RETRACTED: Role of Serum and Urine Biomarkers (PLA2R and THSD7A) in Diagnosis, Monitoring and Prognostication of Primary Membranous Glomerulonephritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Methodology
2.2.1. Data Collection
2.2.2. Cut-Off Points Value for Laboratory Parameters
2.3. Data Analysis
2.4. Flow Chart
3. Results
3.1. General Characteristics of the Subjects at the Time of Renal Biopsy
3.2. General Characteristics of Subjects at the End of Follow-Up
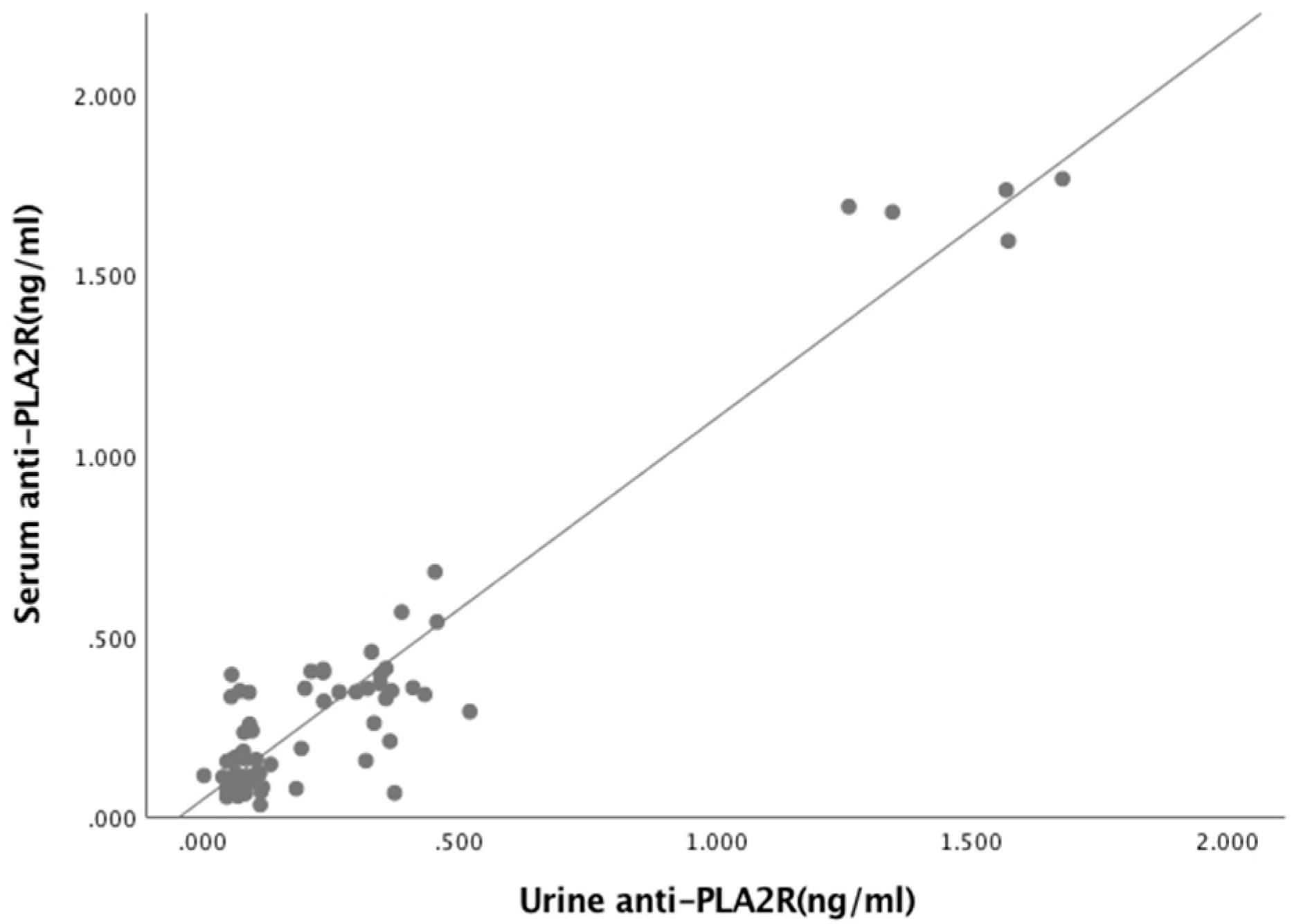
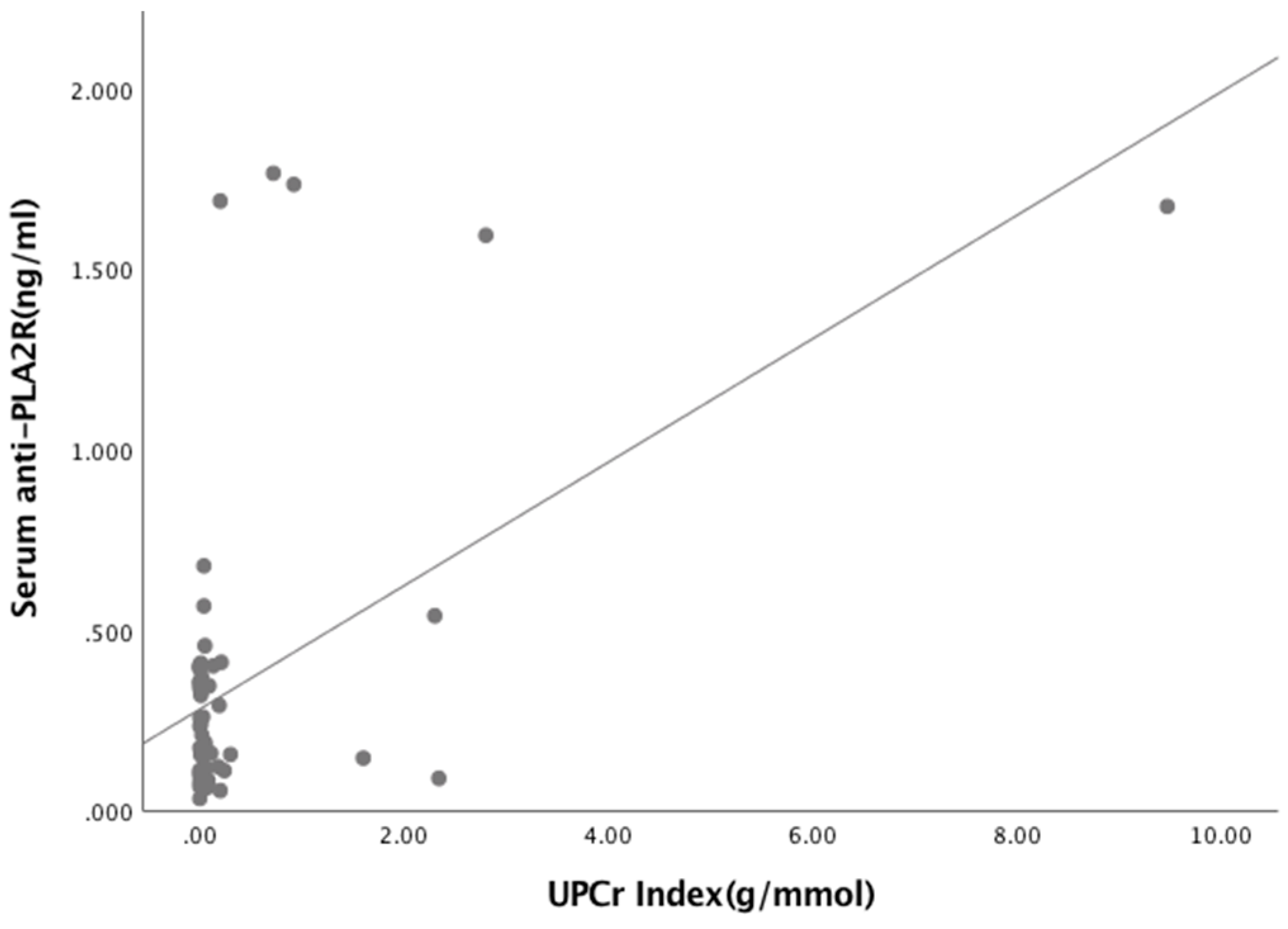
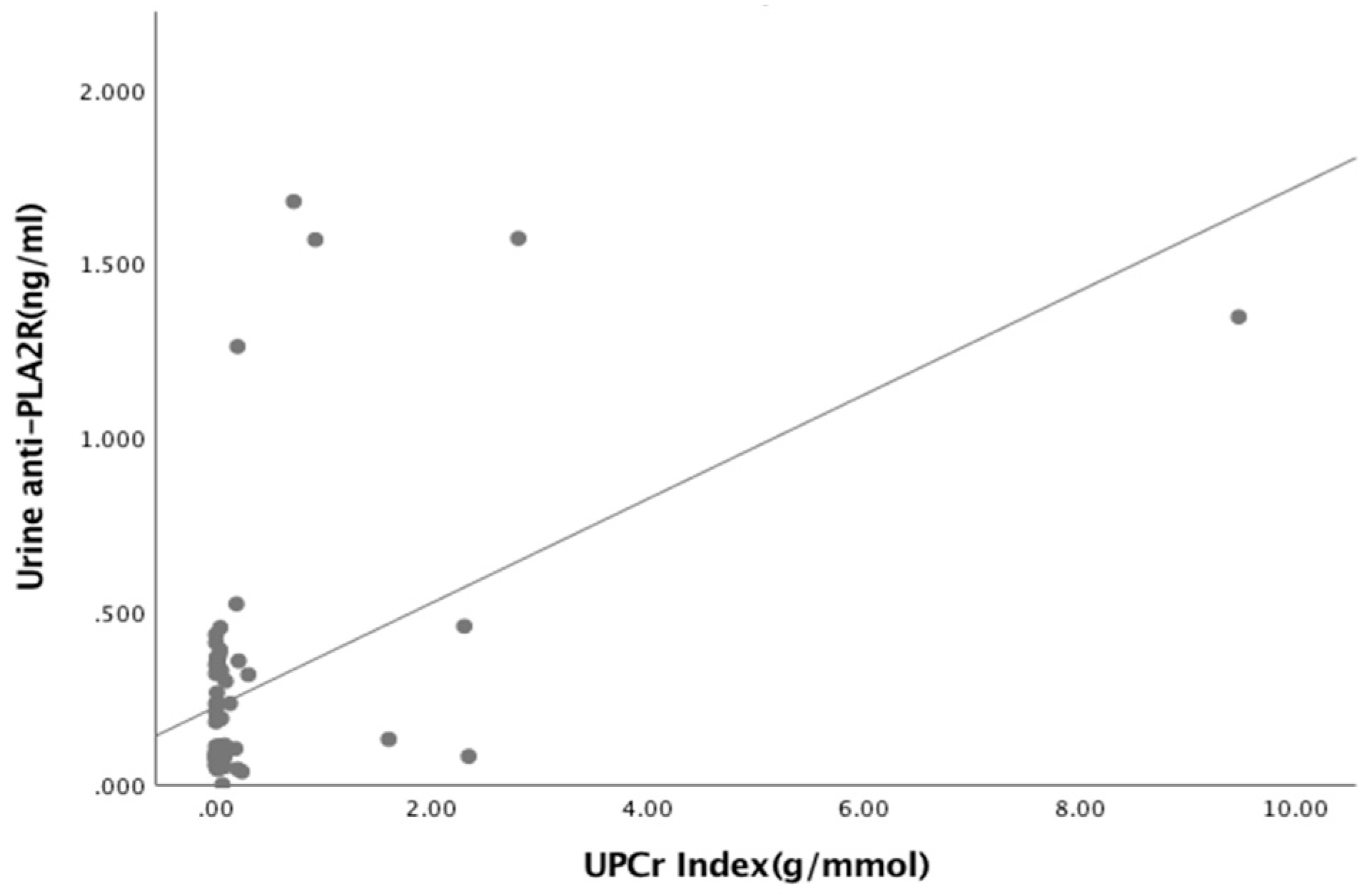
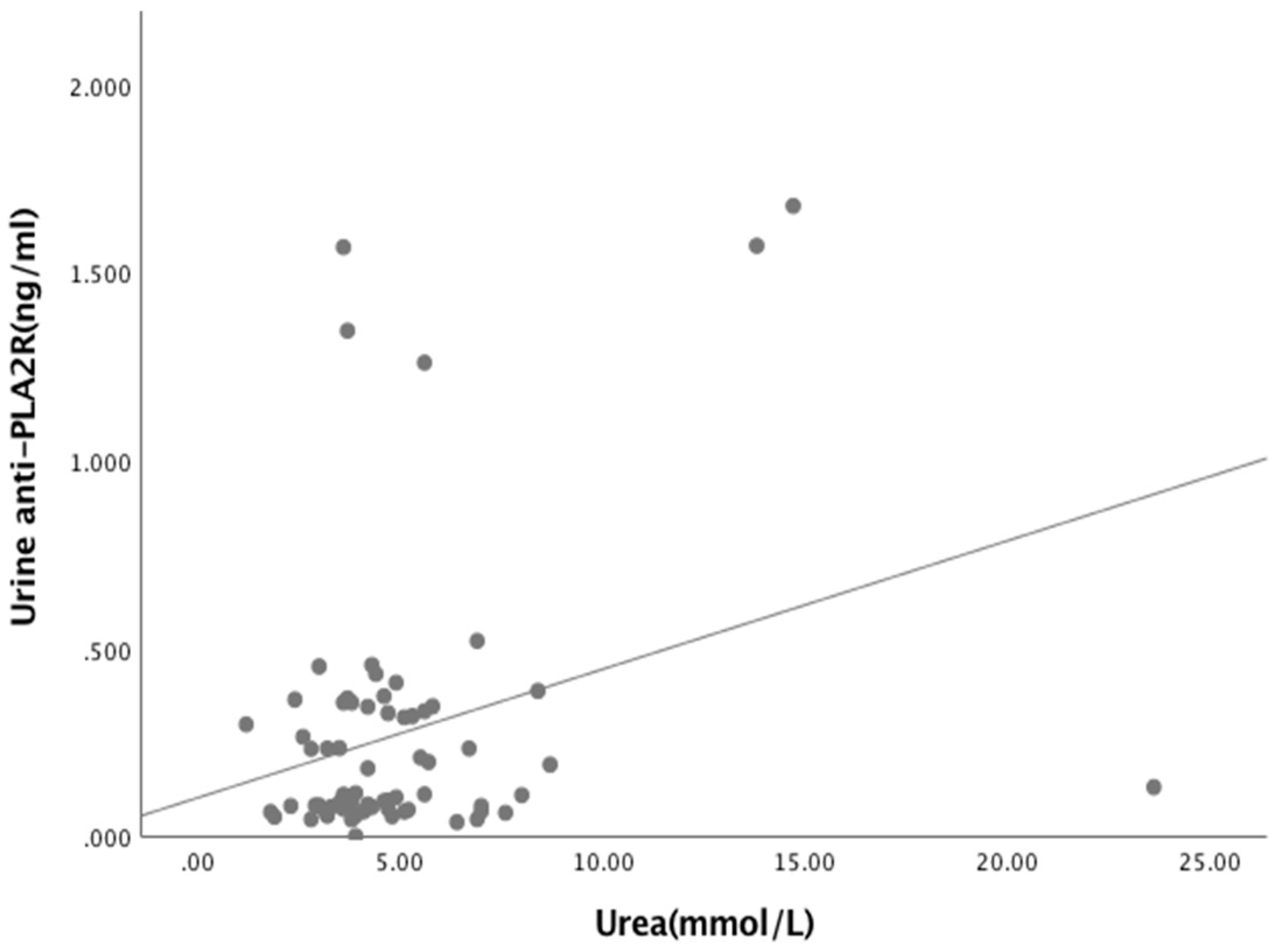
3.3. Relationships Between Biomarkers and Laboratory Parameters
3.4. The Prognostic Outcome of Primary MGN Subjects
3.4.1. The Prognostic Outcome of Primary MGN using eGFR (CKD ≥ 3)
3.4.2. The Prognostic Outcome of Primary MGN using UPCr Index (Remission)
4. Discussion
4.1. Role of Biomarkers (PLA2R and THSD7A) in the Diagnosis of MGN
4.2. Role of Biomarkers in Monitoring MGN Subjects
4.3. Prognosis of MGN
Anti-PLA2R Ab as a Prognostic Biomarker for Primary MGN
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| a | 0.2209 |
| b | −2.203 |
| c | 1.188 |
| d | 1.738 |
| MSE | 0.003579 |
| R² | 0.9886 |
| SS | 0.04294 |
| SYX | 0.07327 |

| a | 0.05272 |
| b | −1.051 |
| c | 0.2162 |
| d | 5.961 |
| MSE | 0.0003364 |
| R² | 0.9995 |
| SS | 0.002018 |
| SYX | 0.03177 |
Appendix B
References
- Hanko, J.B.; O’Rourke, D.M.; McNamee, P.T.; MAxwell, A.P.; Courtney, E.A. The changing pattern of adult primary glomerular disease. Nephrol Dial. Transpl. 2009, 24, 3050–3054. [Google Scholar] [CrossRef]
