Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Line and Macrophages Preparation
2.3. Single-Cell Ca2+ Imaging
2.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.5. α-Bungarotoxin Binding Assay
2.6. Whole-Cell Patch Clamp
2.7. ELISA
2.8. Quantitative Real-Time PCR (q-PCR)
2.9. Data and Statistical Analysis
3. Results
3.1. Expression of nAChR Subunits in THP-1Mϕ and MDMs
3.2. Functional Expression of α7 nAChRs in THP-1 Macrophages (THP-1Mϕ)
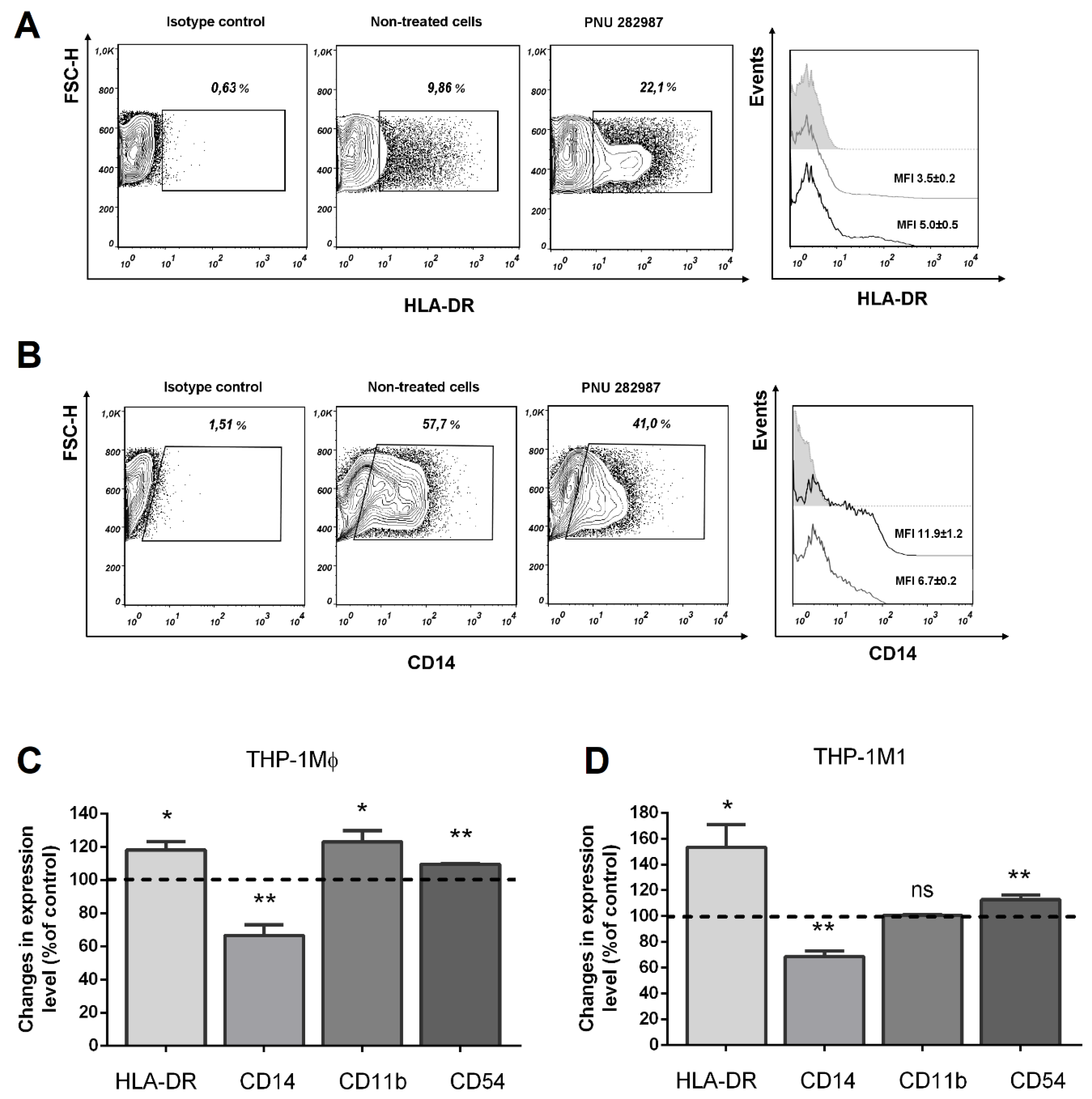
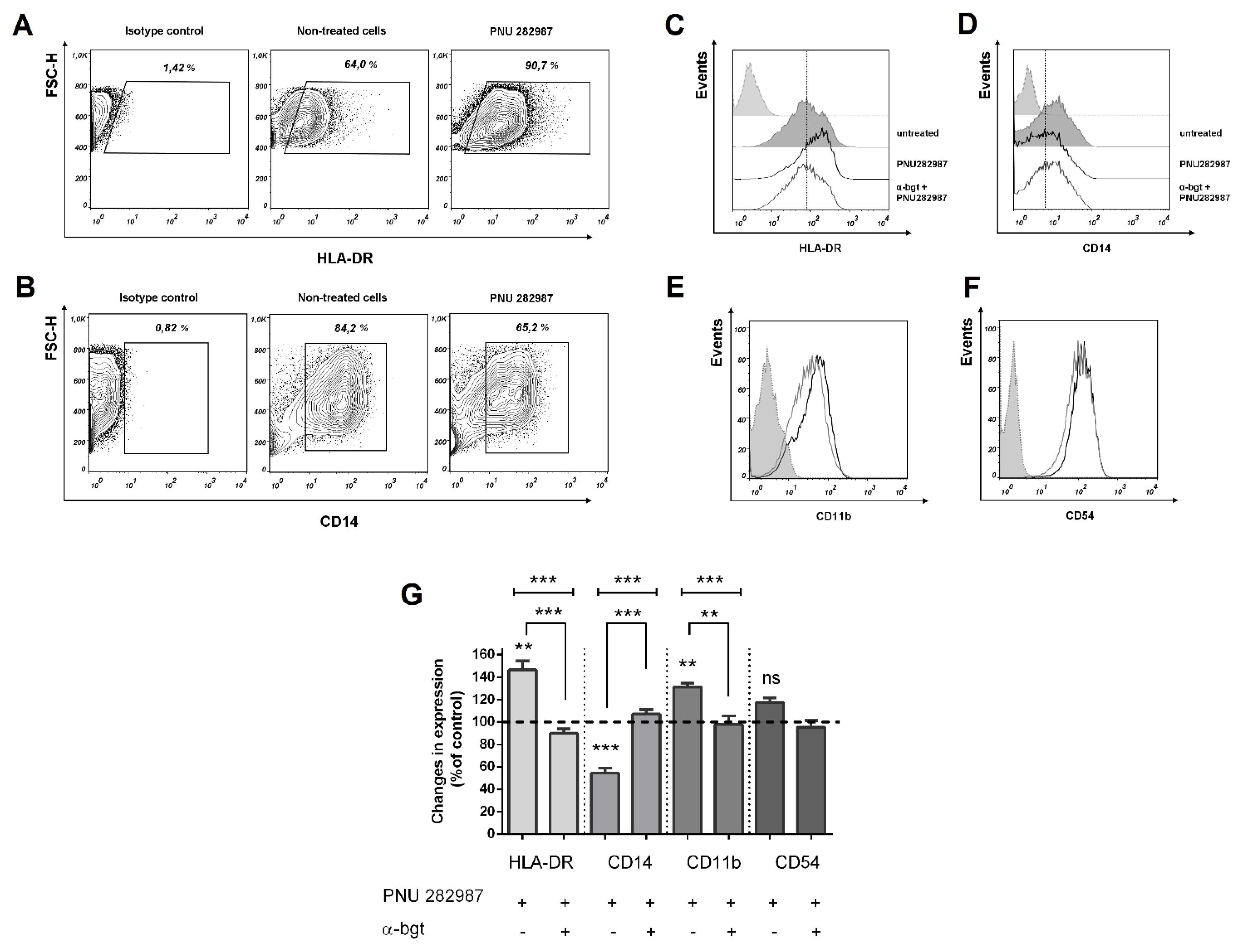
3.3. Activation of α7 nAChRs with PNU 282,987 Regulates the Expression of HLA-DR, CD14, and CD54, CD11b in THP-1Mϕ, THP-1M1, and MDMs
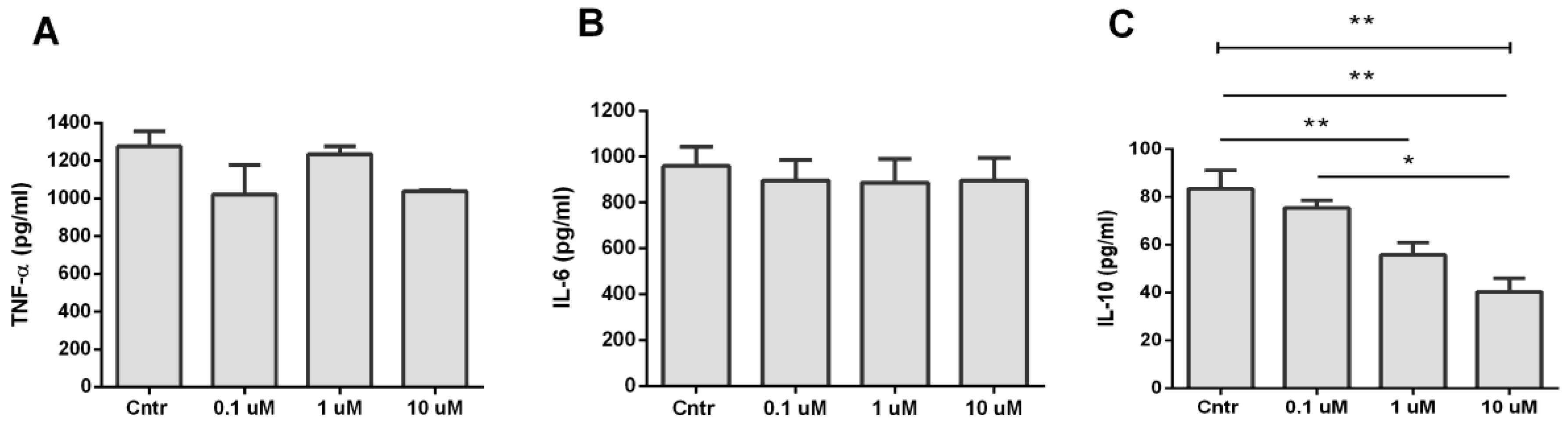
3.4. Analysis of TNF-α, IL-6, and IL-10 Production during Activation of α7 nAChR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouzat, C. New insights into the structural bases of activation of Cys-loop receptors. J. Physiol. Paris 2012, 106, 23–33. [Google Scholar] [CrossRef]
- Pohanka, M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amelia, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, N.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Kalkman, H.O.; Feuerbach, D. Modulatory effects of α7 nAChRs on the immune system and its relevance for CNS disorders. Cell. Mol. Life Sci. 2016, 73, 2511–2530. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Kamochi, H.; Suzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 2006, 146, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, D.L.; Bi, X.Y.; Sun, L.; Yu, X.J.; Fang, H.L.; Miao, Y.; Zhao, M.; He, X.; Liu, J.J.; et al. Acetylcholine Inhibits LPS-Induced MMP-9 Production and Cell Migration via the 7 nAChR-JAK2/STAT3 Pathway in RAW264.7 Cells. Cell. Physiol. Biochem. 2015, 36, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Valdés-Ferrer, S.I.; Patel, N.B.; Chavan, S.; Al-Abed, Y.; Yang, H.; Tracey, K.J. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 2009, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Receptor-Dependent Mechanisms of Cell Stimulation by Bacterial Endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, X.; Su, E.M.; Huang, Y.; Li, L.; Matthay, M.A.; Su, X. Signals of vagal circuits engaging with AKT1 in α7 nAChR + CD11b + cells lessen E. coli and LPS-induced acute inflammatory injury. Cell Discov. 