Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases
Abstract
1. Introduction
2. Pathophysiology
3. Treatment and Management of PUD
4. Monoterpenes, Peptic Ulcers, and H. pylori
5. NSAIDs
6. Ethanol
7. Ischemia-Reperfusion (I/R)
8. Acetic Acid
9. Mechanisms of Action of the Peptic Ulcers
10. Monoterpenes with Anti-H. pylori Effects
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Proctor, M.J.; Deans, C. Complications of peptic ulcers. Surgery 2014, 32, 599–607. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K. Peptic ulcer disease. Lancet 2017, 6736, 1–12. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Schulz, C. Peptic Ulcer: Chapter Closed? Dig. Dis. 2020, 38, 112–116. [Google Scholar] [CrossRef]
- Brown, L.F.; Wilson, D.E. Gastroduodenal ulcers: Causes, diagnosis, prevention and treatment. Compr. Ther. 1999, 25, 30–38. [Google Scholar] [CrossRef]
- Dimaline, R.; Varro, A. Attack and defence in the gastric epithelium - a delicate balance. Exp. Physiol. 2007, 92, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; de Lima Portella, R.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.M.; da Luz, S.C.A.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Júnior, F.E.B.; de Oliveira, D.R.; Boligon, A.A.; Athayde, M.L.; Kamdem, J.P.; Macedo, G.E.; da Silva, G.F.; de Menezes, I.R.A.; Costa, J.G.M.; Coutinho, H.D.M.; et al. Protective effects of Croton campestris A. St-Hill in different ulcer models in rodents: Evidence for the involvement of nitric oxide and prostaglandins. J. Ethnopharmacol. 2014, 153, 469–477. [Google Scholar] [CrossRef][Green Version]
- Wallace, J.L. Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- Wang, G.Z.; Wang, J.F. Effects of Helicobacter pylori and Non-Steroidal Anti-Inflammatory Drugs on Peptic Ulcer. In Peptic Ulcer Disease; InTech: London, UK, 2011; pp. 29–38. [Google Scholar]
- Allen, A.; Flemström, G. Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am. J. Physiol. Physiol. 2005, 288, C1–C19. [Google Scholar] [CrossRef]
- Tarnawski, A.; Ahluwalia, A.; Jones, M.K. Gastric cytoprotection beyond prostaglandins: Cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr. Pharm. Des. 2013, 19, 126–132. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H. Pathophysiology and Risk Factors in Peptic Ulcer Disease. In Introduction to Gastrointestinal Diseases; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 7–20. [Google Scholar]
- Sidahmed, H.M.; Azizan, A.H.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Taha, M.M.; Hadi, H.; Ketuly, K.A.; Hashim, N.M.; Loke, M.F.; et al. Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: Possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylo. BMC Complement. Altern. Med. 2013, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [PubMed]
- Rosso, C.; Fagoonee, S.; Altruda, F.; Pellicano, R. Update on colonization, survival and antibiotic resistance of Helicobacter pylori at the molecular level. Minerva Biotec 2015, 27, 149–157. [Google Scholar]
- Pellicano, R.; Ribaldone, D.G..; Fagoonee, S..; Astegiano, M..; Saracco, G.M.; Mégraud, F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016, 58, 304–317. [Google Scholar]
- Malfertheiner, P.; Chan, F.K.; McColl, K. EL Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Lichtenberger, L.M. The Hydrophobic Barrier Properties of Gastrointestinal Mucus. Annu. Rev. Physiol. 1995, 57, 565–583. [Google Scholar] [CrossRef]
