Tick and Host Derived Compounds Detected in the Cement Complex Substance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ticks
2.2. Collection of Tick Cement Cones and Salivary Glands
2.3. Protein Extraction from Tick Salivary Glands and Cement
2.4. Proteomics Analysis of Tick Sialome and Cementome
2.5. Library Generation, Protein Identification, Data Processing, and Relative Quantitation
2.6. Tick salivary Glands and Cement Physical and Chemical Properties
2.7. Tick Sialome and Cementome α-Gal Content
2.8. Network Analysis of Tick Cementome
2.9. Recombinant Proteins and Antibodies
2.10. Western Blot or Dot Blot Analysis of Recombinant Proteins and the Tick Sialome and Cementome
2.10.1. Recombinant Proteins (Histone H4-MSP1a, Aminopeptidase N, and Glycine-Rich Superfamily Member Protein; Figure S4)
2.10.2. Tick Cementome and Sialome
3. Results and Discussion
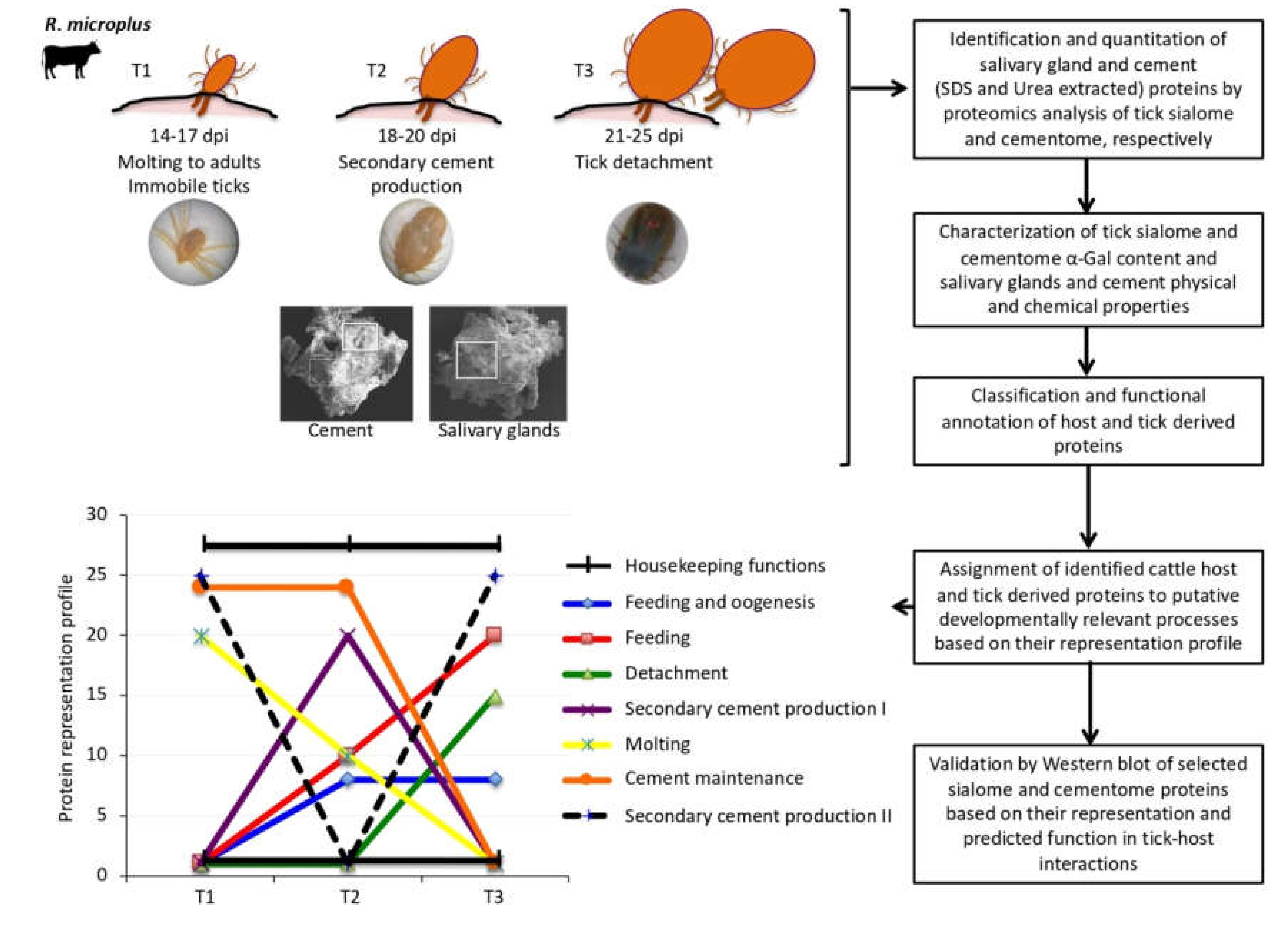
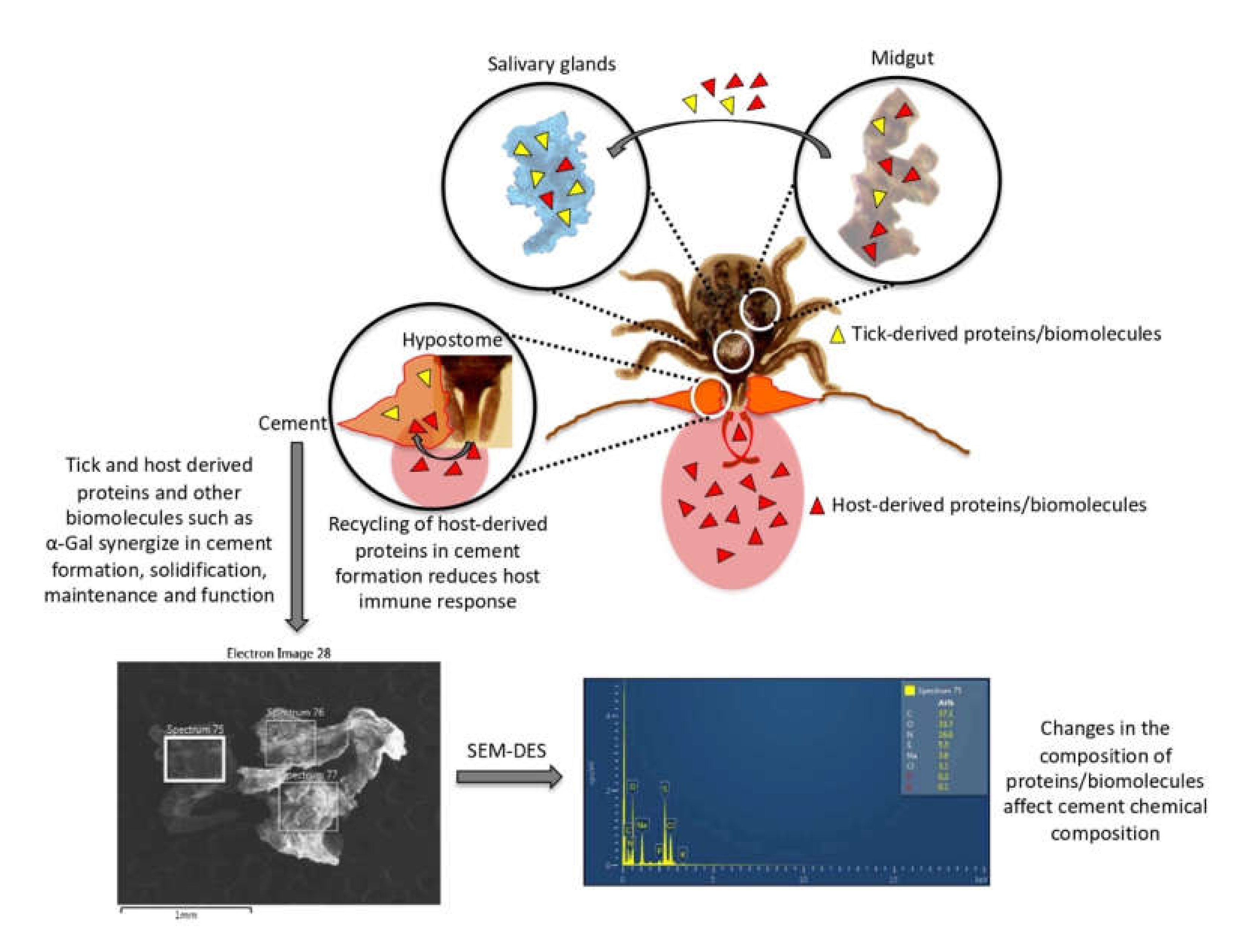
3.1. Experimental Design and Rationale
3.2. The Representation of R. Microplus Tick and Cattle Host Derived Proteins in the Sialome and Cementome Change during Adult Female Parasitic Stages
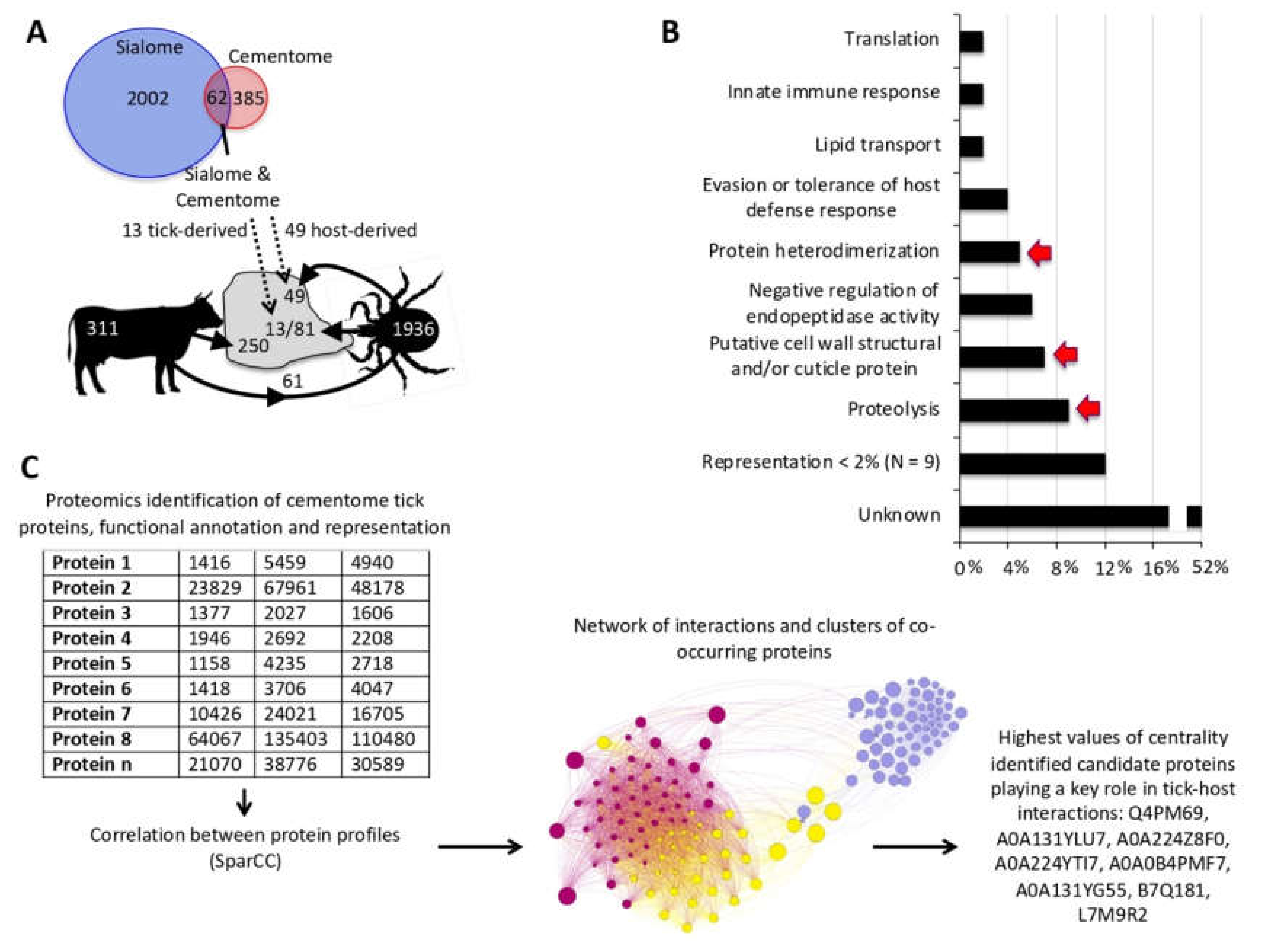
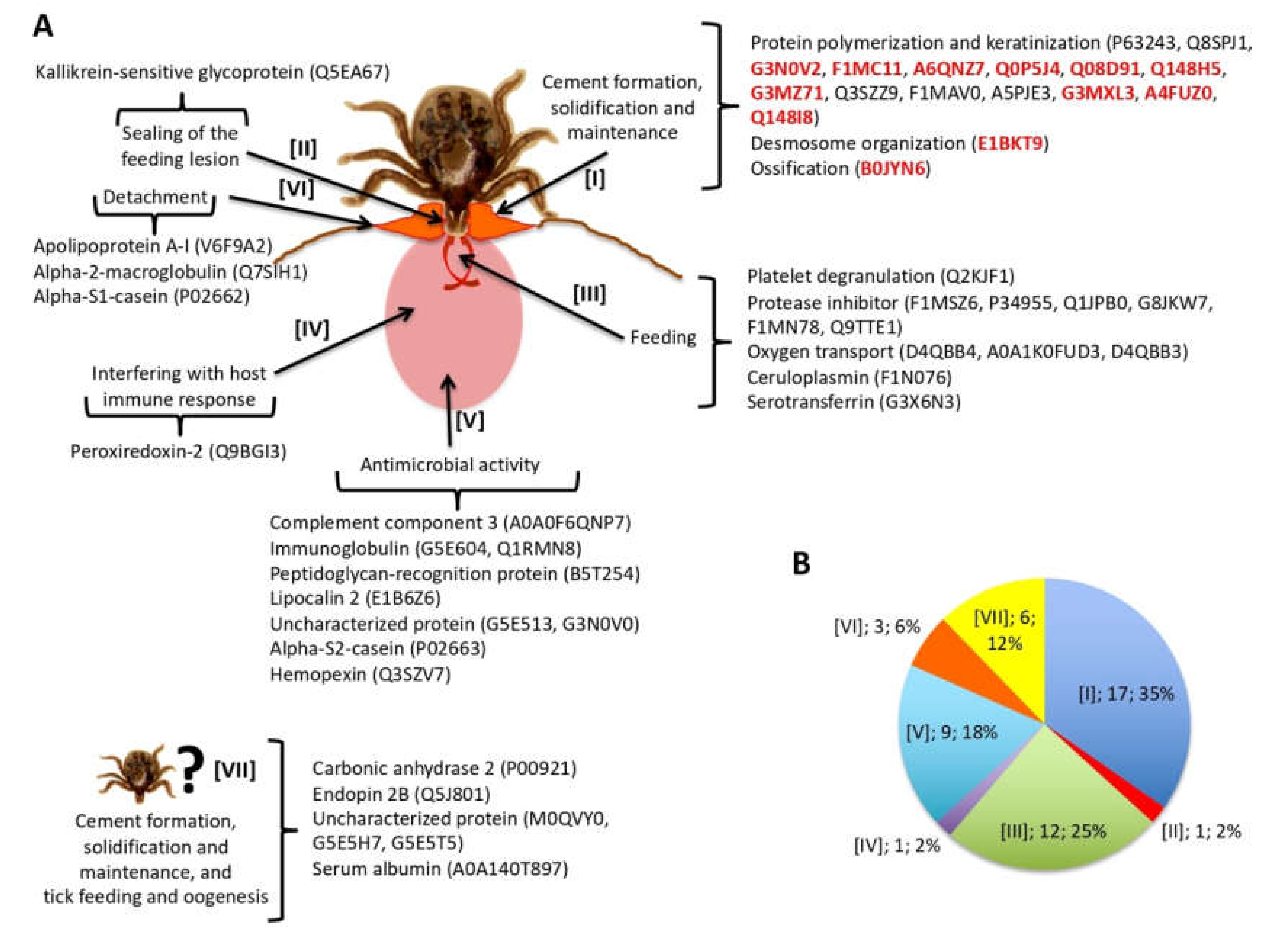
3.3. Tick-Derived Cementome Proteins Appear to be Involved in Cement Formation, Solidification, Maintenance, Feeding, and Interference with Host Immune Response and Detachment
3.4. Host-Derived Cementome Proteins Co-Exist with Tick-Derived Proteins and May Contribute to Cement Structure and Function
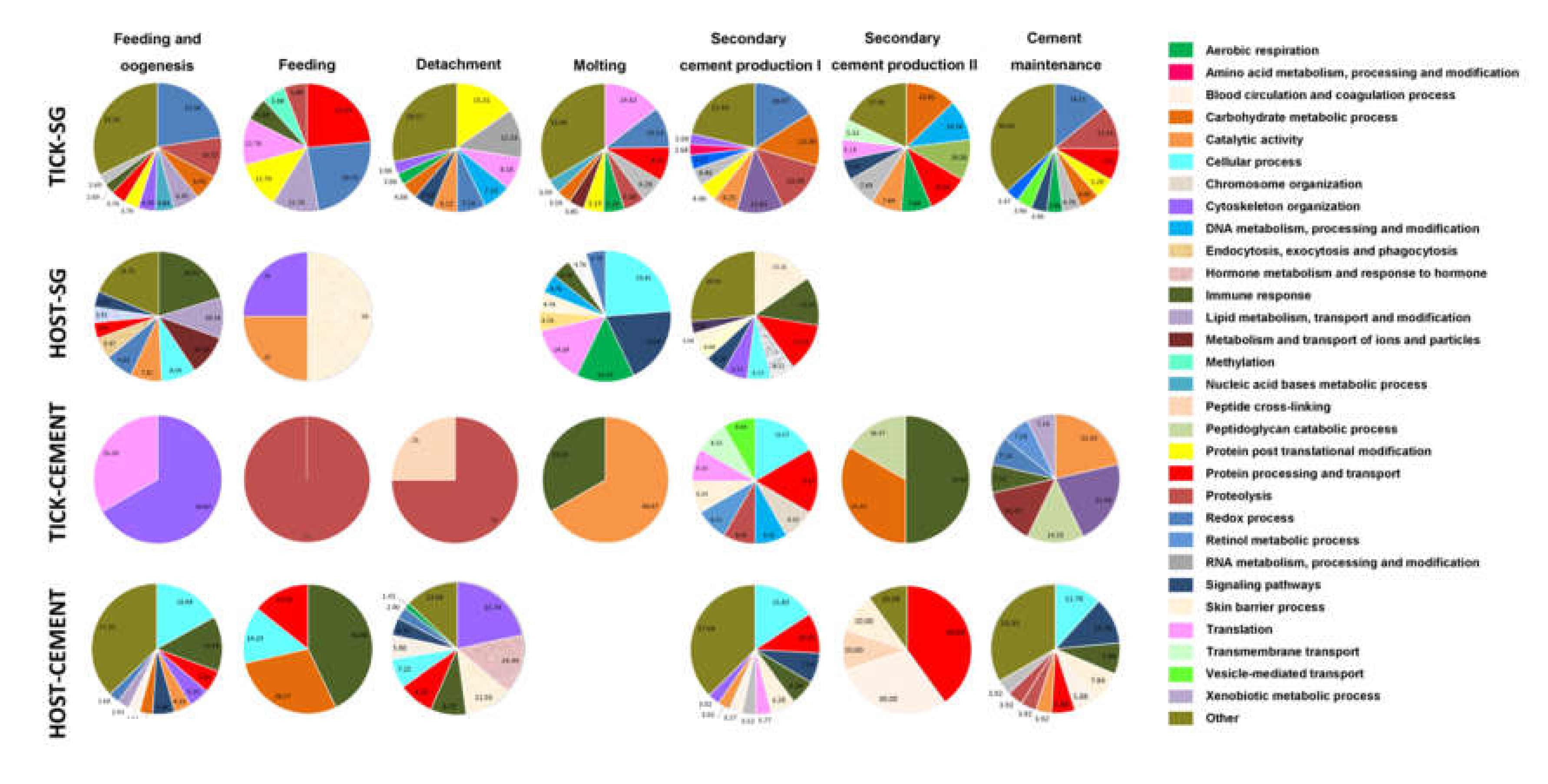
3.5. Biological Processes Represented in the Tick and Host Cementome Support Proposed Complementation and Synergy in Cement Formation and Function between Tick and Host Derived Proteins
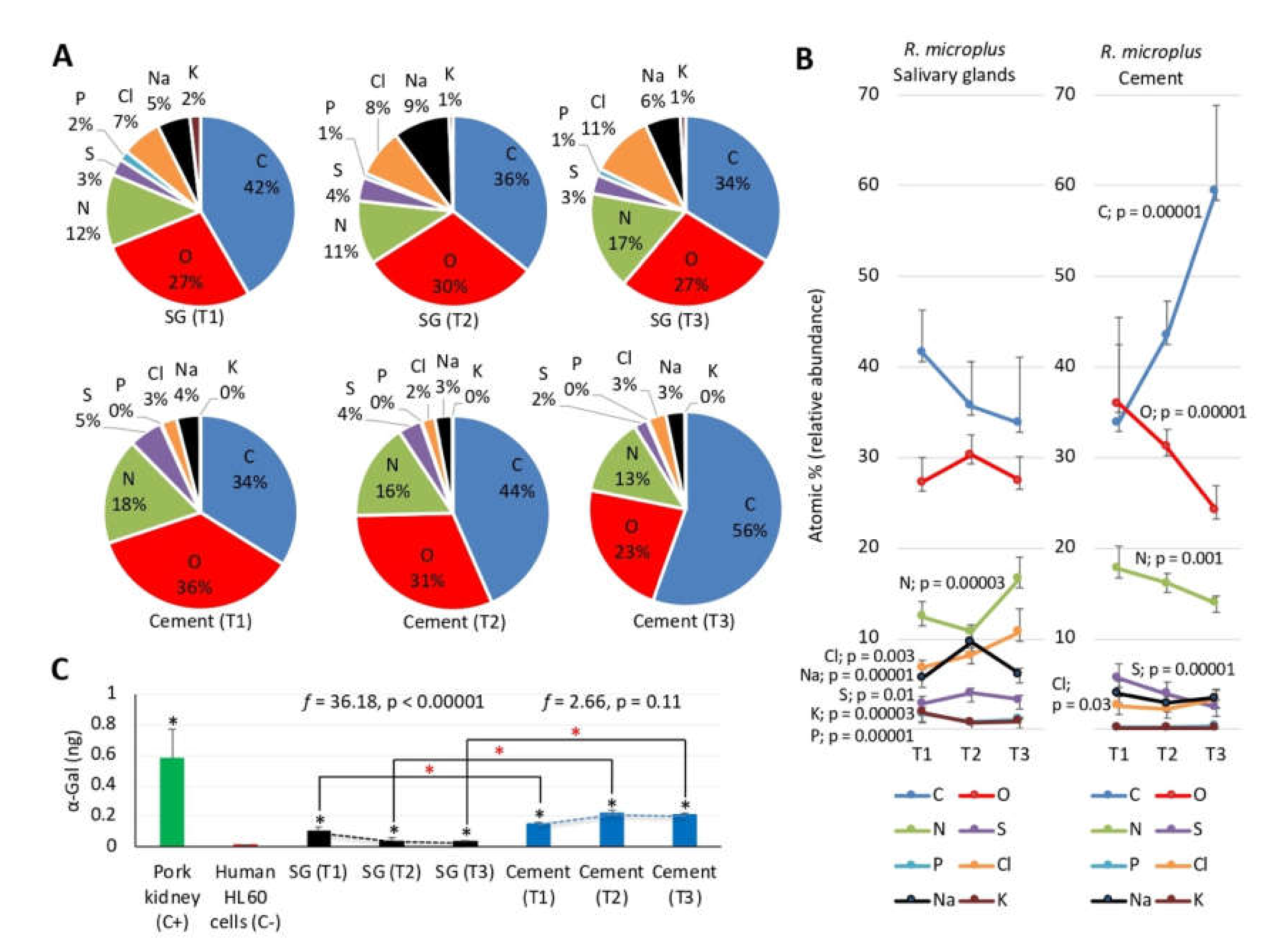
3.6. Physical and Chemical Properties of Tick Salivary Glands and Cement Show Tissue-Specific Differences and Vary during Tick Feeding
3.7. The Glycan α-Gal Content is Higher in the Cementome than in the Sialome throughout Tick Feeding
3.8. Western Blot Analysis of Selected Tick and Cattle Host Derived Cementome Proteins Support Proteomics Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Estrada-Peña, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.; Rashid, M.I.; Akbar, H.; Ahmad, L.; Hassan, M.A.; Ashraf, K.; Saeed, K.; Gharbi, M. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitology 2019, 146, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Marston, H.D.; Bloom, M.E.; Fauci, A.S. Tickborne diseases - confronting a growing threat. N. Engl. J. Med. 2018, 379, 701–703. [Google Scholar] [CrossRef]
- Molaei, G.; Little, E.A.; Williams, S.C.; Stafford, K.C. Bracing for the worst - range expansion of the lone star tick in the northeastern United States. N. Engl. J. Med. 2019, 381, 2189–2192. [Google Scholar] [CrossRef]