2017, 3, 17009. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; O’Dea, K.; Gordon, A. Immune therapy in sepsis: Are we ready to try again? J. Intensive Care Soc. 2018, 19, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Ocklind, G.; Friedrichs, D.; Peters, J.H. Expression of CD54, CD58, CD14, and HLA-DR on macrophages and macrophage-derived accessory cells and their accessory capacity. Immunol. Lett. 1992, 15, 253–258. [Google Scholar] [CrossRef]
- van Spriel, A.B.; Leusen, J.H.W.; Vilé, H.; van de Winkel, J.G.J. Mac-1 (CD11b/CD18) as Accessory Molecule for FcαR (CD89) Binding of IgA. J. Immunol. 2002, 169, 3831–3836. [Google Scholar] [CrossRef]
- Winkler, M.S.; Rissiek, A.; Priefler, M.; Schwedhelm, E.; Robbe, L.; Bauer, A.; Zahrte, C.; Zoellner, C.; Kluge, S.; Nierhaus, A. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PLoS ONE 2017, 13, e0182427. [Google Scholar] [CrossRef] [PubMed]
- Weijer, S.; Lauw, F.N.; Branger, J.; van den Blink, B.; van der Poll, T. Diminished Interferon-γ Production and Responsiveness after Endotoxin Administration to Healthy Humans. J. Infect. Dis. 2002, 12, 1748–1753. [Google Scholar] [CrossRef][Green Version]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Beiter, E.R.; Walton, A.H.; Hall, M.W.; Hotchkiss, R.S. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: A prospective observational study. Crit. Care 2016, 20, 334. [Google Scholar] [CrossRef]
- Kim, O.Y.; Monsel, A.; Bertrand, M.; Coriat, P.; Cavaillon, J.M.; Adib-Conquy, M. Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit. Care 2010, 14, R61. [Google Scholar] [CrossRef]
- Adib-Conquy, M.; Cavaillon, J.M. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 2009, 101, 36–47. [Google Scholar]