- Tziona, P.; Theodosis-Nobelos, P.; Rekka, E.A. Medicinal Chemistry Approaches of Controlling Gastrointestinal Side Effects of Non-Steroidal Anti-Inflammatory Drugs. Endogenous Protective Mechanisms and Drug Design. Med. Chem. 2017, 13, 408–420. [Google Scholar] [CrossRef]
- Scally, B.; Emberson, J.R.; Spata, E.; Reith, C.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Bhala, N.; Hawkey, C.; et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: A meta-analysis of randomised trials. lancet. Gastroenterol. Hepatol. 2018, 3, 231–241. [Google Scholar] [CrossRef]
- Chey, W.D.; Wong, B.C.Y. Practice Parameters Committee of the American College of Gastroenterology American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Fock, K.M.; Katelaris, P.; Sugano, K.; Ang, T.L.; Hunt, R.; Talley, N.J.; Lam, S.K.; Xiao, S.-D.; Tan, H.J.; Wu, C.-Y.; et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2009, 24, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Fischbach, L.; Evans, E.L. Meta-analysis: The effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment. Pharmacol. Ther. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Broutet, N.; Tchamgoué, S.; Pereira, E.; Lamouliatte, H.; Salamon, R.; Mégraud, F. Risk factors for failure of Helicobacter pylori therapy--results of an individual data analysis of 2751 patients. Aliment. Pharmacol. Ther. 2003, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Miwa, H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J. Neurogastroenterol. Motil. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A. We Are Using Too Many PPIs and We Need to Stop: A European Perspective. Am. J. Gastroenterol. 2016, 111, 1085–1086. [Google Scholar] [CrossRef]
- Lehours, P.; Ferrero, R.L. Review: Helicobacter: Inflammation, immunology, and vaccines. Helicobacter 2019, 24. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A. Helicobacter pylori treatment: New perspectives using current experience. J. Glob. Antimicrob. Resist. 2017, 8, 123–130. [Google Scholar] [CrossRef]
- Brierley, S.M.; Kelber, O. Use of natural products in gastrointestinal therapies. Curr. Opin. Pharmacol. 2011, 11, 604–611. [Google Scholar] [CrossRef]
- Kangwan, N.; Park, J.-M.; Kim, E.-H.; Hahm, K.B. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.B. Contribuição da fitoquímica para o desenvolvimento de um país emergente. Quim. Nova 2010, 33, 229–239. [Google Scholar] [CrossRef]
- Barreto, R.; Albuquerque-Júnior, R.; Araújo, A.; Almeida, J.; Santos, M.; Barreto, A.; DeSantana, J.; Siqueira-Lima, P.; Quintans, J.; Quintans-Júnior, L. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014, 19, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes modulating cytokines - A review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Bergonzelli, G.E.; Donnicola, D.; Porta, N.; Corthésy-Theulaz, I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. [Google Scholar] [CrossRef]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 120814114042001. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; de Mello Moraes, T.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.M.; Santos, A.R.S.S.; Hiruma-Lima, C.A. Citral: A monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini Rev. Med. Chem. 2015, 14, 1156–1168. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with Analgesic Activity-A Systematic Review. Phyther. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef]
