Diverse Functions of Polyamines in Virus Infection
Abstract
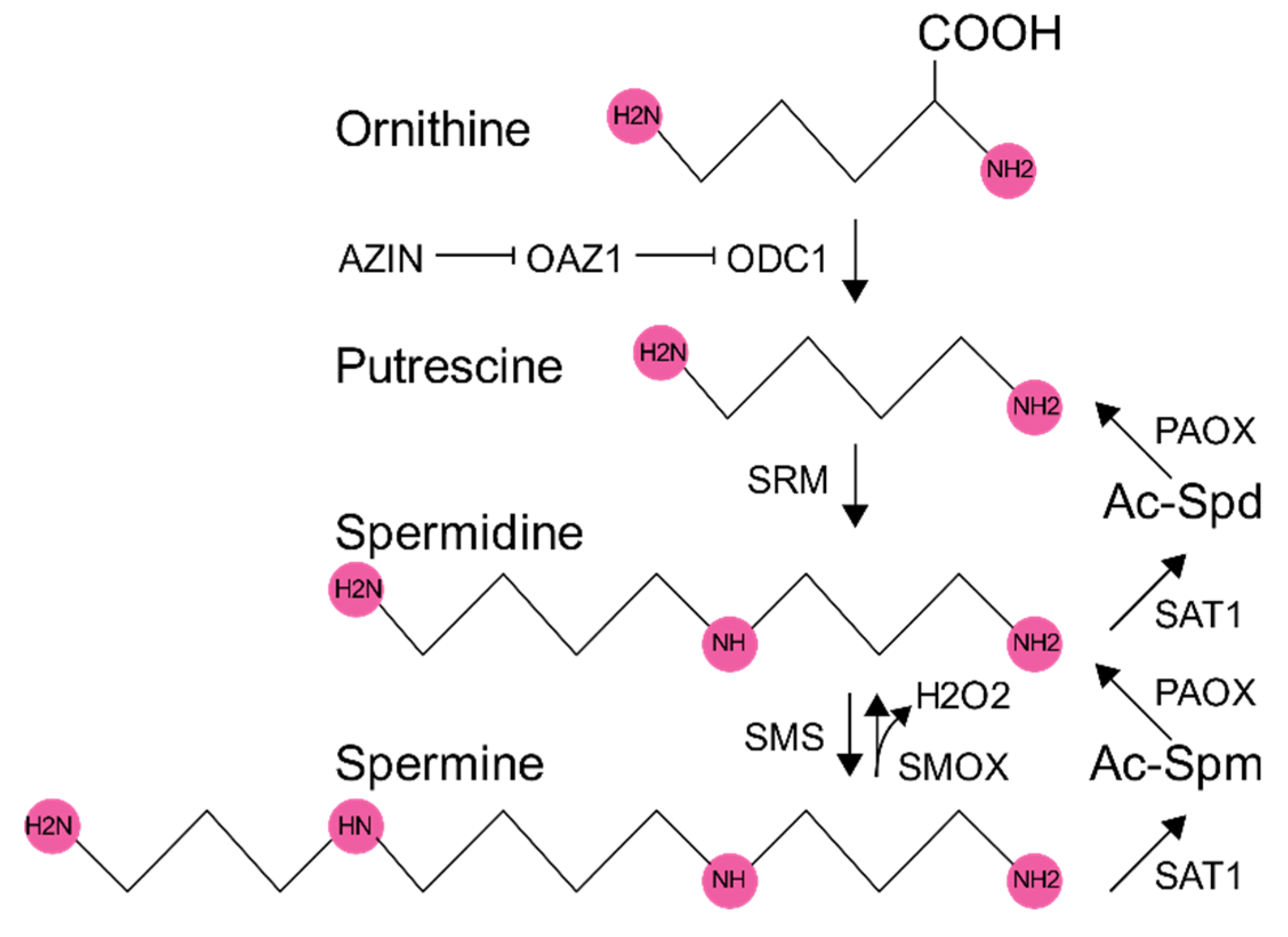
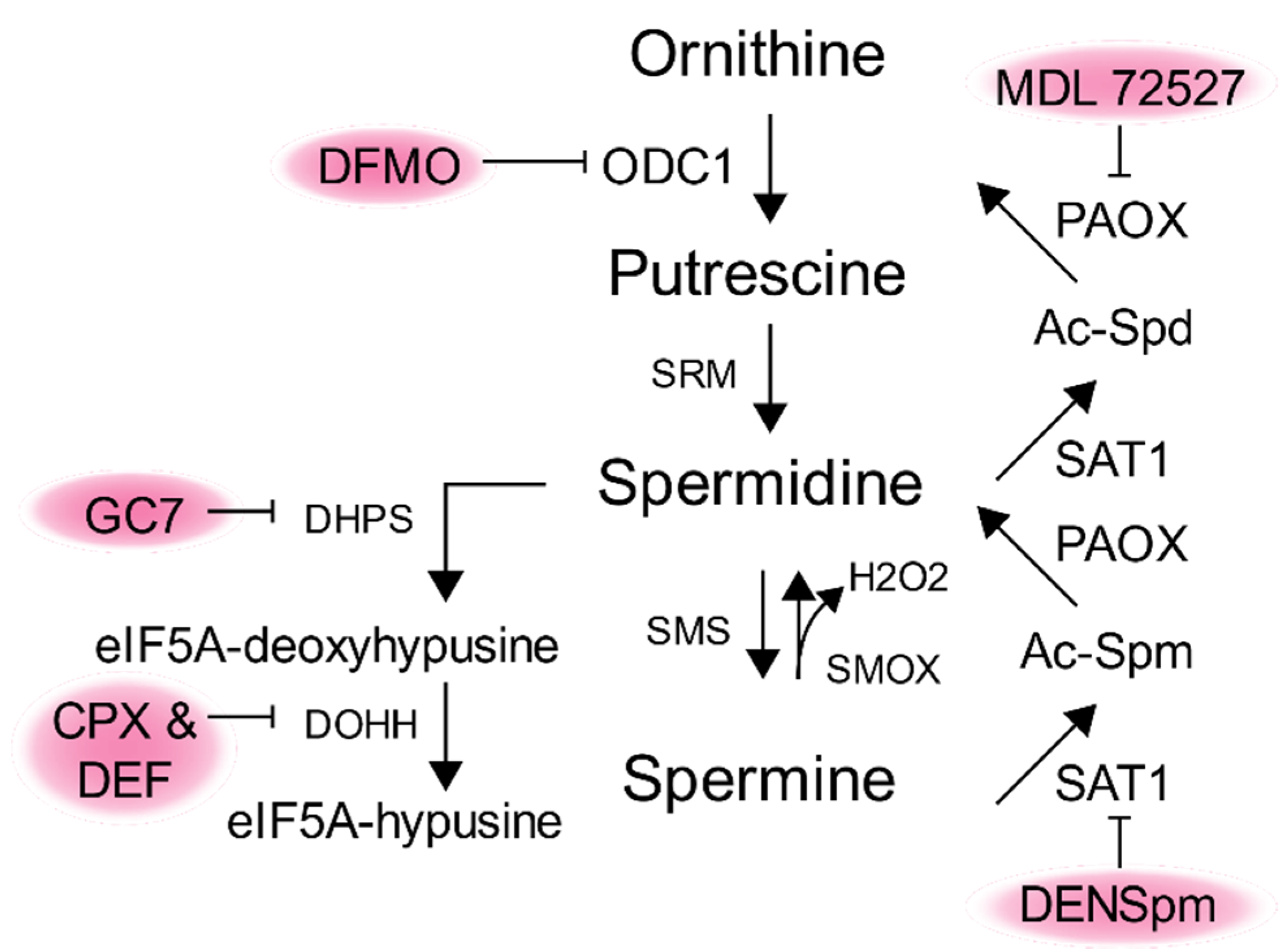
1. Polyamine Synthesis and Regulation
2. Polyamines in Bacteriophages
3. Polyamines in Plant Viruses and the Response to Infection
4. Polyamines in Mammalian Viruses and the Response to Infection
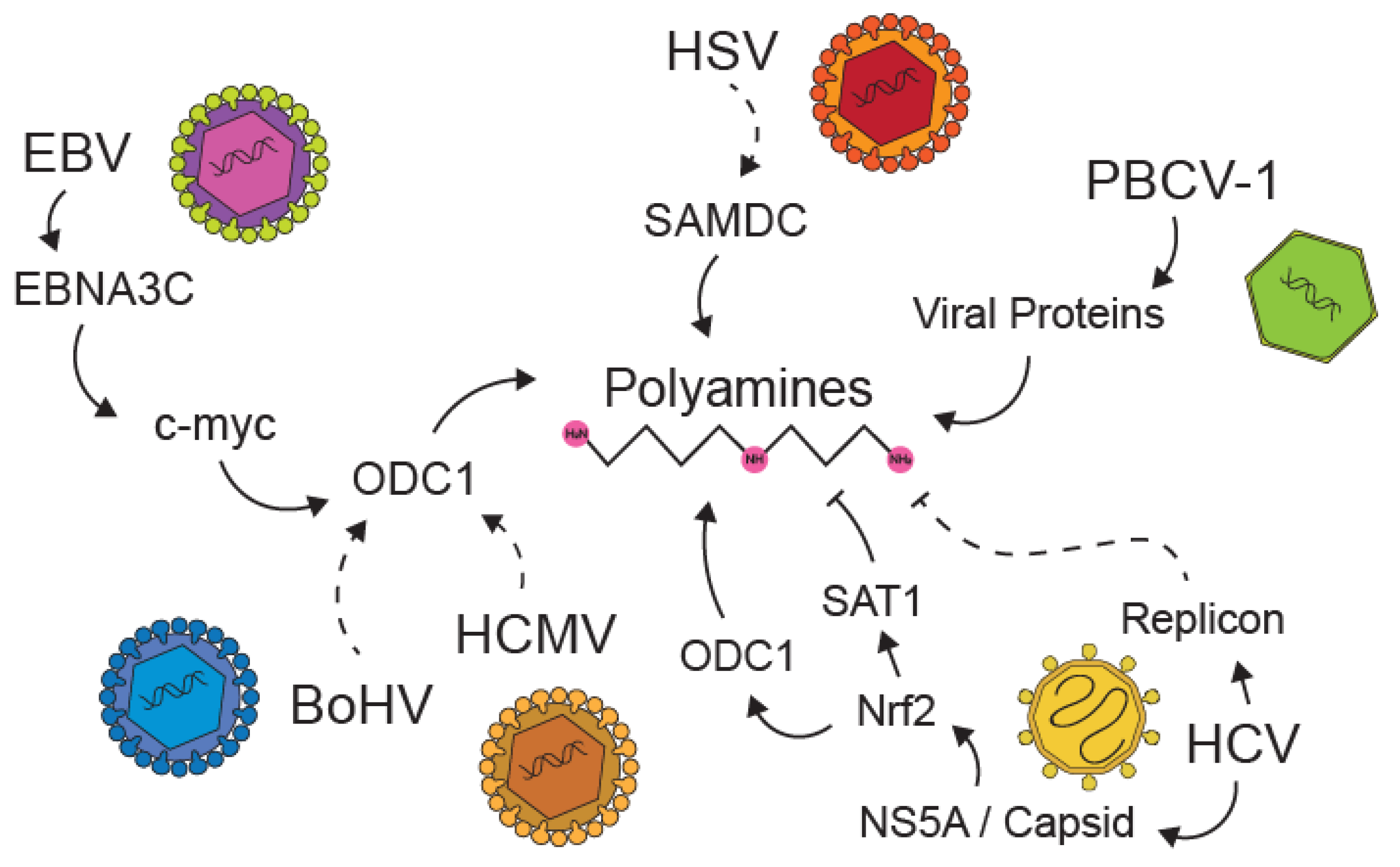
5. Viral Manipulation of Polyamines
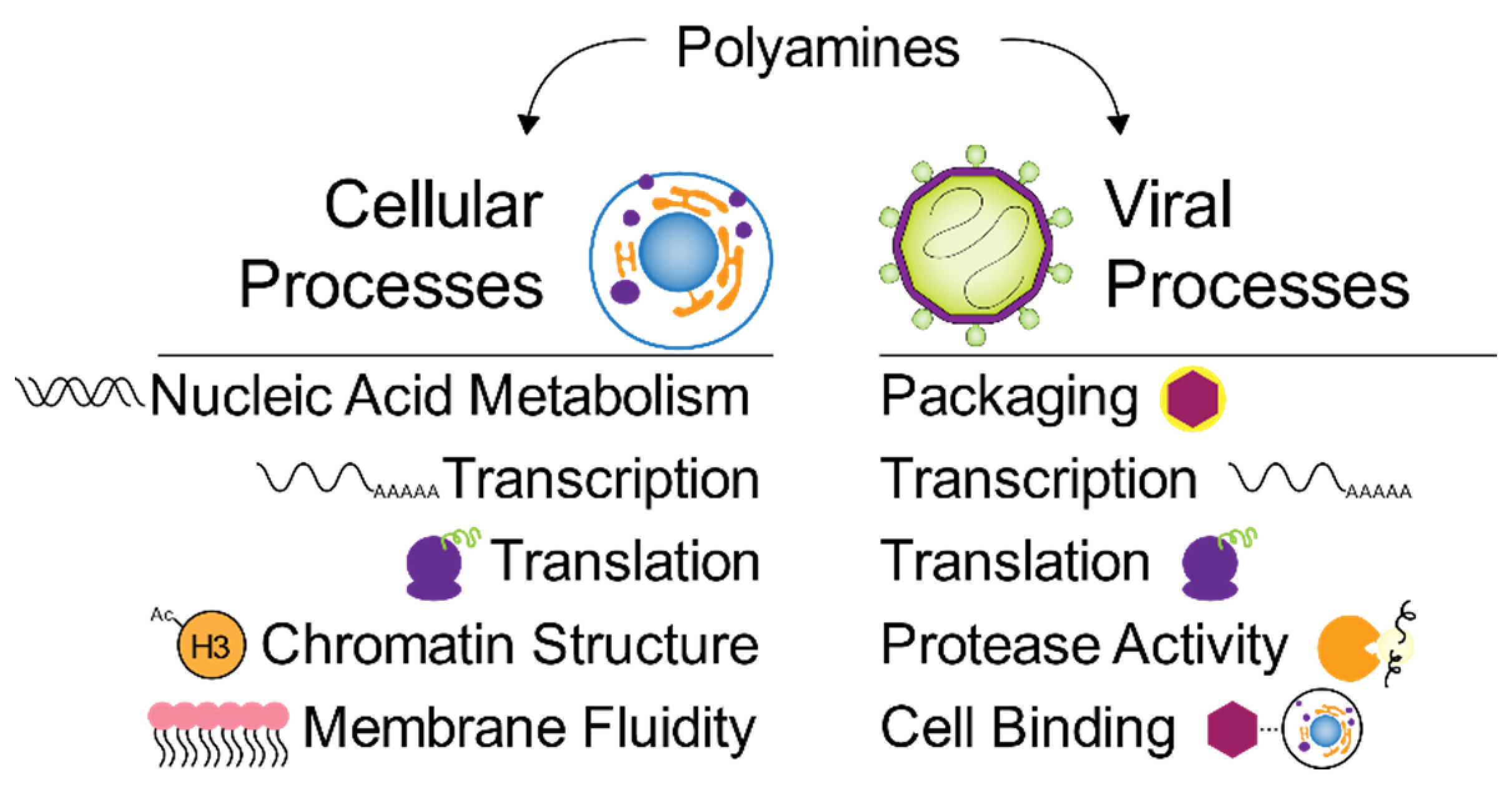
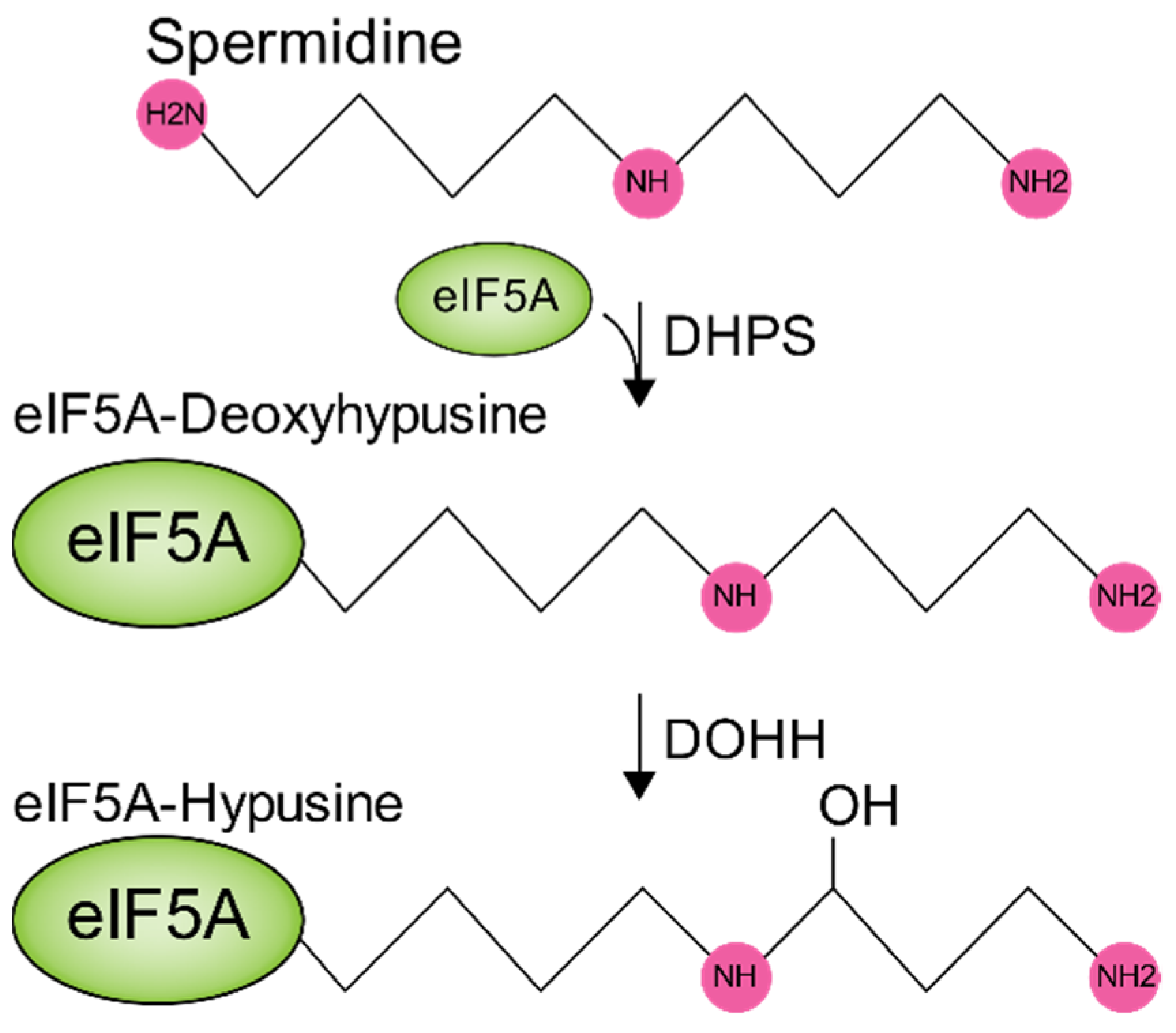
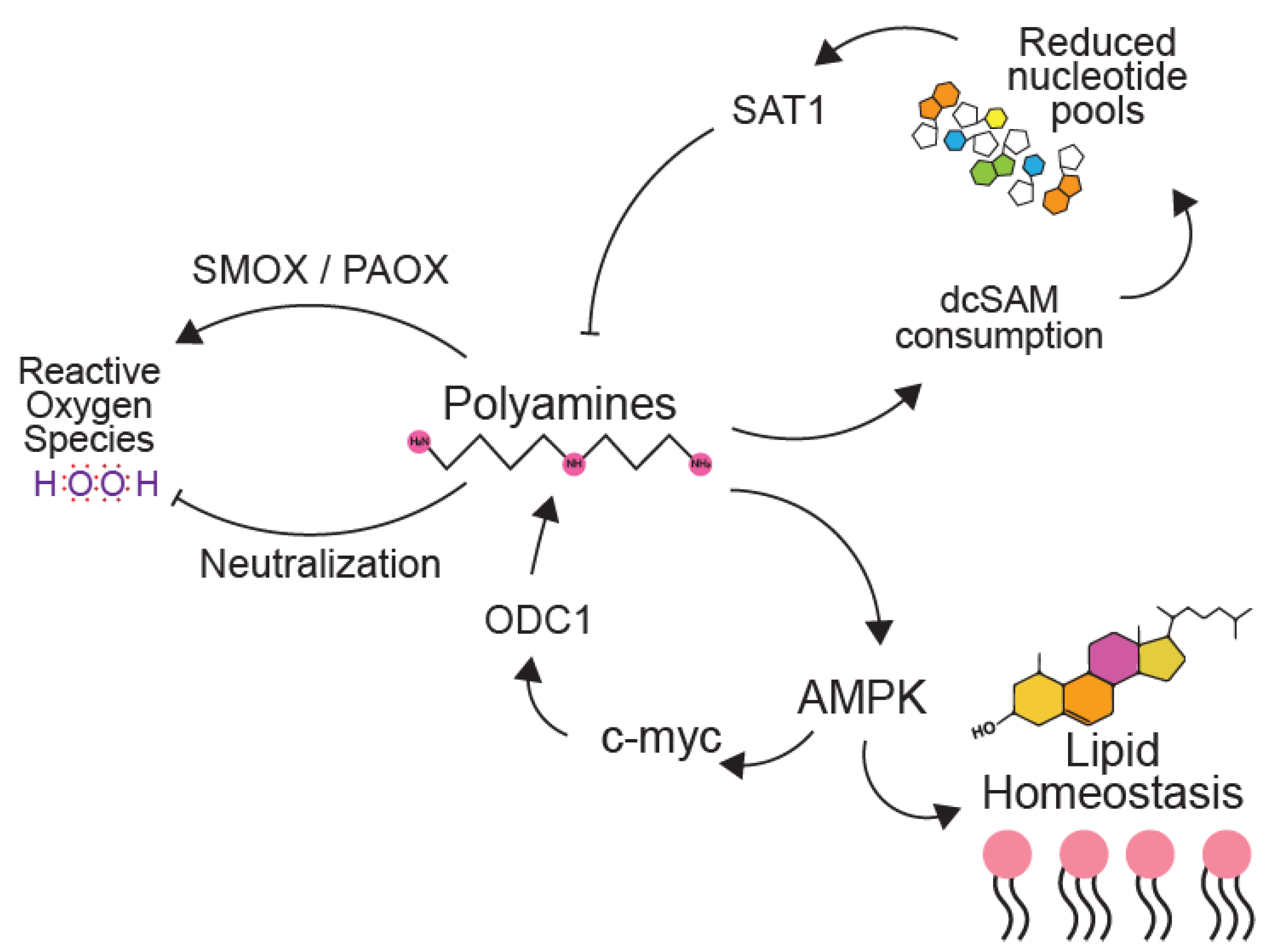
6. Polyamines in Metabolic Pathways Key to Virus Infection
7. Targeting Polyamines as an Antiviral Therapy
8. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Michael, A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 2016, 473, 2315–2329. [Google Scholar] [CrossRef]
- Law, G.L.; Raney, A.; Heusner, C.; Morris, D.R. Polyamine regulation of ribosome pausing at the upstream open reading frame of S-adenosylmethionine decarboxylase. J. Biol. Chem. 2001, 276, 38036–38043. [Google Scholar]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 995–1010. [Google Scholar] [CrossRef]
- Bauer, P.M.; Fukuto, J.M.; Buga, G.M.; Pegg, A.E.; Ignarro, L.J. Nitric Oxide Inhibits Ornithine Decarboxylase by S-Nitrosylation. Biochem. Biophys. Res. Commun. 1999, 262, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, O.A.; Bartosch, B.; Zakirova, N.F.; Kochetkov, S.N.; Ivanov, A.V. Polyamine Metabolism and Oxidative Protein Folding in the ER as ROS-Producing Systems Neglected in Virology. Int. J. Mol. Sci. 2018, 19, 1219. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Basu, H.S.; Sturkenboom, M.C.; Delcros, J.G.; Csokan, P.P.; Szollosi, J.; Feuerstein, B.G.; Marton, L.J. Effect of polyamine depletion on chromatin structure in U-87 MG human brain tumour cells. Biochem. J. 1992, 282, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Gallo, M.A.; Klinge, C.M.; Thomas, T.J. Polyamine-mediated conformational perturbations in DNA alter the binding of estrogen receptor to poly(dG-m5dC).poly(dG-m5dC) and a plasmid containing the estrogen response element. J. Steroid Biochem. Mol. Biol. 1995, 54, 89–99. [Google Scholar] [CrossRef]
- Kumar, N.; Basundra, R.; Maiti, S. Elevated polyamines induce c-MYC overexpression by perturbing quadruplex–WC duplex equilibrium. Nucleic Acids Res. 2009, 37, 3321–3331. [Google Scholar] [CrossRef]