- Alingrin, J.; Coiffard, B.; Textoris, J.; Nicolino-Brunet, C.; Gossez, M.; Jarrot, P.A.; Dignat-George, F.; Monneret, G.; Thomas, P.A.; Leone, M.; et al. Sepsis is associated with lack of monocyte HLA-DR expression recovery without modulating T-cell reconstitution after lung transplantation. Transpl. Immunol. 2018, 51, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohé, J.; Venet, F.; Debard, A.L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Haveman, J. Systemic Inflammation and Monocyte Function after Major Surgery; University Library Groningen: Groningen, The Netherlands, 2009. [Google Scholar]
- Kim, S.Y.; Kang, K.L.; Lee, J.C.; Heo, J.S. Nicotinic acetylcholine receptor α7 and β4 subunits contribute to nicotine-induced apoptosis in periodontal ligament stem cells. Mol. Cells 2012, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, Q.; Leech, C.A.; Yepuri, N.; Zhang, L.; Holz, G.G.; Wang, C.; Cooney, R.N. A7 nicotinic acetylcholine receptor regulates the function and viability of L cells. Endocrinology 2018, 159, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Musso, T.; Calosso, L.; Zucca, M.; Millesimo, M.; Puliti, M.; Bulfone-Paus, S.; Merlino, C.; Savoia, D.; Cavallo, R.; Ponzi, A.N.; et al. Interleukin-15 activates proinflammatory and antimicrobial functions in polymorphonuclear cells. Infect. Immun. 1998, 66, 2640–2647. [Google Scholar] [CrossRef]
- Khan, M.A.S.; Farkhondeh, M.; Crombie, J.; Jacobson, L.; Kaneki, M.; Martyn, J.A.J. Lipopolysaccharide upregulates α7 acetylcholine receptors: Stimulation with Gts-21 mitigates growth arrest of macrophages and improves survival in Burned Mice. Shock 2012, 38, 213–219. [Google Scholar] [CrossRef]
- Lee, R.H.; Vazquez, G. Evidence for a prosurvival role of alpha-7 nicotinic acetylcholine receptor in alternatively (M2)-activated macrophages. Physiol. Rep. 2013, 1, e00189. [Google Scholar] [CrossRef]
- Reichrath, S.; Reichrath, J.; Moussa, A.T.; Meier, C.; Tschernig, T. Targeting the non-neuronal cholinergic system in macrophages for the management of infectious diseases and cancer: Challenge and promise. Cell Death Discov. 2016, 17, 2. [Google Scholar] [CrossRef][Green Version]
- Mikulski, Z.; Hartmann, P.; Jositsch, G.; Zasłona, Z.; Lips, K.S.; Pfeil, U.; Kurzen, H.; Lohmeyer, J.; Clauss, W.G.; Grau, V.; et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir. Res. 2010, 11, 133. [Google Scholar]
