The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases
Abstract
:1. The ADAMTS Protease Family
2. Domain Organization and Posttranslational Modifications of ADAMTS6, 10, 17, and 19
3. Gene Expression Patterns of ADAMTS6, 10, 17, and 19
4. Disorders Associated with Mutations in ADAMTS6, 10, 17, and 19
5. Mouse Models of ADAMTS6, 10, 17, and 19 Deficiency
6. Substrates and Proposed Biological Functions for ADAMTS6, 10, 17, and 19
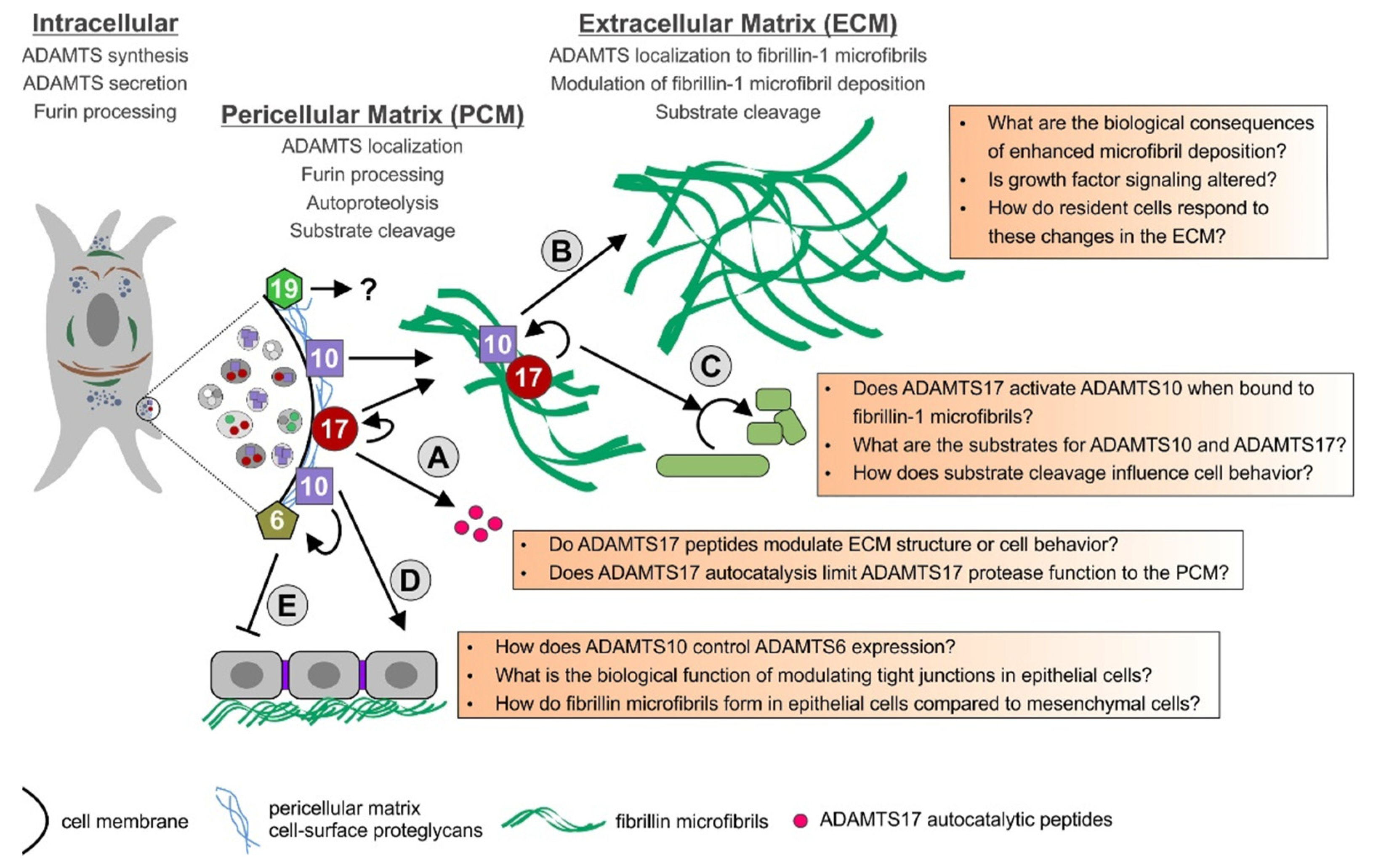
7. Outlook: How ADAMTS6, 10, 17, and 19 May Cooperate in ECM Formation and Remodeling
Author Contributions
Funding
Conflicts of Interest
References
- Apte, S.S. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: Functions and mechanisms. J. Biol. Chem. 2009, 284, 31493–31497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Takeda, S.; Kokame, K.; Takagi, J.; Miyata, T. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc. Natl. Acad. Sci. USA 2009, 106, 19274–19279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, J.; Smith, P.; Wang, S.; Zhang, P.; Zheng, X.L. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J. Biol. Chem. 2005, 280, 29428–29434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortorella, M.; Pratta, M.; Liu, R.Q.; Abbaszade, I.; Ross, H.; Burn, T.; Arner, E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J. Biol. Chem. 2000, 275, 25791–25797. [Google Scholar] [CrossRef] [Green Version]
- Takeda, S. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef] [Green Version]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71, 225–239. [Google Scholar] [CrossRef]
- Apte, S.S. ADAMTS Proteins: Concepts, Challenges, and Prospects. Methods Mol. Biol. 2020, 2043, 1–12. [Google Scholar] [CrossRef]
- Schnellmann, R.; Sack, R.; Hess, D.; Annis, D.S.; Mosher, D.F.; Apte, S.S.; Chiquet-Ehrismann, R. A Selective Extracellular Matrix Proteomics Approach Identifies Fibronectin Proteolysis by A Disintegrin-like and Metalloprotease Domain with Thrombospondin Type 1 Motifs (ADAMTS16) and Its Impact on Spheroid Morphogenesis. Mol. Cell. Proteom. 2018, 17, 1410–1425. [Google Scholar] [CrossRef] [Green Version]
- Mularczyk, E.J.; Singh, M.; Godwin, A.R.F.; Galli, F.; Humphreys, N.; Adamson, A.D.; Mironov, A.; Cain, S.A.; Sengle, G.; Boot-Handford, R.P.; et al. ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome. Hum. Mol. Genet. 2018, 27, 3675–3687. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.W.; Kutz, W.E.; Mead, T.J.; Beene, L.C.; Singh, S.; Jenkins, M.W.; Reinhardt, D.P.; Apte, S.S. Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage. Matrix Biol. 2019, 77, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Colige, A.; Monseur, C.; Crawley, J.T.B.; Santamaria, S.; de Groot, R. Proteomic discovery of substrates of the cardiovascular protease ADAMTS7. J. Biol. Chem. 2019, 294, 8037–8045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmacher, D.; Schneider, M.; Berardinelli, S.J.; Takeuchi, H.; Willard, B.; Reinhardt, D.P.; Haltiwanger, R.S.; Apte, S.S. Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease. Sci. Rep. 2017, 7, 41871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmacher, D.; Apte, S.S. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol. 2015, 47, 34–43. [Google Scholar] [CrossRef]
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFbeta signaling and cell stemness. Matrix Biol. 2018, 71, 82–89. [Google Scholar] [CrossRef]
- Karoulias, S.Z.; Beyens, A.; Balic, Z.; Symoens, S.; Vandersteen, A.; Rideout, A.L.; Dickinson, J.; Callewaert, B.; Hubmacher, D. A novel ADAMTS17 variant that causes Weill-Marchesani syndrome 4 alters fibrillin-1 and collagen type I deposition in the extracellular matrix. Matrix Biol. 2019, 11, 11. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R. Fibrillin protein pleiotropy: Acromelic dysplasias. Matrix Biol. 2019, 80, 6–13. [Google Scholar] [CrossRef]
- Le Goff, C.; Cormier-Daire, V. The ADAMTS(L) family and human genetic disorders. Hum. Mol. Genet. 2011, 20, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Mead, T.J.; McCulloch, D.R.; Ho, J.C.; Du, Y.; Adams, S.M.; Birk, D.E.; Apte, S.S. The metalloproteinase-proteoglycans ADAMTS7 and ADAMTS12 provide an innate, tendon-specific protective mechanism against heterotopic ossification. JCI Insight 2018, 3, 92941. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, H.; Nelson, C.M.; Somerville, R.P.; Mielke, K.; Dixon, L.J.; Powell, K.; Apte, S.S. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development 2010, 137, 4029–4038. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, D.R.; Nelson, C.M.; Dixon, L.J.; Silver, D.L.; Wylie, J.D.; Lindner, V.; Sasaki, T.; Cooley, M.A.; Argraves, W.S.; Apte, S.S. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell 2009, 17, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.; Zha, X.; Zhu, Y.; Lv, J.; Hu, S.; Kong, Y.; Wu, G.; Yang, Y.; He, Y. A novel nonsense mutation in ADAMTS17 caused autosomal recessive inheritance Weill-Marchesani syndrome from a Chinese family. J. Hum. Genet. 2019, 64, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Bhat, V.; Shetty, J.S.; Kumar, A. Whole exome sequencing identifies a novel splice-site mutation in ADAMTS17 in an Indian family with Weill-Marchesani syndrome. Mol. Vis. 2014, 20, 790–796. [Google Scholar] [PubMed]