- Nilsson, J.A.; Keller, U.B.; Baudino, T.A.; Yang, C.; Norton, S.; Old, J.A.; Nilsson, L.M.; Neale, G.; Kramer, D.L.; Porter, C.W.; et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 2005, 7, 433–444. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 Is a Critical Determinant of MYCN Oncogenesis and a Therapeutic Target in Neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef]
- Astrachan, L.; Miller, J.F. Cadaverine in Bacteriophage T4. J. Virol. 1973, 11, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Riemer, S.C.; Bloomfield, V.A. Packaging of DNA in bacteriophage Heads: Some considerations on energetics. Biopolymers 1978, 17, 785–794. [Google Scholar] [CrossRef]
- Young, D.V.; Srinivasan, P.R. Growth of Ribonucleic Acid Bacteriophage f2 in a Conditional Putrescine Auxotroph of Escherichia coli: Evidence for a Polyamine Role in Translation. J. Bacteriol. 1974, 117, 1280–1288. [Google Scholar] [CrossRef]
- Fukuma, I.; Cohen, S.S. Polyamine Synthesis and Accumulation in Escherichia coli Infected with Bacteriophage R17. J. Virol. 1973, 12, 1259–1264. [Google Scholar] [CrossRef]
- Fukuma, I. Synthesis of viral and rRNA in bacteriophage R17 infection of a stringent strain of Escherichia coli. J. Virol. 1975, 15, 1176–1181. [Google Scholar] [CrossRef]
- Dion, A.S.; Cohen, S.S. Polyamines in the Synthesis of Bacteriophage Deoxyribonucleic Acid II. Requirement for Polyamines in T4 Infection of a Polyamine Auxotroph. J. Virol. 1972, 9, 423–430. [Google Scholar] [CrossRef]
- Dion, A.S.; Cohen, S.S. Polyamines in the Synthesis of Bacteriophage Deoxyribonucleic Acid. I. Lack of Dependence of Polyamine Synthesis on Bacteriophage Deoxyribonucleic Acid Synthesis. J. Virol. 1972, 9, 419–422. [Google Scholar] [CrossRef]
- Balint, R.; Cohen, S.S. The incorporation of radiolabeled polyamines and methionine into turnip yellow mosaic virus in protoplasts from infected plants. Virology 1985, 144, 181–193. [Google Scholar] [CrossRef]
- Johnson, M.W.; Markham, R. Nature of the polyamine in plant viruses. Virology 1962, 17, 276–281. [Google Scholar] [CrossRef]
- Virudachalam, R.; Sitaramant, K.; Heusst, K.L.; Argost, P.; Markley, J.L. Carbon-13 and proton nuclear magnetic resonance spectroscopy of plant viruses: Evidence for protein-nucleic acid interactions in Belladonna mottle virus and detection of polyamines in turnip yellow mosaic virus. Virology 1983, 130, 360–371. [Google Scholar] [CrossRef]