- Wang, H.; Nuttall, P.A. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology 1994, 109, 525–530. [Google Scholar] [CrossRef]
- Jasinskas, A.; Jaworski, D.C.; Barbour, A.G. Amblyomma americanum: Specific uptake of immunoglobulins into tick hemolymph during feeding. Exp. Parasitol. 2000, 96, 213–221. [Google Scholar] [CrossRef]
- Jasinskas, A.; Barbour, A.G. The Fc fragment mediates the uptake of immunoglobulin C from the midgut to hemolymph in the ixodid tick Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Kazimírová, M.; Štibrániová, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Hajdušek, O.; Šíma, R.; Ayllón, N.; Jalovecká, M.; Perner, J.; de la Fuente, J.; Kopáček, P. Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Kotál, J.; Langhansová, H.; Lieskovská, J.; Andersen, J.F.; Francischetti, I.M.; Chavakis, T.; Kopecký, J.; Pedra, J.H.; Kotsyfakis, M.; Chmelař, J. Modulation of host immunity by tick saliva. J. Proteomics. 2015, 128, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Tirloni, L.; Pinto, A.F.; Moresco, J.; Yates, J.R., 3rd; da Silva Vaz, I., Jr.; Mulenga, A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis. 2016, 10, e0004323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Moore, I.N.; Nagata, B.M.; Ribeiro, J.M.C.; Valenzuela, J.G.; Sonenshine, D.E. Ticks, Ixodes scapularis, feed repeatedly on white-footed mice despite strong inflammatory response: An expanding paradigm for understanding tick-host interactions. Front. Immunol. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, S.; Couto, J.; Ferrolho, J.; Rodrigues, F.; Nobre, J.; Santos, A.S.; Santos-Silva, M.M.; de la Fuente, J.; Domingos, A. Rhipicephalus bursa sialotranscriptomic response to blood feeding and Babesia ovis infection: Identification of candidate protective antigens. Front. Cell. Infect. Microbiol. 2018, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Perner, J.; Kropáčková, S.; Kopáček, P.; Ribeiro, J.M.C. Sialome diversity of ticks revealed by RNAseq of single tick salivary glands. PLoS Negl. Trop. Dis. 2018, 12, e0006410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollmann, T.; Kim, T.K.; Tirloni, L.; Radulović, Ž.M.; Pinto, A.F.M.; Diedrich, J.K.; Yates, J.R., 3rd; da Silva Vaz, I., Jr.; Mulenga, A. Identification and characterization of proteins in the Amblyomma americanum tick cement cone. Int. J. Parasitol. 2018, 48, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.R.; Pereira, M.C.; Simioni, P.U.; Nodari, E.F.; Paiatto, L.N.; Camargo-Mathias, M.I. Immunomodulatory and morphophysiological effects of Rhipicephalus sanguineus s.l. (Acari: Ixodidae) salivary gland extracts. Vet. Immunol. Immunopathol. 2019, 207, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Booth, C.J.; DePonte, K.; Wu, M.J.; Liang, X.; Mohanty, S.; Kantor, F.; Fikrig, E. Host-specific expression of Ixodes scapularis salivary genes. Ticks Tick-Borne Dis. 2019, 10, 386–397. [Google Scholar] [CrossRef]