- de Cássia da Silveira e Sá, R.; Andrade, L.; de Sousa, D. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Mo, H.; Zhang, L.; Zhang, L.; Zhou, S.; Ma, X.; Zhang, B. Gastroprotective Effects of Ascaridole on Gastric Ulcer in Rats. Chin. Herb. Med. 2012, 4, 58–62. [Google Scholar]
- Rocha Caldas, G.F.; Oliveira, A.R.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; Silva-Neto Jda, C.; Costa-Silva, J.H.; Ferreira, F.; da Costa, J.G.M.; Wanderley, A.G. Gastroprotective Mechanisms of the Monoterpene 1,8-Cineole (Eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar] [CrossRef]
- Siqueira, B.P.J.; Menezes, C.T.; Silva, J.P.; de Sousa, D.P.; Batista, J.S. Antiulcer effect of epoxy-carvone. Rev. Bras. Farmacogn. 2012, 22, 144–149. [Google Scholar] [CrossRef]
- da Silva, F.V.; de Barros Fernandes, H.; Oliveira, I.S.; Viana, A.F.S.C.; da Costa, D.S.; Lopes, M.T.P.; de Lira, K.L.; Quintans-Júnior, L.J.; de Sousa, A.A.; de Cássia Meneses Oliveira, R. Beta-cyclodextrin enhanced gastroprotective effect of (−)-linalool, a monoterpene present in rosewood essential oil, in gastric lesion models. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, Y.; Firempong, C.K.; Xu, X. Preparation, characterization and pharmacokinetic studies of linalool-loaded nanostructured lipid carriers. Pharm. Biol. 2016, 54, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Barocelli, E.; Calcina, F.; Chiavarini, M.; Impicciatore, M.; Bruni, R.; Bianchi, A.; Ballabeni, V. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sci. 2004, 76, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; Meira de Faria, F.; Souza Brito, A.R.; Pellizzon, C.H. The gastroprotective effect of menthol: Involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS ONE 2014, 9, e86686. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; Hiruma-Lima, C.A.; Takahira, R.K.; Padovani, C.R.; Pellizzon, C.H. Effect of menthol in experimentally induced ulcers: Pathways of gastroprotection. Chem. Biol. Interact. 2013, 206, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.F.S.C.; da Silva, F.V.; Fernandes, H.D.B.; Oliveira, I.S.; Braga, M.A.; Nunes, P.I.G.; de Viana, D.A.; de Sousa, D.P.; Rao, V.S.; Oliveira, R.C.M.; et al. Gastroprotective effect of (-)-myrtenol against ethanol-induced acute gastric lesions: Possible mechanisms. J. Pharm. Pharmacol. 2016, 68, 1085–1092. [Google Scholar] [CrossRef]
- González-Ramírez, A.E.; González-Trujano, M.E.; Orozco-Suárez, S.A.; Alvarado-Vásquez, N.; López-Muñoz, F.J. Nerol alleviates pathologic markers in the oxazolone-induced colitis model. Eur. J. Pharmacol. 2016, 776, 81–89. [Google Scholar] [CrossRef]
- Pinheiro, M.A.; Magalhães, R.; Torres, D.; Cavalcante, R.; Mota, F.X.; Oliveira Coelho, E.A.; Moreira, H.; Lima, G.; da Costa Araújo, P.; Cardoso, J.L.; et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn. Mag. 2015, 11, 123. [Google Scholar]
- Souza, R.; Cardoso, M.; Menezes, C.; Silva, J.; De Sousa, D.; Batista, J. Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. Daru 2011, 19, 277–281. [Google Scholar]