- Beer, S.V.; Kosuge, T. Spermidine and spermine—Polyamine components of turnip yellow mosaic virus. Virology 1970, 40, 930–938. [Google Scholar] [CrossRef]
- Savithri, H.S.; Munshi, S.K.; Suryanarayana, S.; Divakar, S.; Murthy, M.R.N. Stability of Belladonna Mottle Virus Particles: The Role of Polyamines and Calcium. J. Gen. Virol. 1987, 68, 1533–1542. [Google Scholar] [CrossRef]
- Virudachalam, R.; Sitaraman, K.; Heuss, K.L.; Markley, J.L.; Argos, P. Evidence for pH-induced release of RNA from belladonna mottle virus and the stabilizing effect of polyamines and cations. Virology 1983, 130, 351–359. [Google Scholar] [CrossRef]
- Marini, F.; Betti, L.; Scaramagli, S.; Biondi, S.; Torrigiani, P. Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible, tobacco. New Phytol. 2001, 149, 301–309. [Google Scholar] [CrossRef]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of Hypersensitive Cell Death by Hydrogen Peroxide Produced through Polyamine Degradation in Tobacco Plants. Plant Physiol. 2003, 132, 1973–1981. [Google Scholar] [CrossRef]
- Baumann, S.; Sander, A.; Gurnon, J.R.; Yanai-Balser, G.M.; Van Etten, J.L.; Piotrowski, M. Chlorella viruses contain genes encoding a complete polyamine biosynthetic pathway. Virology 2007, 360, 209–217. [Google Scholar] [CrossRef]
- Morehead, T.A.; Gurnon, J.R.; Adams, B.; Nickerson, K.W.; Fitzgerald, L.A.; Van Etten, J.L. Ornithine Decarboxylase Encoded by Chlorella Virus PBCV-1. Virology 2002, 301, 165–175. [Google Scholar] [CrossRef][Green Version]
- Charlop-Powers, Z.; Jakoncic, J.; Gurnon, J.R.; Etten, J.L.V.; Zhou, M.-M. Paramecium bursaria Chlorella Virus 1 Encodes a Polyamine Acetyltransferase. J. Biol. Chem. 2012, 287, 9547–9551. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Vollmert, M.; Tholl, D.; Graves, M.V.; Gurnon, J.R.; Xing, W.; Lisec, A.D.; Nickerson, K.W.; Van Etten, J.L. Chlorella Virus PBCV-1 Encodes a Functional Homospermidine Synthase. Virology 1999, 263, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kay, D. The inhibition of bacteriophage multiplication by proflavine and its reversal by certain polyamines. Biochem. J. 1959, 73, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Roizman, B. Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc. Natl. Acad. Sci. USA 1971, 68, 2818–2821. [Google Scholar] [CrossRef]
