Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Turbot by-Products
2.2. Optimization of Protease Hydrolysis of Turbot by-Products
2.3. Production of Fish Protein Hydrolysates (FPHs) of Turbot by-Products
2.4. Chemical and Biological Determinations
2.5. Numerical and Statistical Analyses
3. Results and Discussion
3.1. Optimization of Enzyme Hydrolysis of Turbot by-Products
3.2. Production and Chemical Composition of Turbot FPHs
3.3. Antioxidant and Antihypertensive Properties of Turbot Hydrolysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. 2018. Available online: http://www.fao.org/state-of-fisheries-aquaculture (accessed on 20 July 2019).
- Apromar. La aquicultura en España 2018. Available online: www.apromar.es (accessed on 23 September 2019).
- He, S.; Franco, C.; Zhang, W. Process optimisation and physicochemical characterisation of enzymatic hydrolysates of proteins from co-products of Atlantic Salmon (Salmo salar) and Yellowtail Kingfish (Seriola lalandi). Int. J. Food Sci. Tech. 2012, 47, 2397–2404. [Google Scholar] [CrossRef]
- Fan, B.; Sun, J.; Dong, P.; Xue, C.; Mao, X. Conversion of turbot skin wastes into valuable functional substances with an eco-friendly fermentation technology. J. Cleaner Prod. 2017, 156, 367–377. [Google Scholar]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 24, 6–24. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Tech. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Ruiz-Quesada, C.; Pérez-Morilla, A.I.; Martínez-Agustín, O.; Guadix, A.; Guadix, E.M. Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: Effect of enzymatic treatment and degree of hydrolysis. J. Sci. Food Agric. 2017, 97, 299–308. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Goosen, N.J. Evaluation of fish protein hydrolysates in juvenile African catfish (Clarias gariepinus) diets. Aquaculture 2018, 496, 262–269. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stantone, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef]
- Lapeña, D.; Vuoristo, K.S.; Kosa, G.; Horn, S.J.; Eijsink, V.G.H. Comparative assessment of enzymatic hydrolysis for valorization of different protein-rich industrial byproducts. J. Agric. Food Chem. 2018, 66, 9738–9749. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Amsterdam, The Netherlands, 1986. [Google Scholar]
