Abstract
Tea made from Camellia sinensis leaves is one of the most consumed beverages worldwide. This systematic review aims to update Camellia sinensis pharmacological activity on metabolic and endocrine disorders. Inclusion criteria were preclinical and clinical studies of tea extracts and isolated compounds on osteoporosis, hypertension, diabetes, metabolic syndrome, hypercholesterolemia, and obesity written in English between 2014 and 2019 and published in Pubmed, Science Direct, and Scopus. From a total of 1384 studies, 80 reports met inclusion criteria. Most papers were published in 2015 (29.3%) and 2017 (20.6%), conducted in China (28.75%), US (12.5%), and South Korea (10%) and carried out with extracts (67.5%, especially green tea) and isolated compounds (41.25%, especially epigallocatechin gallate). Most pharmacological studies were in vitro and in vivo studies focused on diabetes and obesity. Clinical trials, although they have demonstrated promising results, are very limited. Future research should be aimed at providing more clinical evidence on less studied pathologies such as osteoporosis, hypertension, and metabolic syndrome. Given the close relationship among all endocrine disorders, it would be of interest to find a standard dose of tea or their bioactive constituents that would be beneficial for all of them.
1. Introduction
The incidence and prevalence of metabolic and endocrine disorders such as obesity and type 2 diabetes mellitus are dramatically increasing due to sedentary lifestyle, food intake, and endocrine disruptors, among others [1,2]. In the year 2030, it is estimated that many of these metabolic and endocrine diseases will be responsible for one of the leading causes of death worldwide [3].
Camellia sinensis (L.) Kuntze (Theaceae family) is a tree that mainly grows in tropical and subtropical climates. Tea made from the leaves of Camellia sinensis is one of the most consumed beverages in the world. Teas can be classified depending on the degree of fermentation as green tea (unfermented tea), white tea and yellow tea (lightly fermented), oolong tea (semi-fermented tea), black tea (fermented tea), and pu-erh tea (post-fermented tea). Black tea is the most produced and consumed tea worldwide (78% of total tea, especially in Western countries) followed by green tea (20%, especially in China, India, and Japan) and oolong tea (<2%) [4]. Flavanols (primary catechin compounds such as epigallocatechin gallate), flavonols and glycosyl derivatives (i.e., apigenin, myricetin, quercetin, rutin), teaflavins and thearubigins have been identified as main bioactive compounds in the leaves of Camellia sinensis. The type and amount of these compounds is determined by the degree of fermentation of the leaves. Epigallocatechin-3-gallate is the major compound in green tea and theaflavins are produced during the processing of black tea, providing the characteristic flavor [5,6,7]. The health benefits of Camellia sinensis teas include antioxidant, anti-inflammatory, anti-cancer, cholesterol lowering, and cardiovascular protection properties, among others [4,8].
The present systematic review aims to update the pharmacological activity of Camellia sinensis (L.) Kuntze on metabolic and endocrine disorders (osteoporosis, hypertension, diabetes, metabolic syndrome, hypercholesterolemia, and obesity).
2. Method
2.1. Search Strategy
The systematic review included preclinical and clinical studies of Camellia sinensis on endocrine and metabolic disorders. The literature search was conducted using combination of the following keywords “Camellia sinensis”, “osteoporosis”, “hypertension”, “diabetes”, “metabolic syndrome”, “hypercholesterolemia”, and “obesity” on Pubmed, Science Direct, and Scopus databases. The search years were from 2014 to 2019.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were preclinical (in vitro and in vivo) and clinical studies, written in English, focused on the pharmacological activity of isolated compounds and extracts of Camellia sinensis on metabolic and endocrine disorders. The excluded criteria were case reports, review articles, conference proceedings, and editorial letters. Moreover, studies involving medicinal plant mixtures, galenic formulations, Camellia species different than Camellia sinensis, functional foods with tea, and comorbidities associated with endocrine and metabolic diseases were excluded.
The literature research was performed by two independent researchers (E.G.-B. and M.S.) and consisted of an initial identification in the above-mentioned databases, followed by duplicated works elimination and, finally, an exclusion of studies that did not meet inclusion criteria established in this systematic review. The research was verified by a third reviewer (M.P.G.-S.) using a predefined spreadsheet designed by the authors.
3. Pharmacological Activity. Description of the Data
Initially, a total of 1384 studies were identified in Pubmed (n = 170), Science Direct (n = 1177), and Scopus (n = 37). However, 40 reports were deleted when appearing in two or more databases (duplicated). Then, 1264 articles were excluded after title and abstract analysis (n = 1237) and after full-text analysis (n = 27), 80 articles finally being included in this systematic review (Figure 1). Five studies of these 80 reports carried out both in vitro and in vivo experiments and one study in vitro and clinical trials.
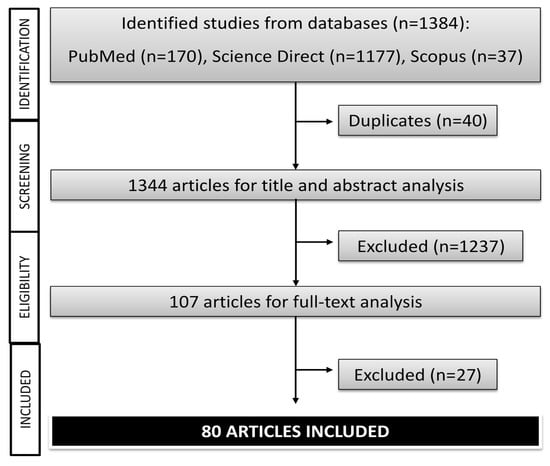
Figure 1.
Flowchart of the literature research (in vitro, in vivo, and clinical trials studies) of Camellia sinensis.
Appendix A (Table A1) and Appendix B (Table A2) include in vitro and in vivo pharmacological studies, respectively, and these studies were grouped based on disease, extract/isolated compound, experimental model, treatments, major findings, and references. Appendix C (Table A3) includes clinical trials and the main information contained was study (author, year, and country), study design, sample size, population, type of plant, intervention, duration of treatments, and results. Most papers were published in 2015 (n = 27, 29.3%) and 2017 (n = 19, 20.6%) (Figure 2A). All works included in this systematic review were conducted by research groups of 23 countries, the majority of them from China (n = 23, 28.75%), United States (n = 10, 12.5%), and South Korea (n = 8, 10%) (Figure 2B). These studies were carried out with extracts (n = 54, 67.5%) and isolated compounds (n = 33, 41.25%) from Camellia sinensis. Particularly, extracts were the part of the plant most studied in in vivo studies and clinical trials whereas isolated compounds were in the in vitro studies. Regarding endocrine and metabolic disorders, diabetes and obesity were the most studies pathologies (n = 35 and n = 33, respectively) followed by hypercholesterolemia (n = 9), osteoporosis (n = 6), hypertension (n = 5) and metabolic syndrome (n = 4). Particularly, diabetes was the most study disease in in vitro studies (n = 16) and obesity in in vivo studies (n = 20), and clinical trials (n = 6). In several in vitro and in vivo studies, the effect of extracts and isolated compounds of Camellia sinensis on two different pathologies were studied in the same research work [9,10,11,12,13,14,15,16]. This review is divided into six sections, based on the pathologies, diabetes, hypercholesterolemia, hypertension, metabolic syndrome, obesity, and osteoporosis. Within each pathology, pharmacological activities of tea isolated compounds and extracts was classified in terms of signal transduction, redox system, and changes of biomarkers.
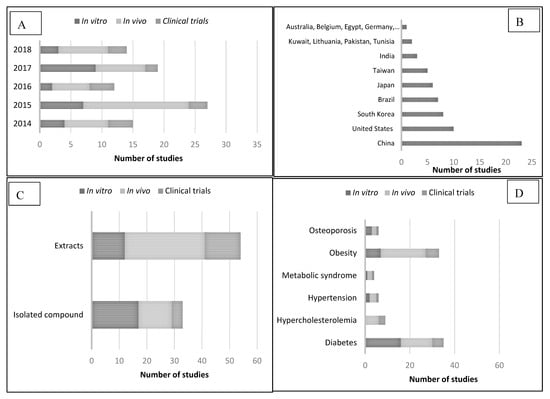
Figure 2.
Main characteristics of papers published on pharmacological activity of Camellia sinensis. (A) Year of publication. (B) Research group country. (C) Part of the plant used for research. (D) Diseases studied in in vitro, in vivo, and clinical trials studies.
3.1. Camellia sinensis and Diabetes
Diabetes mellitus is a chronic metabolic disease that causes abnormally high levels of blood sugar (hyperglycemia) due to a failure in insulin production by pancreas or when the body cannot use insulin effectively [17]. Diabetes mellitus affects about 425 million adults aged between 20 and 79, and it is estimated that in 2025 there will be 629 million. Diabetes mellitus is especially prevalent in low and middle income countries such as those of South-East Asia (82 million) and Western Pacific (159 million) [18]. There are three diabetes mellitus types: type 1, type 2, and gestational. Type 1 diabetes mellitus (insulin-dependent diabetes) is an autoimmune condition that commonly affects individuals during childhood and accounts for around 5% of diabetes mellitus diagnosed cases [17,19]. Type 2 diabetes mellitus (adult onset diabetes) is the most common of the diabetes types (90%–95% of all diagnosed cases worldwide) and it is mainly associated with excess body fat, sedentary lifestyle, and aging [17]. Gestational diabetes mellitus occurs during pregnancy (second or third trimester) because of glucose intolerance; the main risk factors for gestational diabetes mellitus include obesity, ethnicity, age at childbearing, and family history of type 2 diabetes mellitus [20,21].
Most in vitro diabetes studies with Camellia sinensis are based on the ability of their isolated compounds and extracts to inhibit α-amylase and α-glucosidase activity. In addition, there are several in vitro studies with cellular models, mouse 3T3-L1 pre/adipocytes and HepG2 cell lines being the most common. Moreover, for in vivo studies, preclinical diabetic animal models (Kunming mice, Sprague-Dawley, and Wistar rats) commonly used to investigate the anti-diabetic properties of tea are streptozotocin and alloxan-induced diabetic animals [10,16,22,23]. Furthermore, the nematode Caenorhabditis elegans has been also investigated as diabetic model [24].