- Isom, H.C. Stimulation of ornithine decarboxylase by human cytomegalovirus. J. Gen. Virol. 1979, 42, 265–278. [Google Scholar] [CrossRef]
- Tyms, A.S.; Williamson, J.D. Polyamine Metabolism in MRC-5 Cells Infected with Human Cytomegalovirus. J. Gen. Virol. 1980, 48, 183–191. [Google Scholar] [CrossRef]
- Tyms, A.S.; Williamson, J.D. Inhibitors of polyamine biosynthesis block human cytomegalovirus replication. Nature 1982, 297, 690–691. [Google Scholar] [CrossRef]
- Pohjanpelto, P.; Sekki, A.; Hukkanen, V.; von Bonsdorff, C.-H. Polyamine depletion of cells reduces the infectivity of herpes simplex virus but not the infectivity of sindbis virus. Life Sci. 1988, 42, 2011–2018. [Google Scholar] [CrossRef]
- Tuomi, K.; Mäntyjärvi, R.; Raina, A. Inhibition of Semliki Forest and herpes simplex virus production in alpha-difluoromethylornithine-treated cells: Reversal by polyamines. FEBS Lett. 1980, 121, 292–294. [Google Scholar] [CrossRef]
- Mastrodomenico, V.; Esin, J.J.; Graham, M.L.; Tate, P.M.; Hawkins, G.M.; Sandler, Z.J.; Rademacher, D.J.; Kicmal, T.M.; Dial, C.N.; Mounce, B.C. Polyamine depletion inhibits bunyavirus infection via generation of noninfectious interfering virions. J. Virol. 2019, 93, e00530-19. [Google Scholar] [CrossRef]
- Lanzer, W.; Holowczak, J.A. Polyamines in vaccinia virions and polypeptides released from viral cores by acid extraction. J. Virol. 1975, 16, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Fout, G.S.; Medappa, K.C.; Mapoles, J.E.; Rueckert, R.R. Radiochemical determination of polyamines in poliovirus and human rhinovirus 14. J. Biol. Chem. 1984, 259, 3639–3643. [Google Scholar] [PubMed]
- Mounce, B.C.; Poirier, E.Z.; Passoni, G.; Simon-Loriere, E.; Cesaro, T.; Prot, M.; Stapleford, K.A.; Moratorio, G.; Sakuntabhai, A.; Levraud, J.-P.; et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Korovina, A.N.; Tunitskaya, V.L.; Khomutov, M.A.; Simonian, A.R.; Khomutov, A.R.; Ivanov, A.V.; Kochetkov, S.N. Biogenic polyamines spermine and spermidine activate RNA polymerase and inhibit RNA helicase of hepatitis C virus. Biochem. Biokhimiia 2012, 77, 1172–1180. [Google Scholar] [CrossRef]
- Wallace, H.M.; Baybutt, H.N.; Pearson, C.K.; Keir, H.M. The Effect of Polyamines on Herpes Simplex Virus Type 1 DNA Polymerase Purified from Infected Baby Hamster Kidney Cells (BHK-21/C13). J. Gen. Virol. 1980, 49, 397–400. [Google Scholar] [CrossRef]