- Pan, X.; Xu, J.; Ren, H.; Zhang, W.; Xu, Y.; Shen, P.; Li, X.; Wang, W.; Chen, X.; Wu, P.; et al. Changing spectrum of biopsy-proven primary glomerular diseases over the past 15 years: A single-centre study in China. Contrib Nephrol. 2013, 181, 22–30. [Google Scholar] [PubMed]
- Xiaofan, H.; Jing, X.; Chenni, G.; Yifan, W.; Xialian, Y.; Li, L.; Hong, R.; Wen, Z.; Weiming, W.; Xiaoxia, P.; et al. New risk score for predicting progression of membranous nephropathy. J. Transl. Med. 2019, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yahya, R.; Jazilah, W.; Ismail, W. Fifth report of the Malaysian Registry of Renal Biopsy 2012. Malaysian Registry of Renal Biopsy, 2014. Available online: http://www.msn.org.my or https://www.macr.org.my/emrrb (accessed on 30 June 2014).
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.S.D. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Beck, L.H., Jr.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Dabert-Gay, A.; Debayle, D.; et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Maifata, S.M.A.; Hod, R.; Zakaria, N.F.; Ghani, F.A. Membranous Glomerulonephritis: Overview of the Role of Serum and Urine Biomarkers in the Management. Biomedicine 2019, 7, 86. [Google Scholar] [CrossRef]
- Cravedi, P.; Jarque, M.; Angeletti, A.; Favà, À.; Cantarelli, C.; Bastard, O. Immune-Monitoring Disease Activity in Primary Membranous Nephropathy. Front. Med. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Pourcine, F.; Dahan, K.; Mihout, F.; Cachanado, M.; Brocheriou, I.; Debiec, H.; Pierre, R. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: A single-centre study over 14 years. PLoS ONE 2017, 12, e0173201. [Google Scholar] [CrossRef]
- Timmermans, S.A.M.E.G.; Damoiseaux, J.G.M.C.; Heerings-Rewinkel, P.T.J.; Ayalon, R.; Beck, L.H.; Schlumberger, W.; Salant, D.J.; van Paassen, P.; Tervaert, J.W.C. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: A cohort study. Am. J. Clin. Pathol. 2014, 142, 29–34. [Google Scholar] [CrossRef]