- Hecker, A.; Küllmar, M.; Wilker, S.; Richter, K.; Zakrzewicz, A.; Atanasova, S.; Mathes, V.; Timm, T.; Lerner, S.; Klein, J.; et al. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1β Release. J. Immunol. 2015, 195, 2325–2334. [Google Scholar] [CrossRef]
- Suzuki, T.; Hide, I.; Matsubara, A.; Hama, C.; Harada, K.; Miyano, K.; Andrä, M.; Matsubayashi, H.; Sakai, N.; Kohsaka, S.; et al. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006, 83, 1461–1470. [Google Scholar] [CrossRef]
- King, J.R.; Ullah, A.; Bak, E.; Saleet Jafri, M.; Kabbani, N. Ionotropic and metabotropic mechanisms of allosteric modulation of A7 nicotinic receptor intracellular calcium S. Mol. Pharmacol. 2018, 93, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Shelukhina, I.; Spirova, E.; Kudryavtsev, D.; Ojomoko, L.; Werner, M.; Methfessel, C.; Hollmann, M.; Tsetlin, V. Calcium imaging with genetically encoded sensor Case12: Facile analysis of α7/α9 nAChR mutants. PLoS ONE 2017, 12, e0181936. [Google Scholar] [CrossRef] [PubMed]
- Barykin, E.P.; Garifulina, A.I.; Kruykova, E.V.; Spirova, E.N.; Anashkina, A.A.; Adzhubei, A.A.; Shelukhina, I.V.; Kasheverov, I.E.; Mitkevich, V.A.; Kozin, S.A.; et al. Isomerization of Asp7 in Beta-Amyloid Enhances Inhibition of the α7 Nicotinic Receptor and Promotes Neurotoxicity. Cells 2019, 8, 771. [Google Scholar] [CrossRef] [PubMed]
- Shelukhina, I.; Paddenberg, R.; Kummer, W.; Tsetlin, V. Functional expression and axonal transport of α7 nAChRs by peptidergic nociceptors of rat dorsal root ganglion. Brain Struct. Funct. 2015, 220, 1885–1899. [Google Scholar] [CrossRef]
- Báez-Pagán, C.A.; Delgado-Vélez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kang, K.M.; Kim, J.H.; Bahn, J.J.; Jeon, D.; Kim, M.; Lee, S.K.; Roh, J.K. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010, 1309, 164–171. [Google Scholar] [CrossRef]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. Basic Clin. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Sato, K.; Nagayama, H.; Tadokoro, K.; Juji, T.; Takahashi, T.A. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J. Immunol. 1999, 162, 3865–3872. [Google Scholar]