Molecular targets in signaling pathways is one of the most successful therapeutic approaches in antidiabetic therapy [25]. Recent studies have determined that tea and its active metabolites can have a therapeutic effect against diabetes through different signaling pathways. Hence, studies on rat islet RIN-5F cell tumor demonstrated that a type II arabinogalactan (200 µg/mL) isolated from green tea leaves increased glucose-stimulated insulin secretion targeting cAMP/PKA [26]. This cAMP/PKA signaling pathway plays a key role in the regulation of glucose homeostasis through gluconeogenesis process and glycogen synthesis and breakdown [25]. Another signal transduction pathway on which tea polysaccharides from green tea (200, 400, and 800 mg/kg b.w. per day for 4 weeks) have been shown to act is the PI3K/Akt signal pathway which stimulates GLUT 4 translocation and activation [10]. Moreover, epigallocatechin gallate promoted glucose uptake by increasing GLUT4 translocation via PI3K/AKT in L6 skeletal muscle cells [15]. Another anti-hyperglycemic strategy is to inhibit sodium glucose transporters such as intestinal SGLT1 and renal SGLT2 that are involved in glucose absorption and to promote GLUT2 and GLUT 4 transporters which facilitate glucose movement across membranes. The acute administration (30 min) and chronic administration (6 weeks) of green tea decoction (50 g/L) and a combination of 4 mg epigallocatechin gallate (EGCG) and 2 mg epigallocatechin (EGC) inhibited SGLT-1 activity and increased GLUT2 mRNA levels in the jejunum mucosa and GLUT4 mRNA levels in adipose tissue in Wistar rats fed a high fat diet [12]. Moreover, epigallocatechin gallate inhibited GLUT4-dependent insulin-like growth factor I and II, and stimulated glucose transport in 3T3-L1 adipocytes [27]. Another antidiabetic mechanism has been shown for tea polypeptides from green tea (1000 mg/kg bw/day, p.o. for 5 weeks) which reduced blood glucose and ameliorated diabetic nephropathy in a streptozocin-induced mice model by stimulating the AGEs/RAGE/TGF-β1 signaling pathway and inhibiting the NF-κB pathway [28]. Moreover, pu-erh tea ameliorated insulin resistance by inhibiting IL-6 induction via signal transducer and activator of transcription 3 (STAT3) in C57BL/6J mice [9]. Finally, Chen et al. (2019) found that non-catechin flavonoids (500, 1000, 2000 ppm, for 72 h) ameliorated TNF-α induced insulin resistance by stimulating glucose uptake and inhibiting p38 and JNK pathways in HepG2 cells [29].
The ability of different types of teas and their bioactive compounds to inhibit the enzymes α-amylase and α-glucosidase has been extensively studied in recent years. The enzyme α-amylase, found in saliva and pancreas, catalyzes the hydrolysis of alpha 1–4 bonds of glycogen and starch to form simple sugars (oligosaccharides and disaccharides). Then, α-glucosidase enzyme catalyzes alpha 1–4 bonds of oligosaccharides and disaccharides to form glucose in the small intestine. Both enzymes are a therapeutic target for diabetes mellitus treatment [30]. Yang and Kong (2016) [31] investigated the α-glucosidase inhibitory activity of green tea, black tea, and oolong tea, oolong tea having the lowest IC50 value (1.38 µg/mL). Moreover, Oh et al. (2015) [32] compared α-glucosidase inhibitory activity of tea water extracts and tea pomace extracts obtained from green, oolong, and black tea; this research demonstrated that there were no differences between tea water extracts and tea pomace extracts and that green tea was the most active of all assayed type teas (IC50 = 2040 µg/mL for tea water extracts and IC50 = 1950 µg/mL for tea pomace extracts). Furthermore, the aqueous extract of black tea leaves inhibited α-glucosidase enzyme activity (IC50 = 2400 µg/mL for sucrose and IC50 = 2800 µg/mL for maltase) but not α-amylase activity [33]. Additionally, black and green teas inhibited α-amylase activity with IC50 = 589.86 μg/mL and IC50 = 947.80 μg/mL, respectively, and α-glucosidase activity with IC50 = 72.31 μg/mL and IC50 = 100.23 μg/mL, respectively. Differing chemical composition of these three teas may explain, at least in part, their different effects on diabetes-related enzyme activity. Oolong tea stands out for having dimeric flavan-3-ols (theasinensins), green tea has epigallocatechin-3-gallate as major catechin, and black tea is rich in theaflavins and thearubigins [34,35]. Moreover, the differences in activity for the same type of tea may be due to the fact that the chemical composition is highly influenced by the nature of the green shoots and the procedures to manufacture tea in the producing countries [36]. Apart from studies on black, green, and oolong tea, different ages of pu-erh tea polysaccharide have demonstrated inhibition of α-glucosidase activity, specially 3-year old and 5-year old tea (IC50 = 0.583 and 0.438 μg/mL, respectively), however no inhibitory activity was found against α-amylase [37]. In a similar work, Xu et al. (2014) [38] found that pu-erh tea polysaccharides with aging for 3 years and 5 years resulted in inhibition of α-glucosidase enzyme activity with same potency as acarbose (3 years aging) and three times more potently than acarbose (5 years aging). Besides, water extract of pu-erh tea moderately inhibited sucrose activity (IC50 = 14.4 μg/mL) and maltase (IC50 = 11.4 μg/mL), the compound epigallo-catechin-3-O-gallate having the greatest inhibitory activity with IC50 = 32.5 μM against sucrose and IC50 = 1.3 μM against maltase [14]. In another study, the ethyl acetate fraction from Qingzhuan tea extracts showed significant α-glucosidase inhibitory potential (IC50 = 0.26 μg/mL), attributing this activity to the compounds epigallocatechin gallate and epicatechin gallate. Epicatechin gallate has shown to inhibit α-amylase activity (IC50 = 45.30 μg/mL) and α-glucosidase activity (IC50 = 4.03 μg/mL) and epigallocatechin gallate inhibited α-glucosidase with IC50 = 19.5 μM [15,39]. Moreover, the isolated compound amelliaone A from YingDe black tea inhibited more potently α-glucosidase enzyme activity (IC50 = 10.2 μM) than the reference compound acarbose (IC50 = 18.2 μM) [40]. Furthermore, Hua et al. [41] investigated the inhibitory activity of flavone and flavone glycosides of green tea (Lu’an GuaPian) on α-glucosidase and α-amylase enzymes; 7 kaempferol monoglycoside was the most active against α-glucosidase (IC50 = 40.02 µM) and kaempferol diglycoside against α-amylase (IC50 = 0.09 µM). Based on IC50 values of the isolated compounds, epigallocatechin gallate and 7 kaempferol monoglycoside resulted as the most promising α-glucosidase inhibitory agents and kaempferol diglycoside the most interesting α-amylase inhibitor.
Oxidative stress (reactive oxygen species/antioxidant imbalance) contributes to the development of diabetes mellitus and its associated complications. Black tea aqueous extract (2.5%) reduced lipid peroxidation levels and increased GSH content in diabetic rats [22]. Moreover, tea polysaccharides from green tea (200, 400, and 800 mg/kg b.w. per day for 4 weeks) increased superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities in diabetic Kunming mice [10]. Furthermore, in another study epigallocatechin-3-gallate demonstrated reduction of lipid peroxidation, protein oxidation, and superoxide level and increased antioxidant enzymatic activity and GSH content in diabetic rats [23].
Moreover, several studies have identified changes in relevant biomarkers for diabetes mellitus after tea extract supplementation. Hence, epigallocatechin-3-gallate (2 mg/kg, p.o., alternative days, 1 month) reduced glucose levels and glycosylated hemoglobin and increased insulin [23]. Moreover, green tea powder (10%) and ethanolic extract of green tea (5%) for 8 weeks reduced glucose levels in Sprague-Dawley rats [16]. Furthermore, green tea extract and pu-erh tea extract (both at doses of 0.8 g/kg with a content of 30% catechin and 10% caffeine) but not epigallocatechin-3-gallate (at a dose of 0.24 g/kg) reduced blood glucose levels in BALB/c mice which suggests that caffeine is essential in the hypoglycemic effect of tea [41]. The doses and time treatments could explain the differences in the effectiveness of epigallocatechin-3-gallate [23,41]. Finally, both black and green teas suppressed the increased production of advanced glycosylation end products in 3T3-L1 preadipocytes [42].
Clinical trials were randomized, double-blind, and placebo-controlled and they evaluated the hypoglycemic effect of green tea (mainly) and black tea. Most of these works included patients of both sexes (except one with overweight women) and aged between 30 and 80 years. The duration of the treatments varied from weeks to months and the doses/day administered were also different in each clinical trial (i.e., 1 g/day; 2.5 g/three times day; 560 mg tea polyphenols/two times day; 200 mg tea extract/day). The parameters measured were different, being analyzed from biochemical parameters such as blood glucose levels to oxidative stress markers. Doses of 1 g of dry extract of green tea and 2.5 g/three times day of black tea for 12 weeks were effective to improve glycemic control even better than the reference drug metformin [43,44]. Moreover, both 560 mg tea polyphenols/two times day for 20 weeks and 200 mg tea extract/day for 9–18 months had an antioxidant effect as evidenced in an increase of superoxide dismutase activity and a decrease of lipid peroxidation [45,46].
3.2. Camellia sinensis and Hypercholesterolemia
Hypercholesterolemia (blood cholesterol values > 200 mg/dL) affects over 39% of people worldwide, Europe and America being the most affected continents [44].
Green tea extracts have demonstrated in in vivo studies reduced total cholesterol, LDL, and tryglicerides [13,16,47] which is mainly attributed to epigallocatechin gallate and flavonols [48,49]. Moreover, Chungtaejeon aqueous extracts, which is a Korean fermented tea, has shown to decrease hepatic cholesterol, total serum cholesterol, and LDL cholesterol in high fat atherogenic Wistar rats [50].
Clinical trials on the anti-hypercholesterolemia action of black tea and green tea were investigated in patients with high cholesterol levels in randomized, double-bind, and placebo studies. The cholesterol-lowering effect of tea extracts was evaluated by measuring biochemical parameters (i.e., LDL content and total cholesterol) and antioxidant content. Both clinical studies with black tea demonstrated its effectiveness of reducing LDL/HDL ratio, total cholesterol, apolipoprotein B, and oxidative stress. In one of these clinical trials, the effective dose was 2.5 g black tea and phytosterol mixture which contains 1 g plant sterols for 4 weeks [51]. However, for the other study, a specific dose is not specific, but five cups of black tea per day for two 4-week treatment periods [52]. On the other hand, the consumption of “Benifuuki” green tea, which is rich in methylated catechins (3 g of green tea extract/three times daily for 12 weeks) contributed significantly to reduce serum total cholesterol and serum LDL cholesterol compared to “Yabukita” green tea or barley infusion (placebo tea) consumers [53].
3.3. Camellia sinensis and Hypertension
Hypertension (blood pressure of ≥ 130/85 mm Hg) is one of the most common cardiovascular diseases which affects around 1.13 billion people worldwide. Endocrine hypertension occurs when there is a hormone imbalance as example in Cushing syndrome, primary aldosteronism, and pheochromocytoma [54,55].