- Zeren, S.; Bayhan, Z.; Kocak, F.E.; Kocak, C.; Akcılar, R.; Bayat, Z.; Simsek, H.; Duzgun, S.A. Gastroprotective effects of sulforaphane and thymoquinone against acetylsalicylic acid–induced gastric ulcer in rats. J. Surg. Res. 2016, 203, 348–359. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva, F.V.; Viana, A.F.S.C.; dos Santos, M.R.V.; Quintans-Júnior, L.J.; Martins, M.C.C.; Nunes, P.H.M.; Oliveira, F.A.; Oliveira, R.C.M. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012, 385, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Shane, G.T.; Yang, C.H.; Chen, K.Y. Composition for the treatment and prevention of peptic ulcer 11/612,549, 2008.
- De Carvalho, K.I.M.; Bonamin, F.; Dos Santos, R.C.; Périco, L.L.; Beserra, F.P.; De Sousa, D.P.; Filho, J.M.B.; Da Rocha, L.R.M.; Hiruma-Lima, C.A. Geraniol - A flavoring agent with multifunctional effects in protecting the gastric and duodenal mucosa. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Venzon, L.; Mariano, L.N.B.; Somensi, L.B.; Boeing, T.; de Souza, P.; Wagner, T.M.; de Andrade, S.F.; Nesello, L.A.N.; da Silva, L.M. Essential oil of Cymbopogon citratus (lemongrass) and geraniol, but not citral, promote gastric healing activity in mice. Biomed. Pharmacother. 2018, 98, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bonamin, F.; Moraes, T.M.; Dos Santos, R.C.; Kushima, H.; Faria, F.M.; Silva, M.A.; Junior, I.V.; Nogueira, L.; Bauab, T.M.; Souza Brito, A.R.M.; et al. The effect of a minor constituent of essential oil from Citrus aurantium: The role of B-myrcene in preventing peptic ulcer disease. Chem. Biol. Interact. 2014, 212, 11–19. [Google Scholar] [CrossRef]
- De Monte, C.; Bizzarri, B.; Gidaro, M.C.; Carradori, S.; Mollica, A.; Luisi, G.; Granese, A.; Alcaro, S.; Costa, G.; Basilico, N.; et al. Bioactive compounds of Crocus sativus L. and their semi-synthetic derivatives as promising anti- Helicobacter pylori, anti-malarial and anti-leishmanial agents. J. Enzyme Inhib. Med. Chem. 2015, 30, 1027–1033. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Therapeutic potential and mechanism of thymol action against ethanol-induced gastric mucosal injury in rat model. Alcohol 2015, 49, 739–745. [Google Scholar] [CrossRef]
- Ribeiro, A.R.S.; Diniz, P.B.F.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.; Thomazzi, S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K+ channels, and gastric mucus secretion. Chem. Biol. Interact. 2016, 244, 121–128. [Google Scholar] [CrossRef]
- Chen, Y.; Bedson, J.; Hayward, R.A.; Jordan, K.P. Trends in prescribing of non-steroidal anti-inflammatory drugs in patients with cardiovascular disease: Influence of national guidelines in UK primary care. Fam. Pract. 2018. [Google Scholar] [CrossRef]
- Takeuchi, K.; Izuhara, C.; Takayama, S.; Momode, T.; Kojo, M.; Hara, D.; Amagase, K. Animal Models of Gastric Bleeding Induced by Dual Antiplatelet Therapy Using Aspirin and Clopidogrel -Prophylactic Effect of Antiulcer Drugs. Curr. Pharm. Des. 2014, 20, 1139–1148. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Gomes Silva, M.I.; de Sous, F.C.F. Gastric Ulcer Etiology. In Peptic Ulcer Disease; InTech: London, UK, 2011. [Google Scholar]
- Vane, J.R.; Botting, R.M. A better understanding of anti-inflammatory drugs based on isoforms of cyclooxygenase (COX-1 and COX-2). Adv. Prostaglandin. Thromboxane. Leukot. Res. 1995, 23, 41–48. [Google Scholar] [PubMed]