- Frugier, M.; Florentz, C.; Hosseini, M.W.; Lehn, J.M.; Giegé, R. Synthetic polyamines stimulate in vitro transcription by T7 RNA polymerase. Nucleic Acids Res. 1994, 22, 2784–2790. [Google Scholar] [CrossRef]
- Masalova, O.V.; Lesnova, E.I.; Samokhvalov, E.I.; Permyakova, K.Y.; Ivanov, A.V.; Kochetkov, S.N.; Kushch, A.A. Low-molecular-weight regulators of biogenic polyamine metabolism affect cytokine production and expression of hepatitis C virus proteins in Huh7.5 human hepatocarcinoma cells. Mol. Biol. 2017, 51, 453–464. [Google Scholar] [CrossRef]
- Olsen, M.E.; Filone, C.M.; Rozelle, D.; Mire, C.E.; Agans, K.N.; Hensley, L.; Connor, J.H. Polyamines and Hypusination Are Required for Ebolavirus Gene Expression and Replication. mBio 2016, 7, e00882-16. [Google Scholar] [CrossRef]
- Olsen, M.E.; Cressey, T.N.; Mühlberger, E.; Connor, J.H. Differential Mechanisms for the Involvement of Polyamines and Hypusinated eIF5A in Ebola Virus Gene Expression. J. Virol. 2018, 92, e01260-18. [Google Scholar] [CrossRef]
- Kicmal, T.M.; Tate, P.M.; Dial, C.N.; Esin, J.J.; Mounce, B.C. Polyamine depletion abrogates enterovirus cellular attachment. J. Virol. 2019, 93, e01054-19. [Google Scholar] [CrossRef]
- Dial, C.N.; Tate, P.M.; Kicmal, T.M.; Mounce, B.C. Coxsackievirus B3 Responds to Polyamine Depletion via Enhancement of 2A and 3C Protease Activity. Viruses 2019, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Moratorio, G.; Hooikaas, P.J.; Yakovleva, A.; Werneke, S.W.; Smith, E.C.; Poirier, E.Z.; Simon-Loriere, E.; Prot, M.; et al. Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J. Virol. 2016, 90, 9683–9692. [Google Scholar] [CrossRef] [PubMed]
- Raniga, K.; Liang, C. Interferons: Reprogramming the Metabolic Network against Viral Infection. Viruses 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Atmar, V.J.; Joshi, A.R.; Krim, M.; Kuehn, G.D. Inhibition of ornithine decarboxylase in human fibroblast cells by type I and type II interferons. Biochem. Biophys. Res. Commun. 1983, 114, 950–954. [Google Scholar] [CrossRef]