Angiotensin I-Converting Enzyme converts angiotensin I into angiotensin II (vasoconstrictor properties). Infusions and decoctions of four black tea samples (Doors tea, Siliguri tea, Guwahati tea, and Nilgiri tea) (15 μg/mL) were investigated for their ability to inhibit angiotensin I converting enzyme. In general, decoctions were more active than infusions and Nilgiri tea showed the highest inhibitory activity. Antihypertension properties are mainly attributed to thearubigin and theaflavin [56,57]. In another in vitro study, pretreatments with black tea extract (0.3–5 μg/mL) and theaflavin-3,3’-digallate (0.03–0.5 μg/mL) for 30 min improved endothelium dependent relaxations in homocysteine (endoplasmic reticulum stress inductor) treated cultured rat aortic endothelial cells [58]. Moreover, San Cheang et al. (2015) [58] also investigated the effect of black tea extract (15 mg/kg/day for 2 weeks) in a rat model of angiotensin II. This study revealed that black tea extract prevented elevated plasma homocysteine levels and downregulated endoplasmic reticulum stress markers. Furthermore, Nomura et al. (2017) [59] investigated the protective effect of three different cultivars of Camellia sinensis ("Yabukita", "Sofu" and "Sunrouge") in a model of hypertensive rats fed with a high salt diet. All these tea cultivars reduced urinary NO metabolite and, moreover, "Yabukita" and "Sofu" increased soluble guanylate cyclase expression.
Finally, a single clinical trial has been identified in which the effect of tea, compared with coffee, on blood pressure was evaluated. This study (1352 subjects aged 18–69 years) stratified population in three groups (non-consumers, ≤3 dL/d consumers, and >3 dL/d consumers of tea or coffee). Results showed that consumption of 1 dL/day of tea was associated with lower systolic blood pressure (by 0.6 mm Hg) and lower pulse pressure (by 0.5 mm Hg) [60].
3.4. Camellia sinensis and Metabolic Syndrome
The metabolic syndrome is a cluster of metabolic disorders (obesity, hypertension, hypercholesterolemia, and diabetes) that favor cardiovascular disease development [61,62]. It is estimated that around a billion people worldwide suffer from metabolic syndrome [63].
Yang et al. (2014) [64] demonstrated that green tea extract (0.2%–0.5%, w/v) inhibited lipid accumulation during adipogenesis in 3T3-L1 preadipocytes by reducing expression of transcription factors C/EBPα and PPARγ.
The atypical antipsychotic drug olanzapine is associated with severe metabolic side effects through H1 receptor antagonism, 5-HT2 C receptor antagonism, D2 receptor antagonism, and muscarinic (M3) receptor antagonism [65,66]. Green tea aqueous extract (25, 50, and 100 mg/kg/day for 11 days) showed to reduce body weight gain, hypertension, and hyperleptinemia, to decrease blood glucose, triglycerides, total cholesterol, and LDL, and to increase HDL in adult male Wistar rats olanzapine induced [67]. In another in vivo study, Xu et al. (2018) [68] investigated the effect of large yellow tea manufactured in the Anhui Province of China on metabolic syndrome in high fat diet treated C57BL/6 mice. This work revealed that yellow tea improved metabolic abnormalities (changes in lipid profile, hyperglycemia, and body weight).
In a clinical trial, patients with metabolic syndrome who received decaffeinated green tea extracts capsules (500 mg green tea extract providing 400 mg catechins; two capsules/time/day for 12 weeks) had lower adiponectin and visfatin concentration levels than control patients who received water [64].
3.5. Camellia sinensis and Obesity
Obesity (body mass index ≥ 30) and overweight (body mass index ≥ 25) are increasing due to an augmented intake of energy-dense foods and sedentary lifestyle; it affects more than 1.9 billion adults worldwide. This disease causes about 4 million deaths globally [69] and it is related to other prevalent pathologies including hypertension, type 2 diabetes mellitus, stroke, obstructive sleep apnea, and several cancers [70].
The mouse adipocyte 3T3-L1 cell line has been extensively used to study the in vitro effect of tea extracts and its isolated compounds [71,72,73]. In obesity in vivo studies, C57BL/6J mice are the most widely animal model since they are susceptible to high fat diet-induced obesity [74,75]. Moreover, other experimental animal models have been used to investigate the effects of different kind of teas and isolated compounds on obesity including Wistar rats, Sprague-Dawley rats, and Swiss mice fed with a high fat diet [76,77].
Several signaling pathways associated with obesity development have been described. The cAMP/PKA pathway participates in adipogenesis process regulation. The stimulation of the serine/threonine kinase protein kinase A (PKA) activity inhibits adipogenesis whereas the inhibition of PKA activity favors the adipogenic process [78]. Green tea polyphenols (epigallocatechin gallate, epigallocatechin, epicatechin gallate, epicatechin, gallocatechin, catechin, and gallocatechin gallate) increased norepinephrine-induced lipolysis via protein kinase A-dependent pathway in the differentiated mouse adipocyte 3T3-L1 cell line [71]. Moreover, the mitogen activated protein kinases (MAPKs) signaling pathways (extracellular signal regulated kinases (ERKs), Jun amino terminal kinases (JNKs), and stress activated protein kinases (p38/SAPKs)) are involved in adipocyte differentiation and in adipogenesis regulation [79]. Green tea polyphenols, gallocatechin gallate, and epigallocatechin-3-gallate decreased MAPK pathway activation as evidenced in downregulation of adipogenic factor expression (CCAAT element binding protein α (C/EBPα), peroxisome proliferator-activated receptor gamma (PPARγ), and sterol regulatory element-binding protein-1c (SREBP-1c)) in a differentiated mouse adipocyte 3T3-L1 cell line [72,73]. Furthermore, the compound gallocatechin gallate has also demonstrated inhibition of inflammation through NF-κB signaling; adipose tissue inflammation is involved in several pathologies associated with obesity such as diabetes mellitus type 2 [73].
In addition to acting on these signaling pathways, several in vitro studies focused on the inhibitory effect on the digestive enzyme pancreatic lipase which hydrolyzes triglycerides into monoglycerides and free fatty acids. The ethanol extract of Camellia sinensis has demonstrated to suppress lipase activity (IC50 = 0.5 mg/mL) [80] and among isolated compounds, highlight in order of pharmacological potency theaflavin-3,3′-digallate (IC50 = 1.9 μM), theaflavin-3-gallate (IC50 = 3.0 μM), theaflavin-3′-gallate (IC50 = 4.2 μM), theaflavin (IC50 > 10 μM), epigallo-catechin-3-O-gallate (IC50 = 13.3 μM), and catechin-3-O-gallate (IC50 = 13.6 μM) [81,82].
Oxidative stress plays a key role in the development of comorbidities (insulin resistance, cardiovascular problems, diabetes) associated with obesity. The compounds gallocatechin gallate and epigallocatechin-3-gallate at a concentration of 10 μg/mL act as potent antioxidant by decreasing ROS production [73].
Moreover, using experimental in vivo models, the effect of tea extracts and their bioactive compounds on changes in relevant biomarkers for obesity have been demonstrated. Green tea extract supplementation has anti-obesity effects by reducing body weight, white adipose tissue fat, liver fat accumulation, and serum triglyceride levels and by increasing lysophospholipids levels and energy expenditure [83,84,85,86,87,88,89,90,91]. These anti-obesity properties of green tea are mainly attributed to its polyphenols (i.e., epigallocatechin gallate and epigallocatechin) and polysaccharides [12,15]. Moreover, Heber et al. (2014) [74] compared the effects of decaffeinated polyphenol extracts from green tea, black tea, and oolong tea in in male C57BL/6J mice fed a high fat diet. This study revealed that all teas reduced body weight, total visceral fat volume, and liver lipid weight and, that green tea polyphenols and oolong tea polyphenols also increased adiponectin gene expression. Besides, oolong tea reduced body weight and fat accumulation by regulating fatty acid oxidation and energy expenditure [75]. Moreover, oolong tea, white tea, yellow tea, pu-erh tea, and black tea have also showed to decrease body weight, plasma triglycerides, white fat accumulation, and fatty acid synthesis and to increase energy expenditure, fatty acid oxidation, and fecal triglycerides excretion in Wistar rats, Sprague-Dawley rats and Swiss mice fed with a high fat diet [76,77]. Furthermore, other studies showed that pu-erh tea and Traditional Korean Chungtaejeon, which are fermented teas, possess anti-obesity properties by ameliorating hepatic lipid metabolism and decreasing fat mass [9,92]. Studies on bioactive compounds isolated from tea extracts have shown that epigallocatechin-3-gallate is responsible of reducing body weight, fat infiltration in liver tissue, and of increasing serum lipid profiles [11,93] and teasaponin reduced body weight gain and improved gut microbiota alteration and cognitive decline in obese C57BL/6J mice [14].
Clinical studies evaluate the anti-obesity activity of green tea and its main secondary metabolite epigallocatechin-3-gallate. These clinical trials were mainly randomized, double-blind and placebo-controlled, except one which was single-blind and another one which was longitudinal. The duration of these clinical trials was mainly weeks (6, 8, and 12 weeks), although there is a study with 12-month intervention. There is also variability in the administered doses from 300 mg/day of EGCG to 856.8 mg/day. Analyzing the different clinical trials, it has been observed that high doses of EGCG (856.8 mg for 6–12 weeks) do have anti-obesity activity, reducing weight in women and men with a body mass index (BMI) ≥ 27 kg/m2 [94,95]. However, in another study in which a dose of 843 mg of EGCG was administered for 12 months, it had no effect on reductions in adiposity nor body mass index. Compared with the previous cited studies, this lack of efficacy can be attributed to the fact that this last study has only been performed with postmenopausal obese women [96]. On the other hand, low doses of EGCG (300 mg/day and 560 g/day for 12 weeks) do not have any beneficial effect on weight control [97,98]. Finally, another clinical trial elucidated that the possible mechanism of action by which EGCG exerts its anti-obesity activity is by increasing HIF1-alpha (HIF1-α) and rapamycin-insensitive companion of mTOR (RICTOR) [99].
3.6. Camellia sinensis and Osteoporosis
Osteoporosis is a very common metabolic skeletal disorder characterized by bone formation reduction (decrease in osteoblast number) and bone resorption increase (sex hormone lack). As a consequence of this disequilibrium between both processes, there is a bone mass loss and a bone tissue deterioration leading to an increase risk of fractures especially hip and vertebral fractures [100]. Osteoporosis is a multifactorial disease, with age being the most common risk. Other risk factors include environmental (i.e., alcohol consumption, smoking, vitamin D and calcium deficiencies, low physical activity), metabolic (estrogen deficiency), and genetic factors (i.e., cathepsin K, sclerostin, chloride channel 7, high-risk ethnic groups) [101]. Osteoporosis affects around 200 million people worldwide (30% of women, 12% of men) [101].