- AOAC. Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1997. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulfonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Miller, E.L.; Bimbo, A.P.; Walters, D.E.; Barlow, S.M.; Sheridan, B. Determination of nitrogen solubility in dilute pepsin hydrochloric acid solution of fishmeal: Interlaboratory study. J. AOAC Int. 2002, 85, 1374–1381. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidant activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Prieto, M.A.; Vázquez, J.A.; Murado, M.A. Crocin bleaching antioxidant assay revisited. Application to microplate to analyse antioxidant and prooxidant activities. Food Chem. 2015, 167, 299–310. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioproc. Tech. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G.H. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Proc. Biochem. 2005, 40, 1957–1966. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R.I. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Ramos, P.; Valcarcel, J.; Antelo, L.T.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I. An integral and sustainable valorisation strategy of squid pen byproducts. J. Clean. Prod. 2018, 201, 207–218. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Mirón, J.; Valcárcel, J.; Pérez-Martín, R.I.; Antelo, L.T. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. J. Clean. Prod. 2020, 246, 119027. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: Process optimization, chemical characterization and evaluation of bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Pourashouri, P.; Shabanpour, B.; Alishahi, A. Amino acid composition, antioxidant and functional properties of protein hydrolysates from the roe of rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Tech. 2018, 53, 313–319. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; DiNicolantonio, J.J. Mediterranean diet: ω-6 and ω-3 fatty acids and diabetes. Am. J. Clin. Nutr. 2017, 106, 953–954. [Google Scholar]
- World Health Organization (WHO). Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Farvin, K.H.S.; Jacobsen, C.; Baron, C.P. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 2014, 142, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Gavlighi, H.A.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing byproduct with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT-Food Sci. Tech. 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Geirsdottir, M.; Sigurgisladottir, S.; Hamaguchi, P.Y.; Thorkelsson, G.; Johannsson, R.; Kristinsson, H.G.; Kristjansson, M.M. Enzymatic hydrolysis of blue whiting (Micromesistius poutassou); functional and bioactive properties. J. Food Sci. 2011, 76, C14–C20. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Abidi, F.; Hardouin, J.; Abdelkafi, Z.; Marrakchi, N.; Jouenne, T.; Marzouki, M.N. Two novel peptides with angiotensin I converting enzyme inhibitory and antioxidative activities from Scorpaena notata muscle protein hydrolysate. Biotech. Appl. Biochem. 2017, 64, 201–210. [Google Scholar] [CrossRef]
