Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process
Abstract
1. Introduction
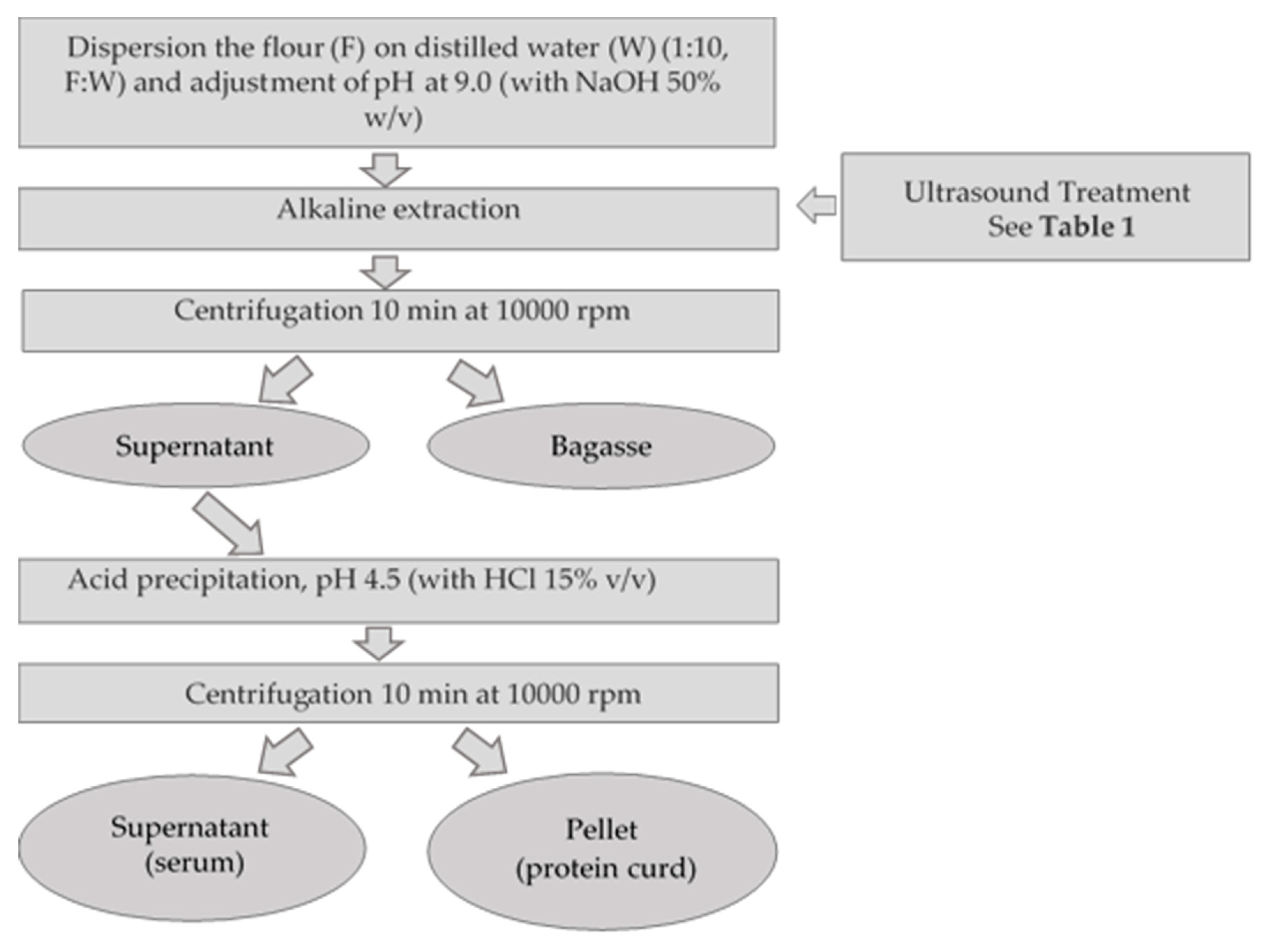
2. Materials and Methods
2.1. Raw Material
2.2. Seeds Quality
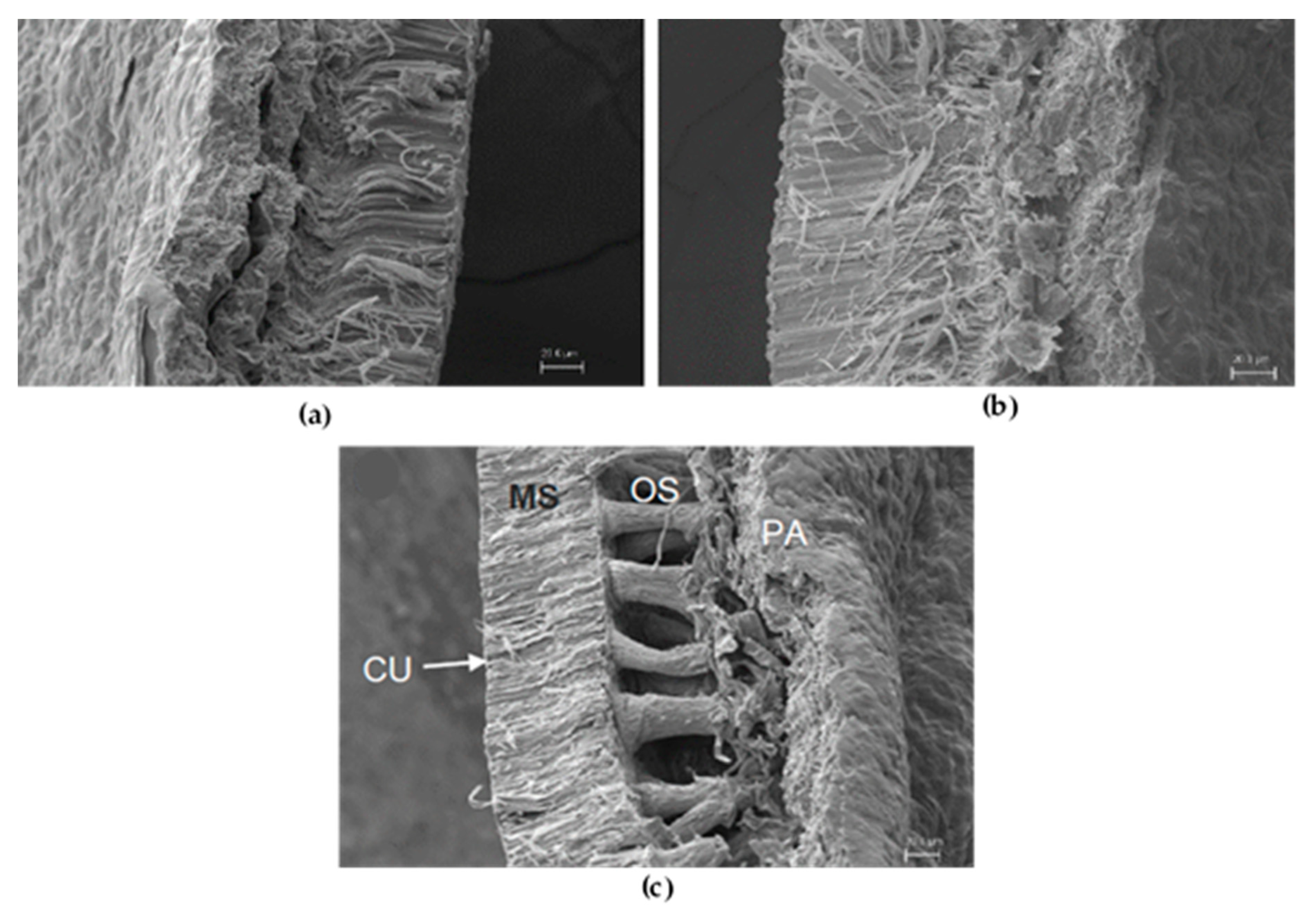
2.3. Scanning Electron Microscopy
2.4. Flour Characterization
2.4.1. Particle Size
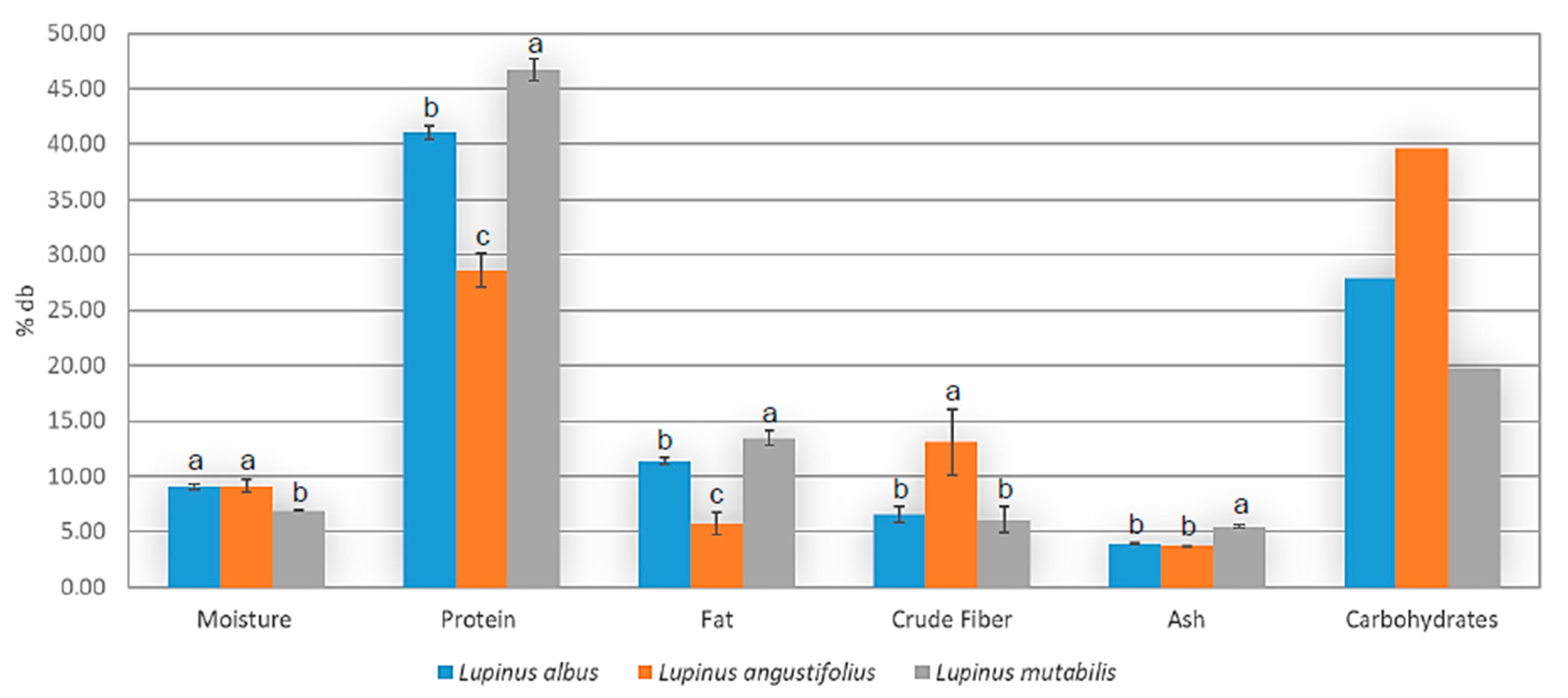
2.4.2. Proximate Analysis
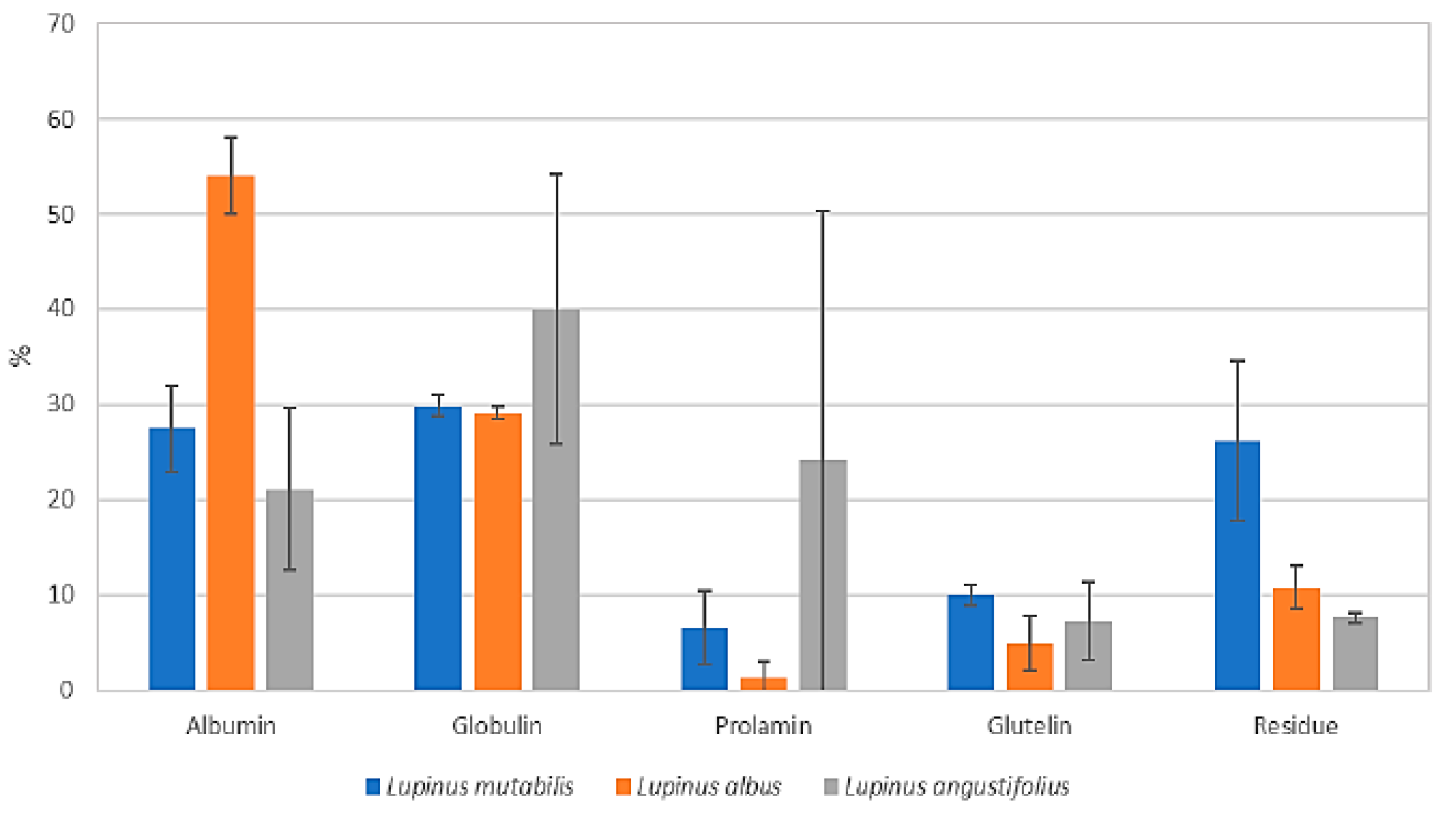
2.4.3. Osborne’s Solubility Profile
2.4.4. Amino Acid Profile
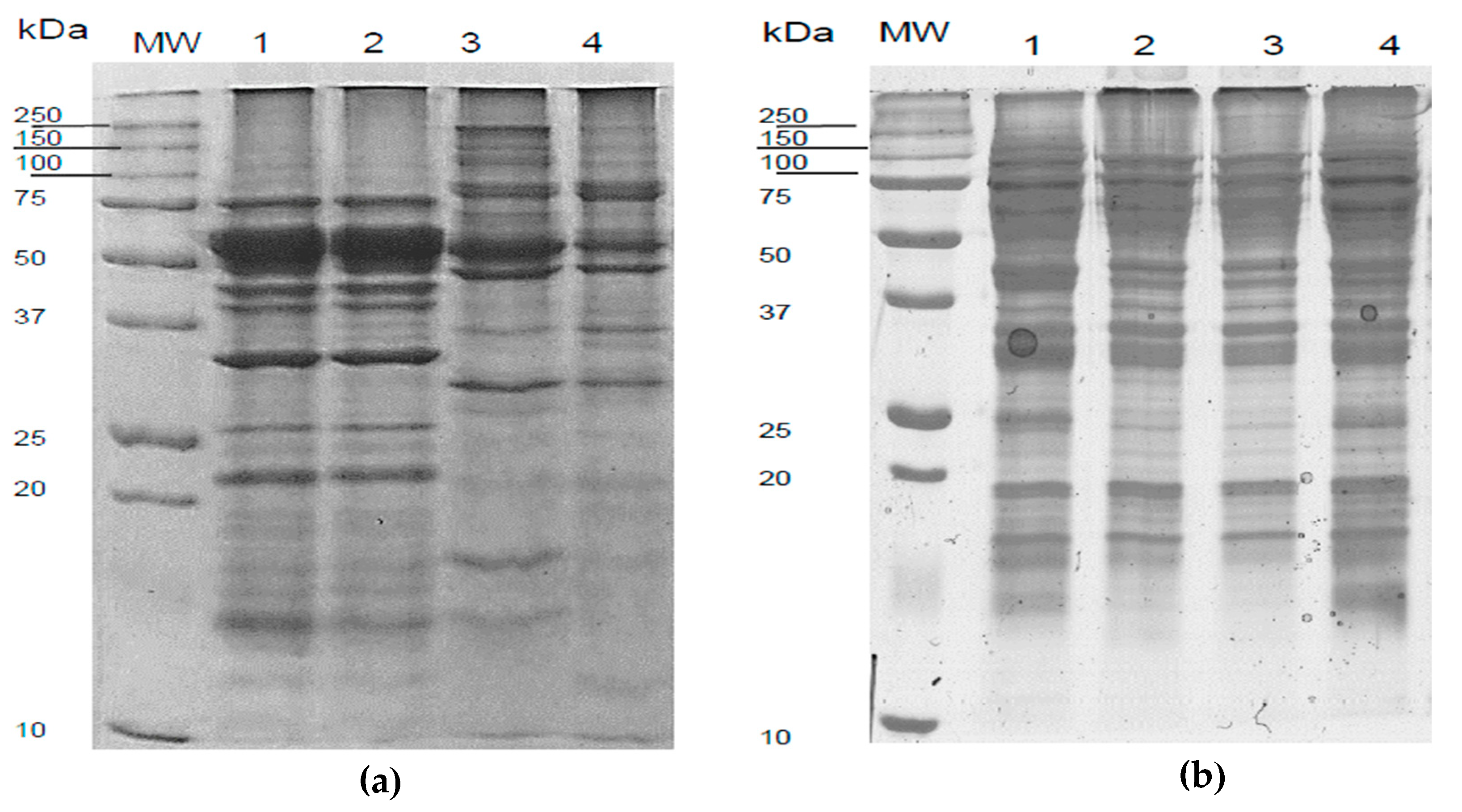
2.4.5. Electrophoretic Profile
2.4.6. Quinolizidine Alkaloids Concentration
2.4.7. Secondary Structure Analysis by FTIR
2.5. Effect of Ultrasound in Lupins Protein Extraction and the Properties of Isolates Produced under Alkaline Conditions
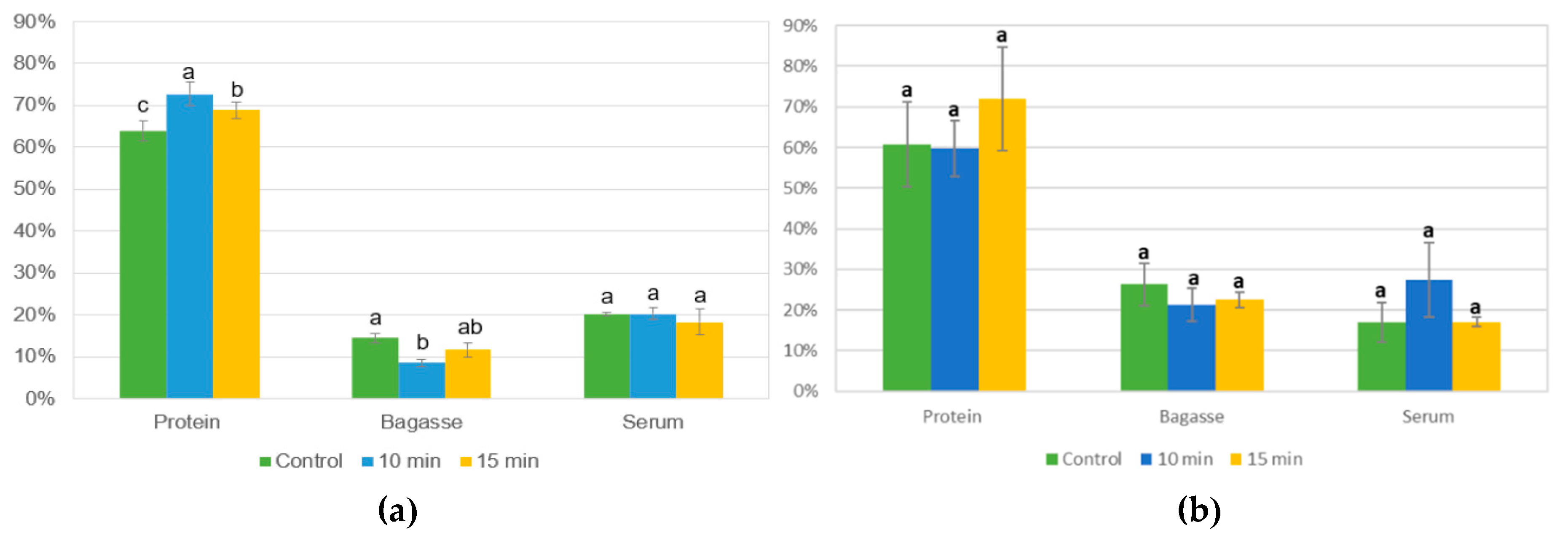
2.5.1. Protein Yield of Alkaline Extraction Process Assisted by Ultrasound
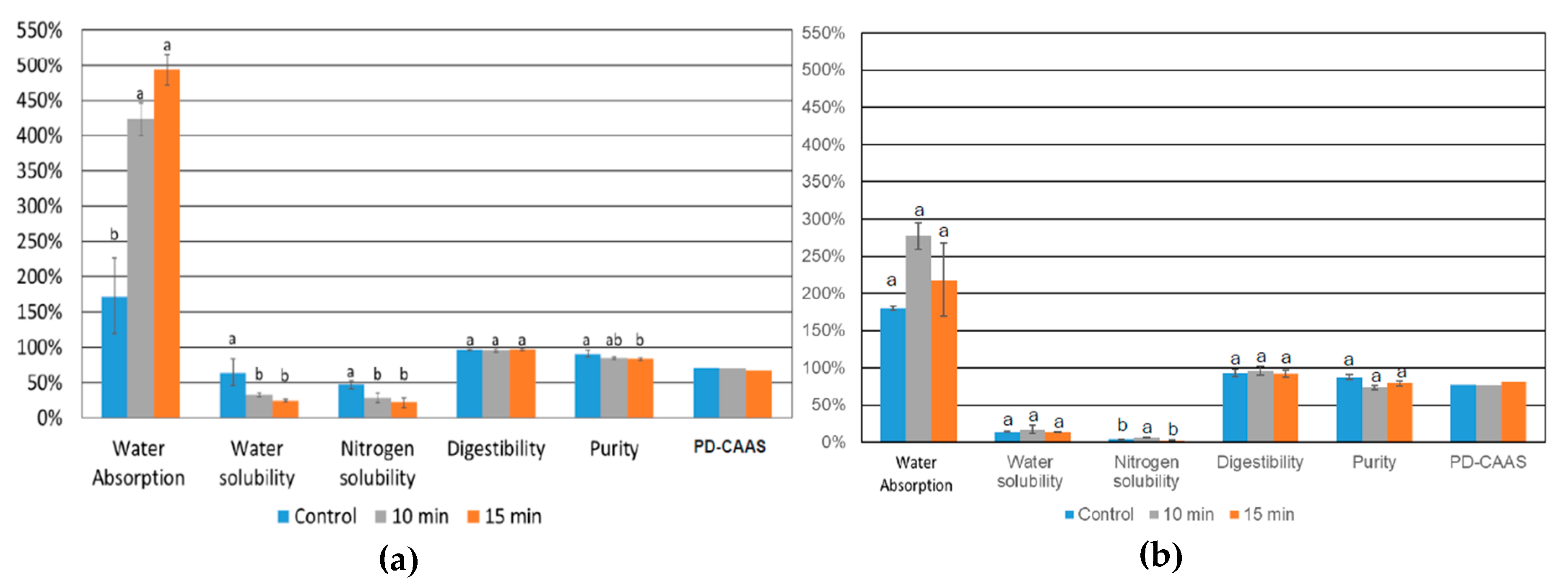
2.5.2. Functional Properties of Protein Isolates Obtained from Ultrasound Assisted Alkaline Extraction Process