- Shi, M.; Gan, Y.; Davis, T.O.; Scott, R.S. Downregulation of the Polyamine Regulator Spermidine/Spermine N1-Acetyltransferase by Epstein-Barr Virus in a Burkitt’s Lymphoma Cell Line. Virus Res. 2013, 177, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, B.G.; Murakami, M.; Cai, Q.; Verma, S.C.; Lan, K.; Robertson, E.S. Epstein-Barr Virus Nuclear Antigen 3C Interacts with and Enhances the Stability of the c-Myc Oncoprotein. J. Virol. 2008, 82, 4082–4090. [Google Scholar] [CrossRef]
- Greco, A.; Callé, A.; Morfin, F.; Thouvenot, D.; Cayre, M.; Kindbeiter, K.; Martin, L.; Levillain, O.; Diaz, J.-J. S-adenosyl methionine decarboxylase activity is required for the outcome of herpes simplex virus type 1 infection and represents a new potential therapeutic target. FASEB J. 2005, 19, 1128–1130. [Google Scholar] [CrossRef]
- Jia, J.; Delhon, G.; Tulman, E.; Diel, D.; Osorio, F.; Wen, X.; Kutish, G.; Rock, D. Novel Gammaherpesvirus Functions 1 Encoded by Bovine herpesvirus (Bovine Lymphotropic virus). J. Gen. Virol. 2014, 95, 1790–1798. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Keinanen, T.A.; Ivanova, O.N.; Hyvonen, M.T.; Khomutov, A.R.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Hepatitis C virus alters metabolism of biogenic polyamines by affecting expression of key enzymes of their metabolism. Biochem. Biophys. Res. Commun. 2017, 483, 904–909. [Google Scholar] [CrossRef]
- Oredsson, S.M.; Kanje, M.; Mamont, P.S.; Wagner, J.; Heby, O. Polyamine depletion increases cellular ribonucleotide levels. Mol. Cell. Biochem. 1986, 70, 89–96. [Google Scholar] [CrossRef]
- Witherspoon, M.; Chen, Q.; Kopelovich, L.; Gross, S.S.; Lipkin, S.M. Unbiased metabolite profiling indicates that a diminished thymidine pool is the underlying mechanism of colon cancer chemoprevention by alpha-difluoromethylornithine. Cancer Discov. 2013, 3, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-Spectrum Antiviral Activity of Virazole: 1-f8- D-Ribofuranosyl- 1,2,4-triazole- 3-carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef]
- Tate, P.M.; Mastrodomenico, V.; Mounce, B.C. Ribavirin Induces Polyamine Depletion via Nucleotide Depletion to Limit Virus Replication. Cell Rep. 2019, 28, 2620–2633.e4. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, L.; Rao, J.N.; Marasa, B.S.; Chen, J.; Xiao, L.; Zhou, H.; Gorospe, M.; Wang, J.-Y. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin α1. Biochem. J. 2008, 409, 389–398. [Google Scholar] [CrossRef]