- Varghese, S.A.; Powell, T.B.; Budisavljevic, M.N.; Oates, J.C.; Raymond, J.R.; Almeida, J.S.; Arthur, J.M. Urine Biomarkers Predict the Cause of Glomerular Disease. Oncotarget 2007, 7, 200–205. [Google Scholar] [CrossRef]
- KDIGO. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012, 2, 142. [Google Scholar]
- Hofstra, J.M.; Beck, L.H.; Beck, D.M.; Wetzels, J.F.; Salant, D.J. Anti-phospholipase a2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nagai, Y.; Mikami, T.; Akasaka, Y.; Shibuya, K.; Urita, Y. Anti-phospholipase A2 receptor antibody-positive hepatitis B virus-associated membranous nephropathy remitted with entecavir after relapse with lamivudine. J. Nephropathol. 2018, 7, 93–97. [Google Scholar] [CrossRef][Green Version]
- Dong, H.R.; Wang, Y.Y.; Cheng, X.H.; Wang, G.Q.; Sun, L.J.; Cheng, H.; Chen, Y.P. A retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in Chinese patients with membranous nephropathy. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Zaghrini, C.; Seitz-Polski, B.; Justino, J.; Dolla, G.; Payré, C.; Jourde-Chiche, N.; Van de Logt, A.E.; Booth, C.; Rigby, E.; Lonnbro-Widgren, J.; et al. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Int. Soc. Nephrol. 2019, 95, 666–679. [Google Scholar]
- Pozdzik., A.; Brochériou, I.; David, C.; Touzani, F.; Goujon, J.M.; Wissing, K.M. Membranous Nephropathy and Anti-Podocytes Antibodies: Implications for the Diagnostic Workup and Disease Management. Biomed. Res. Int. 2018, 1–19. [Google Scholar] [CrossRef]