2.5.3. Amino Acid Profile, Electrophoretic Profile, and Secondary Structure of Protein Isolates Obtained from the Ultrasound Assisted Alkaline Extraction Process
2.5.4. Quinolizidine Alkaloids Concentration in Serum, Bagasse, and Protein Isolates Obtained from Ultrasound Assisted Alkaline Extraction Process
2.6. Statistical Analysis
3. Results
3.1. Seed Quality
3.2. Scanning Electron Microscopy of Lupinus Seeds
3.3. Characterization of Flours
3.3.1. Particle Size
3.3.2. Proximate Analysis
3.3.3. Profile of Osborne Solubility
3.3.4. Amino Acid Profile
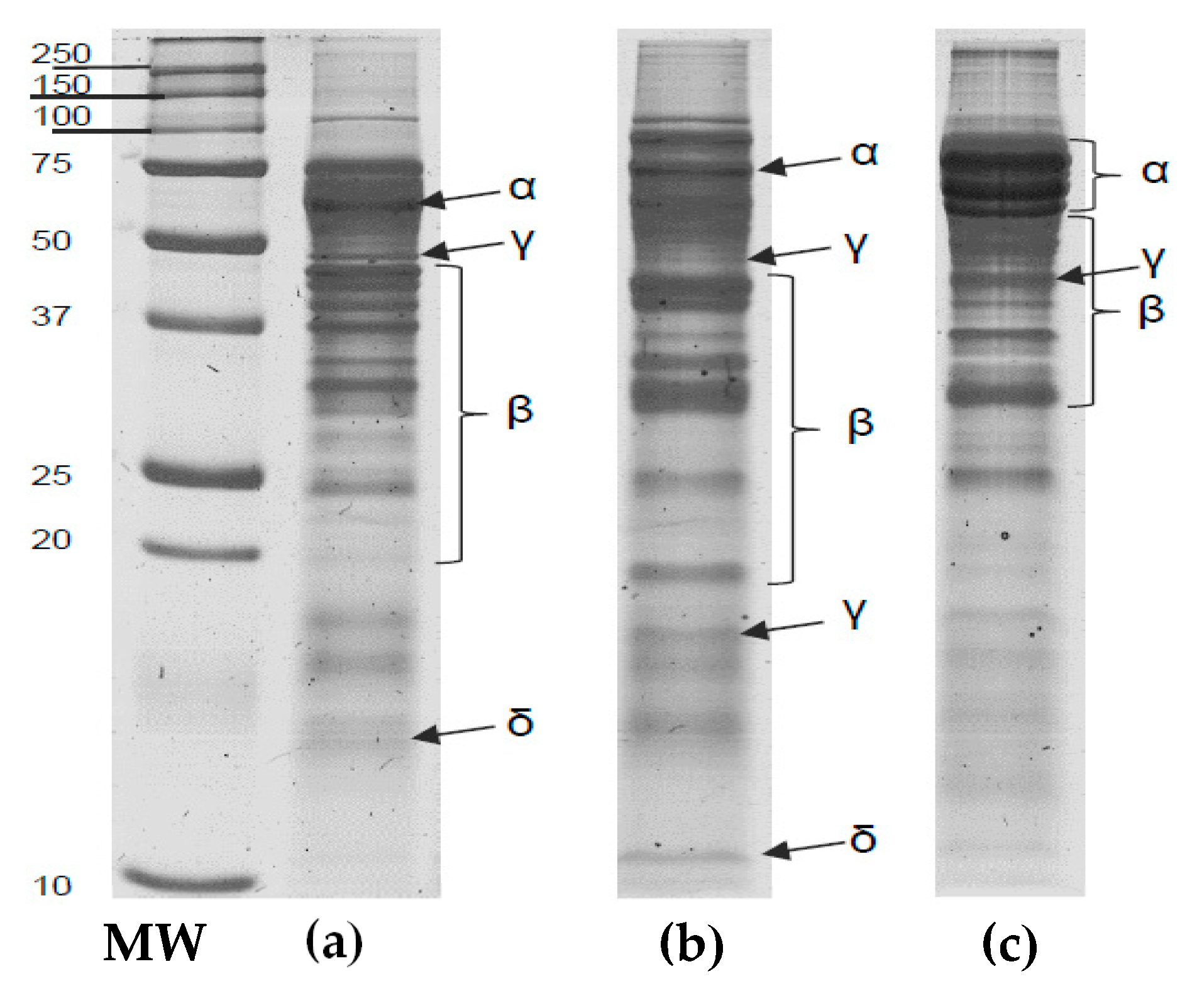
3.3.5. Electrophoretic Profile
3.3.6. Quinolizidine Alkaloids Concentration
3.3.7. Trypsin Inhibitor Activity
3.3.8. Secondary Structure by FTIR
3.4. Ultrasound Effects in Lupin Flours Protein Extraction Process, and Properties of the Obtained Isolates
3.4.1. Protein Yield of US Alkaline Extraction Using L. mutabilis and L. angustifolius
3.4.2. Functional Properties of Protein Isolates from L. mutabilis, and L. angustifolius Obtained from US-Assisted Alkaline Extraction Process
3.4.3. Electrophoretic Profile of L. mutabilis and L. angustifolius Isolates Obtained from US-Assisted Alkaline Extraction Process