- Zanetti, S.R.; Ziblat, A.; Torres, N.I.; Zwirner, N.W.; Bouzat, C. Expression and functional role of α7 nicotinic receptor in human cytokine-stimulated natural killer (NK) cells. J. Biol. Chem. 2016, 291, 16541–16552. [Google Scholar] [CrossRef] [PubMed]
- Razani-Boroujerdi, S.; Boyd, R.T.; Dávila-García, M.I.; Nandi, J.S.; Mishra, N.C.; Singh, S.P.; Pena-Philippides, J.C.; Langley, R.; Sopori, M.L. T Cells Express α7-Nicotinic Acetylcholine Receptor Subunits That Require a Functional TCR and Leukocyte-Specific Protein Tyrosine Kinase for Nicotine-Induced Ca 2+ Response. J. Immunol. 2007, 179, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Skok, M.V.; Kalashnik, E.N.; Koval, L.N.; Tsetlin, V.I.; Utkin, Y.N.; Changeux, J.P.; Grailhe, R. Functional nicotinic acetylcholine receptors are expressed in B lymphocyte-derived cell lines. Mol. Pharmacol. 2003, 64, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.F.; Chruscinski, A.; De Jesus Perez, V.A.; Singh, H.; Pitsiouni, M.; Rabinovitch, M.; Utz, P.J.; Cooke, J.P. Cholinergic modulation of angiogenesis: Role of the 7 nicotinic acetylcholine receptor. J. Cell. Biochem. 2009, 108, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Emilie, L.; Françoise, L.R.; Frank, A. Lymphocytes prime activation is required for nicotine-induced calcium waves. Front. Biosci. Elit. 2010, 2, 928–939. [Google Scholar]
- Padilla, A.; Keating, P.; Hartmann, J.X.; Marí, F. Effects of α-conotoxin ImI on TNF-α, IL-8 and TGF-β expression by human macrophage-like cells derived from THP-1 pre-monocytic leukemic cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Thorgersen, E.B.; Pischke, S.E.; Barratt-Due, A.; Fure, H.; Lindstad, J.K.; Pharo, A.; Hellerud, B.C.; Mollnes, T.E. Systemic CD14 inhibition attenuates organ inflammation in porcine Escherichia coli sepsis. Infect. Immun. 2013, 81, 3173–3181. [Google Scholar] [CrossRef]
- Berenger, B.M.; Hamill, J.; Stack, D.; Montgomery, E.; Huston, S.M.; Timm-McCann, M.; Epelman, S.; Mody, C.H. Membrane CD14, but not soluble CD14, is used by exoenzyme S from P. aeruginosa to signal proinflammatory cytokine production. J. Leukoc. Biol. 2011, 90, 189–198. [Google Scholar] [CrossRef]
