Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
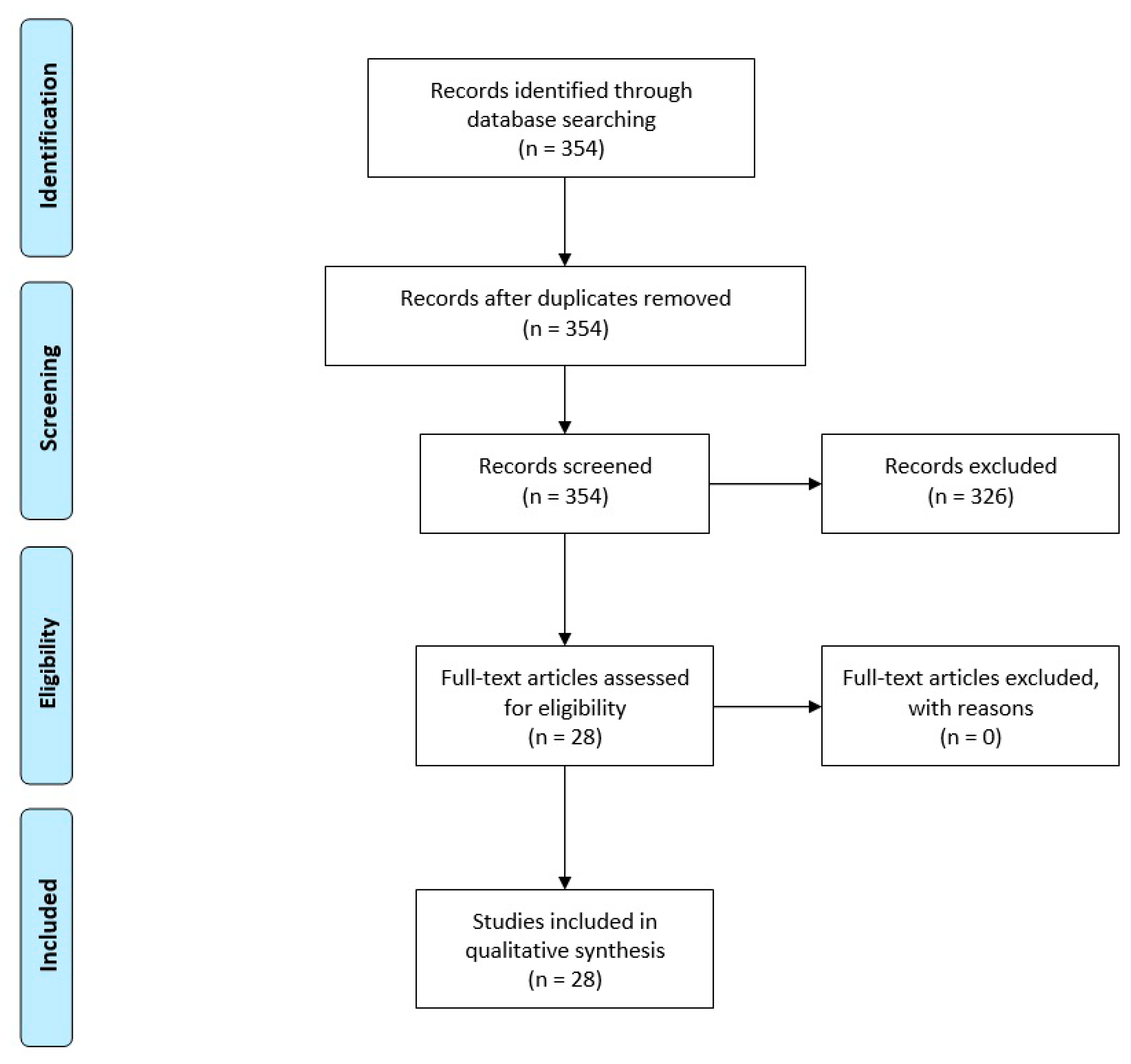
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6OHDA | 6-hydroxydopamine |
| α-syn | alpha synuclein |
| CPu | caudate putamen |
| CXCR4 | chemokine receptor 4 |
| CW | continuous wave |
| DMS | delayed match-to-sample |
| GDNF | glial cell line-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GFAP+ | glial fibrillary acidic protein positive |
| GSH-Px | glutathione peroxidase |
| IBA1 | ionized calcium-binding adapter molecule 1 |
| IFN-γ | interferon-gamma |
| IL2 | Interleukin 2 |
| LED | light-emitting diode |
| MDA | malondialdehyde |
| MPTP | 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine |
| NA | not available |
| NR | not reported |
| PaG | periaqueductal grey matter |
| PBM | photobiomodulation |
| PD | Parkinson’s disease |
| PVT | psychomotor vigilance task |
| SNc | substantia nigra pars compacta |
| STN | subthalamic nucleus |
| TH | tyrosine hydroxylase |
| TNF-α | tumor necrosis factor-alpha |
| WT | wild type |
| ZI | zona incerta |
| ZI-Hyp | zona incerta-hypothalamus |
References
- Elsworth, J.D. Parkinson’s Disease Treatment: Past, Present, and Future. J. Neural Transm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Toffoli, M.; Vieira, S.R.L.; Schapira, A.H.V. Genetic causes of PD: A pathway to disease modification. Neuropharmacology 2020, 170, 108022. [Google Scholar] [CrossRef] [PubMed]
- Zaia, A.; Maponi, P.; Zannotti, M.; Casoli, T. Biocomplexity and Fractality in the Search of Biomarkers of Aging and Pathology: Mitochondrial DNA Profiling of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1758. [Google Scholar] [CrossRef] [PubMed]
- Bullich, C.; Keshavarzian, A.; Garssen, J.; Kraneveld, A.; Perez-Pardo, P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov. Disord. Clin. Pr. 2019, 6, 639–651. [Google Scholar] [CrossRef]
- Parkinson’s Disease Economic Burden On Patients, Families And The Federal Government Is $52 Billion, Doubling Previous Estimates. Available online: https://www.prnewswire.com/news-releases/parkinsons-disease-economic-burden-on-patients-families-and-the-federal-government-is-52-billion-doubling-previous-estimates-300867192.html (accessed on 16 March 2020).