- Passariello, C.L.; Gottardi, D.; Cetrullo, S.; Zini, M.; Campana, G.; Tantini, B.; Pignatti, C.; Flamigni, F.; Guarnieri, C.; Caldarera, C.M.; et al. Evidence that AMP-activated protein kinase can negatively modulate Ornithine decarboxylase activity in cardiac myoblasts. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Kim, M.-M. Inhibitory Effect of Spermidine with Antioxidant Activity on Oxidative Stress in Human Dermal Fibroblasts. J. Life Sci. 2011, 21, 693–699. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- de Mochel, N.S.R.; Seronello, S.; Wang, S.H.; Ito, C.; Zheng, J.X.; Liang, T.J.; Lambeth, J.D.; Choi, J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology 2010, 52, 47–59. [Google Scholar] [CrossRef]
- Pal, S.; Polyak, S.J.; Bano, N.; Qiu, W.C.; Carithers, R.L.; Shuhart, M.; Gretch, D.R.; Das, A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J. Gastroenterol. Hepatol. 2010, 25, 627–634. [Google Scholar] [CrossRef]
- Akaike, T.; Noguchi, Y.; Ijiri, S.; Setoguchi, K.; Suga, M.; Zheng, Y.M.; Dietzschold, B.; Maeda, H. Pathogenesis of influenza virus-induced pneumonia: Involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 1996, 93, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dosal, R.; Horan, K.A.; Rahbek, S.H.; Ichijo, H.; Chen, Z.J.; Mieyal, J.J.; Hartmann, R.; Paludan, S.R. HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6. PLoS Pathog. 2011, 7, e1002250. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.I.; Heider, H.; Schroeder, C. Different modes of inhibition by adamantane amine derivatives and natural polyamines of the functionally reconstituted influenza virus M2 proton channel protein. J. Gen. Virol. 1997, 78, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Meyskens, F.L. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Lianos, G.D.; Ragos, V.; Galani, V.; Kyritsis, A.P. Difluoromethylornithine in cancer: New advances. Future Oncol. 2017, 13, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Brun, R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003, 90 (Suppl. 1), S49–S52. [Google Scholar] [CrossRef]
- Bouteille, B.; Buguet, A. The detection and treatment of human African trypanosomiasis. Res. Rep. Trop. Med. 2012, 3, 35–45. [Google Scholar] [CrossRef]
- Milord, F.; Pépin, J.; Loko, L.; Ethier, L.; Mpia, B. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 1992, 340, 652–655. [Google Scholar] [CrossRef]
