Structural and Functional Comparison of Salmonella Flagellar Filaments Composed of FljB and FliC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Salmonella Strains
2.2. Swimming Motility Assay
2.3. Fluorescence Labeling of Flagellin Antibodies
2.4. Immunofluorescent Staining of the Flagellar Filament
2.5. Flagellar Filament Purification
2.6. Negative Staining
2.7. Electron Cryomicroscopy and Image Processing
3. Results
3.1. Motility Difference between Cells with the FljB and FliC Filaments
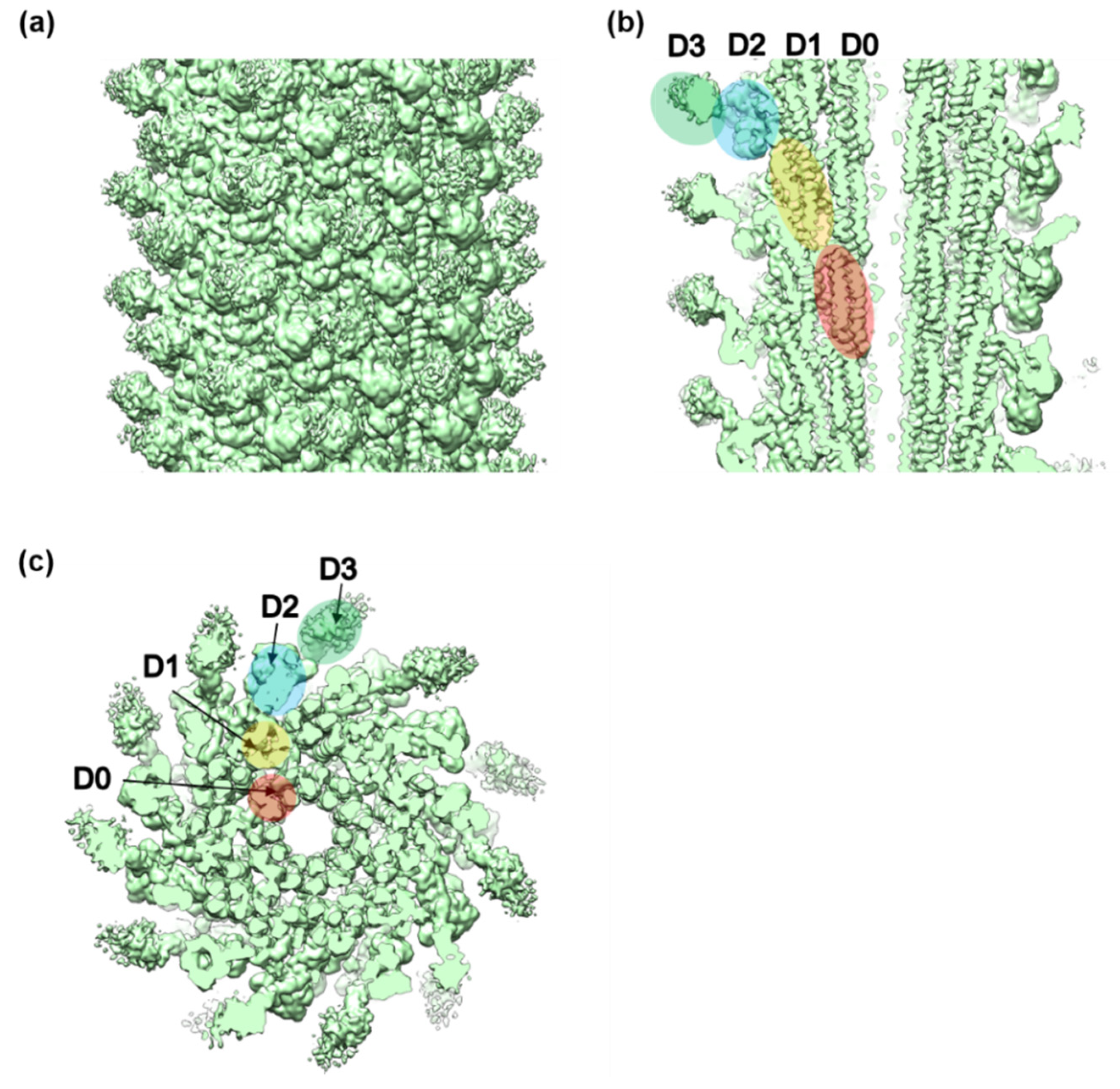
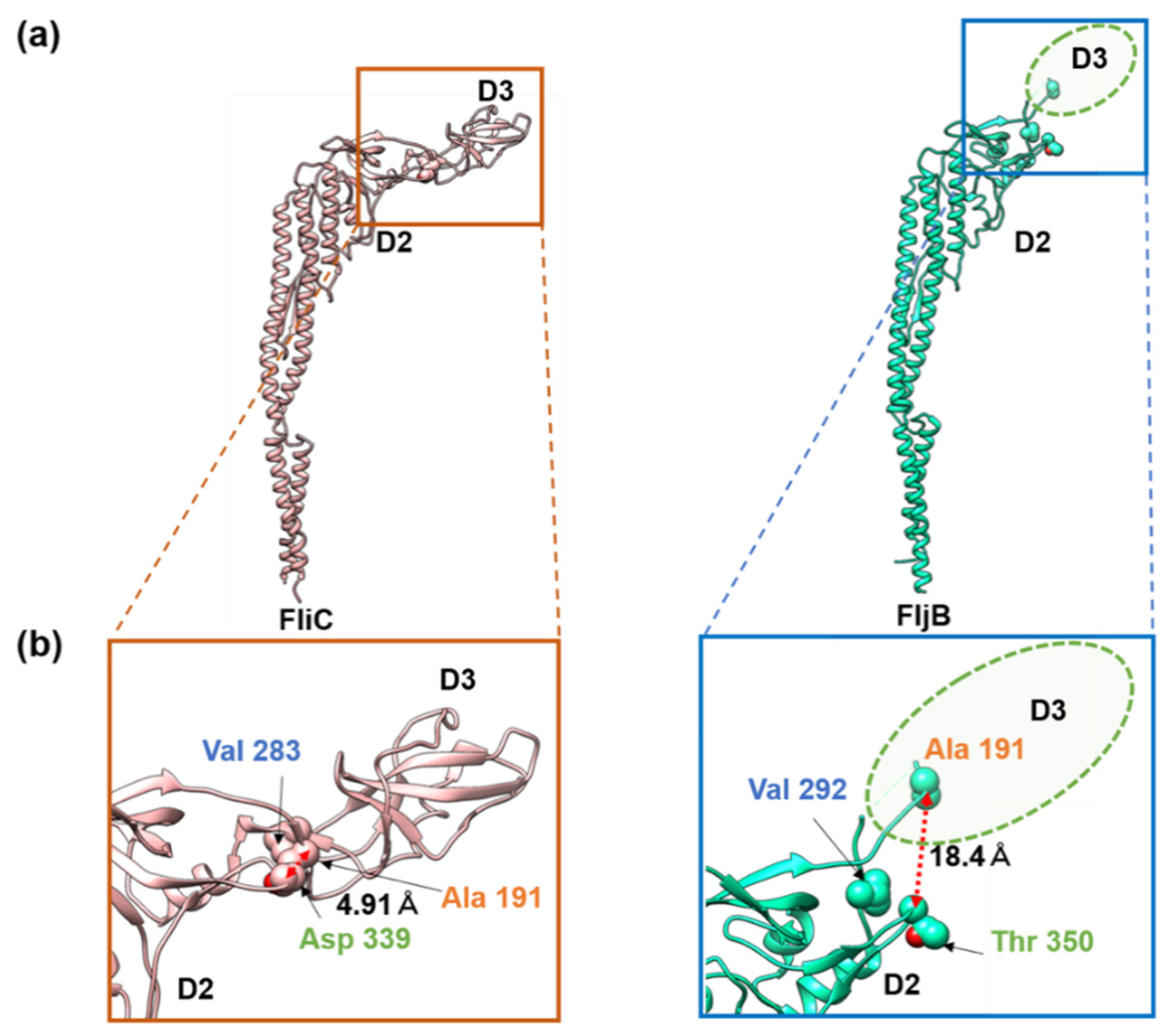
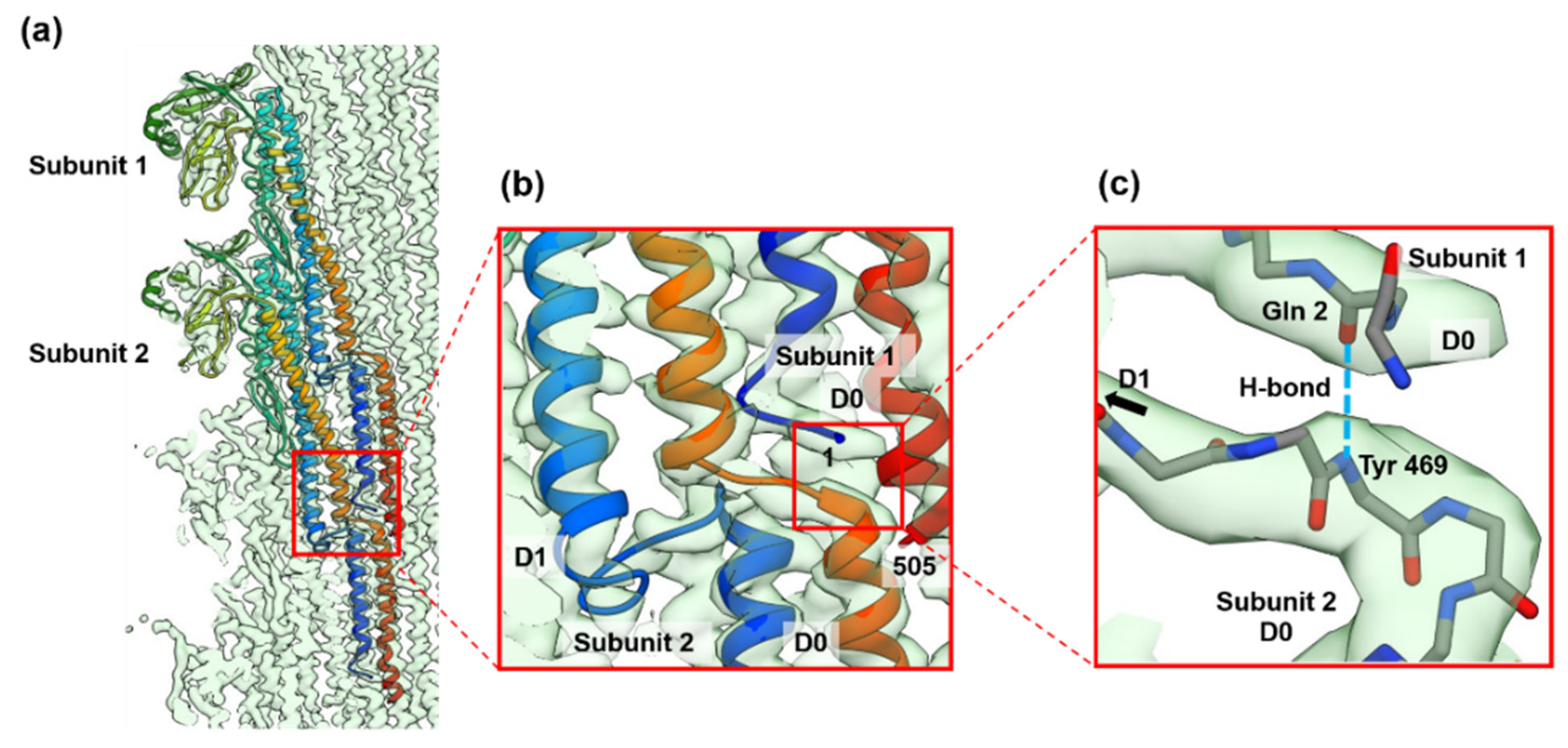
3.2. Structures of the FljB and FliC Filaments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haiko, J.; Westerlund-Wikström, B.; Haiko, J.; Westerlund-Wikström, B. The Role of the Bacterial Flagellum in Adhesion and Virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossez, Y.; Wolfson, E.B.; Holmes, A.; Gally, D.L.; Holden, N.J. Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms. PLoS Pathog. 2015, 11, e1004483. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C. The Rotary Motor of Bacterial Flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, F.W. Studies in group agglutination. II.—The absorption of agglutinin in the diphasic salmonellas. J. Pathol. Bacteriol. 1925, 28, 345–359. [Google Scholar] [CrossRef]