- Redasani, V.K.; Bari, S.B. Synthesis and evaluation of mutual prodrugs of ibuprofen with menthol, thymol and eugenol. Eur. J. Med. Chem. 2012, 56, 134–138. [Google Scholar] [CrossRef]
- WHO Global Status Report on Alcohol and Health 2014; WHO Library Cataloguing-in-Publication Data; World Health Organization, Ed.; WHO: Geneva, Switzerland, 2014; ISBN 978 92 4 069276 3. [Google Scholar]
- Kwiecień, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar]
- Gardes-Albert, M.; Ferradini, C.; Sekaki, A. Oxygen-centered free radicals and their interaction with EGb 761 or CP202. In Advances in Ginkgo biloba extract research; Ferradini, C., Droy-Lefaix, M.T., Christen, Y., Eds.; Elsevier: Paris, France, 1993; pp. 1–11. [Google Scholar]
- Rajasekaran, A.; Sivakumar, V.; Darlinquine, S. Role of Blepharis maderaspatensis and Ammannia baccifera plant extracts on in vitro oxygen radical scavenging, secretion of gastric fluid and gastroprotection on ulcer induced rats. Pharm. Biol. 2012, 50, 1085–1095. [Google Scholar] [CrossRef]
- Hoshino, T.; Takano, T.; Tsutsumi, S.; Tomisato, W.; Tsuchiya, T.; Mizushima, T. Effects of prostaglandin E2 on gastric irritant-induced apoptosis. Dig. Dis. Sci. 2002, 47, 2370–2379. [Google Scholar] [CrossRef]
- Das, A.K.; Bigoniya, P.; Verma, N.K.; Rana, A.C. Gastroprotective effect of Achyranthes aspera Linn. leaf on rats. Asian Pac. J. Trop. Med. 2012, 5, 197–201. [Google Scholar] [CrossRef]
- Pan, J.-S.; He, S.-Z.; Xu, H.-Z.; Zhan, X.-J.; Yang, X.-N.; Xiao, H.-M.; Shi, H.-X.; Ren, J.-L. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J. Gastroenterol. 2008, 14, 5857–5867. [Google Scholar] [CrossRef]
- Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Chamoun, F.; Burne, M.; O’Donnell, M.; Rabb, H. Pathophysiologic role of selectins and their ligands in ischemia reperfusion injury. Front. Biosci. 2000, 5, 103–109. [Google Scholar] [CrossRef]
- Piper, H.M.; Meuter, K.; Schäfer, C. Cellular mechanisms of ischemia-reperfusion injury. Ann. Thorac. Surg. 2003, 75, S644–S648. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Tarnawski, A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 2005, 50, 24–33. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar]
- Cnubben, N.H.P.; Rietjens, I.M.C.M.; Wortelboer, H.; Van-Zanden, J.; Van Bladeren, P.J. The interplay of glutathione related processes in antioxidant defense. Environ. Toxicol. Pharmacol. 2001, 10, 141–152. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Koo, J.-R.; Roberts, C.K.; Vaziri, N.D. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: Response to insulin and antioxidant therapies. Clin. Exp. Hypertens. 2004, 26, 43–53. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Handa, O.; Yoshikawa, T. Lipid Hydroperoxide-Derived Modification of Proteins in Gastrointestinal Tract; Springer: Dordrecht, The Netherlands, 2014; pp. 137–148. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Basak, S.; Hoffmann, A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008, 19, 187–197. [Google Scholar] [CrossRef]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef]
- Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010, 21, 315–324. [Google Scholar] [CrossRef]
- Kruglov, A.A.; Kuchmiy, A.; Grivennikov, S.I.; Tumanov, A.V.; Kuprash, D.V.; Nedospasov, S.A. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: Mouse models. Cytokine Growth Factor Rev. 2008, 19, 231–244. [Google Scholar] [CrossRef]