- Hoxha, E.; Beck, L.H., Jr.; Wiech, T.; Nicola, M.T.; Probst, C.; Mindorf, S.; Meyer-Schwesinger, C.; Zahner, G.; Stahl, P.R.; Schopper, R.; et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J. Am. Soc. Nephrol. 2016, 28, 520–531. [Google Scholar] [CrossRef]
- Xian, L.; Dong, D.; Luo, J.; Zhuo, L.; Li, K.; Zhang, P.; Xu, Y.; Xu, G.; Wang, L.; Li, G. Expression of THSD7A in neoplasm tissues and its relationship with proteinuria. BMC Nephrol. 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Wu, X.; Liu, L.; Guo, Y.; Yang, L. Clinical value of a serum anti-PLA2R antibody in the diagnosis and monitoring of primary membranous nephropathy in adults. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 241–247. [Google Scholar] [CrossRef]
- Jullien, P.; Polski, B.S.; Maillard, N.; Thibaudin, D.; Laurent, B.; Ollier, E.; Alamartine, E.L.; Mariat, C. Anti-phospholipase A2 receptor antibody levels at diagnosis predict spontaneous remission of idiopathic membranous nephropathy. Clin. Kidney J. 2017, 10, 209–214. [Google Scholar] [CrossRef]
- Mastroianni-Kirsztajn, G.; Hornig, N.; Schlumberger, W. Autoantibodies in renal diseases—Clinical significance and recent developments in serological detection. Front. Immunol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.X.; Diao, T.T.; Wei, S.Y.; Qi, W.R.; Wang, C.C.; Song, S.M.; Bi, M.; Li, C.M.; Zhnag, C.X.; et al. Urine anti-PLA2R antibody is a novel biomarker of idiopathic membranous nephropathy. Oncotarget 2018, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Quintana, L.F.; Miguel Seras, N.S.; Perez, M.; Lopez-Hoyos, P.; Villarroel, E.R.; Odette, V.; Guadalupe, E.; Fritz, D.; Jose, J.G.R.; Gema, F.F.; et al. Antiphospholipase A2 antibody predicts the risk of post-transplantation recurrence of membranous nephropathy. Transplantation 2015, 99, 1709–1714. [Google Scholar] [CrossRef]