- De Bie, R.M.A.; Clarke, C.E.; Espay, A.J.; Fox, S.H.; Lang, A.E. Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet Neurol. 2020. [Google Scholar] [CrossRef]
- Merola, A.; Romagnolo, A.; Krishna, V.; Pallavaram, S.; Carcieri, S.; Goetz, S.; Mandybur, G.; Duker, A.P.; Dalm, B.; Rolston, J.D.; et al. Current Directions in Deep Brain Stimulation for Parkinson’s Disease-Directing Current to Maximize Clinical Benefit. Neurol. Ther. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2011, 40, 516–533. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Mester, A.; Mester, A. The History of Photobiomodulation: Endre Mester (1903–1984). Photomed. Laser Surg. 2017, 35, 393–394. [Google Scholar] [CrossRef]
- Moskvin, S.V. Only lasers can be used for low level laser therapy. Biomedicine 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Whelan, H.T.; Eells, J.T.; Meng, H.; Buchmann, E.; Lerch-Gaggl, A.; Wong-Riley, M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience 2006, 139, 639–649. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. Photomedicine and Stem Cells; Morgan & Claypool Publishers: San Rafael, CA, USA, 2017. [Google Scholar]
- De Sousa, K.; Rodrigues, M.; de Santos, D.; Mesquita-Ferrari, R.A.; Nunes, F.D.; da Silva, D.d.T.; Bussadori, S.K.; Fernandes, K.P.S. Differential expression of inflammatory and anti-inflammatory mediators by M1 and M2 macrophages after photobiomodulation with red or infrared lasers. Lasers Med Sci. 2019, 35, 337–343. [Google Scholar] [CrossRef]
- Xuan, W.; Agrawal, T.; Huang, L.; Gupta, G.K.; Hamblin, M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics 2015, 8, 502–511. [Google Scholar] [CrossRef]
- Xuan, W.; Vatansever, F.; Huang, L.; Hamblin, M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014, 19, 108003. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 2017, 96, 731–743. [Google Scholar] [CrossRef]
- Thunshelle, C.; Hamblin, M.R. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomed. Laser Surg. 2016, 34, 587–598. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.L.; el Khoury, H.; Hamilton, D.; Nicklason, F.; Mitrofanis, J. “Buckets”: Early Observations on the Use of Red and Infrared Light Helmets in Parkinson’s Disease Patients. Photobiomodul. Photomed. Laser Surg. 2019, 37, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.; el Massri, N.; Moro, C.; Spana, S.; Wang, X.; Torres, N.; Chabrol, C.; de Jaeger, X.; Reinhart, F.; Purushothuman, S. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism–an abscopal neuroprotective effect. Neuroscience 2014, 274, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Dache, Z.A.A.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef]
- Salehpour, F.; Cassano, P.; Rouhi, N.; Hamblin, M.R.; de Taboada, L.; Farajdokht, F.; Mahmoudi, J. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul. Photomed. Laser Surg. 2019, 37, 581–595. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef]
- Shaw, V.E.; Spana, S.; Ashkan, K.; Benabid, A.L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010, 518, 25–40. [Google Scholar] [CrossRef]
- Peoples, C.; Shaw, V.E.; Stone, J.; Jeffery, G.; Baker, G.E.; Mitrofanis, J. Survival of dopaminergic amacrine cells after near-infrared light treatment in MPTP-treated mice. ISRN Neurol. 2012, 2012, 850150. [Google Scholar] [CrossRef]
- Shaw, V.E.; Peoples, C.; Spana, S.; Ashkan, K.; Benabid, A.-L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Patterns of cell activity in the subthalamic region associated with the neuroprotective action of near-infrared light treatment in MPTP-treated mice. Park. Dis. 2012, 2012, 296875. [Google Scholar] [CrossRef]
- Peoples, C.; Spana, S.; Ashkan, K.; Benabid, A.-L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson’s disease. Park. Relat. Disord. 2012, 18, 469–476. [Google Scholar] [CrossRef]
