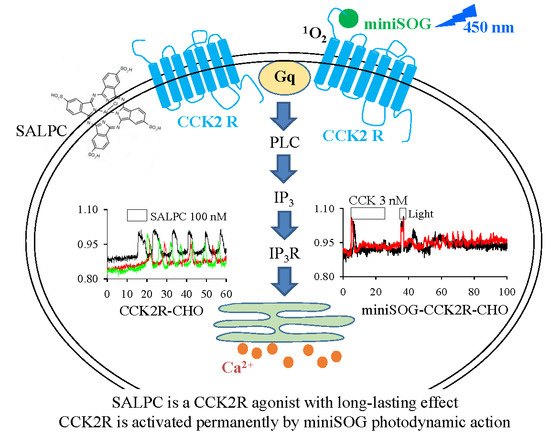
Permanent Photodynamic Activation of the Cholecystokinin 2 Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vector Constructs and CHO-K1 Cell Transfection
2.3. Cell Culture (CHO-K1, Escherichia coli)
2.4. Immunocytochemistry
2.5. Measurement of Cytosolic Calcium Concentration
2.6. Statistical Analyses
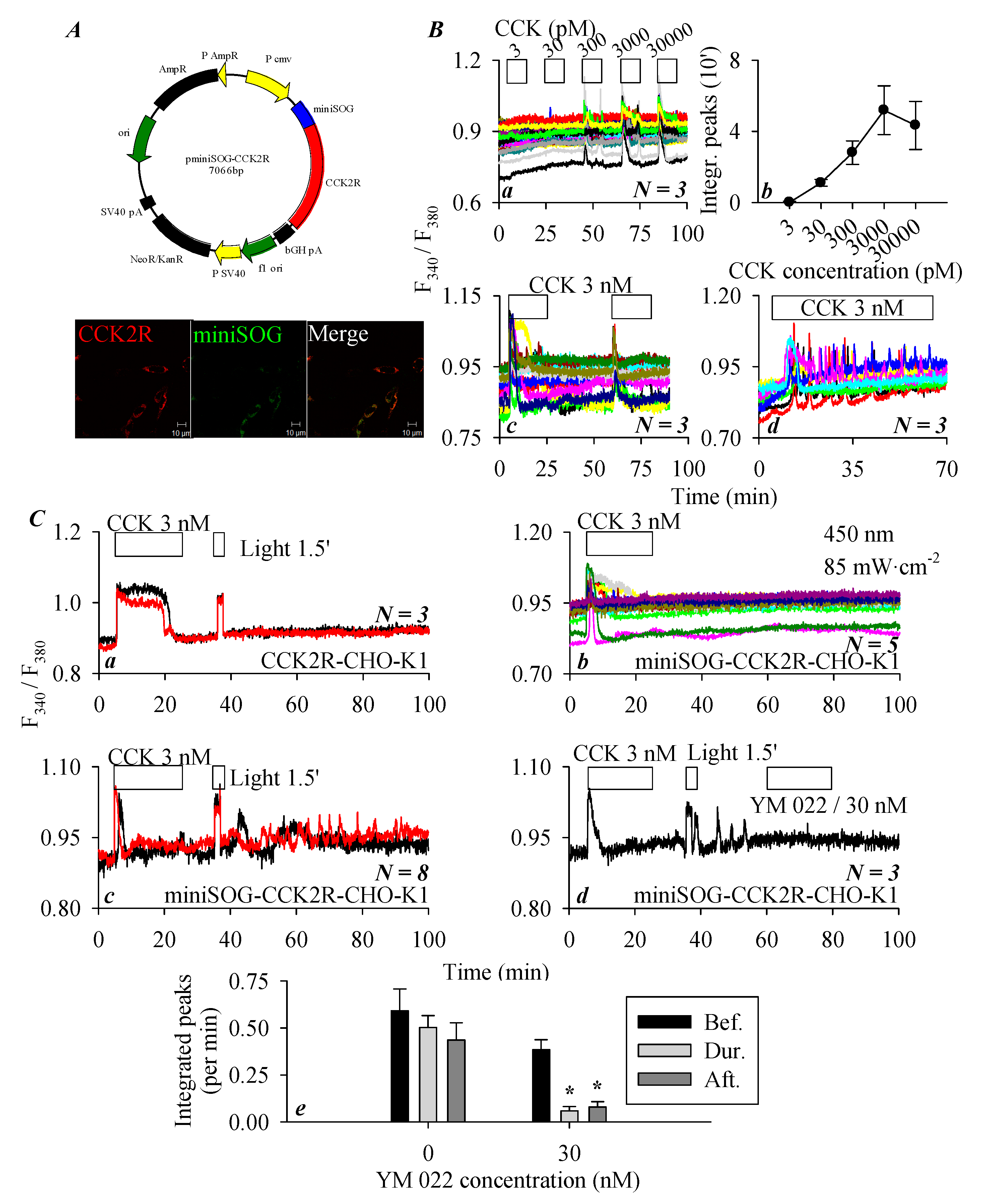
3. Results
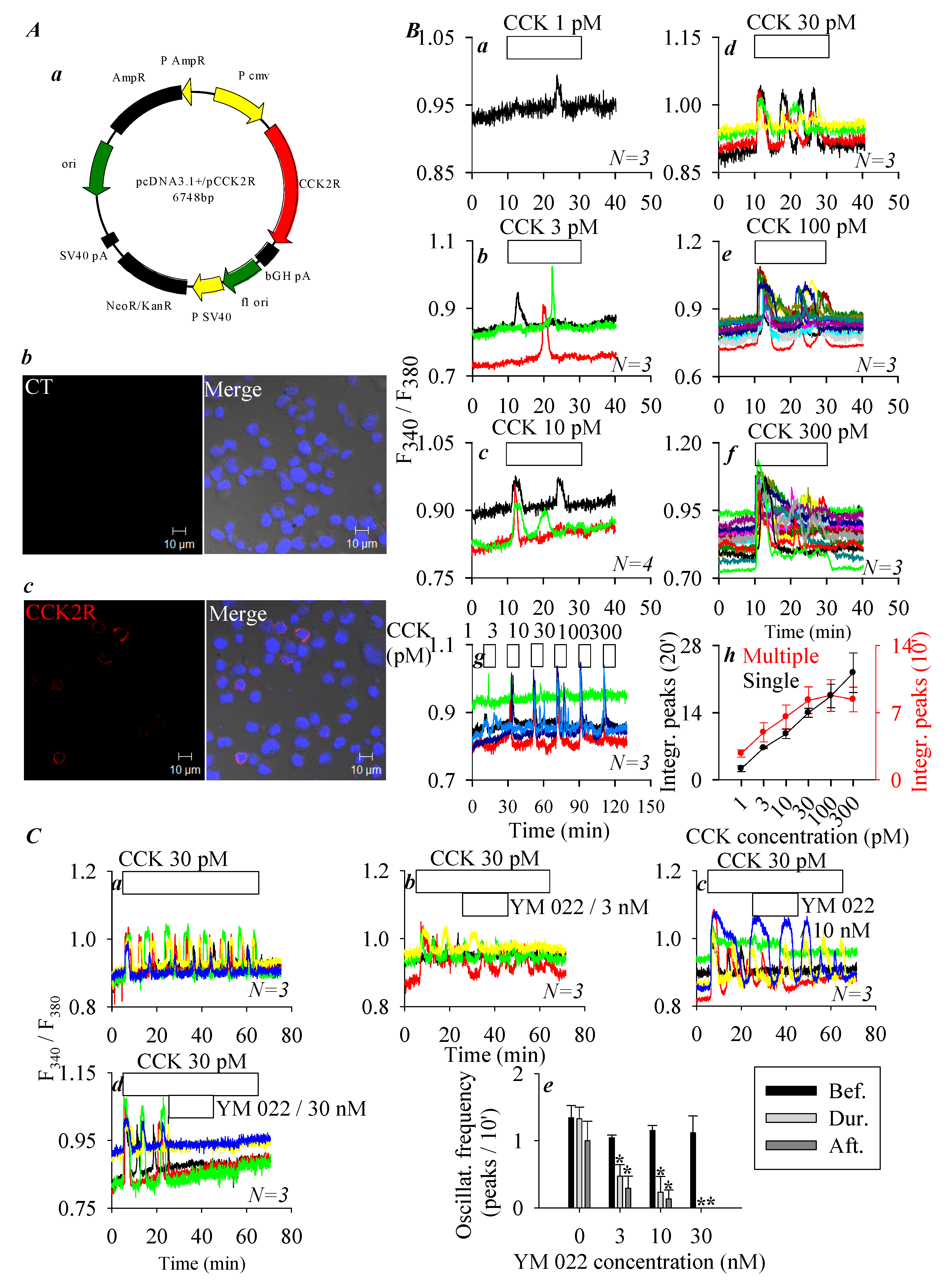
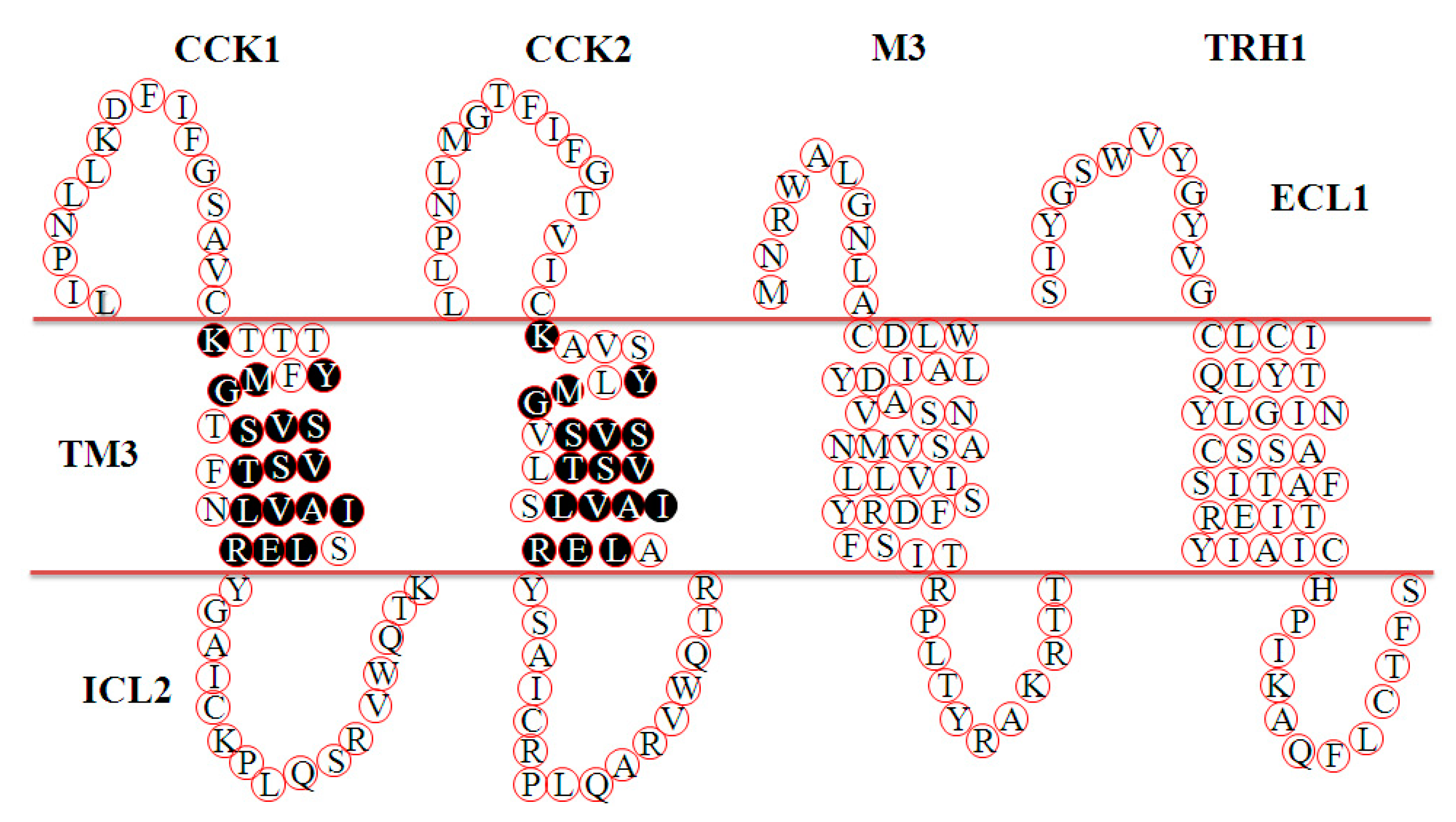
3.1. CCK2R Expression in CHO-K1 Cells
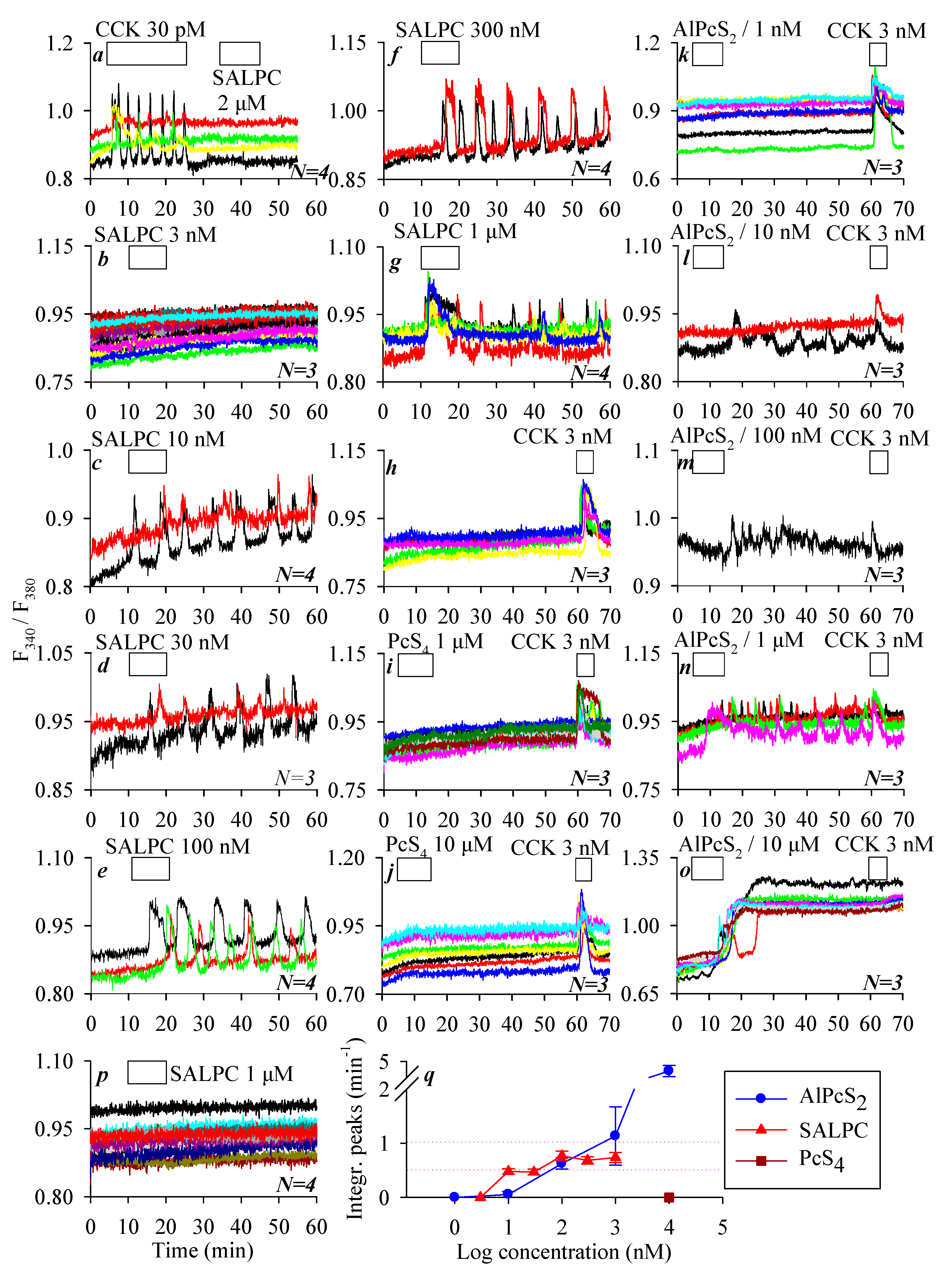
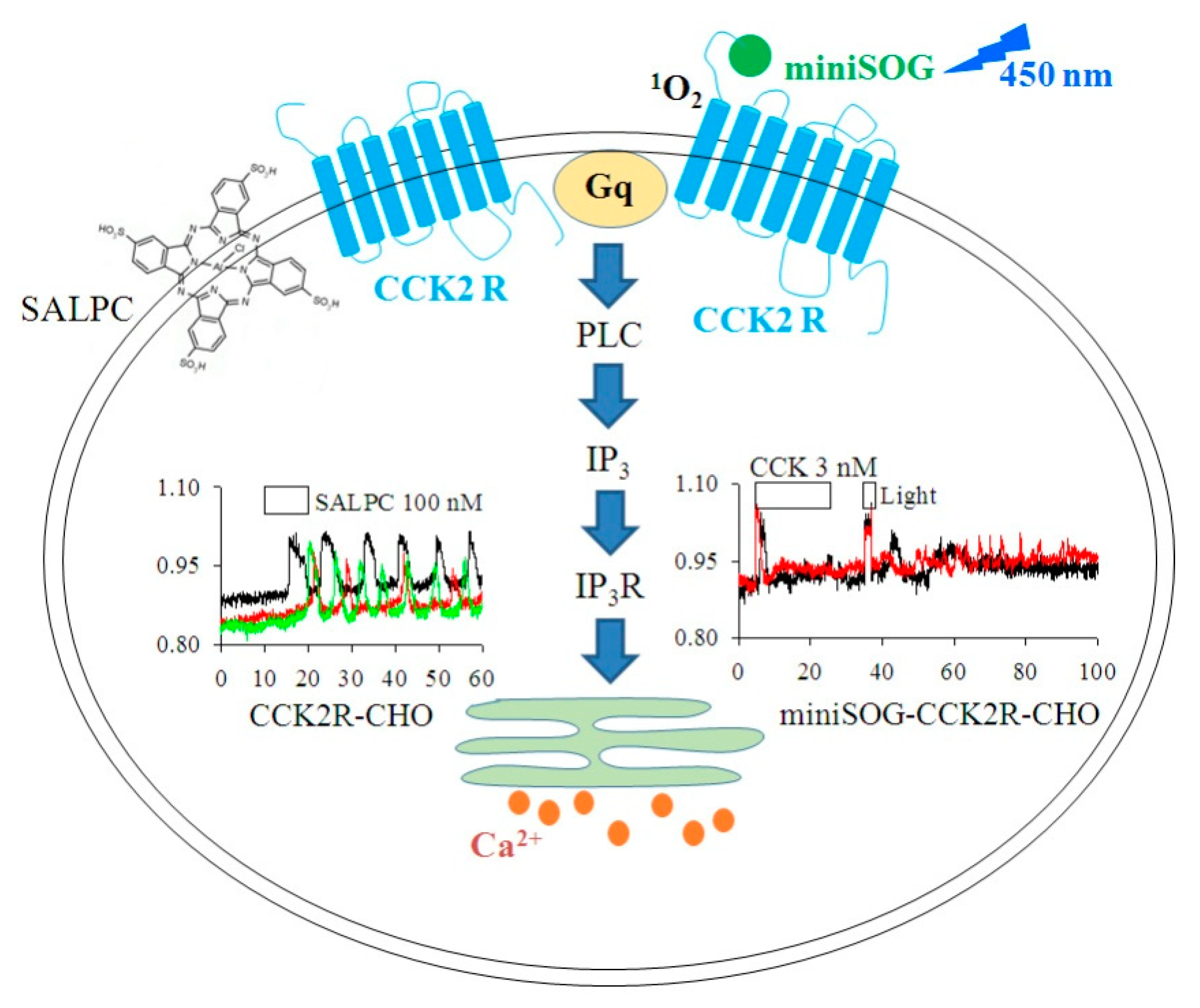
3.2. Permanent Activation of CCK2R by SALPC and an Analogue in the Dark
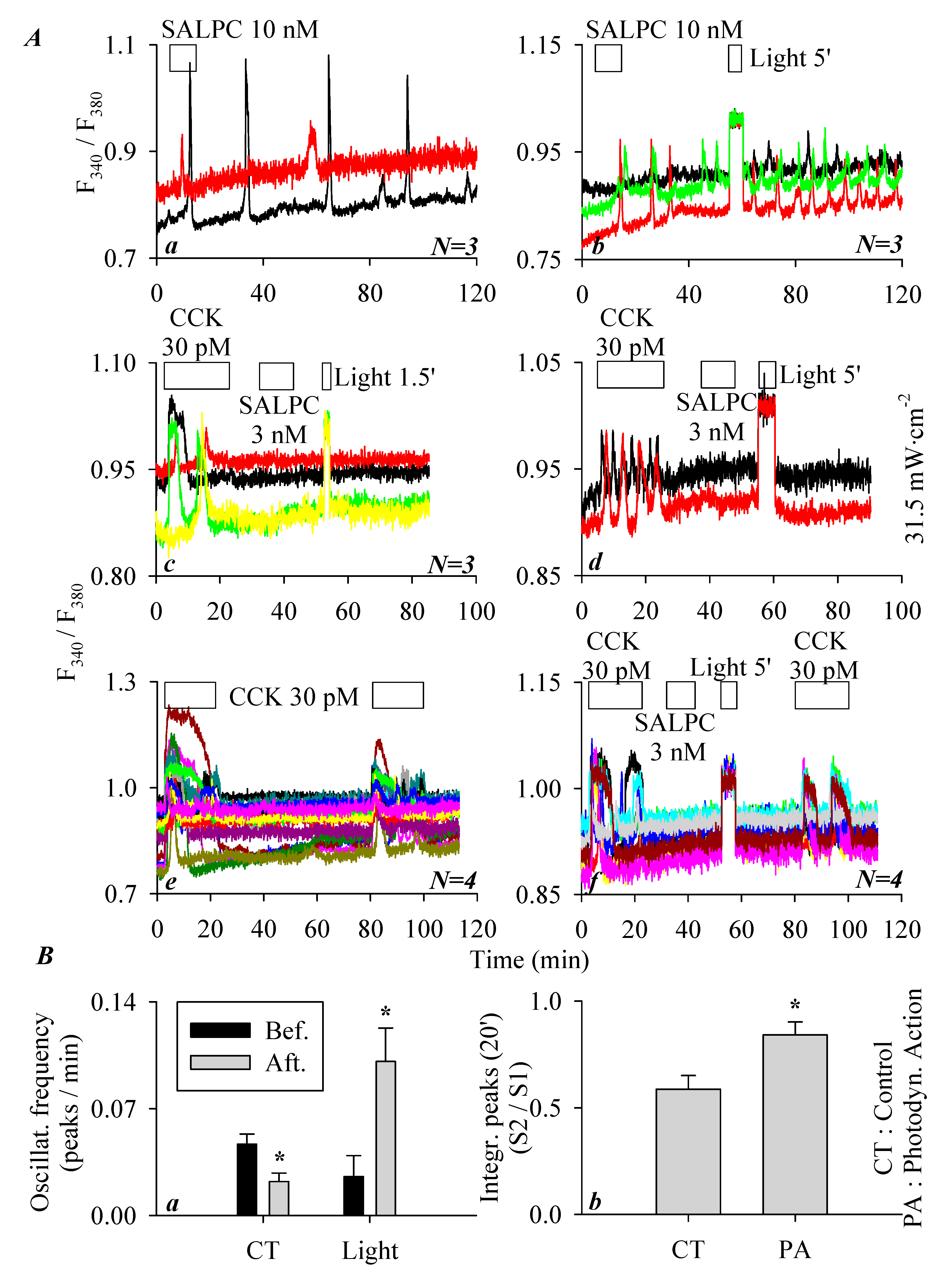
3.3. SALPC Photodynamic Sensitization of CCK2R in CCK2R-CHO-K1 Cells
3.4. miniSOGPM Photogenetic/Photodynamic Activation of CCK2R in miniSOG-CCK2R-CHO-K1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Noble, F.; Roques, B.P. CCK-B receptor: Chemistry, molecular biology, biochemistry and pharmacology. Prog. Neurobiol. 1999, 58, 349–379. [Google Scholar] [CrossRef]
- Sherrin, T.; Heng, K.Y.C.; Zhu, Y.Z.; Tang, Y.M.; Lau, G.; Tan, C.H. Cholecystokinin-B receptor gene expression in cerebellum, pre-frontal cortex and cingulate gyrus and its association with suicide. Neurosci. Lett. 2004, 357, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F.; Friis-Hansen, L.; Goetze, J.P.; Hansen, T.V.O. The biology of cholecystokinin and gastrin peptides. Curr. Top. Med. Chem. 2007, 7, 1154–1165. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, X.; Wang, S.; Qi, J.; Tian, Z.; Wang, B.; Chen, H.; Wu, Y.; Wang, M.; Xu, S.; et al. UCN3 suppresses food intake in coordination with CCK and the CCK2R in Siberian sturgeon (Acipenser baerii). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 234, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gómez, A.M.; Aguilar-Roblero, R.; De La Mora, M.P. Role of cholecystokinin-A and cholecystokinin-B receptors in anxiety. Amino Acids 2002, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hattori, E.; Shimizu, M.; Sugaya, A.; Shibuya, H.; Yoshikawa, T. Association studies of the cholecystokinin B receptor and A2a adenosine receptor genes in panic disorder. J. Neural Transm. 2001, 108, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Markie, D.; Fitches, A. Cholecystokinin system genes: Associations with panic and other psychiatric disorders. J. Affect. Disord. 2012, 136, 902–908. [Google Scholar] [CrossRef]
- Quattrone, A.; Dewaele, B.; Woźniak, A.; Bauters, M.; Vanspauwen, V.; Floris, G.; Schöffski, P.; Chibon, F.; Coindre, J.-M.; Sciot, R.; et al. Promoting role of cholecystokinin 2 receptor (CCK2R) in gastrointestinal stromal tumour pathogenesis. J. Pathol. 2012, 228, 565–574. [Google Scholar] [CrossRef]
- Ashurst, H.L.; Varro, A.; Dimaline, R. Regulation of mammalian gastrin/CCK receptor (CCK2R) expression in vitro and in vivo. Exp. Physiol. 2008, 93, 223–236. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Zhang, Q.; Hu, J.; Wang, W. Human gastric tissues simultaneously express the classical and alternative splicing cholecystokinin-B/gastrin receptors. Recept. Channels 2004, 10, 185–188. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Chen, M.-L.; Zhang, Q.-Z.; Hu, J.-K.; Wang, W.-L. Coexpression of cholecystokinin-B/gastrin receptor and gastrin gene in human gastric tissues and gastric cancer cell line. World J. Gastroenterol. 2004, 10, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Göke, M.N.; Otte, J.-M.; Schrader, H.; Reimann, B.; Kruse, M.-L.; Siegel, E.G.; Peters, J.; Herzig, K.-H.; Fölsch, U.R.; et al. Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul. Pept. 2001, 102, 101–110. [Google Scholar] [CrossRef]
- Miyasaka, K.; Ohta, M.; Kanai, S.; Yoshida, Y.; Sato, N.; Nagata, A.; Matsui, T.; Noda, T.; Jimi, A.; Takiguchi, S.; et al. Enhanced gastric emptying of a liquid gastric load in mice lacking cholecystokinin-B receptor: A study of CCK-A,B, and AB receptor gene knockout mice. J. Gastroenterol. 2004, 39, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; Colucci, R.; Antonioli, L.; Crema, F.; Buccianti, P.; Chiarugi, M.; Baschiera, F.; Ghisu, N.; Tuccori, M.; Blandizzi, C.; et al. Cholecystokinin CCK2 receptors mediate the peptide’s inhibitory actions on the contractile activity of human distal colon via the nitric oxide pathway. Br. J. Pharmacol. 2007, 151, 1246–1253. [Google Scholar] [CrossRef]
- Pagliocca, A.; Wroblewski, L.E.; Ashcroft, F.J.; Noble, P.J.; Dockray, G.J.; Varró, A. Stimulation of the gastrin-cholecystokininBreceptor promotes branching morphogenesis in gastric AGS cells. Am. J. Physiol. Liver Physiol. 2002, 283, G292–G299. [Google Scholar] [CrossRef][Green Version]