- Gupta, G.; Fattah, H.; Ayalon, R.; Kidd, J.; Gehr, T.; Quintana, L.F.; Sadruddin, S.; Massey, D.H.; Kumar., D.; King, A.L.; et al. Pretransplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin. Transpl. 2016, 30, 461–469. [Google Scholar] [CrossRef]
- Leon, J.; Pérez-Sáez, M.J.; Batal, I.; Beck, L.H.; Rennke, H.; Canaud, G.; Legendre, C.; Pascual, J.; Reilla, L. Membranous Nephropathy Post-Transplantation. Transplantation 2019, 103, 1. [Google Scholar]
- Diaz, M.; Agraz, I.; Soler, M.J. Anti-phospholipase A2 receptor antibody and spontaneous remission in membranous nephropathy. Clin. Kidney J. 2019, 12, 33–35. [Google Scholar] [CrossRef]
- Rodas, L.M.; Matas-García, A.; Barros, X.; Blasco, M.; Vinas, O.; Llobell, A.; Martin, N.; Quintana, L.F. Antiphospholipase 2 receptor antibody levels to predict complete spontaneous remission in primary membranous nephropathy. Clin. Kidney J. 2019, 12, 36–41. [Google Scholar] [CrossRef]
- Ayalon, R.L.H.B., Jr.; Schlumberger, W. Evaluation of Anti-PLA2R1 as Measured by a Novel ELISA in Patients With Idiopathic Membranous Nephropathy: A Cohort Study. Am. J. Clin. Pathol. 2014, 29–34. [Google Scholar]

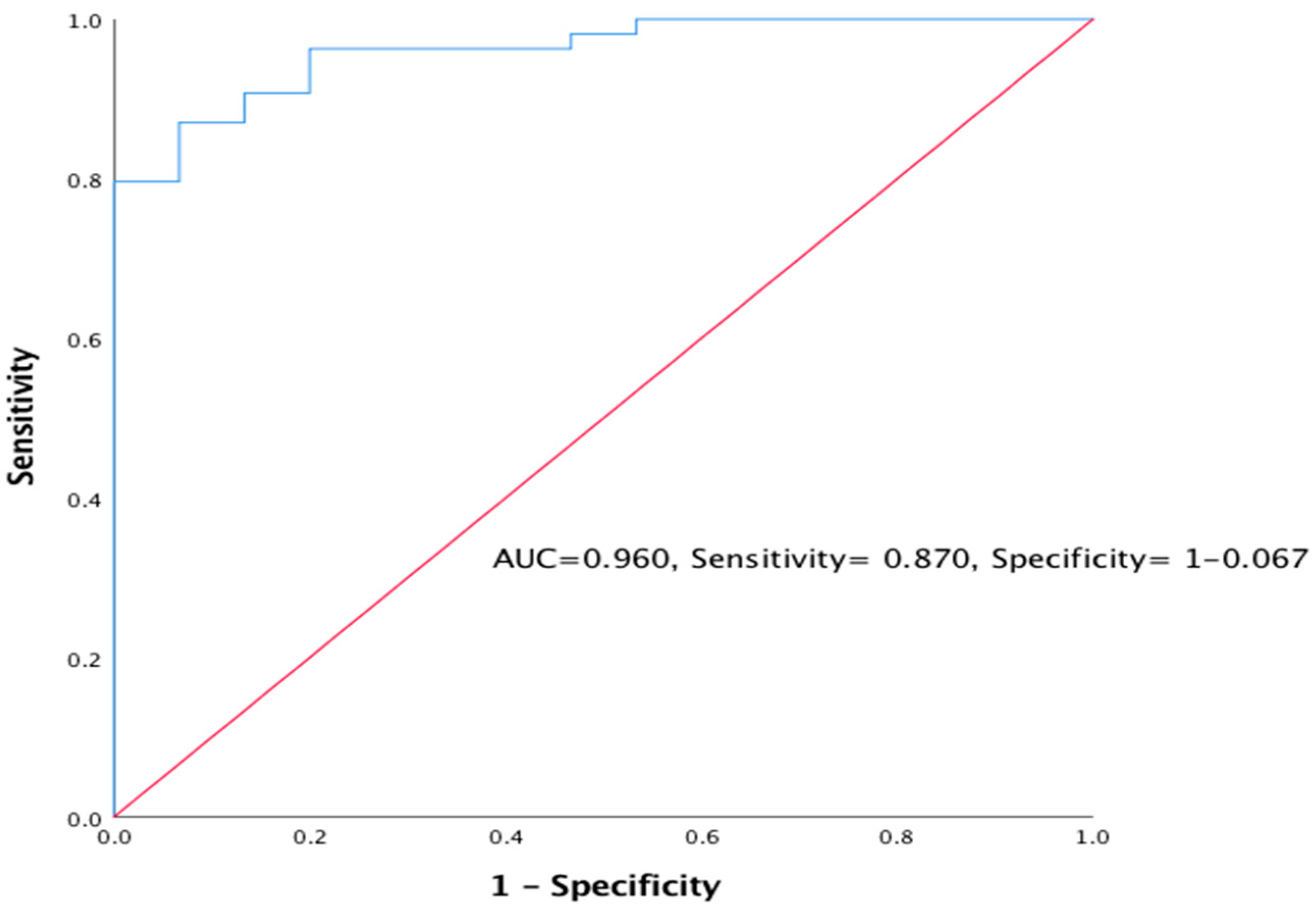
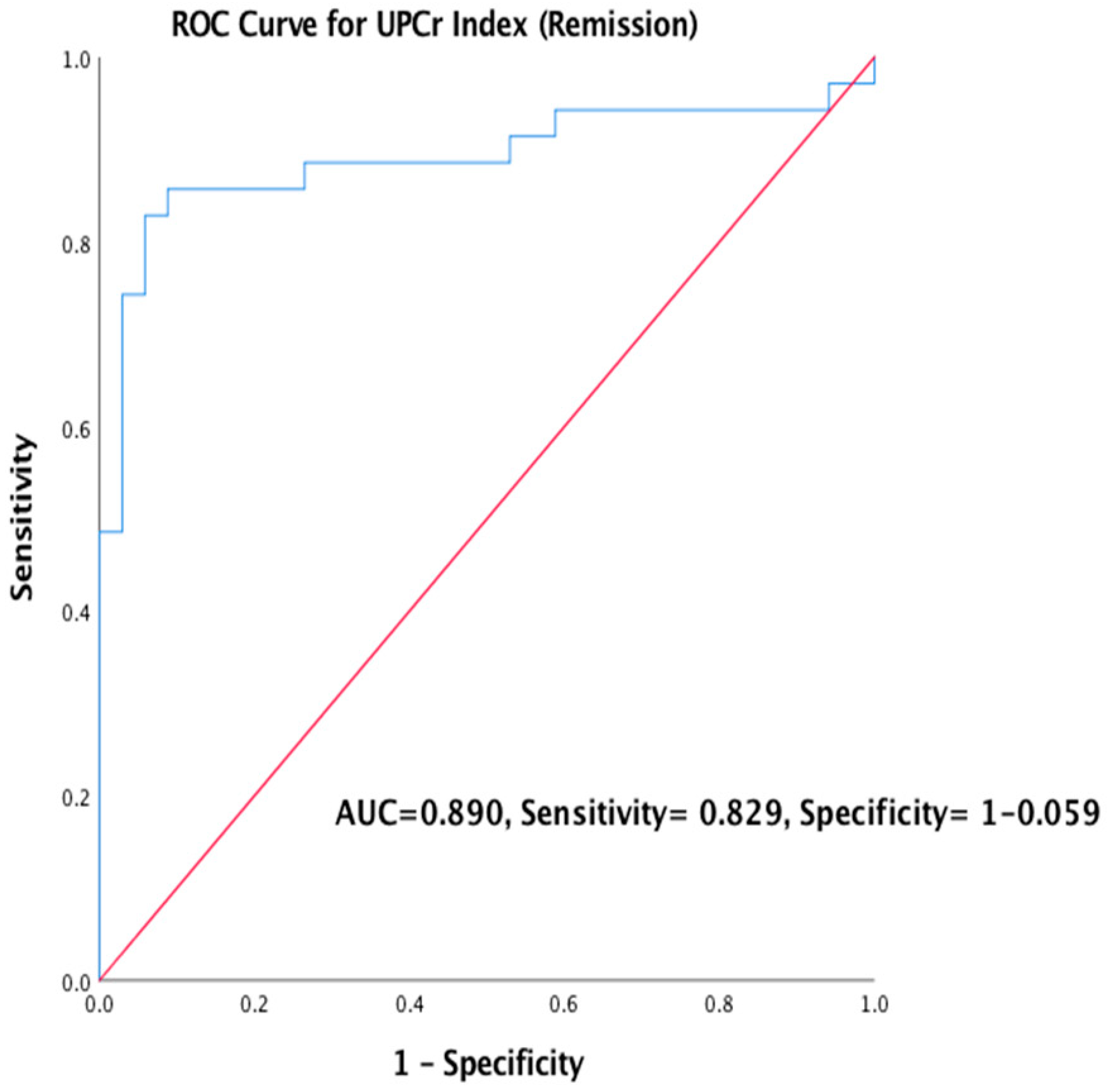
| Variable | Primary MGN (n = 47) Frequency Percentage (%) | Secondary MGN (n = 22) Frequency Percentage (%) | ||
|---|---|---|---|---|
| Age at Diagnosis: ≤30 31–40 41–50 51–60 >60 | 16 8 8 8 7 | 34.1 17.0 17.0 17.0 14.9 | 7 4 1 4 6 | 31.8 18.2 4.5 18.2 27.3 |
| Sex: Male Female | 21 26 | 44.7 55.3 | 8 14 | 36.4 63.6 |
| Ethnicity: Malay Chinese Indian Others | 24 12 9 2 | 51.1 25.5 19.1 4.3 | 16 5 0 1 | 72.8 22.7 0 4.5 |
| Nephrotic Syndrome: Present absent | 38 9 | 80.8 19.1 | 13 9 | 59.1 40.9 |