Osteoporosis in vitro models commonly employ mouse macrophage-like RAW264.7cell lines. Receptor activator of nuclear factor-κB ligand (RANKL) is used to differentiate macrophage cells into mature osteoclasts [68]. Moreover, for in vivo studies, the most common animal model is ovariectomized rats. Ovariectomized rat bone loss has many similarities to those of postmenopausal bone loss such as bone resorption increases more than bone formation and intestinal calcium absorption reduction; these related characteristics make ovariectomized rats an ideal animal model to study osteoporosis pathogenesis [102].
Green tea extract (25, 50, and 100 µg/mL, 48 h) has shown to inhibit RANKL-induced osteoclast formation in the mouse macrophage-like cell line RAW264.7 related to NFATc1, cathepsin K, C-Fos, and MMP-9 gene levels reduction [103]. Moreover, the isolated compound gallocatechin gallate (oxidation product of epigallocatechin-3-gallate) at 10 µM concentration inhibited osteoclastogenesis more potently than epigallocatechin-3-gallate through gene and protein downregulation (TRAP, c-Src, β3-Integrin, cathepsin K, and MMP-9) and master transcriptional regulators downregulation (NFATc1 and c-Fos) in RAW264.7 cells [104].
Redox imbalance is also involved in the pathogenesis of bone loss. Overproduction of ROS increases osteoclast activity and inhibits mineralization [105]. Flavones from tea have demonstrated to act as antioxidants. Particularly, epicatechin isolated from Huangshan Maofeng tea (green tea produced in Anhui province of China) has shown to protect against oxidative stress in a hydrogen peroxide-induced model on C2C12 mouse myoblast cells [106].
Moreover, in vivo evidence has demonstrated that tea exerts a protective effect on osteoporosis as evidenced in relevant biomarkers. Hence, green tea extracts (dose of 370 mg/kg for 13 weeks) increase cortical and trabecular bone mass in ovariectomized female Wistar rats [107]. Furthermore, green tea polyphenols supplementation (4 months) improved bone properties (alleviate bone loss and favored bone microstructure restructuring) in obese rats fed with a high fat diet and a high fat diet followed by a caloric restricted diet [108].
Patients with diabetes mellitus have low bone mass which increase fracture risk. Therefore, de Amorim et al. (2018) [104] conducted a double-blind, randomized, placebo-controlled clinical trial to evaluate the effect of green tea extract on bone mass of diabetic patients. This clinical trial revealed that those subjects with diabetes who received 1120 mg of green tea extract containing 560 mg of polyphenols/day for 20 weeks increased their bone mineral content.
4. Conclusion
This systematic review unified publications on the pharmacological effect (preclinical and clinical studies) of teas made from the leaves of Camellia sinensis and its isolated compounds on metabolic and endocrine disorders. Most pharmacological studies were in vitro and in vivo studies focused on diabetes and obesity. Clinical trials, although they have demonstrated promising results, are very limited. The most studied tea and isolated compounds have been green tea and epigallocatechin gallate, respectively. For almost each pathology, research has been focused on investigating the effect on different signaling pathways, oxidative stress, and relevant biomarkers. Among the types of teas, differences in pharmacological action may be explained by differing chemical compositions. Moreover, differences in activity for the same type of tea can be observed because chemical composition is highly influenced by the nature of the green shoots and the procedures to manufacture tea in the producing countries. Regarding clinical trials, the different doses, treatment duration, and subjects included in each study explain the differences in the activity of tea and bioactive compounds. Future research should be aimed at providing more clinical evidence on less studied pathologies such as osteoporosis, hypertension, and metabolic syndrome. Given the close relationship among all endocrine disorders, it would be of interest to find a standard dose of tea or their bioactive constituents that would be beneficial for all of them.
Author Contributions
All authors contributed to the conceptualization, investigation, supervision, and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
In vitro pharmacological studies for Camellia sinensis.
Table A1.
In vitro pharmacological studies for Camellia sinensis.
| Disease | Extract/Isolated Compound | Experimental Model | Treatments | Major Findings | References |
|---|---|---|---|---|---|
| Diabetes | Amelliaone A | α-Glucosidase model | - | α-Glucosidase inhibition: IC50 = 10.2 µg/mL | [40] |
| Arabinogalactan | Rat islet tumor RIN-5F cells | 50 or 200 μg/mL, 2 h | ↑ Insulin secretion | [26] | |
| Black and green teas | Mouse 3T3-L1 preadipocytes | 10 µg/mL, 24 h | ↑ SOD, CAT, and GPx activities ↓ Protein glycation ↓ α-Amylase and α-glucosidase activities | [42] | |
| Black tea aqueous extract | α-Glucosidase model α-Amylase model Caco-2 cells | - | ↓ α-Glucosidase activity No effect on GLUT2 and SGLT1 uptake | [33] | |
| Black, green, and dark tea extracts | Human liver HepG2 cancer cells | - | ↓ α-Glucosidase, aldose reductase, advanced glycation end-products ↑ Glucose uptake (dark tea extracts) | [109] | |
| Epicatechin gallate | α-Amylase model α-Glucosidase models | - | ↓ α-Amylase activity (IC50 = 45.30 μg/mL) ↓ α-Glucosidase activity (IC50 = 4.03 μg/mL) | [39] | |
| Epigallocatechin gallate | Mouse 3T3-L1 adipocytes | 20 μM, 2 h | ↓ IGF-I and IGF-II stimulation | [27] | |
| Epigallocatechin-3-O-gallate | Rat skeletal muscle L6 cells | 0, 20, 40, 50, and 60 μM, 48 h | ↓ α-Glucosidase activity (IC50 = 19.5 μM) ↑ Glucose uptake Promotion GLUT4 translocation to plasma | [15] | |
| Flavanols | α-Glucosidase model | - | ↓ Sucrase activity and maltase activity (EGCG IC50 = 32.5 and 1.3 μM, respectively) | [14] | |
| Flavone and flavone glycosides | α-Glucosidase model α-Amylase model | - | ↓ α-Glucosidase activity (kaempferol monoglycoside IC50 = 40.02 μM) ↓ α-Amylase activity (kaempferol diglycoside IC50 = 0.09 μM) | [41] | |
| Green tea polyphenols Green, black, and oolong tea extracts | α-Glucosidase model | - | ↓ α-Glucosidase activity (green tea polyphenols IC50 = 2.33 µg/mL, green tea IC50 = 2.82 µg/mL, black tea IC50 = 2.25 µg/mL, and oolong tea IC50 = 1.38 µg/mL) | [31] | |
| Diabetes (continued) | Green, oolong, and black water and pomace tea extracts | Rat intestinal α-glucosidase activity | - | ↓ α-glucosidase activity (tea water extract IC50 = 2040 µg/mL and tea pomace extract IC50 = 1950 µg/mL) | [32] |
| Non-catechin flavonoids | Human liver HepG2 cancer cells | Insulin (5 µM) Insulin + TNF-α (30 ng/mL) Insulin + TNF-α + NCF (2000 ppm) Insulin + TNF-α + NCF (1000 ppm) Insulin + TNF-α + NCF (500 ppm) 2, 4, and 6 h | ↑ TNF-α induced insulin resistance ↓ Glucose levels | [29] | |
| Pu-erh tea polysaccharides | α-Glucosidase model α-Amylase model | - | ↓ α-glucosidase activity No α-amylase inhibition | [37] | |
| Qingzhuan dark tea | α-Glucosidase model | IC50 2270 µg/mL for ethyl acetate fraction | ↓ α-Glucosidase activity (ethyl acetate fraction, EGCG, ECG) | [110] | |
| Tea polysaccharides | α-Glycosidase model | - | ↑ α-Glycosidase inhibitory activities (polysaccharides with 5 years aging) | [38] | |
| Hypertension | Black tea aqueous extracts Thearubigin, theaflavin, catechin, epicatechin, epigallocatechin gallate, gallic acid, caffeine | Angiotensin converting enzyme model | Aqueous tea extract (15 µg/mL) Isolated compounds (37 µM) | ↑ ACE inhibitory activity (Thearubigin, theaflavin, catechin) | [56] |
| Black tea extract Theaflavin-3,3’-digallate | Endothelial cells from rat thoracic artery | Black tea (0.3–5 μg/mL), 30 min TF3 (0.03–0.5 μg/mL), 30 min | Endothelium dependent relaxations restored ↓ ROS production | [57] | |
| Metabolic Syndrome | Green tea extract | Mouse 3T3-L1 preadipocytes | Green tea extract (0.2%–0.5%, w/v), 2 days | ↓ Adipogenesis induced lipid accumulation ↓ C/EBPα and PPARγ expression | [64] |
| Obesity | Black tea theaflavins | Pancreatic lipase model | - | Pancreatic lipase inhibition Theaflavin-3,3′-digallate IC50 = 1.9 μM Theaflavin-3′-gallate IC50 = 4.2 μM Theaflavin-3-gallate IC50 = 3.0 μM Theaflavin IC50 > 10 μM | [81] |
| Ethanol tea extracts | Porcine pancreatic lipase type II | 5 mg/mL ethanol | Antilipase activity (IC50 = 500 µg/mL) | [80] | |
| Flavanols | Lipase model | - | ↓ Lipase activity ECG (IC50 = 16.0 μM) CG (IC50 = 13.6 μM) Epiafzelechin-3-O-gallate (IC50 = 19.8 μM) EGCG (IC50 = 13.3 μM ) | [14] | |
| Gallocatechin gallate Epigallocatechin gallate | Mouse 3T3-L1 preadipocytes | Gallates 0–20 μg/mL | Anti-adipogenic activity ↓ Intracellular lipid droplets (GCG, EGCG) ↓ PPAR γ, SREBP-1c and C/EBP α adipogenic transcription factors (GCG, EGCG) ↓ ROS levels (GCG) ↓ NF-κB activation (GCG) ↓ IL-6 production (GCG) | [73] | |
| Green tea catechins | Mouse 3T3-L1 preadipocytes | Green tea catechins with/without norepinephrine (0.1 or 1 μM) for 6 or 24 h | ↑ Lipolysis via PKA-dependent pathway | [71] | |
| Green tea polyphenols Epigallocatechin-3-gallate | Mouse 3T3-L1 preadipocytes | Green tea polyphenols (1, 10, and 100 μg/mL) EGCG (6.8 μg/mL) | ↓ Triglyceride accumulation ↓ Adipogenic factor C/EBPα, SREBP-1c, and PPARγ expression | [72] | |
| Traditional Korean Chungtaejeon | Mouse 3T3-L1 preadipocytes | Traditional Korean Chungtaejeon (250 μg/mL) | ↓ Lipid accumulation ↓ PPARγ expression ↓ Adipocyte lipid-binding protein | [92] | |
| Osteoporosis | Green tea extract | Mouse macrophage RAW 264.7 cells treated with RANKL (50 ng/mL) | 25, 50, or 100 μg/mL for 48 h | ↓ mRNA expression osteoclast-associated genes ↓ NFATc1, c-Fos, c-src and cath-K protein levels | [103] |
| Gallocatechin gallate Epigallocatechin-3-gallate | Mouse macrophage RAW 264.7 cells | 10 μM, 20 min | ↓ RANKL-induced osteoclast differentiation ↓ F-actin ring formation ↓ Osteoclastogenesis-related marker genes and proteins expression, especially gallocatechin gallate | [104] | |
| Flavones | Rat osteoblastic cells C2C12 mouse myoblast cell line | From 3.125 to 50 μg/mL, 48 h | ↑ Alkaline phosphatase activity (epicatechin) ↑ Hydroxyproline content (epicatechin) ↑ Area of mineralized bone nodules (epiafzelechin) | [106] |
Appendix B

Table A2.