- Egge, K.H.; Thorgersen, E.B.; Lindstad, J.K.; Pharo, A.; Lambris, J.D.; Barratt-Due, A.; Mollnes, T.E. Post challenge inhibition of C3 and CD14 attenuates Escherichia coli-induced inflammation in human whole blood. Innate Immun. 2014, 20, 68–77. [Google Scholar] [CrossRef]
- Theodorakis, E.; Diamantaki, E.; Tsatsanis, C.; Georgopoulos, D.; Vaporidi, K. Macrophage phenotype in sepsis immunosuppression. Crit. Care 2015, 19, P44. [Google Scholar] [CrossRef]
- Cheng, Y.; Marion, T.N.; Cao, X.; Wang, W.; Cao, Y. Park 7: A novel therapeutic target for macrophages in sepsis-induced immunosuppression. Front. Immunol. 2018, 9, 2631. [Google Scholar] [CrossRef]
- Zhuang, Y.; Peng, H.; Chen, Y.; Zhou, S.; Chen, Y. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front. Biosci. Landmark 2017, 22, 1344–1354. [Google Scholar]
- Lebedeva, T.; Dustin, M.L.; Sykulev, Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr. Opin. Immunol. 2005, 17, 251–258. [Google Scholar] [CrossRef]
- Hu, S.X.; Sui, H.X.; Jin, H.J.; Ni, X.Y.; Liu, X.X.; Xue, M.Q.; Zhang, Y.; Gao, F.G. Lipopolysaccharide and dose of nicotine determine the effects of nicotine on murine bone marrow-derived dendritic cells. Mol. Med. Rep. 2012, 5, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Heeschen, C.; Mohaupt, M.; Cooke, J.P.; Zeiher, A.M.; Dimmeler, S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: Potential role for progression of atherosclerotic lesions. Circulation 2003, 107, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.; Moldawer, L.L. Sepsis and the Dendritic Cell. Shock 2003, 20, 386–401. [Google Scholar] [CrossRef]
- Wu, D.D.; Li, T.; Ji, X.Y. Dendritic Cells in Sepsis: Pathological Alterations and Therapeutic Implications. J. Immunol. Res. 2017. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Jin, H.; Yan, J.; Liang, H.P. Alterations of dendritic cells in sepsis: Featured role in immunoparalysis. Biomed Res. Int. 2015. [Google Scholar] [CrossRef]
- Oberholzer, A.; Oberholzer, C.; Moldawer, L.L. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Critical Care Medicine. 2002, 30, S58–S63. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Pang, X.; Huang, Y.; Yang, B.; Yang, Y.; Chen, K.; Liu, X.; Mao, P.; Li, Y. Interleukin-10/lymphocyte ratio predicts mortality in severe septic patients. PLoS ONE 2017, 12, e0179050. [Google Scholar] [CrossRef]