- Mans, B.J. Chemical equilibrium at the tick-host feeding interface: A critical examination of biological relevance in hematophagous behavior. Front. Physiol. 2019, 10, 530. [Google Scholar] [CrossRef] [Green Version]
- Bullard, R.; Sharma, S.R.; Das, P.K.; Morgan, S.E.; Karim, S. Repurposing of glycine-rich proteins in abiotic and biotic stresses in the Lone-Star tick (Amblyomma americanum). Front. Physiol. 2019, 10, 744. [Google Scholar] [CrossRef]
- Chmelař, J.; Kotál, J.; Kovaříková, A.; Kotsyfakis, M. The use of tick salivary proteins as novel therapeutics. Front. Physiol. 2019, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Wonders of tick saliva. Ticks Tick-Borne Dis. 2019, 10, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, J.; Contreras, M.; Estrada-Peña, A.; Cabezas-Cruz, A. Targeting a global health problem: Vaccine design and challenges for the control of tick-borne diseases. Vaccine 2017, 35, 5089–5094. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J. Controlling ticks and tick-borne diseases...looking forward. Ticks Tick Borne Dis. 2018, 9, 1354–1357. [Google Scholar] [CrossRef]
- Wikel, S.K. Ticks and tick-borne infections: Complex ecology, agents, and host interactions. Vet. Sci. 2018, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, J.; Estrada-Peña, A. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Vancová, M.; Bílý, T.; Šimo, L.; Touš, J.; Horodyský, P.; Růžek, D.; Novobilský, A.; Salát, J.; Strnad, M.; Sonenshine, D.E.; et al. Three-dimensional reconstruction of the feeding apparatus of the tick Ixodes ricinus (Acari: Ixodidae): A new insight into the mechanism of blood-feeding. Sci. Rep. 2020, 10, 165. [Google Scholar] [CrossRef]
- Jaworski, D.C.; Rosell, R.; Coons, L.B.; Needham, G.R. Tick (Acari: Ixodidae) attachment cement and salivary gland cells contain similar immunoreactive polypeptides. J. Med. Entomol. 1992, 29, 305–309. [Google Scholar] [CrossRef]
- Suppan, J.; Engel, B.; Marchetti-Deschmann, M.; Nürnberger, S. Tick attachment cement- reviewing the mysteries of a biological skin plug system. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1056–1076. [Google Scholar] [CrossRef] [Green Version]
- Bullard, R.; Allen, P.; Chao, C.C.; Douglas, J.; Das, P.; Morgan, S.E.; Ching, W.M.; Karim, S. Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick-Borne Dis. 2016, 7, 880–892. [Google Scholar] [CrossRef] [Green Version]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Estrada-Peña, A.; Kocan, K.M.; de la Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, N.; Villar, V.; Galindo, R.C.; Kocan, K.M.; Šíma, R.; López, J.A.; Vázquez, J.; Alberdi, P.; Cabezas-Cruz, A.; Kopáček, P.; et al. Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS Genetics 2015, 11, e1005120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, M.; Ayllón, N.; Alberdi, P.; Moreno, A.; Moreno, M.; Tobes, R.; Mateos-Hernández, L.; Weisheit, S.; Bell-Sakyi, L.; de la Fuente, J. Integrated metabolomics, transcriptomics and proteomics identifies metabolic pathways affected by Anaplasma phagocytophilum infection in tick cells. Mol. Cell. Proteomics. 2015, 14, 3154–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillet, L.C.; Navarro, P.; Tate, S.; Rost, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy Server. Humana Press 2005. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Villar, M.; Artigas-Jerónimo, S.; López, V.; Alberdi, P.; Cabezas-Cruz, A.; de la Fuente, J. Use of graph theory to characterize human and arthropod vector cell protein response to infection. Front. Cell. Infect. Microbiol. 2018, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling interactions in the microbiome: A network perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef]
- Opsahl, T.; Agneessens, F.; Skvoretz, J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc. Networks 2010, 32, 245–251. [Google Scholar] [CrossRef]
- Barthelemy, M. Betweenness centrality in large complex networks. Eur. Phys. J. B. 2004, 38, 163–168. [Google Scholar] [CrossRef]
- Almazán, C.; Moreno-Cantú, O.; Moreno-Cid, J.A.; Galindo, R.C.; Canales, M.; Villar, M.; de la Fuente, J. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine 2012, 30, 265–272. [Google Scholar] [CrossRef]
- Canales, M.; Almazán, C.; Pérez de la Lastra, J.M.; de la Fuente, J. Anaplasma marginale major surface protein 1a directs cell surface display of tick BM95 immunogenic peptides on Escherichia coli. J. Biotechnol. 2008, 135, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Marinela, C.; Moreno-Cid, J.A.; Domingos, A.; Canales, M.; Díez-Delgado, I.; Pérez de la Lastra, J.M.; Sánchez, E.; Merino, O.; López Zavala, R.; Ayllón, N.; et al. Bacterial membranes enhance the immunogenicity and protective capacity of the surface exposed tick Subolesin-Anaplasma marginale MSP1a chimeric antigen. Ticks Tick-Borne Dis. 2015, 6, 2820–2828. [Google Scholar]
- Merino, M.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Villar, M.; Jiménez, M.; Pinal, R.; Estrada- Peña, A.; Molina, R.; Lucientes, J.; Gortázar, C.; de la Fuente, J. Control of multiple arthropod vector infestations with subolesin/akirin vaccines. Vaccine 2013, 31, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Lima-Barbero, J.F.; Prado, E.; Pacheco, I.; Alberdi, P.; Villar, M. Anaplasma pathogen infection alters chemical composition of the exoskeleton of hard ticks (Acari: Ixodidae). Comput. Struct. Biotechnol. J. 2020, 18, 253–257. [Google Scholar] [CrossRef]
- Senbill, H.; Kanta Hazarika, L.; Baruah, A.; Kumar Borah, D.; Bhattacharyya, B.; Rahman, S. Life cycle of the southern cattle tick, Rhipicephalus (Boophilus) microplus Canestrini 1888 (Acari: Ixodidae) under laboratory conditions. Syst. Appl. Acarol. 2018, 23, 1169–1179. [Google Scholar] [CrossRef]