- Khan, A.O.; Aldahmesh, M.A.; Al-Ghadeer, H.; Mohamed, J.Y.; Alkuraya, F.S. Familial spherophakia with short stature caused by a novel homozygous ADAMTS17 mutation. Ophthalmic Genet. 2012, 33, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Al-Sharif, L.; Khalil, D.S.; Shinwari, J.M.; Bavi, P.; Al-Mahrouqi, R.A.; Al-Rajhi, A.; Alkuraya, F.S.; Meyer, B.F.; Al Tassan, N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 2009, 85, 558–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutz, W.E.; Wang, L.W.; Dagoneau, N.; Odrcic, K.J.; Cormier-Daire, V.; Traboulsi, E.I.; Apte, S.S. Functional analysis of an ADAMTS10 signal peptide mutation in Weill-Marchesani syndrome demonstrates a long-range effect on secretion of the full-length enzyme. Hum. Mutat. 2008, 29, 1425–1434. [Google Scholar] [CrossRef]
- Dagoneau, N.; Benoist-Lasselin, C.; Huber, C.; Faivre, L.; Megarbane, A.; Alswaid, A.; Dollfus, H.; Alembik, Y.; Munnich, A.; Legeai-Mallet, L.; et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 2004, 75, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Steinkellner, H.; Etzler, J.; Gogoll, L.; Neesen, J.; Stifter, E.; Brandau, O.; Laccone, F. Identification and molecular characterisation of a homozygous missense mutation in the ADAMTS10 gene in a patient with Weill-Marchesani syndrome. Eur. J. Hum. Genet. 2015, 23, 1186–1191. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jia, X.; Li, S.; Fang, S.; Guo, X. Mutation survey of candidate genes in 40 Chinese patients with congenital ectopia lentis. Mol. Vis. 2014, 20, 1017–1024. [Google Scholar]
- Brunet, F.G.; Fraser, F.W.; Binder, M.J.; Smith, A.D.; Kintakas, C.; Dancevic, C.M.; Ward, A.C.; McCulloch, D.R. The evolutionary conservation of the A Disintegrin-like and Metalloproteinase domain with Thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish. BMC Evol. Biol. 2015, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.C.; Malik, S.B.; Logsdon, J.M., Jr.; Van Meir, E.G. Functional evolution of ADAMTS genes: Evidence from analyses of phylogeny and gene organization. BMC Evol. Biol. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevitt, D.J.; Li, Z.; Lindrop, J.L.; Barker, M.D.; Clarke, M.P.; McKie, N. Analysis of full length ADAMTS6 transcript reveals alternative splicing and a role for the 5′ untranslated region in translational control. Gene 2005, 359, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.P.; Jungers, K.A.; Apte, S.S. Discovery and characterization of a novel, widely expressed metalloprotease, ADAMTS10, and its proteolytic activation. J. Biol. Chem. 2004, 279, 51208–51217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prins, B.P.; Mead, T.J.; Brody, J.A.; Sveinbjornsson, G.; Ntalla, I.; Bihlmeyer, N.A.; van den Berg, M.; Bork-Jensen, J.; Cappellani, S.; Van Duijvenboden, S.; et al. Exome-chip meta-analysis identifies novel loci associated with cardiac conduction, including ADAMTS6. Genome Biol. 2018, 19, 87. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.H.; Longpre, J.M.; Somerville, R.P.; Alexander, J.P.; Leduc, R.; Apte, S.S. Regulation of ADAMTS9 secretion and enzymatic activity by its propeptide. J. Biol. Chem. 2007, 282, 16146–16154. [Google Scholar] [CrossRef] [Green Version]
- Kutz, W.E.; Wang, L.W.; Bader, H.L.; Majors, A.K.; Iwata, K.; Traboulsi, E.I.; Sakai, L.Y.; Keene, D.R.; Apte, S.S. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 2011, 286, 17156–17167. [Google Scholar] [CrossRef] [Green Version]
- Holdener, B.C.; Haltiwanger, R.S. Protein O-fucosylation: Structure and function. Curr. Opin. Struct. Biol. 2019, 56, 78–86. [Google Scholar] [CrossRef]
- Vasudevan, D.; Takeuchi, H.; Johar, S.S.; Majerus, E.; Haltiwanger, R.S. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr. Biol. 2015, 25, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Al-Shareffi, E.; Haltiwanger, R.S. Biological functions of fucose in mammals. Glycobiology 2017, 27, 601–618. [Google Scholar] [CrossRef] [Green Version]
- Hurskainen, T.L.; Hirohata, S.; Seldin, M.F.; Apte, S.S. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J. Biol. Chem. 1999, 274, 25555–25563. [Google Scholar] [CrossRef] [Green Version]
- Cal, S.; Obaya, A.J.; Llamazares, M.; Garabaya, C.; Quesada, V.; Lopez-Otin, C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene 2002, 283, 49–62. [Google Scholar] [CrossRef]
- Wunnemann, F.; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; Scharfenberg, F.; et al. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2020, 52, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, E.C.; Oliver, J.A.C.; Ricketts, S.L.; Gould, D.J.; Mellersh, C.S. Glaucoma-causing ADAMTS17 mutations are also reproducibly associated with height in two domestic dog breeds: Selection for short stature may have contributed to increased prevalence of glaucoma. Canine Genet. Epidemiol. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.A.C.; Rustidge, S.; Pettitt, L.; Jenkins, C.A.; Farias, F.H.G.; Giuliano, E.A.; Mellersh, C.S. Evaluation of ADAMTS17 in Chinese Shar-Pei with primary open-angle glaucoma, primary lens luxation, or both. Am. J. Vet. Res. 2018, 79, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.A.; Forman, O.P.; Pettitt, L.; Mellersh, C.S. Two Independent Mutations in ADAMTS17 Are Associated with Primary Open Angle Glaucoma in the Basset Hound and Basset Fauve de Bretagne Breeds of Dog. PLoS ONE 2015, 10, e0140436. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.; Pettitt, L.; McLaughlin, B.; Holmes, N.; Forman, O.; Thomas, A.; Ahonen, S.; Lohi, H.; O’Leary, C.; Sargan, D.; et al. ADAMTS17 mutation associated with primary lens luxation is widespread among breeds. Vet. Ophthalmol. 2011, 14, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.H.; Johnson, G.S.; Taylor, J.F.; Giuliano, E.; Katz, M.L.; Sanders, D.N.; Schnabel, R.D.; McKay, S.D.; Khan, S.; Gharahkhani, P.; et al. An ADAMTS17 splice donor site mutation in dogs with primary lens luxation. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4716–4721. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, A.; Ng, M.; Schmid, A.B.; Smillie, R.W.; Baskozos, G.; Holmes, M.V.; Kunnapuu, K.; Magi, R.; Bennett, D.L.; Furniss, D. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat. Commun. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Karlsson, T.; Ek, W.E.; Johansson, A. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat. Commun. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Oichi, T.; Taniguchi, Y.; Soma, K.; Oshima, Y.; Yano, F.; Mori, Y.; Chijimatsu, R.; Kim-Kaneyama, J.R.; Tanaka, S.; Saito, T. Adamts17 is involved in skeletogenesis through modulation of BMP-Smad1/5/8 pathway. Cell. Mol. Life Sci. 2019, 76, 4795–4809. [Google Scholar] [CrossRef]
- Shi, Y.; Tu, Y.; De Maria, A.; Mecham, R.P.; Bassnett, S. Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2504–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojuri, J.; Razeghinejad, M.R.; Aslani, A. Cardiac findings in Weill-Marchesani syndrome. Am. J. Med. Genet. Part A 2007, 143, 2062–2064. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemeier, K.M.; Schjerling, P.; Ohlenschlaeger, T.F.; Eismark, C.; Olsen, J.; Kjaer, M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. FASEB J. 2018, 32, 4763–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Moller, M.B.; Krogsgaard, M.R.; Grum-Schwensen, T.; Petersen, M.M.; Kjaer, M. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci. Transl. Med. 2016, 8, 346ra390. [Google Scholar] [CrossRef]
- Cain, S.A.; Mularczyk, E.J.; Singh, M.; Massam-Wu, T.; Kielty, C.M. ADAMTS-10 and -6 differentially regulate cell-cell junctions and focal adhesions. Sci. Rep. 2016, 6, 35956. [Google Scholar] [CrossRef] [Green Version]
- Sengle, G.; Tsutsui, K.; Keene, D.R.; Tufa, S.F.; Carlson, E.J.; Charbonneau, N.L.; Ono, R.N.; Sasaki, T.; Wirtz, M.K.; Samples, J.R.; et al. Microenvironmental regulation by fibrillin-1. PLoS Genet. 2012, 8, e1002425. [Google Scholar] [CrossRef] [Green Version]