| Hypertension: Present Absent | 28 19 | 59.6 40.4 | 10 12 | 45.5 54.5 |
| Haematuria: Present Absent | 9 38 | 19.1 80.9 | 2 20 | 9.1 90.9 |
| Albumin: Normal Low | 14 33 | 29.8 70.2 | 8 14 | 36.4 63.6 |
| Serum Urea(mmol/L) Normal High | 26 21 | 55.3 44.7 | 13 9 | 59.1 40.9 |
| Serum Creatinine(µmol/L) Normal High | 6 41 | 12.8 87.2 | 8 14 | 36.4 63.6 |
| eGFR (mL/min/1.73m2) Low risk High risk | 33 14 | 70.2 29.8 | 15 7 | 68.2 31.8 |
| UPCr Index(g/mmol) Normal High | 20 27 | 42.6 57.4 | 5 17 | 22.7 77.3 |
| Variable | Primary MGN (n = 47) Frequency Percentage (%) | Secondary MGN (n = 22) Frequency Percentage (%) | ||
|---|---|---|---|---|
| Median (Interquartile) | ||||
| Follow-Up (months) | 39.0(17.5–59.5) | 27.5(13.0–49.8) | ||
| eGFR(mL/min/1.73m2) Low risk High risk | 33 14 | 70.2 29.8 | 15 7 | 68.2 31.8 |
| UPCr Index (g/mmol) Remission No remission | 20 27 | 42.6 57.4 | 5 17 | 22.7 77.3 |
| Biomarkers | ||||
| Serum anti-PLA2R Ab (ng/mL) Negative Positive | 34 13 | 72.3 27.7 | 21 1 | 95.5 4.5 |
| Urine anti-PLA2R Ab (ng/mL) Negative Positive | 34 13 | 71.1 28.9 | 21 1 | 95.5 4.5 |
| Serum Anti-THSD7A Ab (ng/mL) Negative Positive | 43 4 | 91.5 8.5 | 20 2 | 90.9 9.1 |
| Urine Anti-THSD7A Ab (ng/mL) Negative Positive | 47 0 | 100.0 0.0 | 22 0 | 100.0 0.0 |
| Covariate | R | p-Value |
|---|---|---|
| Serum anti-PLA2R Ab (ng/mL) Urine anti-PLA2R Ab (ng/mL) | 0.902 | <0.05 * |
| Albumin (g/L) Urea (mmol/L) Creatinine (μmol/L) eGFR (mL/min/1.73m2) UPCr Index (g/mmol) | 0.056 0.226 0.034 0.216 0.502 | 0.647 0.062 0.782 0.075 <0.05 * |
| Urine anti-PLA2R Ab (ng/mL) Albumin (g/L) Urea (mmol/L) Creatinine (μmol/L) eGFR (mL/min/1.73m2) UPCr Index (g/mmol) | 0.040 0.251 0.550 −0.08 0.437 | 0.746 0.038 * 0.652 0.514 <0.05 * |