3.4.4. Amino Acid Profile of L. mutabilis and L. angustifolius Protein Isolates Obtained from US-Assisted Alkaline Extraction Process
3.4.5. Trypsin Inhibitor Activity of L. mutabilis and L. angustifolius Protein Isolates Obtained from US-Assisted Alkaline Extraction Process
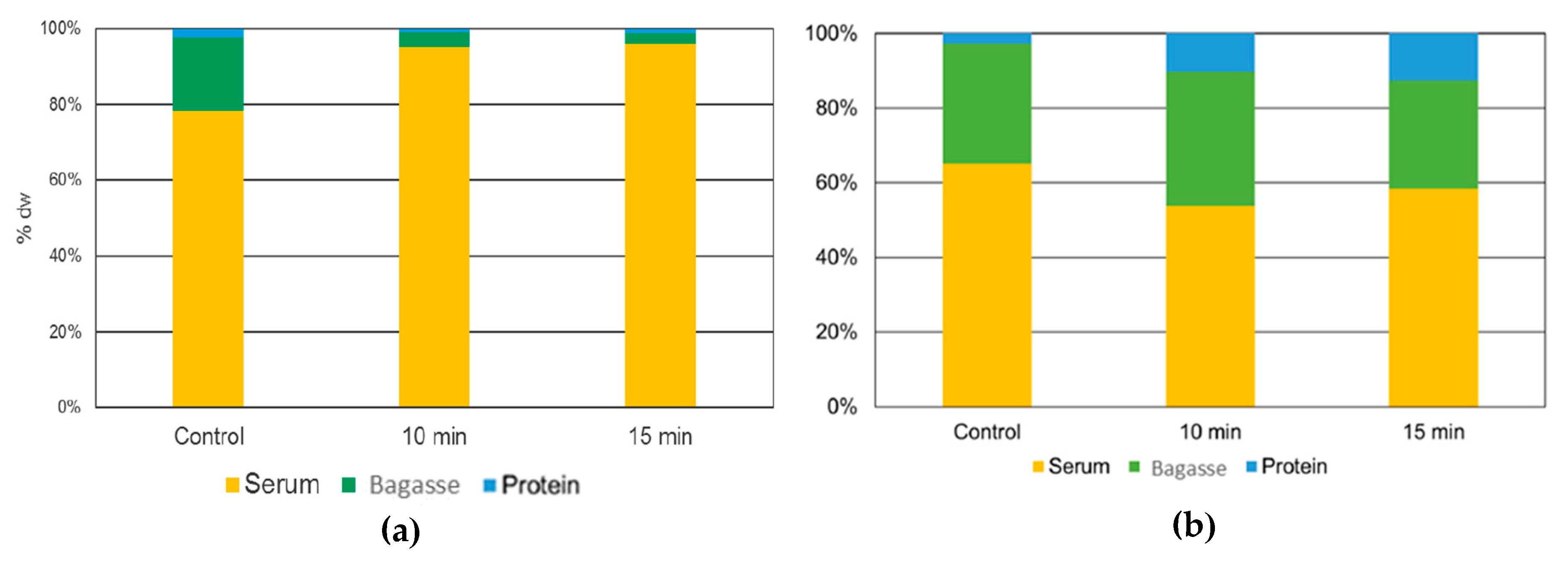
3.4.6. The fate of Alkaloids during L. mutabilis and L. angustifolius Ultrasound Process for Protein Extraction
3.4.7. Secondary Structure of Protein Isolates from L. mutabilis, and L. angustifolius Produced with Ultrasound-Assisted Extraction Procedure
4. Discussion
4.1. Seeds Quality and Morphology
4.2. Flour Characterization
4.3. Effects of the US Treatment in the Protein Isolates Yield and Functional Properties
4.4. Effects of the US Treatment in the Protein Isolates Electrophoretic Profile
4.5. Effects of the US Treatment in the Protein Isolates Amino Acid Profile
4.6. Effects of the US Treatment in the Protein Isolates Trypsin Inhibitor Activity
4.7. Effects of the US Treatment in the Fate of Quinolizidine Alkaloid Presents in the Protein Isolates