- Augusto, A.C.; Miguel, F.; Mendonça, S.; Pedrazzoli, J.; Gurgueira, S.A. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin. Biochem. 2007, 40, 615–622. [Google Scholar] [CrossRef]
- Li, S.-L.; Zhao, J.-R.; Ren, X.-Y.; Xie, J.-P.; Ma, Q.-Z.; Rong, Q.-H. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J. Gastroenterol. 2013, 19, 4590–4595. [Google Scholar] [CrossRef]
- Vaday, G.G.; Hershkoviz, R.; Rahat, M.A.; Lahat, N.; Cahalon, L.; Lider, O. Fibronectin-bound TNF-alpha stimulates monocyte matrix metalloproteinase-9 expression and regulates chemotaxis. J. Leukoc. Biol. 2000, 68, 737–747. [Google Scholar]
- Ganguly, K.; Swarnakar, S. Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: Alleviation by melatonin. Biochimie 2012, 94, 2687–2698. [Google Scholar] [CrossRef]
- Szallasi, A. Small molecule vanilloid TRPV1 receptor antagonists approaching drug status: Can they live up to the expectations? Naunyn. Schmiedebergs. Arch. Pharmacol. 2006, 373, 273–286. [Google Scholar] [CrossRef]
- Tulassay, Z.; Herszényi, L. Gastric mucosal defense and cytoprotection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 99–108. [Google Scholar] [CrossRef]
- Peskar, B.M.; Ehrlich, K.; Peskar, B.A. Role of ATP-Sensitive Potassium Channels in Prostaglandin-Mediated Gastroprotection in the Rat. J. Pharmacol. Exp. Ther. 2002, 301, 969–974. [Google Scholar] [CrossRef]
| Compound | Experimental Model: Treatment (Acute or Chronic) and Doses | Effect | Mechanism |
|---|---|---|---|
| Ascaridole [50] 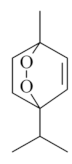 | NSAID - Acute *10 mg/kg (p.o.) ~ ↓ 52% *20 mg/kg (p.o.) ~ ↓ 44% | Gastroprotective and healing effects | ↓Acid secretion (↑pH) ↓ Pepsin |
| Acetic Acid (20 %) - Chronic (7 days): 20 mg/kg (p.o.) - ↓ 57% | |||
| Vehicle: Sodium carboxymethyl cellulose | |||
| Citral [45] 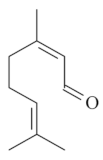 | NSAID – Acute 25 mg/kg (p.o.) - ↓ 74.0% 100 mg/kg (p.o.) - ↓ 35.0%300 mg/kg (p.o.) - ↓ 48.0 % | Gastroprotective effect | |
| Vehicle: Tween 80 - 1% | |||
| Eucalyptol or 1,8-Cineole [51]  | NSAID – Acute 50 mg/kg (p.o.) - ↓ 58.2% 100 mg/kg (p.o.) - ↓ 61.2% 200 mg/kg (p.o.) - ↓ 74.1% | Gastroprotective and healing effect | ↑ Mucus (89.3%), ↑SH, ↓LPO and ↓MPO |
| Absolute Ethanol - Acute 50 mg/kg (p.o.) - ↓ 88.1% 100 mg/kg (p.o.)- ↓ 98.5% 200 mg/kg (p.o.)- ↓ 99.2% | ↑ Cell proliferation | ||
| Acetic Acid (30%) - Chronic (14 days) 100 mg/kg (p.o.)- ↓ 43.1% | |||
| Vehicle: Tween 80 - 1% | |||
| Epoxy-carvone [52] 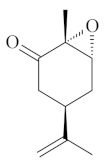 | NSAID - Acute 10 mg/kg (p.o.) – ↓ 60.4 % 30 mg/kg (p.o.) – ↓ 47.9 % 50 mg/kg (p.o.) – ↓ 62.7 % Absolute Ethanol - Acute 10 mg/kg (p.o.) - ↓ 77.7% 30 mg/kg (p.o.) - ↓ 69.2% 50 mg/kg (p.o.) - ↓ 61.4% Vehicle: Tween 80 - 5% | Gastroprotective effect | |
| Linalool [53,54,55] 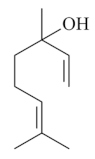 | Ethanol 90% - acute: 33 mg/kg (p.o.) - ↓ 56.0% Vehicle: methylcellulose 0.1% | Gastroprotective effect | |
| Absolute Ethanol - acute: 10 mg/kg (p.o) - ↓ 85.5% 20 mg/kg (p.o) - ↓ 76.2% 40 mg/kg (p.o) - ↓ 89.3% | |||
| Acetic Acid (80%) - chronic (14 days): 40 mg/kg (p.o) - ↓ 48.0% | Gastroprotective and healing effects | ↓MPO and ↓ LPO | |
| Vehicle: Saline | |||
| Linalyl acetate [55] 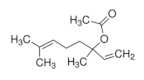 | Ethanol 90% - acute: 36 mg/kg (p.o.) - ↓49.0% Vehicle: methylcellulose 0.1% | Gastroprotective effect | |