- Moro, C.; Torres, N.; el Massri, N.; Ratel, D.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; Benabid, A.-L. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: Evidence from two mouse strains. BMC Neurosci. 2013, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Purushothuman, S.; Nandasena, C.; Johnstone, D.M.; Stone, J.; Mitrofanis, J. The impact of near-infrared light on dopaminergic cell survival in a transgenic mouse model of parkinsonism. Brain Res. 2013, 1535, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Lovisa, B.; Geens, A.; Morais, V.A.; Wagnières, G.; van den Bergh, H.; Ginggen, A.; de Strooper, B.; Tardy, Y.; Verstreken, P. Near-infrared 808 nm light boosts complex IV-dependent respiration and rescues a Parkinson-related pink1 model. PLoS ONE 2013, 8, e78562. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Sutalangka, C. Laser acupuncture at HT7 acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of parkinson’s disease. Evidence-Based Complement. Altern. Med. 2014, 2014, 937601. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, F.; El Massri, N.; Darlot, F.; Torres, N.; Johnstone, D.M.; Chabrol, C.; Costecalde, T.; Stone, J.; Mitrofanis, J.; Benabid, A.-L. 810 nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neurosci. Res. 2015, 92, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Darlot, F.; Moro, C.; El Massri, N.; Chabrol, C.; Johnstone, D.M.; Reinhart, F.; Agay, D.; Torres, N.; Bekha, D.; Auboiroux, V. Near-infrared light is neuroprotective in a monkey model of P arkinson disease. Ann. Neurol. 2016, 79, 59–75. [Google Scholar] [CrossRef]
- Oueslati, A.; Lovisa, B.; Perrin, J.; Wagnières, G.; van den Bergh, H.; Tardy, Y.; Lashuel, H.A. Photobiomodulation suppresses alpha-synuclein-induced toxicity in an AAV-based rat genetic model of Parkinson’s disease. PLoS ONE 2015, 10, e0140880. [Google Scholar] [CrossRef]
- Moro, C.; el Massri, N.; Darlot, F.; Torres, N.; Chabrol, C.; Agay, D.; Auboiroux, V.; Johnstone, D.M.; Stone, J.; Mitrofanis, J. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016, 1648, 19–26. [Google Scholar] [CrossRef]
- Salgado, A.S.; Ribeiro, L.G.; Oliveira, T.B.; Rolão, M.P.; Gomes, J.C.; Carraro, E.; Perreira, M.C.; Suckow, P.T.; Kerppers, I.I. Effects of Light Emitting Diode and Low-intensity Light on the immunological process in a model of Parkinson’s disease. Med Res. Arch. 2017, 4. Issue 8, December, 2016. [Google Scholar] [CrossRef]
- Reinhart, F.; el Massri, N.; Chabrol, C.; Cretallaz, C.; Johnstone, D.M.; Torres, N.; Darlot, F.; Costecalde, T.; Stone, J.; Mitrofanis, J. Intracranial application of near-infrared light in a hemi-parkinsonian rat model: The impact on behavior and cell survival. J. Neurosurg. 2016, 124, 1829–1841. [Google Scholar] [CrossRef]
- Reinhart, F.; El Massri, N.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; Benabid, A.-L.; Moro, C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp. Brain Res. 2016, 234, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- El Massri, N.; Johnstone, D.M.; Peoples, C.L.; Moro, C.; Reinhart, F.; Torres, N.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. The effect of different doses of near infrared light on dopaminergic cell survival and gliosis in MPTP-treated mice. Int. J. Neurosci. 2015, 126, 76–87. [Google Scholar] [CrossRef] [PubMed]
- El Massri, N.; Lemgruber, A.P.; Rowe, I.J.; Moro, C.; Torres, N.; Reinhart, F.; Chabrol, C.; Benabid, A.-L.; Mitrofanis, J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res. 2017, 235, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, F.; El Massri, N.; Torres, N.; Chabrol, C.; Molet, J.; Johnstone, D.M.; Stone, J.; Benabid, A.-L.; Mitrofanis, J.; Moro, C. The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2017, 117, 42–47. [Google Scholar] [CrossRef]
- El Massri, N.; Cullen, K.M.; Stefani, S.; Moro, C.; Torres, N.; Benabid, A.-L.; Mitrofanis, J. Evidence for encephalopsin immunoreactivity in interneurones and striosomes of the monkey striatum. Exp. Brain Res. 2018, 236, 955–961. [Google Scholar] [CrossRef]
- Kim, B.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Remote tissue conditioning is neuroprotective against MPTP insult in mice. IBRO Rep. 2018, 4, 14–17. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Austin, P.J. Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat. Biomolecules 2019, 9, 381. [Google Scholar] [CrossRef]
- Miguel, M.S.; Martin, K.L.; Stone, J.; Johnstone, D.M. Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP. Biomolecules 2019, 9, 564. [Google Scholar] [CrossRef]
- Ganeshan, V.; Skladnev, N.V.; Kim, J.Y.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Pre-conditioning with remote photobiomodulation modulates the brain transcriptome and protects against MPTP insult in mice. Neuroscience 2019, 400, 85–97. [Google Scholar] [CrossRef]