- Jorgensen, W.K.; Kemp, D.H. Continued functioning of the feeding apparatus during moulting of Boophilus microplus as an adaptation of one-host ticks. J. Parasitol. 1986, 72, 846–851. [Google Scholar] [CrossRef]
- Kim, T.K.; Curran, J.; Mulenga, A. Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability. J. Exp. Biol. 2014, 217, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Chinery, W.A. The nature and origin of the “cement” substance at the site of attachment and feeding of adult Haemaphysalis spinigera (Ixodidae). J. Med. Entomol. 1973, 10, 355–362. [Google Scholar] [CrossRef]
- Marhabaie, M.; Leeper, T.C.; Blackledge, T.A. Protein composition correlates with the mechanical properties of spider (Argiope trifasciata) dragline silk. Biomacromolecules 2014, 15, 20–29. [Google Scholar] [CrossRef]
- Pham, T.; Chuang, T.; Lin, A.; Joo, H.; Tsai, J.; Crawford, T.; Zhao, L.; Williams, C.; Hsia, Y.; Vierra, C. Dragline silk: A fiber assembled with low-molecular-weight cysteine-rich proteins. Biomacromolecules 2014, 15, 4073–4081. [Google Scholar] [CrossRef] [PubMed]
- Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [PubMed]
- Huang, H.J.; Chen, W.Y.; Wu, J.H. Total protein extraction for metaproteomics analysis of methane producing biofilm: The effects of detergents. Int. J. Mol. Sci. 2014, 15, 10169–10184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.K.; Tirloni, L.; Pinto, A.F.M.; Diedrich, J.K.; Moresco, J.J.; Yates, J.R., 3rd; da Silva Vaz, I., Jr.; Mulenga, A. Time-resolved proteomic profile of Amblyomma americanum tick saliva during feeding. PLoS Negl. Trop. Dis. 2020, 14, e0007758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landulfo, G.A.; Li, A.Y.; Lima, A.S.; Silva, N.C.S.; Vale, T.L.; Costa-Junior, L.M. Feeding and respiratory gas exchange of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Exp. Appl. Acarol. 2019, 78, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Maksymowicz, K.; Marycz, K.; Szotek, S.; Kaliński, K.; Serwa, E.; Łukomski, R.; Czogała, J. Chemical composition of human and canine fascia lata. Acta Biochim. Pol. 2012, 59, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Ragaisiene, A.; Rusinavičiūtė, J.; Milašiene, D.; Ivanauskas, R. Comparison of selected chemical properties of fibres from different breeds of dogs and German blackface sheep. Fibres & textiles in Eastern Europe 2016, 24, 21–28. [Google Scholar]
- Heylen, D.; Schmidt, O.; Dautel, H.; Gern, L.; Kampen, H.; Newton, J.; Gray, J. Host identification in unfed ticks from stable isotope compositions (δ13 C and δ15 N). Med. Vet. Entomol. 2019, 33, 360–366. [Google Scholar] [CrossRef]
- Analysis of Trace Elements in Cattle. BioCheck - Labor fur Veterinardiagnostik und Umwelthygiene GmbH. 2014. Available online: https://www.biocheck-leipzig.de/images/stories/biocheck/pdf/News_2_14_e.pdf (accessed on 26 February 2020).