| Menthol [56,57] 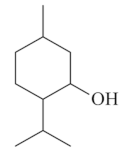 | NSAID - Acute: 50 mg/kg (p.o) - ↓ 73.0 % | Gastroprotective effect | ↓ Acid secretion ↑ Mucus and PGE2 ↑ Compounds SH |
| Absolute Ethanol - Acute: 50 mg/kg (p.o) - ↓ 88.6–92.0% 100 mg/kg (p.o) - ↓ 98.4% | ↑ ATP-sensitive potassium channel | ||
| Vehicle: Tween 80 - 8% | ↓ MPO,↑ GSH, ↑GSH-Px, ↑GR ↓ TNF-α, ↓ IL-6,↑IL-10 Anti-apoptotic effect (HSP-70, Bax) | ||
| Myrtenol [58] 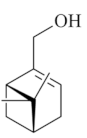 | Absolute Ethanol - Acute: 25 mg/kg (p.o)- ↓ 40.2% 50 mg/kg (p.o)- ↓ 83.0% 100 mg/kg (p.o) - ↓ 83.2% | Gastroprotective effect | Activation of GABA-A receptors ↓ LPO |
| Vehicle: Tween 80 - 2% | |||
| Nerol [59] 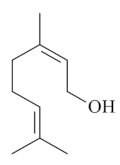 | Absolute Ethanol - acute: *10 mg/kg (p.o) - ↓~94% *30 mg/kg (p.o) - ↓~82% *100 mg/kg (p.o) - ↓~92% *300 mg/kg (p.o) - ↓~94% Vehicle: Tween 80 - 0.5% | Gastroprotective effect | |
| α-pinene [60] 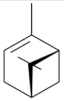 | Absolute Ethanol - Acute: 10 mg/kg (p.o) - ↓ 48.6% 30 mg/kg (p.o) - ↓ 43.9% 100 mg/kg (p.o) - ↓ 42.1% Vehicle: Tween 80 - 0.1% | Gastroprotective effect | ↓ Acid secretion ↑ Mucus |
| α-terpineol [61] 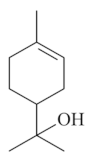 | NSAID - Acute: 30 mg/kg (p.o)- ↓ 63.9% 50 mg/kg (p.o)- ↓ 81.3% Ethanol 70% - Acute: 10 mg/kg (p.o)- ↓ 66.7% 30 mg/kg (p.o)- ↓ 81.0% 50 mg/kg (p.o)- ↓ 94.1% Vehicle: Tween 80 - 10% | Gastroprotective effect | |
| Thymoquinone [62]  | NSAID - Acute: * 20 mg/kg (p.o) - ~↓ 46% | ↑ SOD, ↑GPx, ↑NO, ↓ apoptosis | |
| Vehicle: Corn oil 10% | ↓iNOS,↓TOS, ↓OSI, ↓ NF-κβ, ↓ TNF-α, ↑TAS, ↑TT, ↑ADMA, ↑ DDAH-1, ↑DDAH-2 |
| Compound | Experimental Model: Treatment (Acute or Chronic), Route, and Doses | Effect(s) | Mechanism(s) |
|---|---|---|---|
| Carvacrol [42,63]  | NSAID - Acute: 25 mg/kg (p.o) - ↓43.0% 50 mg/kg (p.o) - ↓42.0% | Gastroprotective and bactericidal effects | ↑ Mucus, ↑ SH compounds,↑ NO, ↑catalase and ↑PGE2 level KATP channel |
| Absolute Ethanol - Acute: 16.6 mM (p.o) - ↓48.0% 33.3 mM (p.o) - ↓41.0% | |||
| Ethanol/HCl - Acute: 8.3 mM (p.o.) - ↓ 28.0% 16.6 mM (p.o) - ↓70.0% 33.3 mM (p.o) - ↓63.0% | |||
| I/R - Acute: 16.6 mM (p.o) - ↓51.0% 33.3 mM (p.o) - ↓38.0% | |||
| Acetic Acid (80%) – Chronic (14 days): 25 mg/kg (p.o) - ↓60.0% 50 mg/kg (p.o) - ↓91.0% 100 mg/kg (p.o) - ↓81.0% | |||
| Minimal Bactericidal Concentration against H. pylori: 0.04 g/L | |||
| Vehicle: Saline, Tween 80 -1%, saline, propylene glycol or distilled water | |||
| Citronellol [64] 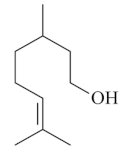 | Absolute Ethanol - Acute: 30 mg/kg (p.o) - ↓ 72.0% 100 mg/kg (p.o) - ↓89.0% Bactericidal activity in vivo against H. pylori - Chronic (7 days; 2 x day): 12.5 mg/kg (i.p.) - ↓ 87.0% 12.5 mg/kg (p.o.) - ↓53.0% 25 mg/kg (p.o.) - ↓ 80.0% 50 mg/kg (p.o) - ↓87.0% Vehicle: Tween 80 - 2% | Gastroprotective and bactericidal effects | |
| Geraniol [65,66] 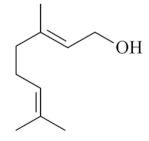 | Absolute Ethanol - Acute: 3 mg/kg (p.o) - ↓ 55.7% 7.50 mg/kg (p.o) - ↓ 70.0%10 mg/kg (p.o) - ↓ 84.8% 200 mg/kg (p.o) - ↓ 99.0% Acetic Acid 10% - Chronic (5 days) 3 mg/kg (p.o) - ↓ 80.5% Bactericidal activity in vitro against H. pylori: 2 mg/L - ↓ 92.0% Vehicle: DMSO 10% or Tween 80 - 8% | Gastroprotective, bactericidal and healing effects | ↑ Mucus (↑ mucin levels 88.5%) ↑ GSH ↓ MPO ↑ Compounds SH, NO, and PGE2 level ↑ CGRP and TRPV-1 activation |