4.8. Effects of the US Treatment in the Protein Isolates Secondary Structure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Hettiarachchy, N.S.; Ziegler, G.R. Protein Functionality in Food Systems, 1st ed.; CRC PRESS: Boca Raton, FL, USA, 1994; ISBN 9780367402051. [Google Scholar]
- Deshpande, S.S.; Damodaran, S. Structure-digestibility relationship of legume 7S proteins. J. Food Sci. 1989, 54, 108–113. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Garcia, H.D. Protein extraction from lupin seeds: A mathematical model. Int. J. Food Sci. Technol. 1989, 24, 17–27. [Google Scholar] [CrossRef]
- Schwartzberg, G.H.; Chao, R.Y. Solute diffusivities in leaching processes. Food Technol. 1982, 36, 73–86. [Google Scholar]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; del Refugio Rocha-Pizaña, M.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of thermal processing and reducing agents on trypsin inhibitor activity and functional properties of soybean and chickpea protein concentrates. Lwt 2018, 98, 629–634. [Google Scholar] [CrossRef]
- D’Agostina, A.; Antonioni, C.; Resta, D.; Arnoldi, A.; Bez, J.; Knauf, U.; Wäsche, A. Optimization of a pilot-scale process for producing lupin protein isolates with valuable technological properties and minimum thermal damage. J. Agric. Food Chem. 2006, 54, 92–98. [Google Scholar] [CrossRef]
- Wäsche, A.; Müller, K.; Knauf, U. New processing of lupin protein isolates and functional properties. Nahrung Food 2001, 45, 393–395. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawey, A.A.; Gafar, A.F. Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates. Food Chem. 2001, 74, 455–462. [Google Scholar] [CrossRef]
- Australia New Zealand Food Authority. Lupin Alkaloids in Food. A Toxicological Review and Risk Assessment; Technical Report Series; Australia-New Zealand Food Authority: Canberra, Australia, 2001.
- Ortiz, J.G.F.; Mukherjee, K.D. Extraction of alkaloids and oil from bitter lupin seed. J. Am. Oil Chem. Soc. 1982, 59, 241–244. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.A.J.S. Lupinus mutabilis: Composition, uses, toxicology, and debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Alberta Agriculture and Food. Using 1000 Kernel Weight for Calculating Seeding Rates and Harvest Losses; Alberta Agriculture and Forestry: Red Deer, AB, Canada, 2001.
- AACC International AACC Approved Methods of Analysis, 11th Edition-AACC Method 55-10.01. Test Weight per Bushel. Available online: https://methods.aaccnet.org/summaries/55-10-01.aspx (accessed on 4 November 2019).
- Serna Saldívar, S.R.O. Cereal Grains: Laboratory Reference and Procedures Manual; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439855652. [Google Scholar]
- American Organization of Analytical Chemists International (AOAC). Official Methods 962.09, 978.02, 925.10, 923.03, 982.30, 984.13. Determination of Crude Fiber, Total Nitrogen, Moisture, Ash, Amino Acids Profile, Aplha-Amino Nitrogen; AOAC International: Washington, DC, USA, 1992. [Google Scholar]
- AACC International. AACC Approved Methods of Analysis, 11th Edition—AACC Method 30-20.01. Crude Fat in Grain and Stock Feeds. Available online: https://methods.aaccnet.org/summaries/30-20-01.aspx (accessed on 4 November 2019).