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742. [Google Scholar] [CrossRef]
- Alberdi, P.; Cabezas-Cruz, A.; Espinosa, P.J.; Villar, M.; Artigas-Jerónimo, S.; de la Fuente, J. The redox metabolic pathways function to limit Anaplasma phagocytophilum infection and multiplication while preserving fitness in tick vector cells. Sci. Rep. 2019, 9, 13236. [Google Scholar] [CrossRef]
- Della Noce, B.; Carvalho Uhl, M.V.; Machado, J.; Waltero, C.F.; de Abreu, L.A.; da Silva, R.M.; da Fonseca, R.N.; de Barros, C.M.; Sabadin, G.; Konnai, S.; et al. Carbohydrate metabolic compensation coupled to high tolerance to oxidative stress in ticks. Sci. Rep. 2019, 9, 4753. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.; Hochberg, R. Elemental characterization of the exoskeleton in the whipscorpions Mastigoproctus giganteus and Typopeltis dalyi (Arachnida: Thelyphonida). Invertebrate Biol. 2017, 136, 345–359. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2016, 27, 3–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nunen, S.; O’Connor, K.S.; Clarke, L.R.; Boyle, R.X.; Fernando, S.L. The association between Ixodes holocyclus tick bite reactions and red meat allergy. Intern. Med. J. 2007, 39, A132. [Google Scholar]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.D.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Saleh, H.; Embry, S.; Nauli, A.; Atyia, S.; Krishnaswamy, G. Anaphylactic reactions to oligosaccharides in red meat: A syndrome in evolution. Clin. Mol. Allergy. 2012, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Steinke, J.W.; Platts-Mills, T.A.; Commins, S.P. The alpha-gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015, 135, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Platts-Mills, T.A.; Schuyler, A.J.; Tripathi, A.; Commins, S.P. Anaphylaxis to the carbohydrate side chain alpha-gal. Immunol. Allergy Clin. North. Am. 2015, 35, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Hernández, L.; Villar, M.; Moral, A.; Rodríguez, C.G.; Arias, T.A.; de la Osa, V.; Brito, F.F.; Fernández de Mera, I.G.; Alberdi, P.; Ruiz-Fons, F.; et al. Tick-host conflict: Immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget 2017, 8, 20630–20644. [Google Scholar] [CrossRef]
- Galili, U. Evolution in primates by “catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anticarbohydrate antibodies. Am. J. Phys. Anthropol. 2018, 168, 352–363. [Google Scholar] [CrossRef]