In vivo pharmacological studies for Camellia sinensis.
Table A2.
In vivo pharmacological studies for Camellia sinensis.
| Disease | Extract/Isolated Compound | Experimental Model | Treatments | Major Findings | References |
|---|---|---|---|---|---|
| Diabetes | Black tea aqueous extract | GK rats | Group 1: black tea 31.3, 62.5, and 250 mg/kg Group 2: acarbose 0.1, 0.3, and 3.0 mg/kg Group 3: acarbose 0.3 mg/kg + black tea 31.3 mg/kg | ↓ Plasma glucose levels | [33] |
| Black tea aqueous extract | Alloxan-induced diabetic rats | Group 1: control Group 2: alloxan Group 3: black tea extract (1 mL/100 g body w/d for 10 days before alloxan injection and 35 days after alloxan injection) Group 4: black tea extract (35 days) Group 5: diabetic insulin group (twice a day/subcutaneous injection of three units of insulin) | ↑ Plasma antioxidant potential ↓ Lipid peroxidation levels ↑ GSH levels | [22] | |
| Epigallocatechin-3-gallate | C57BL/6J mice | Group 1: low fat diet Group 2: high fat diet Group 3: high fat diet + EGCG (25 mg/kg) Group 4: high fat diet + EGCG (75 mg/kg) | ↓ Plasma glucose ↓ Insulin level ↓ Advanced glycation end products | [11] | |
| Epigallocatechin-3-gallate | Wistar rats streptozotocin-nicotinamide-induced diabetic rats | Group 1: control Group 2: EGCG (2 mg/kg body wt) Group 3: diabetic control group Group 4: diabetic control group + EGCG 1 month | ↓ Glucose, glycosylated hemoglobin, HOMA-IR and lipid profile level ↑ Insulin levels ↑ GSH levels and SOD and CAT activities | [23] | |
| Green tea decoctions Epigallocatechin gallate Epigallocatechin | Wistar rats | Group 1: water Group 2: green tea decoctions Group 3: EGCG, EGC | ↓ SGLT-1 activity ↑ GLUT2 activity ↑ Glucose tolerance | [12] | |
| Green tea ethanol extracts | Sprague-Dawley rats | Group 1: hyperglycemic rats Group 2: hyperglycemic rats + tea extract 10% Group 3: hyperglycemic rats + tea extract 5% 8 weeks | ↓ Serum glucose | [16] | |
| Green tea extract | Nematode Caenorhabditis elegans | 0.1%, 48 h | ↓ Glucose induced damage | [24] | |
| Diabetes (continued) | Green tea extract | Rat model High sodium diet | Group 1: high sodium diet Group 2: high sodium diet + 2 g green tea extract in kg diet Group 3: high sodium diet + 4 g green tea extract in kg diet 6 weeks | ↓ Insulin level and homeostatic model assessment | [13] |
| Green tea polysaccharides | Kunming mice | Group 1: high fat diabetic control Group 2: rosiglitazone Groups 3, 4, and 5: green Tea polysaccharides (200, 400, and 800 mg/kgb.w. per day) 4 weeks | ↓ Insulin resistance PI3K/Akt signal pathway | [10] | |
| Pu-erh tea and green tea | BALB/c mice | Group 1: glucose (2000 mg/kg) Group 2: glucose (2000 mg/kg) + pu-erh tea (800 mg/kg) Group 3: glucose (2000 mg/kg) + green tea (800 mg/kg) Group 4: glucose (2000 mg/kg) + EGCG (240 mg/kg) Group 5: glucose (2000 mg/kg) + EGCG (240 mg/kg) + caffeine (80 mg/kg) Group 6: caffeine (80 mg/kg) | ↓ Blood glucose levels | [111] | |
| Pu-erh tea polysaccharides (TPS) | ICR mice | Group 1: control Group 2: acarbose (5 mg kg−1) Group 3: TPS (1 mg kg−1) Group 4: TPS (5 mg kg−1) | ↓ Blood glucose levels | [37] | |
| Pu-erh tea extract | C57BL/6J mice | Group 1: normal chow diet Group 2: high fat diet Group 3: normal chow diet + pu-erh tea extract (5 mg/mL, 17 weeks) Group 4: high fat diet + pu-erh tea extract (5 mg/ml, 17 weeks) | ↓ Gluconeogenesis related genes expression | [9] | |
| Tea polypeptides from green tea | High fat diet/streptozocin induced (30 mg/kg bw) diabetic mice | 1000 mg/kg bw/day, p.o., 5 weeks | ↓ Total urinary protein, creatinine, and urine nitrogen | [28] | |
| Tea water extract and tea pomace extract of green and black tea | Sprague-Dawley rats | Group 1: sucrose Group 2: tea extracts (0.5 g/kg body wt) | ↓ Glucose level | [32] | |
| Hypercholes-terolemia | Chungtaejeon aqueous extracts | Wistar rats high fat atherogenic diet (HFAD) | Group 1: normal basal diet Group 2: HFAD Group 3: 100 mg/kg day tea extract + HFAD Group 4: 200 mg/kg day tea extract + HFAD Group 5: 400 mg/kg day tea extract + HFAD | ↓ LDL cholesterol ↓ Total serum cholesterol ↓ Hepatic cholesterol | [50] |
| Epigallocatechin-gallate | Wistar rats Fluoride-induced oxidative stress mediated cardiotoxicity | Group 1: normal saline Group 2: EGCG (40 mg/kg BW/day) Group 3: sodium fluoride (25 mg/kg body weight/day, 4 weeks) Group 4: EGCG (40 mg/kg BW/day) + sodium fluoride (25 mg/kg body weight/day, 4 weeks) | ↓ Lipid peroxidative markers ↓ Plasma total cholesterol ↓ Triglycerides ↓ Phospholipids ↓ LDL cholesterol ↑ HDL cholesterol | [48] | |
| Green tea ethanol extracts | Sprague-Dawley rats | Group 1: hypercholesterolemic rats Group 2: hypercholesterolemic rats + diet containing green tea extracts 5% Group 3: hypercholesterolemic rats + diet containing tea powder 10% 8 weeks | ↓ LDL ↓ Triglycerides ↓ Cholesterol | [16] | |
| Green tea extracts | Rat model High sodium diet | Group 1: high sodium diet Group 2: high sodium diet + 2 g green tea extract in kg diet Group 3: high sodium diet + 4 g green tea extract in kg diet 6 weeks | ↓ Total cholesterol, LDL, cholesterol serum concentrations | [13] | |
| Green tea polysaccharides | Kunming mice | Group 1: high fat diabetic control Group 2: rosiglitazone Groups 3, 4, and 5: green tea polysaccharides (200, 400, and 800 mg/kgb.w. per day) 4 weeks | ↓ Total cholesterol ↓ LDL cholesterol | [47] | |
| Tea flavonols (“Sofu” green tea leaves and “Yabukita” tea leaves) | Mice model High cholesterol diet induced | Group 1: high cholesterol diet Group 2: high cholesterol diet + water Group 3: high cholesterol diet + “Sofu” green tea Group 4: high cholesterol diet + “Yabukita” tea | ↓ Plasma oxidized LDL level | [59] | |
| Hypertension | Black tea extract | Sprague-Dawley rats Angiotensin II induced | Group 1: control Group 2: angiotensin II (50 ng/kg/min) Group 3: angiotensin II + black tea extract (15 mg/kg/day, 14 days) | ↑ Endothelium-dependent relaxations ↓ Endoplasmic reticulum stress markers levels ↓ ROS production | [57] |
| Green tea from three cultivars “Yabukita”, “Sofu” and “Sunrouge” | Hypertensive rats High salt diet | Group 1: high salt water Group 2: high salt water + Yabukita Group 3: high salt water + Sofu Group 4: high salt water + Sunrouge | ↓ Urinary NO metabolite ↑ Soluble guanyilate cyclase expression (Yabukita and Sofu) | [59] | |
| Metabolic Syndrome | Green tea aqueous extract | Olanzapine induced Wistar rats | Group 1: control Group 2: olanzapine (5 mg/kg/day) Groups 3, 4, and 5: green tea aqueous extract (25, 50, and 100 mg/kg/day) + olanzapine Groups 6, 7, and 8: green tea aqueous extract (25, 50, and 100 mg/kg/day) | ↓ Body weight gain ↓ Average food and water intake Improved changes in lipid profile ↓ Hyperleptinemia and hypertension | [67] |
| Yellow tea | C57BL/6 male mice High fat diet | Group 1: low fat diet Group 2: high fat diet Group 3: high fat diet + 2.5% yellow tea Group 4: high fat diet + 0.5% yellow tea 12 weeks | ↓ Body weight, liver weight, and adipose tissue weight ↓ Serum glucose, TC, TG, LDL-C, and HDL-C ↓ Glucose intolerance and insulin resistance | [68] | |
| Obesity | Black tea and green tea decoctions | Male Wistar rats | Group 1: high fat diet Group 2: green tea decoction Group 3: black tea decoction 10 weeks | ↑ Fecal triglycerides excretion ↓ Liver triglycerides ↓ Plasma triglycerides ↓ Body weight ↓ Glucose | [76] |
| Decaffeinated green tea extract rich in EGCG | Male Swiss mice | Group 1: control diet + water (0.1 mL/day) Group 2: control diet + EGCG (50 mg/kg/day) Group 3: hyperlipidic diet + water Group 4: hyperlipidic diet + EGCG 16 weeks | ↓ Body weight ↓ Insulin level ↓ Liver fat accumulation ↑ Glucose uptake | [90] | |
| Obesity (continued) | Decaffeinated polyphenol extracts (green tea, black tea, and oolong tea) | C57BL/6J mice High fat/high sucrose | Group 1: low fat/high sucrose diet Group 2: high fat/high sucrose diet Group 3: high fat/high sucrose diet + green tea polyphenols Group 4: high fat/high sucrose diet + black tea polyphenols Group 5: high fat/high sucrose diet + oolong tea polyphenols | ↓ Body weight ↓ Total visceral fat volume ↓ Liver lipid weight ↓ Food intake (green tea polyphenols) | [74] |
| Decaffeinated green tea extract rich in EGCG | Swiss mice High fat diet | Group 1: control diet Group 2: high fat diet Group 3: control diet + placebo Group 4: high fat diet + placebo Group 5: control diet + EGCG Group 6: high fat diet + EGCG 8 weeks | ↓ Body weights ↓ Serum triglyceride levels ↓ Adipocyte area | [91] | |
| Epigallocatechin 3-gallate | C57BL/6J mice | Group 1: low fat diet (negative control) Group 2: high fat diet (positive control) Group 3: high fat diet + EGCG (25 mg/kg) Group 4: high fat diet + EGCG (75 mg/kg) | ↓Body weight ↓ Liver and kidney weight | [11] | |
| Epigallocatechin-3-gallate | C57BL/6 mice | Group 1: high fat diet Group 2: high fat diet + EGCG (20 mg/kg) | ↓ Body weight ↓ Fat infiltration in liver tissue ↑ Serum lipid profiles | [93] | |
| Fermented green tea extract | C57BL/6 mice | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + fermented green tea extract | ↓ Body weight gain ↓ Fat mass ↓ Glucose intolerance ↓ Fatty liver symptoms | [84] | |
| Green tea | C57BL/6J mice | Group 1: normal diet Group 2: high fat (60% energy as fat) Group 3: high fat + 0.25% (w/w) Green tea 12 weeks | ↓ Body weight gain ↑ Energy expenditure ↓ Adiposity | [85] | |
| Obesity (continued) | Green tea | C57BL/6J mice | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + 0.25% (w/w) green tea extract | ↑ Lysophospholipids levels | [87] |