- Bah, I.; Kumbhare, A.; Nguyen, L.; McCall, C.E.; El Gazzar, M. IL-10 induces an immune repressor pathway in sepsis by promoting S100A9 nuclear localization and MDSC development. Cell. Immunol. 2018, 332, 32–38. [Google Scholar] [CrossRef]
- Urbonas, V.; Eidukaitë, A.; Tamulienë, I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine 2012, 57, 313–315. [Google Scholar] [CrossRef]
- Mittal, S.K.; Cho, K.J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-mediated Immunosuppression March-i induction regulates antigen presentation by macrophages but not Dendritic cells. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef]
- Abrams, J.; Figdor, C.G.; De Waal Malefyt, R.; Bennett, B.; De Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar]
- Randow, F.; Syrbe, U.; Meisel, C.; Krausch, D.; Zuckermann, H.; Platzer, C.; Volk, H.D. Mechanism of endotoxin desensitization: Involvement of interhukin 10 and transforming growth factor β. J. Exp. Med. 1995, 181, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Klava, A.; Windsor, A.C.J.; Farmery, S.M.; Woodhouse, L.F.; Reynolds, J.V.; Ramsden, C.W.; Boylston, A.W.; Guillou, P.J. Interleukin-10: A role in the development of postoperative immunosuppression. Arch. Surg. 1997, 132, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage deactivation by interleukln 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Flohé, S.B.; Agrawal, H.; Schmitz, D.; Gertz, M.; Flohé, S.; Schade, F.U. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leukoc. Biol. 2006, 79, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Chen, C.K.; Chung, K.; Tseng, J.C.; Hua, C.C.; Liu, Y.C.; Chuang, D.Y.; Yang, C.H. Serial cytokine levels in patients with severe sepsis. Inflamm. Res. 2009, 58, 385–393. [Google Scholar] [CrossRef]
- Shao, R.; Fang, Y.; Yu, H.; Zhao, L.; Jiang, Z.; Li, C.S. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: A prospective cohort study. Crit. Care 2016, 20, 124. [Google Scholar] [CrossRef]
- Bertrand, D.; Gopalakrishnan, M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007, 97, 408–417. [Google Scholar] [CrossRef]