| Serum anti-THSD7A Ab (ng/mL) Urine anti-THSD7A Ab (ng/mL) | 0.063 | 0.604 |
| Albumin (g/L) Urea (mmol/L) Creatinine (μmol/L) eGFR (mL/min/1.73m2) UPCr Index (g/mmol) | −0.069 −0.137 −0.110 0.136 −0.069 | 0.574 0.999 0.367 0.266 0.395 |
| Variables | B | SE | COR | CI (95%) | p-Value |
|---|---|---|---|---|---|
| Age | 0.062 | 0.023 | 0.940 | 0.898-0.993 | 0.008 * |
| Gender Male female | 0.241 | 0.587 | 1.000 1.273 | 0.403–4.021 | 0.681 |
| Albumin (g/L) Urea (mmol/L) | 0.111 0.883 | 0.077 0.259 | 1.117 2.417 | 0.961–1.299 1.454–4.019 | 0.149 0.002 * |
| Creatinine (μmol/L) | 0.178 | 0.068 | 1.195 | 1.046–1.316 | 0.009 * |
| UPCr Index (g/mmol) Remission No remission | −2.565 | 0.813 | 1.000 0.077 | 0.016–0.378 | 0.002 * |
| Serum anti-PLA2R Ab(ng/mL) Negative Positive | −1.978 | 0.971 | 1.000 0.138 | 0.021–0.928 | 0.042 * |
| Urine anti-PLA2R Ab(ng/mL) Negative Positive | −1.833 | 0.755 | 1.000 0.160 | 0.036–0.703 | 0.015 * |
| Serum anti-THSD7A Ab(ng/mL) Negative Positive | 1.872 | 0.967 | 1.000 6.500 | 0.976–43.289 | 0.053 |
| Variables | B | SE | AOR | C.I. 95% | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis (years) | −0.094 | 0.052 | 0.910 | 0.822–1.009 | 0.534 |
| Serum anti-PLA2R Ab(ng/mL) | −1.447 | 1.467 | 1.000 0.235 | 0.013–4.175 | 0.048 * |
| Urea(mmol/L) | −1.174 | 0.441 | 1.000 0.309 | 0.130–0.734 | 0.008 * |
| Variable | B | SE | COR | 95% C.I. | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis | 0.025 | 0.018 | 1.025 | 0.989–1.063 | 0.181 |
| Sex | 0.308 | 0.490 | 1.360 | 0.521–3.551 | 0.530 |
| Albumin(g/L) | −0.075 | 0.060 | 0.928 | 0.825–1.043 | 0.209 |
| Urea(mmol/L) | 0.257 | 0.136 | 1.293 | 0.991–1.686 | 0.058 |
| Creatinine (μmol/L) | 0.046 | 0.015 | 1.047 | 1.016–1.079 | 0.002 * |