- Hilger, C.; Fischer, J.; Wölbing, F.; Biedermann, T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr. Allergy Asthma Rep. 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezas-Cruz, A.; Valdés, J.; de la Fuente, J. Cancer research meets tick vectors for infectious diseases. Lancet. Infect. Dis. 2014, 10, 916–917. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Cruz, A.; Mateos-Hernández, L.; Alberdi, P.; Villar, M.; Riveau, G.; Hermann, E.; Schacht, A.; Khalife, J.; Correia-Neves, M.; Gortázar, C.; et al. Effect of blood type on anti-α-Gal immunity and the incidence of infectious diseases. Exp. Mol. Med. 2017, 49, e301. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Hodžić, A.; Román-Carrasco, P.; Mateos-Hernández, L.; Duscher, G.G.; Sinha, D.K.; Hemmer, W.; Swoboda, I.; Estrada-Peña, A.; de la Fuente, J. Environmental and molecular drivers of the α-Gal syndrome. Front. Immunol. 2019, 10, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, J.; Pacheco, I.; Villar, M.; Cabezas-Cruz, A. The alpha-Gal syndrome: New insights into the tick-host conflict and cooperation. Parasit. Vectors 2019, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, L.; Luo, J.; Guo, L.; Wang, Z.; Yang, X.; Jin, W.; Fang, Y.; Ye, J.; Shan, B.; et al. SWATH enables precise label-free quantification on proteome scale. Proteomics 2015, 15, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; Pastor Comín, J.J.; Villar, M.; Contreras, M.; Alberdi, P.; León Viera, I.; Soto, L.; Cordero, R.; Valdés, J.J.; Cabezas-Cruz, A.; et al. A novel combined scientific and artistic approach for advanced characterization of interactomes: The Akiri/Subolesin model. Vaccines 2020, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Villar, M.; López, V.; Ayllón, N.; Cabezas-Cruz, A.; López, J.A.; Vázquez, J.; Alberdi, P.; de la Fuente, J. The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit. Vectors 2016, 9, 467. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, J.G.; Francischetti, I.M.; Pham, V.M.; Garfield, M.K.; Mather, T.N.; Ribeiro, J.M. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2002, 205, 2843–2864. [Google Scholar]
- Madden, R.D.; Sauer, J.R.; Dillwith, J.W. A proteomics approach to characterizing tick salivary secretions. Exp. Appl. Acarol. 2002, 28, 77–87. [Google Scholar] [CrossRef]
- Diaz-Martin, V.; Manzano-Roman, R.; Valero, L.; Oleaga, A.; Encinas-Grandes, A.; Perez-Sanchez, R. An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J. Proteomics. 2013, 80, 216–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.J.; Anatriello, E.; de Miranda-Santos, I.K.; Francischetti, I.M.; Sá-Nunes, A.; Ferreira, B.R.; Ribeiro, J.M. Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks Tick-Borne Dis. 2013, 4, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirloni, L.; Reck, J.; Terra, R.M.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R., 3rd; Termignoni, C.; Pinto, A.F.