- Horstmann, J.A.; Zschieschang, E.; Truschel, T.; de Diego, J.; Lunelli, M.; Rohde, M.; May, T.; Strowig, T.; Stradal, T.; Kolbe, M.; et al. Flagellin phase-dependent swimming on epithelial cell surfaces contributes to productive Salmonella gut colonisation. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samatey, F.A.; Imada, K.; Nagashima, S.; Vonderviszt, F.; Kumasaka, T.; Yamamoto, M.; Namba, K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 2001, 410, 331–337. [Google Scholar] [CrossRef]
- Yonekura, K.; Maki-Yonekura, S.; Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 2003, 424, 643–650. [Google Scholar] [CrossRef]
- Maki-Yonekura, S.; Yonekura, K.; Namba, K. Conformational change of flagellin for polymorphic supercoiling of the flagellar filament. Nat. Struct. Mol. Biol. 2010, 17, 417–422. [Google Scholar] [CrossRef]
- Mimori-Kiyosue, Y.; Yamashita, I.; Fujiyoshi, Y.; Yamaguchi, S.; Namba, K. Role of the outermost subdomain of Salmonella flagellin in the filament structure revealed by electron cryomicroscopy. J. Mol. Biol. 1998, 284, 521–530. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Hara, N.; Namba, K.; Minamino, T. Genetic Characterization of Conserved Charged Residues in the Bacterial Flagellar Type III Export Protein FlhA. PLoS ONE 2011, 6, e22417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S.; Fujita, H.; Sugata, K.; Taira, T.; Iino, T. Genetic Analysis of H2, the Structural Gene for Phase-2 Flagellin in Salmonella. Microbiology 1984, 130, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, P. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 1988, 21, 129–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 2016, 193, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zivanov, J.; Nakane, T.; Forsberg, B.O.; Kimanius, D.; Hagen, W.J.H.; Lindahl, E.; Scheres, S.H.W. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 2018, 7, 42166. [Google Scholar] [CrossRef]
- Mimori, Y.; Yamashita, I.; Murata, K.; Fujiyoshi, Y.; Yonekura, K.; Toyoshima, C.; Namba, K. The structure of the R-type straight flagellar filament of Salmonella at 9 Å resolution by electron cryomicroscopy. J. Mol. Biol. 1995, 249, 69–87. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2006, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Biological Crystallography Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkó, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.-W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Cryst. 2019, 75, 861–877. [Google Scholar] [CrossRef] [Green Version]
- Vonderviszt, F.; Aizawa, S.-I.; Namba, K. Role of the disordered terminal regions of flagellin in filament formation and stability. J. Mol. Biol. 1991, 221, 1461–1474. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Houry, A.; Gohar, M.; Deschamps, J.; Tischenko, E.; Aymerich, S.; Gruss, A.; Briandet, R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 13088–13093. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Salmonella Strains | Relevant Characteristics | Source or Reference |
|---|---|---|
| SJW1103 | FliC wild-type Δhin-fljB-fljA | Yamaguchi et al. 1984 [12] |
| SJW1103ΔC | SJW1103 ΔfliC::tetRA | This study |
| SJW1103B | FljB wild-type ΔfliC::fljB | This study |
| SJW590 | FljB_R-type straight filament fljB(A461V), ΔfliC | This study |
| Rotation (Degree) | Axial Rise (Å) | |
|---|---|---|
| FljB | 65.81 | 4.69 |
| FliC | 65.84 | 4.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Toma, S.; Terahara, N.; Miyata, T.; Ashihara, M.; Minamino, T.; Namba, K.; Kato, T. Structural and Functional Comparison of Salmonella Flagellar Filaments Composed of FljB and FliC. Biomolecules 2020, 10, 246. https://doi.org/10.3390/biom10020246
Yamaguchi T, Toma S, Terahara N, Miyata T, Ashihara M, Minamino T, Namba K, Kato T. Structural and Functional Comparison of Salmonella Flagellar Filaments Composed of FljB and FliC. Biomolecules. 2020; 10(2):246. https://doi.org/10.3390/biom10020246
Chicago/Turabian StyleYamaguchi, Tomoko, Shoko Toma, Naoya Terahara, Tomoko Miyata, Masamichi Ashihara, Tohru Minamino, Keiichi Namba, and Takayuki Kato. 2020. "Structural and Functional Comparison of Salmonella Flagellar Filaments Composed of FljB and FliC" Biomolecules 10, no. 2: 246. https://doi.org/10.3390/biom10020246
APA StyleYamaguchi, T., Toma, S., Terahara, N., Miyata, T., Ashihara, M., Minamino, T., Namba, K., & Kato, T. (2020). Structural and Functional Comparison of Salmonella Flagellar Filaments Composed of FljB and FliC. Biomolecules, 10(2), 246. https://doi.org/10.3390/biom10020246