- McCann, P.P.; Bacchi, C.J.; Clarkson, A.B., Jr.; Seed, J.R.; Nathan, H.C.; Amole, B.O.; Hutner, S.H.; Sjoerdsma, A. Further studies on difluoromethylornithine in African trypanosomes. Med. Biol. 1981, 59, 434–440. [Google Scholar]
- WHO|WHO Model Lists of Essential Medicines. Available online: http://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 6 March 2020).
- Meyskens, F.L.; Kingsley, E.M.; Glattke, T.; Loescher, L.; Booth, A. A phase II study of α-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest. New Drugs 1986, 4, 257–262. [Google Scholar] [CrossRef]
- Goyal, L.; Supko, J.G.; Berlin, J.; Blaszkowsky, L.S.; Carpenter, A.; Heuman, D.M.; Hilderbrand, S.L.; Stuart, K.E.; Cotler, S.; Senzer, N.N.; et al. Phase 1 study of N(1),N(11)-diethylnorspermine (DENSPM) in patients with advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2013, 72, 1305–1314. [Google Scholar] [CrossRef]
- Wolff, A.C.; Armstrong, D.K.; Fetting, J.H.; Carducci, M.K.; Riley, C.D.; Bender, J.F.; Casero, R.A.; Davidson, N.E. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003, 9, 5922–5928. [Google Scholar] [PubMed]
- Seiler, N.; Duranton, B.; Raul, F. The polyamine oxidase inactivator MDL 72527. Prog. Drug Res. Fortschritte Arzneimittelforschung Progres Rech. Pharm. 2002, 59, 1–40. [Google Scholar]
- Olsen, M.E.; Connor, J.H. Hypusination of eIF5A as a Target for Antiviral Therapy. DNA Cell Biol. 2017, 36, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Hanauske-Abel, H.M.; Palumbo, P.; Saxena, D.; D’Alliessi Gandolfi, D.; Park, M.H.; Pe’ery, T.; Mathews, M.B. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology 2009, 6, 90. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firpo, M.R.; Mounce, B.C. Diverse Functions of Polyamines in Virus Infection. Biomolecules 2020, 10, 628. https://doi.org/10.3390/biom10040628
Firpo MR, Mounce BC. Diverse Functions of Polyamines in Virus Infection. Biomolecules. 2020; 10(4):628. https://doi.org/10.3390/biom10040628
Chicago/Turabian StyleFirpo, Mason R., and Bryan C. Mounce. 2020. "Diverse Functions of Polyamines in Virus Infection" Biomolecules 10, no. 4: 628. https://doi.org/10.3390/biom10040628
APA StyleFirpo, M. R., & Mounce, B. C. (2020). Diverse Functions of Polyamines in Virus Infection. Biomolecules, 10(4), 628. https://doi.org/10.3390/biom10040628