| Limonene [43,44] 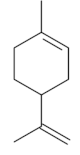 | NSAID - Acute: 177 mg/kg (p.o) - ↓ 50.1% 245 mg/kg (p.o) - ↓ 99.0% Absolute Ethanol - Acute: 50 mg/kg (p.o) 100 mg/kg (p.o) 177 mg/kg (p.o) - ↓100.0% 245 mg/kg (p.o) - ↓ 99.2% Bactericidal activity in vitro against H. pylori: MIC 75 µg/mL Vehicle: Tween 80 - 8% | Gastroprotective and bactericidal effects | ↑ Mucus Maintenance of high PGE2 levels ↑ GPx ↓ MPO ↓ IL-1β, IL-6 and TNF-α ↓ mRNA expression of Nf-κB, IL-1β and MPO ↑ mRNA expression of GPx |
| β-Myrcene [67] 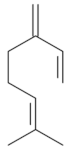 | NSAID – Acute 7.5 mg/kg (p.o) – 74.0% | ↑ Mucus ↓ MDA ↑ GPx ↑ GR | |
| Absolute Ethanol - Acute 7.5 mg/kg (p.o)- 60.0% | Gastroprotective effect | ||
| I/R - Acute 7.5 mg/kg (p.o) – 86.0% | Bacteriostatic effect against H. pylori | ||
| Bactericidal activity in vitro against H. pylori: MIC = 500 µg/mL | |||
| Vehicle: Tween 80 - 8% | |||
| β-pinene [43] 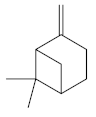 | Absolute Ethanol - Acute: 33 mg/kg (p.o) - absent Bactericidal activity in vitro against H. pylori: MIC = 500 µg/mL Vehicle: Tween 80 - 8% | No gastroprotective and bactericidal effects | |
| Safranal [68] 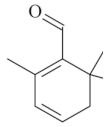 | Bactericidal activity in vitro against H. pylori: MIC50 against H. pylori: 32 µg/mL Vehicle: not described | Bactericidal effect | |
| Thymol [69,70] 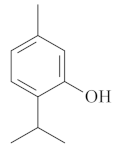 | Absolute Ethanol - acute: *10 mg/kg (p.o) - ~↓ 81.0% Vehicle: DMSO - 5% | ||
| Absolute Ethanol - Acute: *10 mg/kg (p.o) - ~ 37.0% *30 mg/kg (p.o) - ~ 63.0% *100 mg/kg (p.o) - ~95.0% | Gastroprotective effect | ↑SOD, ↑ GSH, ↓MPO, ↓LPO, ↓MMP-9 | |
| NSAID - Acute 30 mg/kg (p.o) – 43.0% 100 mg/kg (p.o) – 49.0% | Gastroprotective effect Healing effect Bactericidal effect Absent | ↑ Mucus PGE2 level KATP channel No involvement of NO No antisecretory action | |
| Acetic Acid 30% - Chronic (7 days) 30 mg/kg (p.o) – 91.8% 100 mg/kg (p.o) – 92.5% | |||
| Bactericidal activity in vitro against H. pylori: 10,000 µg/mL (absent) | |||
| Vehicle: Tween 80 - 0.2% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Périco, L.L.; Emílio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; Rocha, L.R.M.d.; Batista, L.M.; Hiruma-Lima, C.A. Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules 2020, 10, 265. https://doi.org/10.3390/biom10020265
Périco LL, Emílio-Silva MT, Ohara R, Rodrigues VP, Bueno G, Barbosa-Filho JM, Rocha LRMd, Batista LM, Hiruma-Lima CA. Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules. 2020; 10(2):265. https://doi.org/10.3390/biom10020265
Chicago/Turabian StylePérico, Larissa Lucena, Maycon Tavares Emílio-Silva, Rie Ohara, Vinícius Peixoto Rodrigues, Gabriela Bueno, José Maria Barbosa-Filho, Lúcia Regina Machado da Rocha, Leônia Maria Batista, and Clélia Akiko Hiruma-Lima. 2020. "Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases" Biomolecules 10, no. 2: 265. https://doi.org/10.3390/biom10020265
APA StylePérico, L. L., Emílio-Silva, M. T., Ohara, R., Rodrigues, V. P., Bueno, G., Barbosa-Filho, J. M., Rocha, L. R. M. d., Batista, L. M., & Hiruma-Lima, C. A. (2020). Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules, 10(2), 265. https://doi.org/10.3390/biom10020265