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirloni, L.; Islam, M.S.; Kim, T.K.; Diedrich, J.K.; Yates, J.R., 3rd; Pinto, A.F.; Mulenga, A.; You, M.J.; da Silva Vaz, I., Jr. Saliva from nymph and adult females of Haemaphysalis longicornis: A proteomic study. Parasit. Vectors 2015, 8, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickramasekara, S.; Bunikis, J.; Wysocki, V.; Barbour, A.G. Identification of residual blood proteins in ticks by mass spectrometry proteomics. Emerg. Infect. Dis. 2008, 14, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.; Torina, A.; Nuñez, Y.; Zivkovic, Z.; Marina, A.; Alongi, A.; Scimeca, S.; La Barbera, G.; Caracappa, S.; Vázquez, J.; et al. Application of highly sensitive saturation labelling to the analysis of differential protein expression in infected ticks from limited samples. Proteome Sci. 2010, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Willadsen, P.; Smith, D.; Cobon, G.; McKenna, R.V. Comparative vaccination of cattle against Boophilus microplus with recombinant antigen Bm86 alone or in combination with recombinant Bm91. Parasite Immunol. 1996, 18, 241–246. [Google Scholar] [CrossRef]
- Masina, S.; Broady, K.W. Tick paralysis: Development of a vaccine. Int. J. Parasitol. 1999, 29, 535–541. [Google Scholar] [CrossRef]
- Contreras, M.; Alberdi, P.; Fernández de Mera, I.G.; Krull, C.; Nijhof, A.; Villar, M.; de la Fuente, J. Vaccinomics approach to the identification of candidate protective antigens for the control of tick vector infestations and Anaplasma phagocytophilum infection. Front. Cell. Infect. Microbiol. 2017, 7, 360. [Google Scholar] [CrossRef]
- Stewart, R.J.; Ransom, T.C.; Hlady, V. Natural underwater adhesives. Journal of Polymer Science Part. B: Polymer Physics 2011, 49, 757–771. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar, M.; Pacheco, I.; Merino, O.; Contreras, M.; Mateos-Hernández, L.; Prado, E.; Barros-Picanço, D.K.; Lima-Barbero, J.F.; Artigas-Jerónimo, S.; Alberdi, P.; et al. Tick and Host Derived Compounds Detected in the Cement Complex Substance. Biomolecules 2020, 10, 555. https://doi.org/10.3390/biom10040555
Villar M, Pacheco I, Merino O, Contreras M, Mateos-Hernández L, Prado E, Barros-Picanço DK, Lima-Barbero JF, Artigas-Jerónimo S, Alberdi P, et al. Tick and Host Derived Compounds Detected in the Cement Complex Substance. Biomolecules. 2020; 10(4):555. https://doi.org/10.3390/biom10040555
Chicago/Turabian StyleVillar, Margarita, Iván Pacheco, Octavio Merino, Marinela Contreras, Lourdes Mateos-Hernández, Eduardo Prado, Dina Karen Barros-Picanço, José Francisco Lima-Barbero, Sara Artigas-Jerónimo, Pilar Alberdi, and et al. 2020. "Tick and Host Derived Compounds Detected in the Cement Complex Substance" Biomolecules 10, no. 4: 555. https://doi.org/10.3390/biom10040555
APA StyleVillar, M., Pacheco, I., Merino, O., Contreras, M., Mateos-Hernández, L., Prado, E., Barros-Picanço, D. K., Lima-Barbero, J. F., Artigas-Jerónimo, S., Alberdi, P., Fernández de Mera, I. G., Estrada-Peña, A., Cabezas-Cruz, A., & de la Fuente, J. (2020). Tick and Host Derived Compounds Detected in the Cement Complex Substance. Biomolecules, 10(4), 555. https://doi.org/10.3390/biom10040555








