natl. Acad. Sci. USA 1999, 96, 7336–7341. [Google Scholar] [CrossRef]
- Young, T.; Lee, C.; Chiu, H.; Hsu, C.; Lin, S. Biomaterials Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly (ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials 2008, 29, 3521–3530. [Google Scholar] [CrossRef]
- Obokata, H.; Vacanti, C.A. Stem Cells in Tissue Engineering. Principles of Tissue Engineering, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 595–608. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Jahoda, C.A.B.; Whitehouse, C.J.; Reynolds, A.J.; Hole, N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 2003, 12, 849–859. [Google Scholar] [CrossRef]
| Gene | Forward/Reverse | Sequence (5′–3′) | Size (bp) | Amplicon (bp) |
|---|---|---|---|---|
| NCAM | Forward | 5′-TAAGCTCGAAGGGCAGATGG-3′ | 20 | 142 |
| Reverse | 5′-GAGCCTGATCTCTGGTTTCC-3′ | 20 | ||
| CORIN | Forward | 5′-GGCTGTGTCCTCATTGCCAA-3′ | 20 | 146 |
| Reverse | 5′-GTCTTCACAAAGCGTGTCTGC-3′ | 21 | ||
| PROM1 | Forward | 5′-TTCTTGACCGACTGAGACCCA-3′ | 21 | 99 |
| Reverse | 5′-TCATGTTCTCCAACGCCTCTT-3′ | 21 | ||
| PTGS1 | Forward | 5′-GGCACCAACCTCATGTTTGC-3′ | 20 | 146 |
| Reverse | 5′-TGACGCTCCAGATTGTCTCC-3′ | 20 | ||
| ALPL | Forward | 5′-ATGGGATGGGTGTCTCCACA-3′ | 20 | 108 |
| Reverse | 5′-CCACGAAGGGGAACTTGTC-3′ | 19 | ||
| ACTA2 | Forward | 5′-CAGGGCTGTTTTCCCATCCA-3′ | 20 | 108 |
| Reverse | 5′-CCTCTTTTGCTCTGTGCTTCGT-3′ | 22 | ||
| RPL22 | Forward | 5′-TGACATCCGAGGTGCCTTTC-3′ | 20 | 101 |
| Reverse | 5′-GTTAGCAACTACGCGCAACC-3′ | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betriu, N.; Jarrosson-Moral, C.; Semino, C.E. Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold. Biomolecules 2020, 10, 684. https://doi.org/10.3390/biom10050684
Betriu N, Jarrosson-Moral C, Semino CE. Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold. Biomolecules. 2020; 10(5):684. https://doi.org/10.3390/biom10050684
Chicago/Turabian StyleBetriu, Nausika, Claire Jarrosson-Moral, and Carlos E. Semino. 2020. "Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold" Biomolecules 10, no. 5: 684. https://doi.org/10.3390/biom10050684
APA StyleBetriu, N., Jarrosson-Moral, C., & Semino, C. E. (2020). Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold. Biomolecules, 10(5), 684. https://doi.org/10.3390/biom10050684