- Yu, S.; Zhang, Y.; Zhao, X.; Chang, Z.; Wei, Y.; Sun, Y.; Jiang, D.; Jiang, X.; Tao, J. Cholecystokinin type B receptor-mediated inhibition of A-type K+ channels enhances sensory neuronal excitability through the phosphatidylinositol 3-kinase and c-Src-dependent JNK pathway. Cell Commun. Signal. 2019, 17, 68. [Google Scholar] [CrossRef]
- Yin, K.; Deuis, J.R.; Lewis, R.J.; Vetter, I. Transcriptomic and behavioural characterisation of a mouse model of burn pain identify the cholecystokinin 2 receptor as an analgesic target. Mol. Pain 2016, 12. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; He, Q.-H.; Han, J.-S.; Su, L.; Wan, Y. Heteromerization of μ-opioid receptor and cholecystokinin B receptor through the third transmembrane domain of the μ-opioid receptor contributes to the anti-opioid effects of cholecystokinin octapeptide. Exp. Mol. Med. 2018, 50, 64. [Google Scholar] [CrossRef]
- Roca-Lapirot, O.; Fossat, P.; Ma, S.; Egron, K.; Trigilio, G.; López-González, M.-J.; Covita, J.; Bouali-Benazzouz, R.; Favereaux, A.; Gundlach, A.L.; et al. Acquisition of analgesic properties by the cholecystokinin (CCK)/CCK2 receptor system within the amygdala in a persistent inflammatory pain condition. Pain 2019, 160, 345–357. [Google Scholar] [CrossRef]
- Kõks, S.; Fernandes, C.; Kurrikoff, K.; Vasar, E.; Schalkwyk, L.C. Gene expression profiling reveals upregulation of Tlr4 receptors in Cckb receptor deficient mice. Behav. Brain Res. 2008, 188, 62–70. [Google Scholar] [CrossRef]