- Sambrook, J.; Russell, D.W. SDS-Polyacrylamide Gel Electrophoresis of Proteins. CSH Protoc. 2006, 4, 40–45. [Google Scholar] [CrossRef]
- Ruiz, L.P.; White, S.F.; Hove, E.L. The alkaloid content of sweet lupin seed used in feeding trials on pigs and rats. Anim. Feed Sci. Technol. 1977, 2, 59–66. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; González-Ruiz, J.; Chuck-Hernández, C. Alkaline peanut protein extraction assisted with microwaves (MAE). In Proceedings of the Food, Pharmaceutical and Bioengineering Division 2015—Core Programming Area at the 2015 AIChE Meeting, Salt Lake City, UT, USA, 8–13 November 2015; Volume 2. [Google Scholar]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Valdés, A.; De la Rosa Millán, J.; Serna-Saldivar, S.O.; Chuck-Hernández, C. Yield and textural characteristics of Panela cheeses produced with dairy-vegetable protein (soybean or peanut) blends supplemented with transglutaminase. J. Food Sci. 2015, 80, S2950–S2956. [Google Scholar] [CrossRef] [PubMed]
- Cheftel, J.C.; Cuq, J.L.; Lorient, D. Propiedades Funcionales de las Proteínas. In (Chapter 4). Proteínas Alimentarias; Acribia: Zaragoza, Spain, 1989; pp. 49–57. [Google Scholar]
- AOCS. Official and Tentative Methods of the American Oil Chemist’s Society; Method Ba 11-65. Nitrogen Solubility Index; American Oil Chemist’s Society (AOCS): Urbana, IL, USA, 2006. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Statterlee, L.D.; Miller, G.A. A multiezyme techniquefor estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Muzquiz, M. Alkaloids. Gas Chromatography. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1938–1949. [Google Scholar]
- Wink, M. Metabolism of Quinolizidine Alkaloids in Plants and Cell Suspension Cultures: Induction and Degradation; Springer: Berlin/Heidelberg, Germany, 1985; pp. 107–116. [Google Scholar]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Schoeneberger, H.; Gross, R.; Cremer, H.D.; Elmadfa, I. Composition and protein quality of Lupinus mutabilis. J. Nutr. 1982, 112, 70–76. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Analytical nutritional characteristics of seed proteins in six wild Lupinus species from Southern Spain. Food Chem. 2009, 117, 466–469. [Google Scholar] [CrossRef]
- Gdala, J.; Buraczewska, L. Chemical composition and carbohydrate content of seeds from several lupin species. J. Anim. Feed Sci. 1996, 5, 403–416. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press: Washington, DC, USA, 2005; ISBN 030908525X.
- Erbaş, M.; Certel, M.; Uslu, M.K. Some chemical properties of white lupin seeds (Lupinus albus L.). Food Chem. 2005, 89, 341–345. [Google Scholar] [CrossRef]
- Czubinski, J.; Barciszewski, J.; Gilski, M.; Szpotkowski, K.; Debski, J.; Lampart-Szczapa, E.; Jaskolski, M. Structure of γ-conglutin: Insight into the quaternary structure of 7S basic globulins from legumes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Lamsal, B.P.; Jung, S.; van Leeuwen, J.H.; Pometto, A.L., III; Grewell, D.; Khanal, S.K. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J. Food Eng. 2010, 96, 270–278. [Google Scholar] [CrossRef]
- Muranyi, I.S.; Volke, D.; Hoffmann, R.; Eisner, P.; Herfellner, T.; Brunnbauer, M.; Koehler, P.; Schweiggert-Weisz, U. Protein distribution in lupin protein isolates from Lupinus angustifolius L. prepared by various isolation techniques. Food Chem. 2016, 207, 6–15. [Google Scholar] [CrossRef]
- Eaton-Mordas, C.A.; Moore, K.G. Seed glycoproteins of Lupinus angustifolius. Phytochemistry 1978, 17, 619–621. [Google Scholar] [CrossRef]
- Ahmed Rayan, M.; Morsy, N.E. Physicochemical properties, antioxidant activity, phytochemicals and sensory evaluation of rice-based extrudates containing dried Corchorus olitorius L. leaves. J. Food Process. Technol. 2015, 6, 408. [Google Scholar]
- Lampart-Szczapa, E.; Konieczny, P.; Nogala-Kałucka, M.; Walczak, S.; Kossowska, I.; Malinowska, M. Some functional properties of lupin proteins modified by lactic fermentation and extrusion. Food Chem. 2006, 96, 290–296. [Google Scholar] [CrossRef]
- King, J.; Aguirre, C.; De Pablo, S. Functional properties of lupin protein isolates (Lupinus albus cv Multolupa). J. Food Sci. 2006, 50, 82–87. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J. Food Sci. 1982, 47, 491–497. [Google Scholar] [CrossRef]
- Lqari, H.; Vioque, J.; Pedroche, J.; Millán, F. Lupinus angustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem. 2002, 76, 349–356. [Google Scholar] [CrossRef]
- Rozan, P.; Lamghari, R.; Linder, M.; Villaume, C.; Fanni, J.; Parmentier, M.; Méjean, L. In vivo and in vitro digestibility of soybean, lupine, and rapeseed meal proteins after various technological processes. J. Agric. Food Chem. 1997, 45, 1762–1769. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Batistuti, J.P.; da Cruz, R.J.; Saldiva, P.H.N.; Arêas, J.A.G. Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]