| eGFR(mL/min/1.73m2) | −0.047 | 0.013 | 0.954 | 0.931–0.978 | <0.05 * |
| Serum anti-PLA2R Ab (ng/mL) | 1.377 | 0.810 | 3.964 | 0.810–19.399 | 0.015 * |
| Urine anti-PLA2R Ab(ng/mL) | 2.472 | 1.249 | 11.845 | 1.025–136.924 | 0.05 * |
| Serum anti-THSD7A Ab(ng/mL) | −0.937 | 1.022 | 0.392 | 0.530–2.902 | 0.392 |
| Variable | B | SE | AOR | 95% C.I. | p-Value |
|---|---|---|---|---|---|
| Creatinine eGFR (mL/min/1.73m2) Urine anti-PLA2R Ab (ng/mL) | 0.018 0.002 2.917 | 0.044 0.039 2.446 | 1.018 1.002 18.486 | 0.934–1.110 0.928–1.882 0.153–2234.834 | 0.660 0.958 0.038 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maifata, S.M.; Hod, R.; Zakaria, F.; Abd Ghani, F. RETRACTED: Role of Serum and Urine Biomarkers (PLA2R and THSD7A) in Diagnosis, Monitoring and Prognostication of Primary Membranous Glomerulonephritis. Biomolecules 2020, 10, 319. https://doi.org/10.3390/biom10020319
Maifata SM, Hod R, Zakaria F, Abd Ghani F. RETRACTED: Role of Serum and Urine Biomarkers (PLA2R and THSD7A) in Diagnosis, Monitoring and Prognostication of Primary Membranous Glomerulonephritis. Biomolecules. 2020; 10(2):319. https://doi.org/10.3390/biom10020319
Chicago/Turabian StyleMaifata, Sadiq Mu’azu, Rafidah Hod, Fadhlina Zakaria, and Fauzah Abd Ghani. 2020. "RETRACTED: Role of Serum and Urine Biomarkers (PLA2R and THSD7A) in Diagnosis, Monitoring and Prognostication of Primary Membranous Glomerulonephritis" Biomolecules 10, no. 2: 319. https://doi.org/10.3390/biom10020319
APA StyleMaifata, S. M., Hod, R., Zakaria, F., & Abd Ghani, F. (2020). RETRACTED: Role of Serum and Urine Biomarkers (PLA2R and THSD7A) in Diagnosis, Monitoring and Prognostication of Primary Membranous Glomerulonephritis. Biomolecules, 10(2), 319. https://doi.org/10.3390/biom10020319