- Smith, J.P.; Hamory, M.W.; Verderame, M.F.; Zagon, I.S. Quantitative analysis of gastrin mRNA and peptide in normal and cancerous human pancreas. Intl. J. Mol. Med. 1998, 2, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Abraham, T.; Pan, W.H.; Tang, X.M.; Linton, S.S.; McGovern, C.O.; Loc, W.S.; Smith, J.P.; Butler, P.J.; Kester, M.; et al. A cholecystokinin B receptor-specific DNA aptamer for targeting pancreatic ductal adenocarcinoma. Nucleic Acid. Ther. 2017, 27, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Schaer, J.C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar] [PubMed]
- Goetze, J.P.; Nielsen, F.C.; Burcharth, F.; Rehfeld, J.F. Closing the gastrin loop in pancreatic carcinoma: Coexpression of gastrin and its receptor in solid human pancreatic adenocarcinoma. Cancer 2000, 88, 2487–2494. [Google Scholar] [CrossRef]
- Behr, T.M.; Béhé, M.P. Cholecystokinin-B/gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Semin. Nucl. Med. 2002, 32, 97–109. [Google Scholar] [CrossRef]
- Smith, J.P.; Fonkoua, L.K.; Moody, T.W. The role of gastrin and CCK receptors in pancreatic cancer and other malignancies. Intl. J. Boil. Sci. 2016, 12, 283–291. [Google Scholar] [CrossRef]
- Dufresne, M.; Seva, C.; Fourmy, D. Cholecystokinin and gastrin receptors. Physiol. Rev. 2006, 86, 805–847. [Google Scholar] [CrossRef]