- Langston, J.W.; Palfreman, J. The Case of the Frozen Addicts: How the Solution of a Medical Mystery Revolutionized the Understanding of Parkinson’s Disease; IOS Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gubellini, P.; Kachidian, P. Animal models of Parkinson’s disease: An updated overview. Rev. Neurol. 2015, 171, 750–761. [Google Scholar] [CrossRef]
- Bezchlibnyk, Y.B.; Sharma, V.D.; Naik, K.B.; Isbaine, F.; Gale, J.T.; Cheng, J.; Triche, S.D.; Miocinovic, S.; Buetefisch, C.M.; Willie, J.T.; et al. Clinical outcomes of globus pallidus deep brain stimulation for Parkinson disease: A comparison of intraoperative MRI- and MER-guided lead placement. J. Neurosurg. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.; Dastolfo-Hromack, C.; Lipski, W.J.; Kratter, I.H.; Smith, L.J.; Gartner-Schmidt, J.L.; Richardson, R.M. Anterior Sensorimotor Subthalamic Nucleus Stimulation Is Associated With Improved Voice Function. Neurosurgery 2020, 2020, nyaa024. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, L.; Blomstedt, P.; Karlsson, F.; Hartelius, L. The Effects of Deep Brain Stimulation on Speech Intelligibility in Persons With Essential Tremor. J. Speech, Lang. Hear. Res. 2020, 63, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Xuan, W.; Xu, T.; Dai, T.; Sharma, S.K.; Kharkwal, G.B.; Huang, Y.Y.; Wu, Q.; Whalen, M.J.; Sato, S.; et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS ONE 2011, 6, e26212. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.; Hamilton, D.; Nicklason, F.; el Massri, N.; Mitrofanis, J. Exploring the use of transcranial photobiomodulation in Parkinson’s disease patients. Neural Regen. Res. 2018, 13, 1738–1740. [Google Scholar] [PubMed]
- Santos, L.; Olmo-Aguado, S.D.; Valenzuela, P.L.; Winge, K.; Iglesias-Soler, E.; Arguelles-Luis, J.; Alvarez-Valle, S.; Parcero-Iglesias, G.J.; Fernandez-Martinez, A.; Lucia, A. Photobiomodulation in Parkinson’s disease: A randomized controlled trial. Brain Stimul. 2019, 12, 810–812. [Google Scholar] [CrossRef] [PubMed]

| Study/Year | Animal/Species (n) | Gender/Age | PD Model | Light Source | Output Power | Irradiance | Irradiation Time per Session | Fluence or Dose per Session | Total Fluence or Dose | Irradiation Approach/Sites | Number of Treatment Sessions | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shaw et al., (2010) [29] | Mouse Albino BALB/c (n Saline = 20) (n Saline + PBM = 20) (n MPTP = 20) (n MPTP + PBM = 20) | Male 8 weeks old | MPTP Mild: 50 mg/kg per mouse Strong: 100 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | 14.4 J/cm2 (at scalp) | Transcranial Holding probe at 1 cm from the head | 4 simultaneous irradiations over 30 h | Increased TH+ terminals in the caudate-putamen complex; no effect on the overall volume of the SNc and ZI-Hyp; increased TH+ cells in the SNc and ZI-Hyp regions; no effect on the morphology of TH+ cells in both the SNc and ZI-Hyp; increased number of TH+ cells in the SNc (in both 50 and 100 mg/kg MPTP doses); no effect on the number of TH+ cells in the ZI-Hyp (in 50 and 100 mg/kg MPTP doses) |
| Peoples et al., (2012) [30] | Mouse Albino BALB/c (n Saline = 20) (n Saline + PBM = 20) (n MPTP = 20) (n MPTP+PBM = 20) | Male 8 weeks old | MPTP: 200 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Simultaneous group: 36 J/cm2 (at scalp) Post-treatment group: 36 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | Simultaneous group: 10 irradiations over 5 weeks Post-treatment group: 10 irradiations over 3 weeks | For both simultaneous and post-treatment series: increased TH+ cell number in the SNc, but not in the PaG and ZI-Hyp regions |
| Shaw et al., (2012) [31] | Mouse Albino BALB/c (n Saline = 24) (n Saline + PBM = 24) (n MPTP = 24) (n MPTP + PBM = 24) | Male 8 weeks old | MPTP Acute: 100 mg/kg per mouse Chronic: 200 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Acute regimen: 14.4 J/cm2 (at scalp) Chronic regimen: 36 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | Acute regimen: 4 simultaneous irradiations over 30 h Chronic regimen: 10 simultaneous irradiations over 5 weeks | For acute regimen: decreased Fos+ cell number in the STN and ZI regions in group with six-day survival period For chronic regimen: decreased Fos+ cell number in the STN and ZI regions |