- Williams, D.K.; Wang, J.; Papke, R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochemical Pharmacology. 2011, 82, 915–930. [Google Scholar] [CrossRef] [PubMed]
- van Ton, A.M.P.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision immunotherapy for sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016, 274, 330–353. [Google Scholar] [CrossRef] [PubMed]
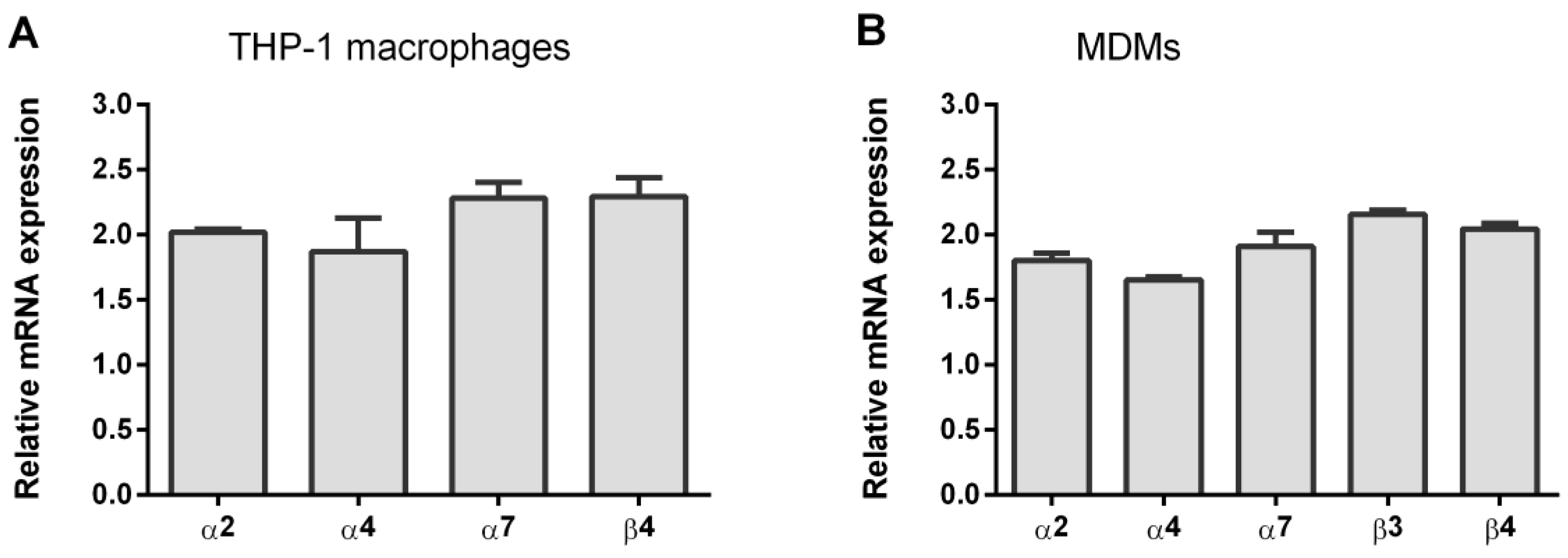
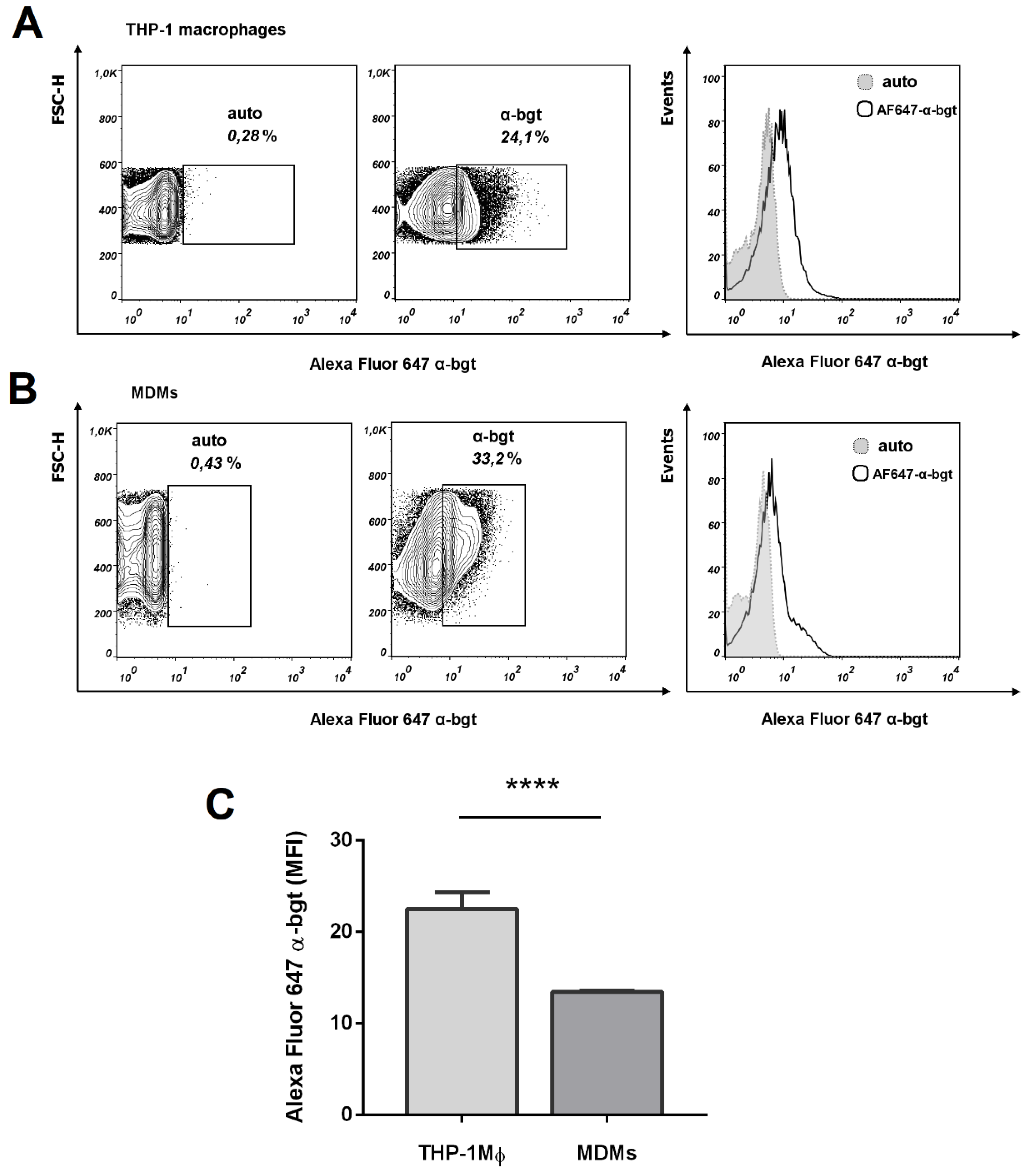
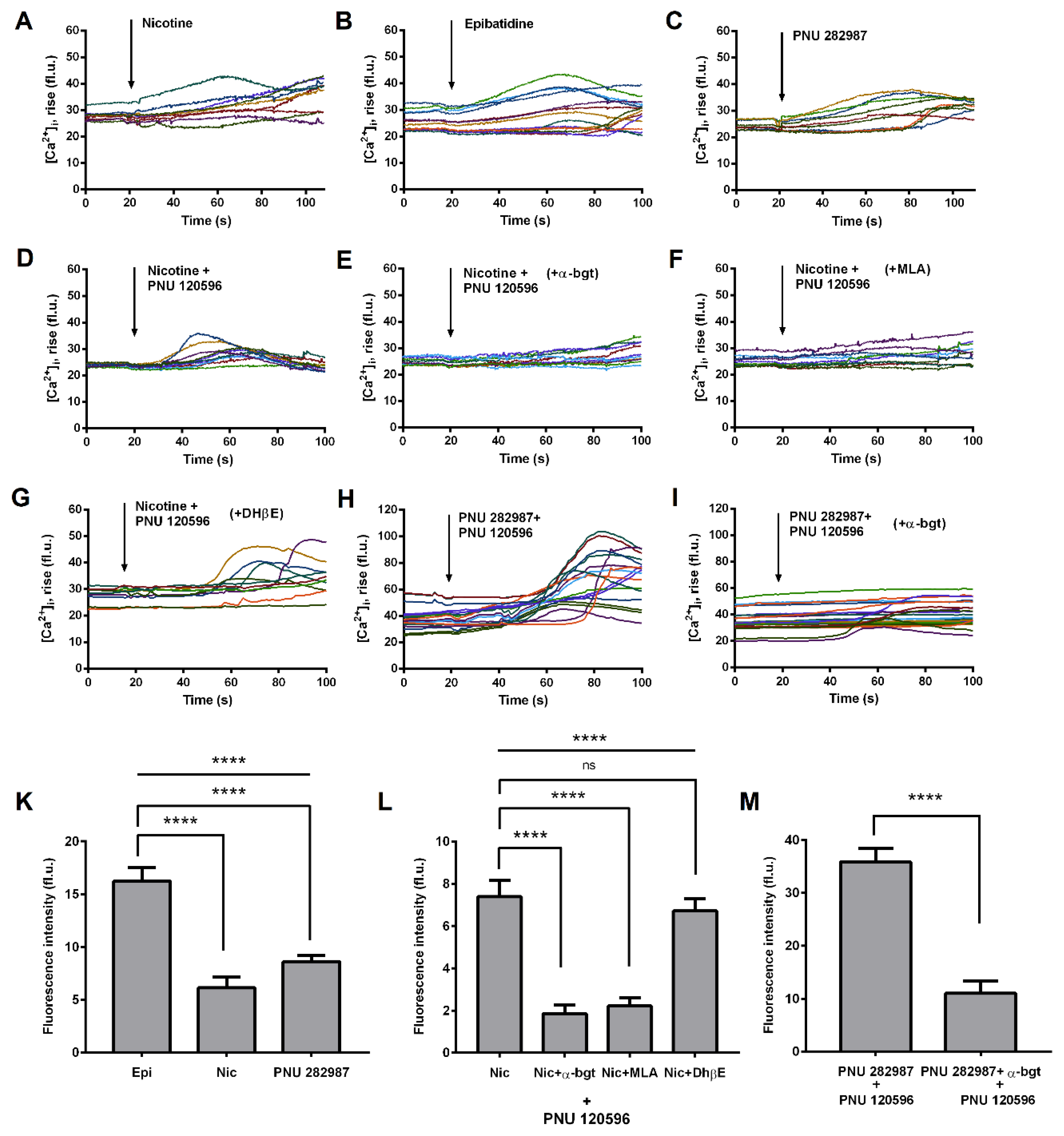
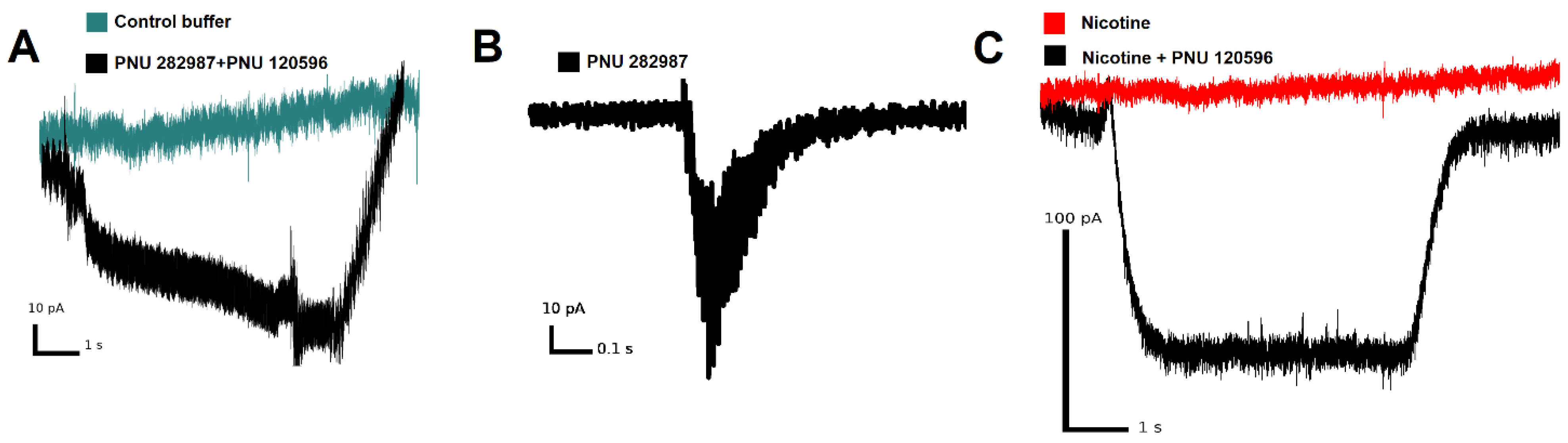
| Gene | Forward | Reverse | Sources |
|---|---|---|---|
| α1 | GGCTCCGAACATGAGACCCG | GCGTGACTTTGGGAGTTCCTTT | [24] |
| α2 | TGACCCACATGACCAAGGCCCA | TGGTGAACAGCAGGTACTCGCC | [24] |
| α3 | CCGAGGCCCCTCTACGGT | CACACAGCTTAGTGCTTA | [24] |
| α4 | CCTCGGCCTGTCCATCGCTCA | AAGACGGTGAGCGACAGCAGC | [24] |
| α7 | CCCGGCAAGAGGAGTGAAAGGT | TGCAGATGATGGTGAAGACC | [24,25] |
| α9 | AGAGCCTGTGAACACCAATGTGG | ATGACTTTCGCCACCTTCTTCC | [3] |
| β2 | GTGTCCTTCTATTCCAAT | AATGATGAAGTCATACGT | [24] |
| β3 | AAGGGGAACAGAAGGGACGG | GAAGCAGTACGTCGCGGACG | [24] |
| β4 | CAACAACCTGATCCGCCCAGC | GAAGGGAAAGTACTTCACCTC | [24] |
| β-actin | GAGCGGGAAATCGTGCGTGACATT | GATGGAGTTGAAGGTAGTTTCGTG | [26] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniavin, A.E.; Streltsova, M.A.; Kudryavtsev, D.S.; Shelukhina, I.V.; Utkin, Y.N.; Tsetlin, V.I. Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules 2020, 10, 507. https://doi.org/10.3390/biom10040507
Siniavin AE, Streltsova MA, Kudryavtsev DS, Shelukhina IV, Utkin YN, Tsetlin VI. Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules. 2020; 10(4):507. https://doi.org/10.3390/biom10040507
Chicago/Turabian StyleSiniavin, Andrei E., Maria A. Streltsova, Denis S. Kudryavtsev, Irina V. Shelukhina, Yuri N. Utkin, and Victor I. Tsetlin. 2020. "Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression" Biomolecules 10, no. 4: 507. https://doi.org/10.3390/biom10040507
APA StyleSiniavin, A. E., Streltsova, M. A., Kudryavtsev, D. S., Shelukhina, I. V., Utkin, Y. N., & Tsetlin, V. I. (2020). Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules, 10(4), 507. https://doi.org/10.3390/biom10040507