- Cinq-Mars, C.D.; Li-Chan, E.C. Optimizing angiotensin I-converting enzyme inhibitory activity of Pacific hake (Merluccius productus) fillet hydrolysate using response surface methodology and ultrafiltration. J. Agric. Food Chem. 2007, 55, 9380–9388. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.H.; Ryu, B.; Kim, S.K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.; Byun, H.; Kim, S. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Proc. Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Ngo, D.; Qian, Z.; Ryu, B.; Park, J.W.; Kim, S. In vitro antioxidant activity of a peptide isolated from Nile Tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Jung, W.K.; Karawita, R.; Heo, S.J.; Lee, B.J.; Kim, S.K.; Jeon, Y.J. Recovery of a novel Ca-binding peptide from Alaska pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Proc. Biochem. 2006, 41, 2097–2100. [Google Scholar] [CrossRef]
- Kim, S.; Je, J.; Kim, S. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]

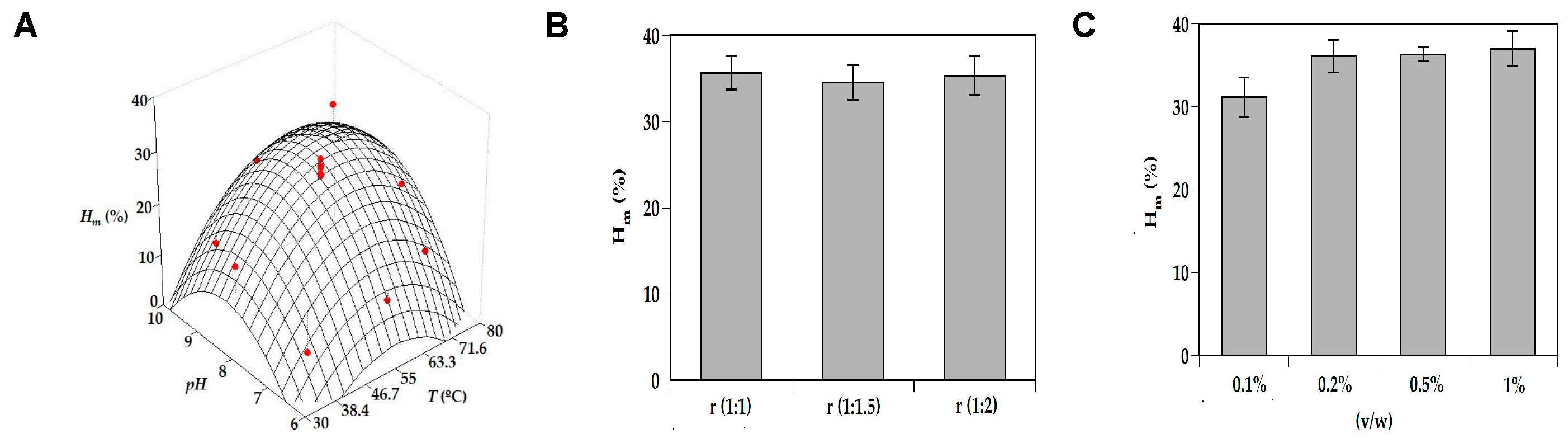


| Parameters | Hm (%) | Vdig (%) | Prs (g/L) |
|---|---|---|---|
| b0 (intercept) | 28.36 ± 1.57 | 88.59 ± 2.13 | 58.69 ± 2.27 |
| b1 (T) | 3.12 ± 1.25 | 3.70 ± 1.69 | 5.28 ± 1.80 |
| b2 (pH) | 4.74 ± 1.24 | 5.05 ± 1.69 | 10.0 ± 1.80 |
| b12 (TxpH) | 2.07 ± 1.76 | NS | 3.25 ± 2.54 |
| b11 (T2) | −5.81 ± 1.34 | −10.26 ± 1.82 | −9.19 ± 1.94 |
| b22 (pH2) | −6.27 ± 1.34 | −3.05 ± 1.82 | −11.8 ± 1.94 |
| 0.879 | 0.888 | 0.904 | |
| 0.803 | 0.832 | 0.835 | |
| F1 | 10.19 | 15.85 | 13.13 |
| F2 | 0.705 | 0.558 | 0.688 |
| F3 | 6.012 | 5.669 | 5.998 |
| Topt (°C) | 61.1 | 58.2 | 61.6 |
| pHopt | 8.62 | 9.17 | 8.68 |
| Ymax | 29.9% | 91.0% | 62.1 g/L |
| FPHs | mb (%) | Voil (%) | Vdig (%) | Prs (g/L) | Pr-tN (g/L) | TS (g/L) | Dig (%) |
|---|---|---|---|---|---|---|---|
| Tu_H | 16.8 ± 1.4 a | 0.25 ± 0.19 a | 86.9 ± 1.2 a | 73.5 ± 4.9 a | 73.9 ± 4.5 a | 1.26 ± 0.14 a | 92.1 ± 4.1 a |
| Tu_TF | 9.7 ± 1.4 b | 4.25 ± 1.09 b | 82.5 ± 1.2 b | 73.9 ± 3.8 a | 72.7 ± 3.9 a | 1.34 ± 0.17 a | 93.8 ± 2.4 a |
| Tu_V | - | 0.46 ± 0.10 a | 95.3 ± 1.0 c | 61.6 ± 2.8 b | 63.6 ± 2.9 b | 1.39 ± 0.25 a | 92.4 ± 1.9 a |
| Amino Acids | Tu_H | Tu_TF | Tu_V |
|---|---|---|---|
| Asp | 8.81 ± 0.16 | 9.63 ± 0.10 | 9.26 ± 0.42 |
| Thr | 3.64 ± 0.16 | 3.90 ± 0.06 | 3.85 ± 0.18 |
| Ser | 5.67 ± 0.14 | 5.37 ± 0.18 | 5.39 ± 0.36 |