| Peoples et al., (2012) [32] | Mouse Albino BALB/c (n Saline = 21) (n Saline + PBM = 19) (n MPTP = 22) (n MPTP + PBM = 18) | Male 8 weeks old | MPTP Acute: 100 mg/kg per mouse Chronic: 200 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Simultaneous acute group: 14.4 J/cm2 (at scalp) Simultaneous chronic group: 36 J/cm2 (at scalp) Post-treatment acute group: 14.4 J/cm2 (at scalp) Post-treatment chronicgroup: 36 J/cm2 (at scalp) | TranscranialJust above the mouse head and in full view of the eyes | Simultaneous group: 4 irradiations over 30 h (acute regimen) or 10 irradiations over 5 weeks (chronic regimen) Post-treatment group: 4 irradiations over 2 days (acute regimen) or 10 irradiations over 3 weeks (chronic regimen) | For all group and regimens: no effect on the retinal areas For all groups except simultaneous group with acute regimen: increased TH+ cell number in the retina |
| Moro et al., (2013) [33] | Mouse Albino BALB/c: (n Saline = 10) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM = 10) Black C57BL/6: (n Saline = 10) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM = 10) | Male 8–10 weeks old | MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | 14.4 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | 4 simultaneous irradiations over 30 h | For Albino BALB/c mice: increased TH+ cell number in the SNc; improved locomotor activities via increase of velocity and high mobility, and decrease of immobility For C57BL/6 mice: no effect on the TH+ cell number in the SNc; no effect on the locomotor activities |
| Purushothuman et al., (2013) [34] | Mouse K3 transgenic model (n WT = 5) (n K3 = 5) (n K3 + PBM = 5) | NR 5 months old | K369I tau transgenic model | LED 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) | 80 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | 20 irradiations over 4 weeks | Decreased markers of oxidative stress, over expression of hyperphosphorylated tau, and increased TH+ cell number in the SNc |
| Vos et al., (2013) [35] | Drosophila Pink1 null mutants | NA | Rotenone (250 μM) | Laser, 808 nm | NR | 25 mW/cm2 | 100 s | 2.5 J/cm2 | 2.5 J/cm2 | Whole-body | One session (single dose) | Improved CCO-dependent oxygen consumption and ATP production; rescued major systemic and mitochondrial defects |
| Wattanathorn and Sutalangka, (2014) [36] | Rat Albino Wistar (n Control = 12) (n 6OHDA = 12) (n 6OHDA + Sham PBM = 12) (n 6OHDA + Sham PBM = 12) | Male 8 weeks old | 6OHDA (6 μg per rat) | Laser, 405 nm | 100 mW | NR | 10 min | NR | NR | Laser acupuncture at HT7 acupoint | Once daily for 14 days | Improved spatial memory in Morris water maze test; attenuated the decreased neuron density in CA3 and dentate gyrus, but not CA1 and CA2 regions; decreased activity of monoamine oxidase-B and acetylcholinesterase in the hippocampus; mitigated the decreased GSH-Px activity and the elevation of MDA level |
| Johnstone et al., (2014) [25] | Mouse Albino BALB/c: 50 mg/kg MPTP: (n MPTP = 36) (n MPTP + Transcranial PBM = 12) (n MPTP + Remote PBM = 11) 75 mg/kg MPTP: (n MPTP = 8) (n MPTP + Transcranial PBM = 8) (n MPTP + Remote PBM = 8) 100 mg/kg MPTP: (n MPTP = 9) (n MPTP + Transcranial PBM = 19) (n MPTP + Remote PBM = 9) | Male 8 weeks old | MPTP: 50 mg/kg per mouse 75 mg/kg per mouse 100 mg/kg per mouse | LED, 670 nm | NR | 50 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) | 50 mg/kg MPTP: 8 J/cm2 (at scalp) 75 mg/kg MPTP: 12 J/cm2 (at scalp) 100 mg/kg MPTP: 16 J/cm2 (at scalp) | Transcranial irradiation to the head Remote irradiation to the dorsum | 50 mg/kg MPTP: 2 irradiations over 2 days 75 mg/kg MPTP: 3 irradiations over 3 days 100 mg/kg MPTP: 4 irradiations over 4 days | In 50 but not 75 or 100 mg/kg MPTP doses: increased TH+ cell number in the SNc with both transcranial and remote irradiations |
| Moro et al., (2014) [25] | Mouse Albino BALB/c (n Saline = 5) (n Saline + Pulse PBM = 5) (n Saline + Continuous PBM = 5) (n MPTP = 5) (n MPTP + Pulse PBM = 5) (n MPTP + Continuous PBM = 5) | Male NR | MPTP: 50 mg/kg per mouse | LEDs, 670 nm | 0.16 mW | Pulse irradiation 1.5 mW/cm2 Continuous irradiation 14.5 mW/cm2 | Pulse irradiation: 90 s Continuous irradiation: 6 days continuously | Pulse irradiation: 0.13 J/cm2 Continuous irradiation: 7516.8 J/cm2 | Pulse irradiation: 0.54 J/cm2 Continuous irradiation: 7516.8 J/cm2 | Intracranial, implanted in the lateral ventricles | Pulse irradiation: 4 simultaneous irradiations over 30 h Continuous irradiation: 6 days continuously | For pulse irradiation group: significantly increased TH+ cell number in the SNc For continuous irradiation group: Non-significantly increased TH+ cell number in the SNc |