| Green tea decoctions Epigallocatechin gallate Epigallocatechin | Wistar rats | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + green tea decoctions | ↓ Body weight ↓ Triglycerides ↓ Cholesterol | [12] | |
| Green tea extract | C57BL/6J mice | Group 1: green tea extract (77 mg/g) Group 2: voluntary exercise Group 3: green tea extract + voluntary exercise | ↑ Adipose tissue conversion into brown fat like adipose | [83] | |
| Green tea extracts | C57BL/6 mice | Group 1: control diet Group 2: high fat diet Group 3: high fat diet + 0.5% polyphenolic green tea extracts 8 weeks | ↓ Adiposity ↓ High diet inflammation ↓ Adipocyte size ↓ Lipid droplet size | [86] | |
| Green tea extract | Sprague–Dawley rats | Group 1: normal diet control Group 2: high fat diet control Group 3: orlistat control (50 mg/kg/d + high fat diet) Group 4: green tea extract (100 mg/kg/d + high fat diet) 50 days | ↓ Body weight ↓ White adipose tissue fat | [89] | |
| Oolong tea water extract | C57BL/6J mice | Group 1: normal diet Group 2: high fat diet Group 3/4/5: oolong tea (different storage years) 6 weeks | ↓ Body weight ↓ Fat accumulation ↓ Triglyceride levels ↓ LDL cholesterol ↑ HDL cholesterol level | [75] | |
| Polyphenol-rich green tea extract | C57BL/6 mice | Group 1: fed a standard diet + gavage with water Group 2: standard diet + gavage with 500 mg/kg GT Group 3: HFD + gavage with water Group 4: HFD+ gavage with GT 16 weeks | ↓ Body weight ↓ Body adiposity ↓ Inflammation ↑ Insulin sensitivity | [88] | |
| Obesity (continued) | Polysaccharides, polyphenols and caffeine from green tea | Sprague-Dawley rats High fat rats | Groups control, polysaccharides, polyphenols, and caffeine at two doses (low and high) | ↓ Body weight and fat accumulation ↑ Antioxidant levels ↓ Leptin levels ↓ Fatty acids absorption | [112] |
| Pu-erh tea extract | C57BL/6J mice | Group 1: normal chow diet Group 2: high fat diet Group 3: normal diet + tea extract (5 mg/mL, 17 weeks) Group 4: high fat diet + tea extract (5 mg/mL, 17 weeks) | ↓ Obesity ↓ Hepatic steatosis and liver inflammation ↓ Liver injury | [9] | |
| Teasaponin | High fat diet C57BL/6 male mice | High fat diet (8 weeks) + oral teasaponin (0.5%) with high diet (6 weeks) | ↓ Neuroinflammation ↑ Brain derived neurotrophic factor ↑ Glucose tolerance ↓ Body weight gain | [82] | |
| Traditional Korean Chungtaejeon | C57BL6J-Lep ob/ob mice | Traditional Korean Chungtaejeon (200 or 400 mg/kg body weight, 10 weeks) | ↓ Body weight gain ↓ Fat mass ↓ Food efficacy ratio ↓ Levels of plasma triglyceride and total cholesterol | [92] | |
| Water extract of white tea, yellow tea, oolong tea, green tea, white tea, and raw pu-erh tea | High fat diet induced obese mice | Group 1: untreated Group 2: atorvastatin-treated (oral daily at 10 mg/kg body weight) Group 3: green tea Group 4: yellow tea Group 5: black tea Group 6: white tea Group 7: raw pu-erh tea Group 8: oolong tea Teas: daily oral 1000 mg/kg body weight for 9 weeks | ↓ Body weight ↓ White fat accumulation ↑ Energy expenditure and fatty acid oxidation (white, yellow, and oolong teas) ↓ Fatty acid synthesis (green, white, and raw pu-erh teas) Best tea: white tea | [77] | |
| Osteoporosis | Green tea aqueous extract | Ovariectomized female rats | GTE (60, 120, and 370 mg/kg, 13 weeks) | ↑ Bone mass ↓ Trabecular bone loss | [103] |
| Green tea polyphenols | Sprague-Dawley | Group 1: high fat diet Group 2: caloric restricted diet Group 3: high fat diet + 0.5% green tea polyphenols Group 4: caloric restricted diet + 0.5% green tea polyphenols | ↑ Femoral mass and strength ↑ Trabecular thickness and number ↑ Cortical thickness of tibia ↓ Trabecular separation ↓ Formation rate and eroded surface at proximal tibia ↓ Insulin-like growth factor-I and leptin | [108] |
Appendix C

Table A3.
Clinical trials for Camellia sinensis.
Table A3.
Clinical trials for Camellia sinensis.
| Study (Author, Year, Country) | Study Design | Sample Size | Population | Type of Plant | Intervention | Duration of Treatment | Results |
|---|---|---|---|---|---|---|---|
| DIABETES | |||||||
| Alves Ferreira et al., 2017 [43] Brazil | Randomized, double-blind, placebo-controlled study | 120 | Women (20–45 years) abnormal glucose values | Green tea capsules | Group 1: control (cellulose) Group 2: green tea (1 g) Group 3: metformin (1 g) Group 4: green tea (1 g) + metformin (1 g) | 12 weeks | Improving glycemic and lipid profile ↓ Fasting glucose ↓ Total cholesterol and LDL |
| Lasaite et al., 2014 [113] Lithuania | Randomized double-blind placebo-controlled study | 56 | Patients (37–78 years) with diabetes mellitus type II and diabetic retinopathy, nephropathy or neuropathy | Green tea extract | Group 1: placebo Group 2: Gingko biloba dry extract Group 3: green tea extract For extracts: one capsule twice a day (9 months) and one capsule three times a day (9 months) | 18 months | No statistically significant differences in HbA1c level, antioxidant state, and psychological data |
| Mahmoud et al., 2016 [44] Kuwait | Randomly assigned | 34 | Male and female type 2 diabetics | Black tea infusions | Group 1: three cups black tea daily (600 mL) Group 2: one cup black tea daily (200 mL) | 12 weeks | ↓ HbA1c levels ↑ Regulatory T cells ↓ Pro-inflammatory |
| Spadiene et al., 2014 [45] Lithuania | Randomized, double-blind, placebo-controlled study | 45 | Patients (35-80 years) with diabetes mellitus type II and diabetic retinopathy, nephropathy or neuropathy | Green tea extract | Group 1: green tea extract Group 2: placebo | 9–18 months | ↓ Lipid peroxidation |
| Vaz et al., 2018 [46] Brazil | Randomized, double-blind, placebo-controlled study | 60 | Patients with diabetes | Green tea extract | Group 1: green tea extract (two capsules/day, containing 560 mg of polyphenols/each) Group 2: cellulose (two capsules/day) | 20 weeks | No effect on total antioxidant capacity, glycemic control markers, and renal function ↑ SOD activity |
| HYPERCHOLESTEROLEMIA | |||||||
| Imbe et al., 2016 [53] Japan | Randomized, double-blind, placebo-controlled trial | 155 | Healthy volunteers High LDL cholesterol levels Aged 20–80 years | “Benifuuki” green tea | Group 1: “Benifuuki” Group 2: “Yabukita” Group 3: barley infusion drinker | 12 weeks | ↓ LDL cholesterol levels ↓ Lectin-like oxidized LDL receptor-1 containing LAB level |
| Orem et al., 2017 [51] Canada | Randomized, double-blind, placebo-controlled study | 125 | Subjects 25–60 years hypercholesterolemia | Black tea | Group 1: placebo Group 2: instant black tea Group 3: functional black tea | 4 weeks | Functional black tea: ↓ Total cholesterol ↓ LDL ↓ Oxidative stress index ↑ Total antioxidant status |
| Troup et al., 2015 [52] United States | Randomized, double-blind, crossover trial | 57 | 45–65 years, hypercholesterolemia | Black tea | Group 1: controlled low flavonoid diet plus five cups per day of black tea Group 2: Placebo | 4 weeks | ↓ LDL/HDL ratio ↓ Total cholesterol |
| HYPERTENSION | |||||||
| Alkerwi et al., 2015 [60] Luxembourg | National cross-sectional stratified sample | 1352 | 18–69 years | Tea | Group 1: nonconsumers Group 2: ≤ 3-dL/d consumers (tea/coffee) Group 3: > 3-dL/d consumers (tea/coffee) | - | ↓ Systolic BP and pulse pressure |
| METABOLIC SYNDROME | |||||||
| Yang et al., 2014 [64] China | - | 134 | Metabolic syndrome | Green tea extract | Group 1: green tea extract (500 mg). Two capsules/time/day Group 2: control (water) | 45 days | ↑ Adiponectin serum concentrations ↓ Visfatin levels |
| OBESITY | |||||||
| Chen et al., 2016 [94] Taiwan | Randomized, double-blind trial | 102 | Women BMI ≥ 27 kg/m2 Waist circumference ≥ 80 cm | EGCG | Group 1: placebo Group 2: high dose green tea | 12 weeks | ↓ Weight ↓ Waist circumference ↓ Total cholesterol and LDL plasma levels |
| Dostal et al., 2016 [96] USA | Randomized, double-blind, placebo-controlled clinical trial | 937 | Postmenopausal women aged 50–70 with high breast density and overweight/obese | Green tea extract | Group 1: placebo Group 2: EGCG (843 mg), four capsules daily | 12 months | No ↓ adiposity No improvements in BMI ↓ Tissue fat and gynoid fat |
| Huang et al., 2018 [95] Taiwan | Randomized, double-blind, crossover, placebo-controlled | 90 | Women (18 - 65 years) BMI ≥ 27 kg/m2 LDL-C ≥ 130 mg/dL | Green tea extract | Group 1: placebo Group 2: one capsule 30 min after meal, three times a day, green tea extract | 6 weeks | ↑ Leptin ↓ LDL |
| Janssens et al., 2015 [98] The Netherlands | Randomized, placebo-controlled, single-blind design | 60 | Caucasian men and women with body mass index from 18 kg/m², age: 18–50 | Green tea extract | Group 1: placebo Group 2: green tea (capsules > 0.06 g EGCG and 0.03–0.05 g caffeine) | 12 weeks | No effect on fecal energy content, fecal fat content, resting energy expenditure, respiratory quotient, and body composition |
| Mielgo-Ayuso et al., 2014 [97] Spain | Randomized, double-blind, parallel design | 83 | Obese (30 kg/m2. BMI, 40 kg/m2) premenopausal women | EGCG | Group 1: placebo (lactose) Group 2: EGCG (300 mg/d) | 12 weeks | No changes in body weight No changes in adiposity |
| Nicoletti et al., 2019 [99] Brazil | Longitudinal interventional study | 11 | Women (18–60 years) (BMI) > 40 kg/m2 | EGCG | Group 1: eutrophic women Group 2: decaffeinated green tea capsules with 450.7 mg of EGCG, two capsules/day | 8 weeks | ↑ RICTOR ↑ HIF1-α expression |
| OSTEOPOROSIS | |||||||