- Hubmacher, D.; Apte, S.S. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: A novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. 2011, 68, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, N.L.; Dzamba, B.J.; Ono, R.N.; Keene, D.R.; Corson, G.M.; Reinhardt, D.P.; Sakai, L.Y. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J. Biol. Chem. 2003, 278, 2740–2749. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Tiedemann, K.; Vollbrandt, T.; Peters, H.; Batge, B.; Brinckmann, J.; Reinhardt, D.P. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J. Biol. Chem. 2002, 277, 50795–50804. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, N.L.; Jordan, C.D.; Keene, D.R.; Lee-Arteaga, S.; Dietz, H.C.; Rifkin, D.B.; Ramirez, F.; Sakai, L.Y. Microfibril structure masks fibrillin-2 in postnatal tissues. J. Biol. Chem. 2010, 285, 20242–20251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmacher, D.; Taye, N.; Balic, Z.; Thacker, S.; Adams, S.M.; Birk, D.E.; Schweitzer, R.; Apte, S.S. Limb- and tendon-specific Adamtsl2 deletion identifies a role for ADAMTSL2 in tendon growth in a mouse model for geleophysic dysplasia. Matrix Biol. 2019, 82, 38–53. [Google Scholar] [CrossRef]
- Collin, G.B.; Hubmacher, D.; Charette, J.R.; Hicks, W.L.; Stone, L.; Yu, M.; Naggert, J.K.; Krebs, M.P.; Peachey, N.S.; Apte, S.S.; et al. Disruption of murine Adamtsl4 results in zonular fiber detachment from the lens and in retinal pigment epithelium dedifferentiation. Hum. Mol. Genet. 2015, 24, 6958–6974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmacher, D.; Wang, L.W.; Mecham, R.P.; Reinhardt, D.P.; Apte, S.S. Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation and bronchial epithelial dysplasia—A novel mouse model providing insights into geleophysic dysplasia. Dis. Model Mech. 2015, 8, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, L.A.; Wang, L.W.; Bader, H.; Ho, J.C.; Majors, A.K.; Hollyfield, J.G.; Traboulsi, E.I.; Apte, S.S. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest. Ophthalmol. Vis. Sci. 2012, 53, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kurokawa, M.; Oda, M.; Oshima, M.; Tsutsui, K.; Kosaka, K.; Nakao, K.; Ogawa, M.; Manabe, R.; Suda, N.; et al. ADAMTSL6beta protein rescues fibrillin-1 microfibril disorder in a Marfan syndrome mouse model through the promotion of fibrillin-1 assembly. J. Biol. Chem. 2011, 286, 38602–38613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, H.L.; Wang, L.W.; Ho, J.C.; Tran, T.; Holden, P.; Fitzgerald, J.; Atit, R.P.; Reinhardt, D.P.; Apte, S.S. A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is a novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing a netrin-like module. Matrix Biol. 2012, 31, 398–411. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, C.; Morice-Picard, F.; Dagoneau, N.; Wang, L.W.; Perrot, C.; Crow, Y.J.; Bauer, F.; Flori, E.; Prost-Squarcioni, C.; Krakow, D.; et al. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 2008, 40, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Ahram, D.; Sato, T.S.; Kohilan, A.; Tayeh, M.; Chen, S.; Leal, S.; Al-Salem, M.; El-Shanti, H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am. J. Hum. Genet. 2009, 84, 274–278. [Google Scholar] [CrossRef] [Green Version]
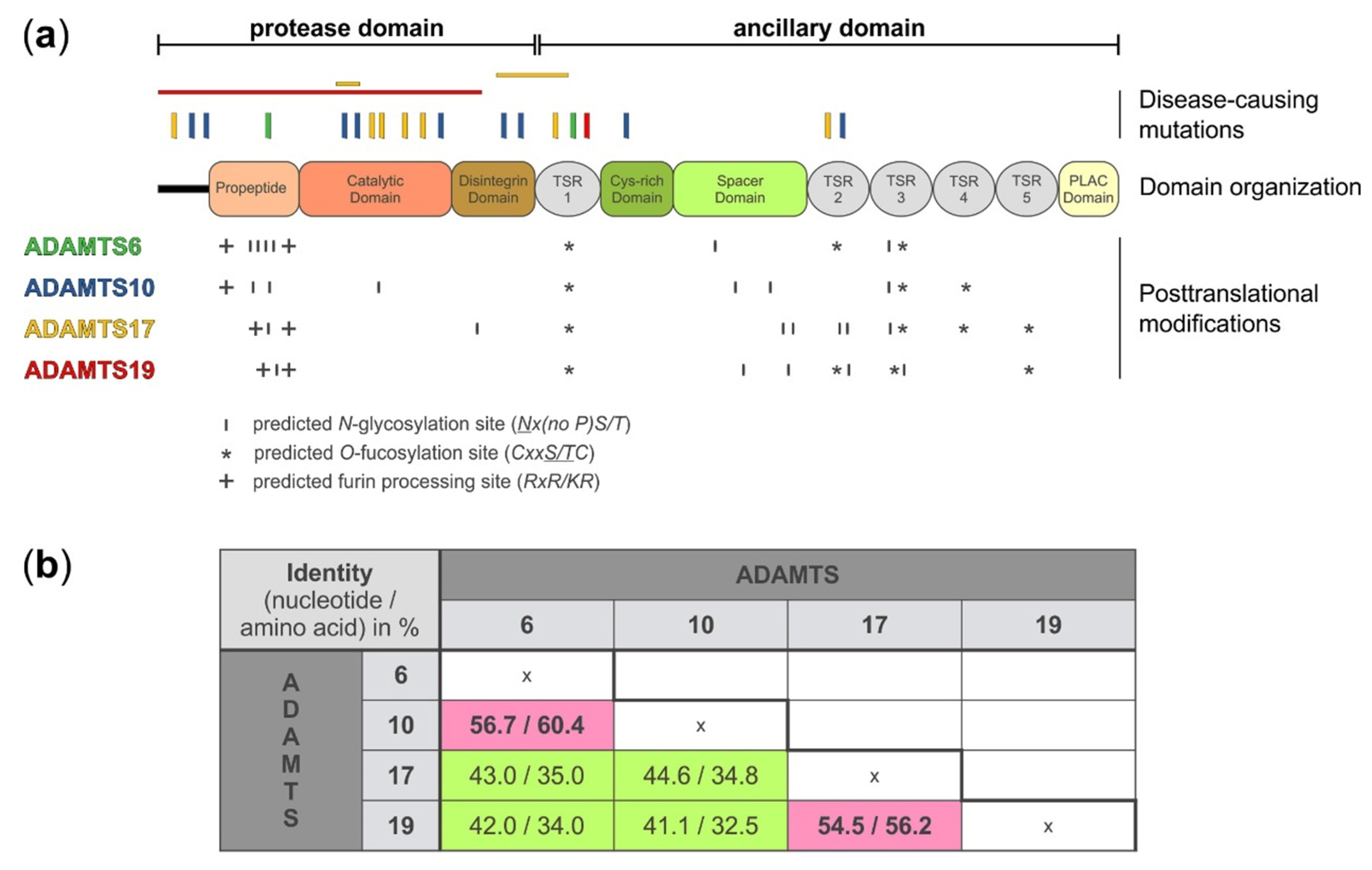
| ADAMTS6 | ADAMTS10 | ADAMTS17 | ADAMTS19 | |
|---|---|---|---|---|
| N-Glycosylation sites 1 | 6 | 6 | 7 | 5 |
| O-Fucosylation sites 2 | 3 | 3 | 4 | 4 |
| Furin consensus sequences 3 | 2 | 1 | 2 | 2 |
| Furin processing 4 | yes | no | yes | yes |
| Autocatalysis | no | no | yes | no |
| Substrates | LTBP1, SDC4 | FBN1, FBN2 | ADAMTS17 | n.d. |
| Alternative splicing | yes | yes | yes | likely |
| Human disorders | Prolonged QRS syndrome | WMS 1 (MIM #277600) | WMS 4 (MIM #613195) | Non-syndromic heart valve disease |
| Disease-causing human mutations | 2 | 9 | 9 | 2 |
| Gene knockout phenotype in mice | Prenatal/neo-natal lethality, double outlet right ventricle, ventricular hypertrophy, atrial and ventricular septal defects | Some prenatal/ neonatal lethality, abnormal ciliary zonule, shorter long bones due to growth plate abnormalities, skeletal muscle abnormalities | Some prenatal/ neonatal lethality, skeletal growth impairment due to growth plate abnormalities, brachydactyly by 8 mos. of age | Aortic valve dysfunction in ~40% of knockout mice |
| Fibrillin-1 binding | n.d. | yes | yes | n.d. |
| Fibrillin microfibril formation 5 | n.d. | Promotes FBN1 deposition | No effect | n.d. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoulias, S.Z.; Taye, N.; Stanley, S.; Hubmacher, D. The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases. Biomolecules 2020, 10, 596. https://doi.org/10.3390/biom10040596
Karoulias SZ, Taye N, Stanley S, Hubmacher D. The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases. Biomolecules. 2020; 10(4):596. https://doi.org/10.3390/biom10040596
Chicago/Turabian StyleKaroulias, Stylianos Z., Nandaraj Taye, Sarah Stanley, and Dirk Hubmacher. 2020. "The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases" Biomolecules 10, no. 4: 596. https://doi.org/10.3390/biom10040596
APA StyleKaroulias, S. Z., Taye, N., Stanley, S., & Hubmacher, D. (2020). The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases. Biomolecules, 10(4), 596. https://doi.org/10.3390/biom10040596