| Reinhart et al., (2015) [37] | Mouse Albino BALB/c (n Saline = 11) (n Saline + PBM = 11) (n MPTP = 11) (n MPTP + PBM = 11) | Male 8–10 weeks old | MPTP: 50 mg/kg per mouse | LEDs, 810 nm | 0.16 mW | NR | 90 s | 14.4 mJ (at scalp) | 57.6 mJ (at scalp) | Transcranial | 4 simultaneous irradiations over 30 h | Improved locomotor activity at different time points including at immediately after first MPTP injection, at after sond PBM, at after fourth PBM, and 6 days after the last MPTP injection; increased TH+ cell number in the SNc |
| Darlot et al., (2015) [38] | Macaque monkey Macaca fascicularis (n Control = 5) (n MPTP (1.5 mg/kg) = 6) (n MPTP (2.1 mg/kg) = 5) (n MPTP (1.5 mg/kg) + PBM = 5) (n MPTP (2.1 mg/kg) + PBM = 4) | Male 4–5 years old | MPTP: 1.5 mg/kg per monkey 2.1 mg/kg per monkey | Laser, 670 nm | 10 mW | NR | MPTP (1.5 mg/kg) continuous irradiation (5 s ON/60 s OFF) for 5 days MPTP (2.1 mg/kg) continuous irradiation (5 s ON/60 s OFF) for 7 days | NA | MPTP (1.5 mg/kg): 25 J MPTP (2.1 mg/kg): 35 J | Intracranial, Implanted 1 to 2 mm to the left side of the midline in the midbrain | MPTP (1.5 mg/kg): continuous irradiation for 5 days MPTP (2.1 mg/kg): continuous irradiation for 7 days | For both irradiation groups: Improved clinical scores and behavioral activities as indicated by locomotive traces and distance moved and velocity as well as increased nigral dopaminergic cells For PBM (25 J) group: increased striatal TH+ terminals |
| Oueslati et al., (2015) [39] | AAV-Based Rat Genetic Model Sprague-Dawley (n α-syn = 9) (n α-syn + PBM (2.5 mW/cm2) = 7) (n α-syn + PBM (5 mW/cm2) = 7) | Female NR | α-syn-induced toxicity: 2 μL of viral suspension per rat | Laser, 808 nm | NR | PBM (2.5 mW/cm2): 20.4 mW/cm2 (at scalp) or 2.5 mW/cm2 (at midbrain) PBM (5 mW/cm2): 40.8 mW/cm2 (at scalp) or 5 mW/cm2 (at midbrain) | 100 s | PBM (2.5 mW/cm2): 4.08 J/cm2 (at scalp) or 0.50 J/cm2 (at midbrain) PBM (5 mW/cm2): 8.16 J/cm2 (at scalp) or 1 J/cm2 (at midbrain) | PBM (2.5 mW/cm2): 114.24 J/cm2 (at scalp) or 14 J/cm2 (at midbrain) PBM (5 mW/cm2): 228.48 J/cm2 (at scalp) or 28 J/cm2 (at midbrain) | Transcranial 2 irradiation spots of about 1 cm2 bilaterally on the head | All groups: once a day for 4 weeks | For both irradiation groups: decreased motor deficits (akinesia) as indicated by improvement of the use of the contralateral forepaw For PBM (5 mW/cm2) group: decreased nigral and striatal dopaminergic fiber loss |
| Moro et al., (2016) [40] | Macaque monkey Macaca fascicularis (n Control = 3) (n MPTP) = 5) (n MPTP + PBM = 7) | Male 4–5 years old | MPTP: 1.8–2.1 mg/kg per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 25 days | NA | 125 J | Intracranial, implanted in region 1–2 mm to the left hand side of the midline in the mid-brain | Continuous irradiation for 25 days | Improved clinical scores as indicated by locomotive traces; increased TH+ cell number in the SNc; no effect on the striatal TH+ terminal density |
| Salgado et al., (2016) [41] | Rat Albino Wistar (n 6OHDA = 20) (n 6OHDA + LED PBM=20) (n 6OHDA + Laser PBM = 20) | NR NR | 6OHDA bilateral microinjections of 15 μg per rat | LEDs, 627 nm Laser, 630 nm | LEDs: 70 mW Laser: 45 mW | LEDs: 70 mW/cm2 (at scalp) Laser: 45 mW/cm2 (at scalp) | LEDs: 57 s Laser: 88 s | LEDs: 4 J/cm2 (at scalp) Laser: 4 J/cm2 (at scalp) | LEDs: 28 J/cm2 (at scalp) Laser: 28 J/cm2 (at scalp) | Transcranial | All groups: once a day for 7 days | For laser and LEDs sources: increased locomotive traces in open field test; decreased TNF-α levels For LEDs source: increased IFN-γ levels For laser source: increased IL-2 levels no effect on the IL-4, IL-6 and IL-10 levels |