- Akagi, K.; Nagao, T.; Urushidani, T. Calcium oscillations in single cultured Chinese hamster ovary cells stably transfected with a cloned human cholecystokinin (CCK)B receptor. Jpn. J. Pharmacol. 1997, 75, 33–42. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Harikumar, K.G.; Holicky, E.L.; Miller, L.J. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J. Biol. Chem. 2003, 278, 52972–52979. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Miller, L.J. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J. Boil. Chem. 2001, 276, 48040–48047. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J.; Matthews, E.K. Photodynamic modulation of cellular function. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1998, 19, 297–303. [Google Scholar]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Photodynamic physiology—Photonanomanipulations in cellular physiology with protein photosensitizers. Front. Physiol. 2017, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Sekkat, N.; Bergh, H.V.D.; Nyokong, T.; Lange, N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules 2011, 17, 98–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shao, J.; Yang, T.; Wang, J.; Jia, L. Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy. J. Pharm. Biomed. Anal. 2014, 87, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for photodynamic therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J.; Kanno, T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J. Physiol. 1997, 504, 47–55. [Google Scholar] [CrossRef]
- An, Y.P.; Xiao, R.; Cui, H.; Cui, Z.J. Selective activation by photodynamic action of cholecystokinin receptor in the freshly isolated rat pancreatic acini. Br. J. Pharmacol. 2003, 139, 872–880. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Li, Y.; Li, Z.Y.; Cui, Z.J. Permanent photodynamic cholecystokinin 1 receptor activation: Dimer-to-monomer conversion. Cell. Mol. Neurobiol. 2018, 38, 1283–1292. [Google Scholar] [CrossRef]
- Jiang, H.N.; Li, Y.; Jiang, W.Y.; Cui, Z.J. Cholecystokinin 1 receptor–A unique G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Front. Physiol. 2018, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, Z.J. Photogenetical activation of cholecystokinin type 1 and other G protein-coupled receptor. 2020; in submission. [Google Scholar]
- Shimmura, T.; Tamura, M.; Ohashi, S.; Sasaki, A.; Yamanaka, T.; Nakao, N.; Ihara, K.; Okamura, S.; Yoshimura, T. Cholecystokinin induces crowing in chickens. Sci. Rep. 2019, 9, 3978. [Google Scholar] [CrossRef] [PubMed]
- Noble, F.A.; Wank, S.; Crawley, J.N.; Bradwejn, J.; Seroogy, K.B.; Hamon, M.; Roques, B.P. International union of pharmacology. XXI. structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999, 51, 745–781. [Google Scholar] [PubMed]
- Charpentier, B.; Durieux, C.; Menant, I.; Roques, B.P. Investigation of peripheral cholecystokinin receptor heterogeneity by cyclic and related linear analogs of CCK26-Synthesis and biological properties. J. Med. Chem. 1987, 30, 962–968. [Google Scholar] [CrossRef]
- Charpentier, B.; Durieux, C.; Pelaprat, D.; Dor, A.; Reibaud, M.; Blanchard, J.-C.; Roques, B.P. Enzyme-resistant CCK analogs with high affinities for central receptors. Peptides 1988, 9, 835–841. [Google Scholar] [CrossRef]