| Glu | 12.86 ± 0.18 | 13.55 ± 0.08 | 13.12 ± 0.32 |
| Gly | 14.50 ± 0.32 | 12.57 ± 0.15 | 13.00 ± 0.50 |
| Ala | 8.38 ± 0.24 | 8.06 ± 0.05 | 7.83 ± 0.22 |
| Cys | 0.62 ± 0.05 | 0.77 ± 0.10 | 0.71 ± 0.11 |
| Val | 2.96 ± 0.09 | 3.21 ± 0.11 | 3.23 ± 0.12 |
| Met | 2.76 ± 0.15 | 2.90 ± 0.04 | 2.84 ± 0.17 |
| Ile | 1.97 ± 0.11 | 2.19 ± 0.08 | 2.29 ± 0.12 |
| Leu | 5.37 ± 0.11 | 5.93 ± 0.11 | 5.86 ± 0.17 |
| Tyr | 2.90 ± 0.16 | 3.18 ± 0.08 | 3.11 ± 0.20 |
| Phe | 4.16 ± 0.18 | 4.51 ± 0.17 | 4.31 ± 0.37 |
| His | 1.61 ± 0.07 | 1.75 ± 0.04 | 1.85 ± 0.11 |
| Lys | 5.57 ± 0.14 | 6.15 ± 0.11 | 6.18 ± 0.23 |
| Arg | 6.53 ± 0.24 | 6.34 ± 0.08 | 6.59 ± 0.36 |
| OHPro | 4.00 ± 0.19 | 3.14 ± 0.18 | 3.42 ± 0.35 |
| Pro | 7.67 ± 0.25 | 6.86 ± 0.13 | 7.14 ± 0.37 |
| Pr (Σaa) (g/L) | 68.62 ± 5.05 | 69.72 ± 4.39 | 61.94 ± 2.84 |
| TEAA/TAA (%) | 28.04 | 30.54 | 30.41 |
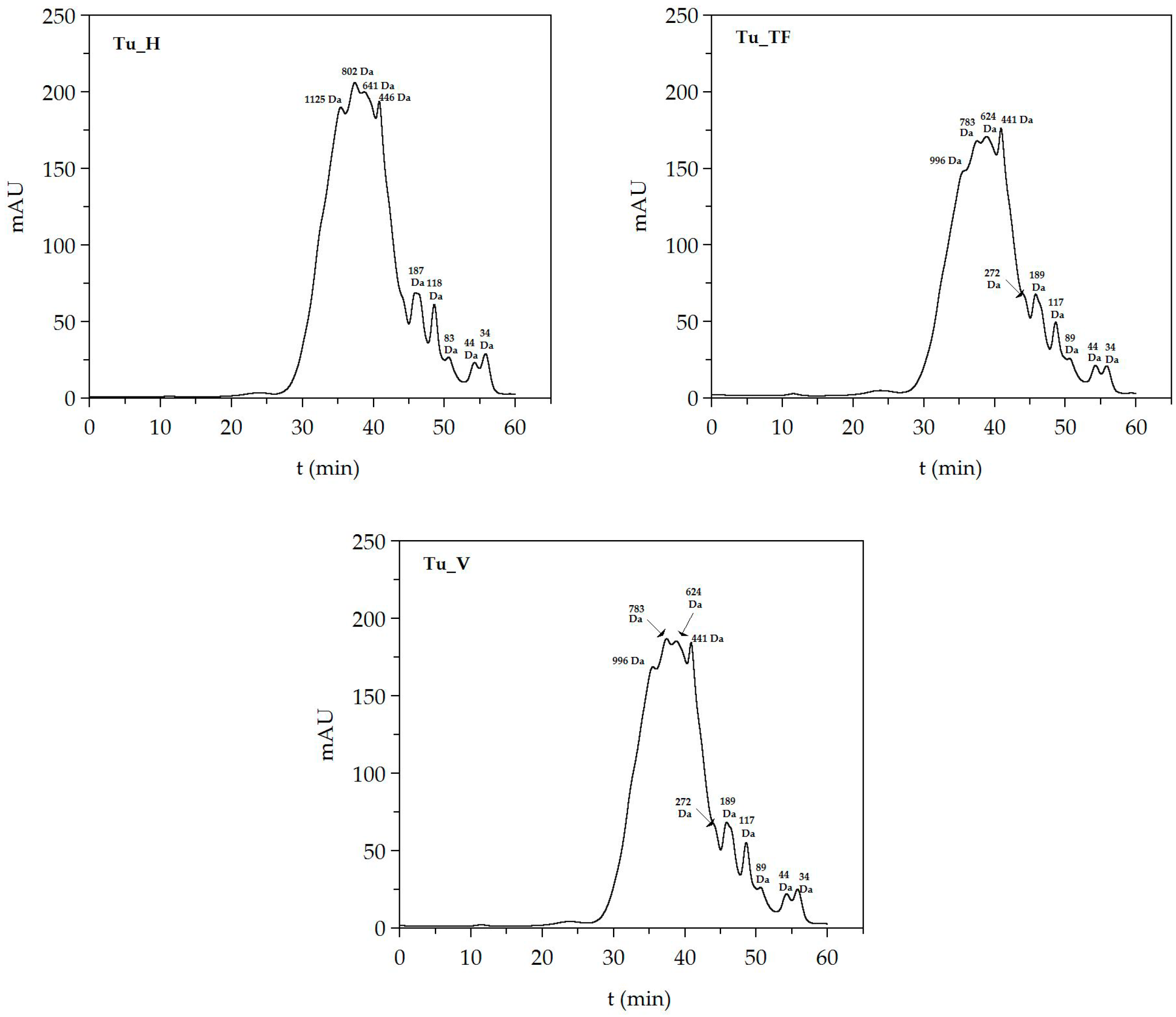
| FPHs | Mn (Da) | Mw (Da) | PDI | 0–0.2 kDa (%) | 0.2–0.5 kDa (%) | 0.5–1 kDa (%) | 1–3 kDa (%) | >3 kDa (%) |
|---|---|---|---|---|---|---|---|---|
| Tu_H | 1062 ± 102 | 1622 ± 146 | 1.527 | 8.1 ± 0.6 | 14.9 ± 0.0 | 23.1 ± 0.5 | 46.0 | 8.0 ± 1.4 |
| Tu_TF | 826 ± 42 | 1200 ± 53 | 1.453 | 7.0 ± 0.9 | 14.1 ± 0.2 | 34.1 ± 1.2 | 40.3 | 4.4 ± 0.9 |
| Tu_V | 878 ± 64 | 2146 ± 144 | 2.444 | 12.3 ± 3.9 | 6.6 ± 0.5 | 16.0 ± 1.2 | 52.9 | 12.21 |
| Antioxidant | Antihypertensive | ||||
|---|---|---|---|---|---|
| FPHs | DPPH (%) | ABTS (μg BHT/mL) | Crocin (μg Trolox/mL) | IACE (%) | IC50 (μg protein/mL) |
| Tu_H | 36.12 ± 2.81 a | 10.01 ± 0.98 a | 7.30 ± 0.92 a | 60.4 ± 5.8 a | 1273.6 ± 74.5 a |
| Tu_TF | 41.09 ± 0.98 b | 11.47 ± 2.14 a | 7.99 ± 0.51 a | 52.6 ± 24.1 a | 1063.4 |
| Tu_V | 65.15 ± 4.08 c | 12.81 ± 1.92 a | 8.03 ± 0.78 a | 81.9 ± 8.9 a,b | 212.7 ± 63.7 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. https://doi.org/10.3390/biom10020310
Vázquez JA, Rodríguez-Amado I, Sotelo CG, Sanz N, Pérez-Martín RI, Valcárcel J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules. 2020; 10(2):310. https://doi.org/10.3390/biom10020310
Chicago/Turabian StyleVázquez, José Antonio, Isabel Rodríguez-Amado, Carmen G. Sotelo, Noelia Sanz, Ricardo I. Pérez-Martín, and Jesus Valcárcel. 2020. "Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes" Biomolecules 10, no. 2: 310. https://doi.org/10.3390/biom10020310
APA StyleVázquez, J. A., Rodríguez-Amado, I., Sotelo, C. G., Sanz, N., Pérez-Martín, R. I., & Valcárcel, J. (2020). Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules, 10(2), 310. https://doi.org/10.3390/biom10020310