| Amorim et al., 2018 [114] Brazil | Double-blind, randomized, controlled clinical trial | 35 | ≥ 18 years old Diabetes for more than 5 years. | Green tea extract | Group 1: cellulose Group 2: 1120 mg of green tea extract contains 560 mg of polyphenols/day | 10 and 20 weeks | ↑ Bone mineral content |
References
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune system: An emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 2017, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.H.; Patnaik, S.K. Incidence of endocrine disorders in Indian adult male population. Indian J. Endocr. Metab. 2017, 21, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; Noreldin, A.E. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharm. 2018, 100, 521–531. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants (Basel). 2019, 8, 180. [Google Scholar] [CrossRef]
- Konieczynski, P.; Viapiana, A.; Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and yerva-mate (Ilex paraguariensis A. St.-Hil.) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Finzer, J.R.D. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Cai, X.; Fang, C.; Hayashi, S.; Hao, S.; Zhao, M.; Tsutsui, H.; Nishiguchi, S.; Sheng, J. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J. Gastroenterol. 2016, 51, 819–829. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharm. 2017, 87, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Bado, A. Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Kujawska-Luczak, M.; Szulinska, M.; Kregielska-Narozna, M.; Skrypnik, D.; Suliburska, J.; Skrypnik, K.; Regula, J.; Bogdanski, P. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in a NaCl-induced hypertensive rat model. J. Physiol. Pharm. 2018, 69, 275–282. [Google Scholar]
- Wang, X.; Liu, Q.; Zhu, H.; Wang, H.; Kang, J.; Shen, Z.; Chen, R. Flavanols from the Camellia sinensis var. Assamica and their hypoglycemic and hypolipidemic activities. Acta Pharm. Sin. B 2017, 7, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef]
- Yousaf, S.; Butt, M.S.; Suleria, H.A.; Iqbal, M.J. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014, 5, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. Metab. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- International Diabetes Federation. Available online: www.idf.org (accessed on 1 December 2019).
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational diabetes mellitus. Endocrinol. Metab. Clin. 2019, 48, 479–493. [Google Scholar] [CrossRef]
- Kumar, D.; Rizvi, S.I. Black tea extract improves anti-oxidant profile in experimental diabetic rats. Arch. Physiol. Biochem. 2015, 121, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharm. 2017, 94, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Deusing, D.J.; Winter, S.; Kler, A.; Kriesl, E.; Bonnländer, B.; Wenzel, U.; Fitzenberger, E. A catechin-enriched green tea extract prevents glucose-induced survival reduction in Caenorhabditis elegans through sir-2.1 and uba-1 dependent hormesis. Fitoterapia 2015, 102, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Bhandari, K.; Chakravorty, N.; Mukherjee, R.; Gundamaraju, R.; Singla, R.K.; Katakam, P.; Adiki, S.K.; Ghosh, B.; Mitra, A. Computational pharmacokinetics and in vitro-in vivo correlation of anti-diabetic synergistic phyto-composite blend. World J. Diabetes 2015, 6, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Bao, B.; Li, X.; Wang, S. Structure characterisation of an arabinogalactam from green tea and its anti-diabetic effect. Carbohydr. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef]
- Ku, H.C.; Tsuei, Y.W.; Kao, C.C.; Weng, J.T.; Shih, L.J.; Chang, H.H.; Kao, Y.H. Green tea (−)-epigallocatechin gallate suppresses IGF-I and IGF-II stimulation of 3T3-L1 adipocyte glucose uptake via the glucose transporter 4, but not glucose transporter 1 pathway. Gen. Comp. Endocr. 2014, 199, 46–55. [Google Scholar] [CrossRef]
- Deng, X.; Sun, L.; Lai, X.; Xiang, L.; Li, Q.; Zhang, W.; Zhang, L.; Sun, S. Tea polypeptide ameliorates diabetic nephropathy through RAGE and NF-κB signaling pathway in type 2 diabetes mice. J. Agr. Food Chem. 2018, 66, 11957–11967. [Google Scholar] [CrossRef]
- Chen, F.C.; Shen, K.P.; Ke, L.Y.; Lin, H.L.; Wu, C.C.; Shaw, S.Y. Flavonoids from Camellia sinensis (L.) O. Kuntze seed ameliorates TNF-α induced insulin resistance in HepG2 cells. Saudi Pharm. J. 2019, 27, 507–516. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharm. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Yang, X.; Kong, F. Evaluation of the in vitro α-glucosidase inhibitory activity of green tea polyphenols and different tea types. J. Sci. Food Agric. 2016, 96, 777–782. [Google Scholar] [CrossRef]
- Oh, J.; Jo, S.H.; Kim, J.S.; Ha, K.S.; Lee, J.Y.; Choi, H.Y.; Yu, S.Y.; Kwon, Y.I.; Kim, Y.C. Selected tea and tea pomace extracts inhibit intestinal α-glucosidase activity in vitro and postprandial hyperglycemia in vivo. Int. J. Mol. Sci. 2015, 16, 8811–8825. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorna, M.; Hung, W.-L.; Pan, M.-H.; Li., S.; Li, D.; Wan, X.; Ho, C.-T. Chemistry and health beneficial effects of oolong tea and theasinensins. FSHW 2015, 4, 133–146. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, No. 51. In Coffee, Tea, Mate, Methylxanthines and Methylglyoxal; IARC Working Group on the Evaluation of Carcinogenic Risk to Humans: Lyon, France, 1991. [Google Scholar]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of pu-erh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Mol. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.-M.; Du, Y.-M.; Yan, R.-A.; Fu, L. C-geranylated flavanones from Ying De black tea and their antioxidant and α-glucosidase inhibition activities. Food Chem. 2017, 235, 227–333. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.Y.; Chu, G.X.; Xie, Z.W.; Bao, G.H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Ramlagan, P.; Rondeau, P.; Planesse, C.; Neergheen-Bhujun, V.S.; Bourdon, E.; Bahorun, T. Comparative suppressing effects of black and green teas on the formation of advanced glycation end products (AGEs) and AGE-induced oxidative stress. Food Funct. 2017, 8, 4194–4209. [Google Scholar] [CrossRef]
- Alves Ferreira, M.; Oliveira Gomes, A.P.; Guimarães de Moraes, A.P.; Ferreira Stringhini, M.L.; Mota, J.F.; Siqueira Guedes Coelho, A.; Borges Botelho, P. Green tea extract outperforms metformin in lipid profile and glycaemic control in overweight women: A double-blind, placebo-controlled, randomized trial. Clin Nutr. 2017, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Al-Ozairi, E.; Haines, D.; Novotny, L.; Dashti, A.; Ibrahim, B.; Abdel-Hamid, M. Effect of Diabetea tea™ consumption on inflammatory cytokines and metabolic biomarkers in type 2 diabetes patients. J. Ethnopharmacol. 2016, 194, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Spadiene, A.; Savickiene, N.; Ivanauskas, L.; Jakstas, V.; Skesters, A.; Silova, A.; Rodovicius, H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J. Food Drug Anal. 2014, 22, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.R.; de Amorim, L.M.N.; de Nascimento, P.V.F.; Veloso, V.S.P.; Nogueira, M.S.; Castro, I.A.; Botelho, P.B. Effects of green tea extract on oxidative stress and renal function in diabetic individuals: A randomized, double-blinded, controlled trial. J. Funct. Foods 2018, 46, 195–201. [Google Scholar] [CrossRef]
- Li, S.B.; Li, Y.F.; Mao, Z.F.; Hu, H.H.; Ouyang, S.H.; Wu, Y.P.; Tsoi, B.; Gong, P.; Kurihara, H.; He, R.R. Differing chemical compositions of three teas may explain their different effects on acute blood pressure in spontaneously hypertensive rats. J. Sci. Food Agric. 2015, 95, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Miltonprabu, S.; Thangapandiyan, S. Epigallocatechin gallate potentially attenuates Fluoride induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. J. Trace. Elem. Med. Biol. 2015, 29, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef]
- Paudel, K.R.; Lee, U.W.; Kim, D.W. Chungtaejeon, a Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef]
- Orem, A.; Alasalvar, C.; Kural, B.V.; Yaman, S.; Orem, C.; Karadag, A.; Zawistowski, J. Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J. Funct. Foods 2017, 31, 311–319. [Google Scholar] [CrossRef]
- Troup, R.; Hayes, J.H.; Raatz, S.K.; Thyagarajan, B.; Khaliq, W.; Jacobs, D.R., Jr.; Gross, M. Effect of black tea intake on blood cholesterol concentrations in individuals with mild hypercholesterolemia: A diet-controlled randomized trial. J. Acad. Nutr. Diet 2015, 115, 264–271. [Google Scholar] [CrossRef]
- Imbe, H.; Sano, H.; Miyawaki, M.; Fujisawa, R.; Miyasato, M.; Nakatsuji, F.; Tachibana, H. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: A double-blind, placebo-controlled randomized trial. J. Funct. Foods 2016, 25, 25–37. [Google Scholar] [CrossRef]
- Gumprecht, J.; Domek, M.; Lip, G.Y.; Shantsila, A. Invited review: Hypertension and atrial fibrillation: Epidemiology, pathophysiology, and implications for management. J. Hum. Hypertens. 2019, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pragle, A. Screening for endocrine hypertension. Clin. Rev. 2019, 29, 5e–7e. [Google Scholar]
- Ray, S.; Dutta, M.; Chaudhury, K.; De, B. GC–MS based metabolite profiling and angiotensin I-converting enzyme inhibitory property of black tea extracts. Rev. Bras. Farm. 2017, 27, 580–586. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020, 128, 108749. [Google Scholar] [CrossRef]