| Reinhart et al., (2016) [42] | Rat Wistar (n Saline = 8) (n 6OHDA = 15) (n 6OHDA + Pulse PBM = 16) (n 6OHDA + Continuous PBM (0.16 mW) = 13) (n 6OHDA + Continuous PBM (333 nW) = 9) | Male 8 weeks old | 6OHDA 7.5 μg/μL per rat | LEDs, 670 nm | Pulse irradiation: 0.16 mW Continuous irradiation (0.16 mW): 0.16 mW Continuous irradiation (333 nW): 333 nW | NR | Pulse irradiation: 90 s Continuous irradiation (0.16 mW): continuous irradiation for 23 days Continuous irradiation (333 nW): continuous irradiation for 23 days | NA | Pulse irradiation: 634 mJ Continuous irradiation (0.16 mW): 304 J Continuous irradiation (333 nW): 634 mJ | Intracranial, implanted in region near the SNc, incorporating the red nucleus and ventral tegmental area, toward the midline | Pulse irradiation: twice a day for 23 days Continuous irradiation (0.16 mW): continuous irradiation for 23 days Continuous irradiation (333 nW): continuous irradiation for 23 days | For pulse irradiation group: decreased rotational behavior at 21 days post-surgery; increased TH+ cell number in the SNc For continuous irradiation (0.16 mW) group: decreased rotational behavior at 14 and 21 days post-surgery; no effect on the TH+ cell number in the SNc For continuous irradiation (333 nW) group: no effect on the rotational behavior; no effect on the TH+ cell number in the SNc |
| Reinhart et al., (2016) [42] | Mouse Albino BALB/c (n Saline = 9) (n MPTP = 9) (n MPTP + Pre-PBM = 9) (n MPTP + Simultaneous PBM = 9) (n MPTP + Post-PBM = 9) (n MPTP + Pre- & Simultaneous PBM = 9) (n MPTP + Post- & Simultaneous PBM = 9) (n MPTP + Pre- & Post- & Simultaneous PBM = 9) | Male 8–10 weeks old | MPTP: 50 mg/kg per mouse | LEDs, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Pre-PBM: 14.4 J/cm2 Simultaneous-PBM: 14.4 J/cm2 Post-PBM: 14.4 J/cm2 Pre- & Simultaneous PBM: 28.4 J/cm2 Post- & Simultaneous PBM: 28.4 J/cm2 Pre- & Post- & Simultaneous PBM: 43.2 J/cm2 | Transcranial | Pre-PBM: twice a day for 2 days Simultaneous-PBM: twice a day for 2 days Post-PBM: twice a day for 2 days Pre- & Simultaneous PBM: twice a day for 4 days Post- & Simultaneous PBM: twice a day for 4 days Pre- & Post- & Simultaneous PBM: twice a day for 6 days | In all irradiation groups: increased locomotor activity in open field test by a similar magnitude and increased TH+ cell number in the SNc |
| El Massri et al., (2016) [43] | Macaque monkey Macaca fascicularis (n Control = 5) (n MPTP) = 11) (n MPTP + PBM = 6) | Male 4–5 years old | MPTP: 1.5–2.1 mg/k per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 5 or 7 days | NA | 25 or 35 J | Intracranial, Implanted in 1 to 2 mm to the left side of the midline in the midbrain | Continuous irradiation for 5 or 7 days | Decreased number of GFAP+ astrocytes and astrocyte cell body size in the SNc and striatum; decreased microglia cell body size in the SNc and striatum |
| El Massri et al., (2016) [44] | Mouse Albino BALB/c: 2 days group (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP+PBM = 10) 7 days group: (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP+PBM=10) 14 days group: (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM (2 J/cm2) = 10) (n MPTP + PBM (4 J/cm2) = 10) | Male 8–10 weeks old | MPTP: 50 or 100 mg/kg per mouse | LEDs, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) or 0.5 J/cm2 (at brain) | 2 days group: 8 J/cm2 (at scalp) or 1 J/cm2 (at brain) 7 days group: 8 J/cm2 (at scalp) or 1 J/cm2 (at brain) 14 days group (2 J/cm2): 16 J/cm2 (at scalp) or 2 J/cm2 (at brain) 14 days group (4 J/cm2): 32 J/cm2 (at scalp) or 4 J/cm2 (at brain) | Transcranial Holding probe at 1 cm from the head | 2 days group: once a day for 2 days 7 days group: once a day for 2 days 14 days group (2 J/cm2): once a day for 4 days 14 days group (4 J/cm2): once a day for 8 days | In 7 days irradiation group: increased TH+ cell number in the SNc In 14 days (4 J/cm2 ) irradiation group: increased TH+ cell number in the SNc; decreased number of GFAP+ cells in the CPu |
| El Massri et al., (2017) [45] | Mouse Albino BALB/c (n Saline = 5) (n Saline + PBM = 3) (n MPTP = 5) (n MPTP + PBM = 4) Rat Wistar (n Saline = 5) (n 6OHDA = 5) (n 6OHDA + PBM = 4) Macaque monkey Macaca fascicularis (n Saline = 3) (n Saline + PBM = 5) (n MPTP = 5) (n MPTP + PBM = 3) | Mouse: ~8 weeks old Rat: ~8 weeks old Monkey: 4–5 years old | Mouse: MPTP (50 mg/kg per mouse) Rat: 6OHDA (7.5 μg/μL) Monkey: MPTP (1.5 mg/kg per monkey) | Laser, 670 nm | Mouse: 0.16 mW Rat: 0.16 mW Monkey: 10 mW | NR | NR | NR | NR | Intracranial, Mouse: implanted in lateral ventricle Rat and Monkey: implanted in midline region of the midbrain | Mouse: Continuous irradiation for 30 h Rat: Continuous irradiation for 23 days Monkey: Continuous irradiation for 6 days | Mouse: no effect Rat: no effect Monkey: increased TH+ cell number and terminal density in the striatum; increased GDNF expression in the striatum |