| Parameter | Unit | Value(s) |
|---|---|---|
| pH | - | 9 |
| Frequency | kHz | 24 |
| Temperature | °C | Monitored |
| Amplitude | μm | 100 |
| Sonotrode diameter | mm | 22 |
| Acoustic power density | W/cm2 | 85 |
| Time | min | 0, 10, 15 |
| Variety | Thousand Seed Weight (g) | Test Weight (kg/hL) | Foreign Material (%) | Damaged Kernels (%) | Foreign Seeds (%) |
|---|---|---|---|---|---|
| Lupinus albus | 311.0 ± 13.64 | 60.23 ± 0.20 | 0.18 | 0.75 | 0.09 |
| Lupinus angustifolius | 140.1 ± 5.23 | 62.14 ± 0.42 | 0.95 | 0.36 | 0.35 |
| Lupinus mutabilis | 214.1 ± 0.90 | 59.01 ± 0.27 | 0.66 | 1.07 | 0.11 |
| Amino Acid | L. albus | L. angustifolius | L. mutabilis |
|---|---|---|---|
| (g/100 g of Crude Protein) | |||
| Aspartic acid | 10.52 | 10.35 | 10.36 |
| Threonine | 3.65 | 3.76 | 3.61 |
| Serine | 4.63 | 4.05 | 4.04 |
| Glutamic acid | 21.66 | 21.20 | 22.45 |
| Proline | 4.38 | 4.43 | 4.23 |
| Glycine | 4.07 | 4.65 | 4.25 |
| Alanine | 3.45 | 3.89 | 3.73 |
| Cysteine | 1.74 | 1.77 | 1.46 |
| Valine | 4.35 | 4.56 | 4.32 |
| Methionine + Cys | 2.50 | 2.58 | 2.20 |
| Methionine | 0.76 | 0.80 | 0.73 |
| Isoleucine | 4.71 | 4.56 | 4.82 |
| Leucine | 7.74 | 7.52 | 6.87 |
| Tyrosine | 4.32 | 3.59 | 4.20 |
| Phenylalanine + Tyr | 8.39 | 7.81 | 8.17 |
| Phenylalanine | 4.07 | 4.22 | 3.97 |
| Lysine | 5.02 | 5.62 | 5.93 |
| Histidine | 2.38 | 2.91 | 2.95 |
| Arginine | 10.86 | 10.56 | 10.67 |
| Tryptophan | 0.76 | 1.06 | 0.97 |
| Amino Acid Score 1 | 0.96 | 0.99 | 0.84 |
| Variety | β-Sheet 1 | 310 Helix | α-Helix | Unordered | β-Sheet | Aggregated Strands |
|---|---|---|---|---|---|---|
| L. albus | 21.4 | 7.1 | 28.6 | 21.4 | 21.4 | 0.0 |
| L. angustifolius | 50.0 | 6.3 | 25.0 | 018.8 | 0.0 | 0.0 |
| L. mutabilis | 34.8 | 13.0 | 17.4 | 8.7 | 26.1 | 0.0 |
| Amino Acid | Control (0 min) | 10 min US Treatment | 15 min US Treatment | |||
|---|---|---|---|---|---|---|
| (g/100 g of Crude Protein) | ||||||
| L. mutabilis | L. angustifolius | L. mutabilis | L. angustifolius | L. mutabilis | L. angustifolius | |
| Aspartic acid | 10.27 | 10.35 | 10.34 | 10.40 | 10.39 | 10.45 |
| Threonine | 3.24 | 3.12 | 3.43 | 3.59 | 3.43 | 3.62 |
| Serine | 5.05 | 4.69 | 4.22 | 4.05 | 4.20 | 3.98 |
| Glutamic acid | 24.08 | 24.15 | 21.78 | 20.88 | 22.03 | 20.59 |
| Proline | 4.17 | 4.41 | 4.37 | 4.57 | 4.37 | 4.52 |
| Glycine | 3.87 | 4.08 | 4.04 | 4.42 | 4.03 | 4.41 |
| Alanine | 3.25 | 3.23 | 3.53 | 3.77 | 3.52 | 3.78 |
| Cysteine | 1.25 | 1.60 | 1.14 | 1.41 | 1.11 | 1.42 |
| Valine | 4.09 | 4.08 | 4.59 | 4.79 | 4.53 | 4.88 |
| Methionine+ Cys | 1.82 | 2.16 | 2.82 | 2.17 | 1.74 | 2.21 |
| Methionine | 0.57 | 0.56 | 0.68 | 0.76 | 0.63 | 0.79 |
| Isoleucine | 5.01 | 4.69 | 5.25 | 4.91 | 5.22 | 4.98 |
| Leucine | 7.08 | 7.61 | 7.38 | 7.94 | 7.31 | 8.08 |
| Tyrosine | 3.81 | 3.63 | 4.26 | 3.93 | 4.24 | 3.92 |
| Phenylalanine+Tyr | 7.75 | 7.84 | 8.57 | 8.53 | 8.51 | 8.61 |
| Phenylalanine | 3.94 | 4.21 | 4.31 | 4.60 | 4.28 | 4.69 |
| Lysine | 5.34 | 4.61 | 5.59 | 5.31 | 5.59 | 5.31 |
| Histidine | 2.69 | 2.60 | 2.72 | 2.73 | 2.72 | 2.76 |
| Arginine | 11.28 | 11.29 | 11.14 | 10.47 | 11.19 | 10.37 |
| Tryptophan | 0.96 | 0.99 | 0.98 | 1.16 | 1.00 | 1.10 |
| Amino Acid Score 1 | 0.70 | 0.83 | 0.70 | 0.83 | 0.67 | 0.85 |
| US Treatment | β-Sheet 1 | 310 Helix | α-Helix | Unordered | β-Sheet | Aggregated Strands |
|---|---|---|---|---|---|---|
| Control (0 min) | 40.00 | 20.0 | 20.0 | 0.0 | 10.0 | 10.0 |
| 10 min | 50.0 | 0.0 | 12.5 | 12.5 | 12.5 | 12.5 |
| 15 min | 57.1 | 0.0 | 14.3 | 14.3 | 0.0 | 14.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Acosta, L.A.; Serna-Saldivar, S.O.; Rodríguez-Rodríguez, J.; Escalante-Aburto, A.; Chuck-Hernández, C. Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process. Biomolecules 2020, 10, 292. https://doi.org/10.3390/biom10020292
Aguilar-Acosta LA, Serna-Saldivar SO, Rodríguez-Rodríguez J, Escalante-Aburto A, Chuck-Hernández C. Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process. Biomolecules. 2020; 10(2):292. https://doi.org/10.3390/biom10020292
Chicago/Turabian StyleAguilar-Acosta, Luis Alberto, Sergio O. Serna-Saldivar, José Rodríguez-Rodríguez, Anayansi Escalante-Aburto, and Cristina Chuck-Hernández. 2020. "Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process" Biomolecules 10, no. 2: 292. https://doi.org/10.3390/biom10020292
APA StyleAguilar-Acosta, L. A., Serna-Saldivar, S. O., Rodríguez-Rodríguez, J., Escalante-Aburto, A., & Chuck-Hernández, C. (2020). Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process. Biomolecules, 10(2), 292. https://doi.org/10.3390/biom10020292