- San Cheang, W.; Yuen Ngai, C.; Yen Tam, Y.; Yu Tian, X.; Tak Wong, W.; Zhang, Y.; Wai Lau, C.; Chen, Z.Y.; Bian, Z.X.; Huang, Y.; et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015, 15, 10340. [Google Scholar] [CrossRef]
- Nomura, S.; Monobe, M.; Ema, K.; Maeda-Yamamoto, M.; Nesumi, A. comparison of the effects of three tea cultivars (Camellia sinensis L.) on nitric oxide production and aortic soluble guanylate cyclase expression in high-salt diet-fed spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2017, 63, 306–314. [Google Scholar] [CrossRef]
- Alkerwi, A.; Sauvageot, N.; Crichton, G.E.; Elias, M.F. Tea, but not coffee consumption, is associated with components of arterial pressure. The observation of cardiovascular risk factors study in Luxembourg. Nutr. Res. 2015, 35, 557–565. [Google Scholar] [CrossRef]
- Dichi, I.; Simão, A.N.; Vannucchi, H.; Curi, R.; Calder, P.C. Metabolic syndrome: Epidemiology, pathophysiology, and nutrition intervention. J. Nutr. Metab. 2012, 2012, 584541. [Google Scholar] [CrossRef]
- Fornari, E.; Maffeis, C. Treatment of metabolic syndrome in children. Front. Endocrinol. 2019, 10, 702. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, L.; Li, T.; Chen, Z. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ. Int. J. Clin. Exp. Med. 2014, 7, 4906–4914. [Google Scholar] [PubMed]
- Gracious, B.L.; Meyer, A.E. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry 2005, 2, 36–42. [Google Scholar] [PubMed]
- Kirk, S.L.; Glazebrook, J.; Grayson, B.; Neill, J.C.; Reynolds, G.P. Olanzapine-induced weight gain in the rat: Role of 5-HT2C and histamine H1 receptors. Psychopharmacology 2009, 207, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Lookian, F.; Hosseinzadeh, H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed. Pharm. 2017, 92, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chu, J.; Wang, M.; Chen, L.; Zhang, L.; Xie, Z.; Zhang, J.; Ho, C.T.; Li, D.; Wan, X. Large yellow tea Attenuates macrophage-related chronic inflammation and metabolic syndrome in high-fat diet treated mice. J. Agr. Food Chem. 2018, 66, 3823–3832. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K. Health effects of overweight and obesity in 195 countries over 25 years. N. Eng. J. Med. 2017, 377, 13–27. [Google Scholar]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 228–298. [Google Scholar] [CrossRef]
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Bioph. Res. Commun. 2015, 461, 1–7. [Google Scholar] [CrossRef]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.; Qu, X. Comparison of cytotoxicity and the anti-adipogenic effect of green tea polyphenols with epigallocatechin-3-gallate in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin gallate (GCG) inhibits 3T3-L1 differentiation and lipopolysaccharide induced inflammation through MAPK and NF-κB signaling. J. Funct. Foods 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Heber, D.; Zhang, Y.; Yang, J.; Ma, J.E.; Henning, S.M.; Li, Z. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. Nutr. J. 2014, 144, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.; Duan, X.; Xiang, L.; Ren, J.; Lai, X.; Li, Q.; Sun, L.; Sun, S. Aged oolong tea reduces high-fat diet-induced fat accumulation and dyslipidemia by regulating the AMPK/ACC signaling pathway. Nutrients 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, M.H.; Snoussi, C.; Dhaouadi, K.; Fattouch, S.; Ducroc, R.; Le Gall, M.; Bado, A. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Intermed. Metab. 2016, 6, 33–40. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef]
- Ray, I.; Mahata, S.K.; De, R.K. Obesity: An immunometabolic perspective. Front. Endocrinol. 2016, 7, 157. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Ali-Shtayeh, M.S. Antiobesity and antioxidant potentials of selected palestinian medicinal plants. Evid. Based Complement. Altern. Med. 2018, 13, 8426752. [Google Scholar] [CrossRef]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Rogers, C.J.; Lambert, J.D. Decaffeinated green tea and voluntary exercise induce gene Changes related to beige adipocyte formation in high fat-fed obese mice. J. Funct. Foods 2015, 14, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, Y.J.; Ryu, R.; Cho, S.J.; Kwon, E.Y.; Choi, M.S. Effect of green tea extract on systemic metabolic homeostasis in diet-induced obese mice determined via RNA-Seq transcriptome profiles. Nutrients 2016, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Ashfaq, U.A. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef]
- Nam, M.; Choi, M.S.; Choi, J.Y.; Kim, N.; Kim, M.S.; Jung, S.; Kim, J.; Ryu, D.H.; Hwang, G.S. Effect of green tea on hepatic lipid metabolism in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Chaudhary, N.; Bhardwaj, J.; Seo, H.J.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Camellia sinensis fruit peel extract inhibits angiogenesis and ameliorates obesity induced by high-fat diet in rats. J. Funct. Foods 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Oyama, L.M. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [CrossRef]
- Santana, A.; Santamarina, A.; Souza, G.; Mennitti, L.; Okuda, M.; Venancio, D.; Seelaender, M.; do Nascimento, C.O.; Ribeiro, E.; Lira, F.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate improves insulin resistance and metabolic profiles in normolipidic diet—But not high-fat diet-fed mice. J. Nutr. Biochem. 2015, 26, 893–902. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, D.W.; Rhyu, D.Y. Korean Chungtaejeon tea extract attenuates body weight gain in C57BL/6J-Lep ob/ob mice and regulates adipogenesis and lipolysis in 3T3-L1 adipocytes. J. Integr. Med. 2017, 15, 56–63. [Google Scholar] [CrossRef]
- Byun, J.K.; Yoon, B.Y.; Jhun, J.Y.; Oh, H.J.; Kim, E.K.; Min, J.K.; Cho, M.L. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol. Lett. 2014, 157, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Liu, C.Y.; Wang, L.Y.; Huang, C.J.; Hsu, C.H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern. Med. 2018, 18, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women. J. Nutr. 2016, 46, 256–264. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. Nutr. J. 2015, 145, 864–870. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Delfino, H.B.P.; Pinhel, M.; Noronha, N.Y.; Pinhanelli, V.C.; Quinhoneiro, D.C.G.; de Oliveira, B.A.P.; Marchini, J.S.; Nonino, C.B. Impact of green tea epigallocatechin-3-gallate on HIF1-α and mTORC2 expression in obese women: Anti-cancer and anti-obesity effects? Nutr. Hosp. 2019, 36, 315–320. [Google Scholar]
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone 2019, 11, 100216. [Google Scholar] [CrossRef]
- Ralston, S.H.; de Crombrugghe, B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006, 20, 2492–2506. [Google Scholar] [CrossRef]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Min. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Wu, X.; Xie, C.Q.; Zhu, Q.Q.; Wang, M.Y.; Sun, B.; Huang, Y.P.; Shen, C.; An, M.F.; Zhao, Y.L.; Wang, X.J.; et al. Green tea (Camellia sinensis) aqueous extract alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro. Food Nutr. Res. 2018, 62, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, T.; Li, J.; Xu, J.; Chen, F.; Hu, L.; Sheng, J. Oxidation derivative of (-)-epigallocatechin-3-gallate (EGCG) inhibits RANKL-induced osteoclastogenesis by suppressing RANK signaling pathways in RAW 264.7 cells. Biomed. Pharm. 2019, 118, 109237. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Min. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tian, J.; Cai, K.; Wu, X.; Wang, Y.; Zheng, Y.; Su, Y.; Cui, L. Promoting osteoblast differentiation by the flavanes from Huangshan Maofeng tea is linked to a reduction of oxidative stress. Phytomedicine 2014, 21, 217–224. [Google Scholar] [CrossRef]
- Xu, H.; Yin, D.; Liu, T.; Chen, F.; Chen, Y.; Wang, X.; Sheng, J. Tea polysaccharide inhibits RANKL-induced osteoclastogenesis in raw264. 7 cells and ameliorates ovariectomy-induced osteoporosis in rats. Biomed. Pharm. 2018, 102, 539–548. [Google Scholar] [CrossRef]
- Shen, C.L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.C.; Cao, J.J. Green tea supplementation benefits body composition and improves bone properties in obese female rats fed with high-fat diet and caloric restricted diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef]
- Wu, Y.T.; Du, W.H.; Shi, L.; Liang, Q.; Zou, X.Q. Vasculoprotective effects of water extracts of black, green and dark tea in vitro. Nat. Prod. Commun. 2017, 12, 387–390. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Z.; Zhu, H.; Zhang, W.; Chen, Y. In vitro α-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement. Altern. Med. 2016, 16, 378. [Google Scholar] [CrossRef]
- Fang, C.Y.; Wang, X.J.; Huang, Y.W.; Hao, S.M.; Sheng, J. Caffeine is responsible for the blood glucose-lowering effects of green tea and Pu-erh tea extracts in BALB/c mice. Chin. J. Nat. Med. 2015, 13, 595–601. [Google Scholar]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Lasaite, L.; Spadiene, A.; Savickiene, N.; Skesters, A.; Silova, A. The effect of Ginkgo biloba and Camellia sinensis extracts on psychological state and glycemic control in patients with type 2 diabetes mellitus. Nat. Prod. Commun. 2014, 9, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, L.M.N.; Vaz, S.R.; Cesário, G.; Coelho, A.S.G.; Botelho, P.B. Effect of green tea extract on bone mass and body composition in individuals with diabetes. J. Funct. Foods. 2018, 40, 589–594. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).