| Reinhart et al., (2017) [46] | Mouse Albino BALB/c (n Saline = 8) (n MPTP = 8) (n MPTP + 670 nm PBM = 8) (n MPTP + 810 nm PBM = 8) (n MPTP + Sequentially 670 & 810 nm PBM (15 mW) = 8) (n MPTP + Sequentially 670 & 810 nm PBM (30 mW) = 8) (n MPTP + Concurrently 670 & 810 nm PBM (15 mW) = 8) (n MPTP + Concurrently 670 & 810 nm PBM (30 mW) = 8) | Male 8–10 weeks old | MPTP: 50 mg/kg per mouse | LED, 670 or 810 nm | 15 or 30 mW | NR | 45 or 90 s | 2.7 J (at scalp) | 670 nm PBM: 11 J 810 nm PBM: 11 J Sequentially 670 & 810 nm PBM (15 mW): 11 J Sequentially 670 & 810 nm PBM (30 mW): 22 J Concurrently 670 & 810 nm PBM (15 mW): 11 J Concurrently 670 & 810 nm PBM (30 mW): 22 J | Transcranial | All groups: twice a day for 2 days | In all irradiation groups: increased locomotor activity in open field test and increased TH+ cell number in the SNc Note: combination treatment groups exhibited a greater overall beneficial outcome |
| El Massri et al., (2018) [47] | Macaque monkey Macaca fascicularis (n Control = 3) (n Control + PBM = 3) (n MPTP = 3) (n MPTP + PBM = 3) | Male 4–5 years old | MPTP: 1.5 mg/kg per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 5 days | NA | 25 J | Intracranial, Implanted in 1 to 2 mm to the left side of the midline in the midbrain | Continuous simultaneous irradiation for 5 days | No effect on the number and somal sizes of encephalopsin +cells in the striatum |
| Kim et al., (2018) [48] | Mouse C57BL/6: (n Saline = 10) (n MPTP = 10) (n MPTP + PBM = 10) | Male 10 weeks old | MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 50 mW/cm2 (at skin) | 180 s | 9 J/cm2 (at skin) | 18 J/cm2 (at skin) | Remotely; irradiation to the dorsum | Twice (24 h apart) | Increased TH+ cell number in the SNc; no effect on the density of TH+ terminations in the dorsal CPu |
| O’Brien and Austin (2019) [49] | Rat Sprague–Dawley (n Vehicle = various) (n Lipopolysaccharide = various) (n Lipopolysaccharide + PBM = various) | Male NR | Lipopolysaccharide 10 μg per rat 20 μg per rat | LED, 675 nm | 500 mW | 40 mW/cm2 (at scalp) | 88 s | 3.6 J/cm2 (at scalp) | 46.8 J/cm2 (at scalp) | Transcranial Holding probe at 1 cm from the head | Thirteen (once 2 h following the completion of the lipopolysaccharide injection + twice daily for 6 days) | With 10 µg lipopolysaccharide: increased TH+ cell number in the SNc; no effect on the IBA1+ cell densities in the SNc With 20 µg lipopolysaccharide: no significant effect on the motor behavior in the cylinder, rotarod and adjusted stepping tests |
| Miguel et al., (2019) [50] | Mouse C57BL/6: (n Saline = 8) (n MPTP = 6) (n MPTP + PBM = 6) | Male 12 weeks old | MPTP: 80 mg/kg per mouse | LED, 675 nm | NR | 50 mW/cm2 (at scalp) | 180 s | 9 J/cm2 (at scalp) | 63 J/cm2 (at scalp) | Transcranial | Once a day for 7 days | Decreased vascular leakage in the SNc and CPu |
| Ganeshan et al., (2019) [51] | Mouse Albino BALB/c (n Saline = 10) (n MPTP = 10) (n MPTP + PBM (2 days) = 10) (n MPTP + PBM (5 days) = 10) (n MPTP + PBM (10 days) = 10) | Male 10 weeks old | MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 50 mW/cm2 (at skin) | 90 s | 4.5 J/cm2 (at skin) | PBM (2 days): 9 J/cm2 (at skin) PBM (5 days): 22.5 J/cm2 (at skin) PBM (10 days): 45 J/cm2 (at skin) | Remotely; irradiation to the dorsum and hind limbs | Once a day for 2, 5 or 10 days | In PBM (2 days) group: decreased Fos+ cell number in the CPu In PBM (5 days) group: decreased Fos+ cell number in the CPu In PBM (10 days) group: increased TH+ cell number in the SNc; decreased Fos+ cell number in the CPu; upregulated cell signaling and migration (including CXCR4+ stem cell and adipocytokine signaling), oxidative stress response pathways and modulated blood-brain barrier |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehpour, F.; Hamblin, M.R. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules 2020, 10, 610. https://doi.org/10.3390/biom10040610
Salehpour F, Hamblin MR. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules. 2020; 10(4):610. https://doi.org/10.3390/biom10040610
Chicago/Turabian StyleSalehpour, Farzad, and Michael R Hamblin. 2020. "Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review" Biomolecules 10, no. 4: 610. https://doi.org/10.3390/biom10040610
APA StyleSalehpour, F., & Hamblin, M. R. (2020). Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules, 10(4), 610. https://doi.org/10.3390/biom10040610