- Bellier, B.; Million, M.-E.; Garbay, C.; Crété, D.; Beslot, F.; Bado, A.; Daugé, V. New CCK2 agonists confirming the heterogeneity of CCK2 receptors: Characterisation of BBL454. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 404–413. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Drugs 2008, 2008, 1–23. [Google Scholar] [CrossRef]
- Desai, A.J.; Lam, P.C.H.; Orry, A.; Abagyan, R.; Christopoulos, A.; Sexton, P.M.; Miller, L.J. Molecular mechanism of action of triazolobenzodiazepinone agonists of the Type 1 cholecystokinin receptor. Possible Cooperativity across the Receptor Homodimeric Complex. J. Med. Chem. 2015, 58, 9562–9577. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Guo, Y.; Liao, J.; Tu, Z.; Lu, Y.; Ding, K.; Tortorella, M.D.; He, J. Identification and synthesis of low-molecular weight cholecystokinin B receptor (CCKBR) agonists as mediators of long-term synaptic potentiation. Med. Chem. Res. 2019, 28, 387–393. [Google Scholar] [CrossRef]
- Vummidi, B.R.; Noreen, F.; Alzeer, J.; Moelling, K.; Luedtke, N.W. Photodynamic agents with anti-metastatic activities. ACS Chem. Boil. 2013, 8, 1737–1746. [Google Scholar] [CrossRef]
- Shisaka, Y.; Iwai, Y.; Yamada, S.; Uehara, H.; Tosha, T.; Sugimoto, H.; Shiro, Y.; Stanfield, J.K.; Ogawa, K.; Watanabe, Y.; et al. Hijacking the heme acquisition system of pseudomonas aeruginosa for the delivery of phthalocyanine as an antimicrobial. ACS Chem. Boil. 2019, 14, 1637–1642. [Google Scholar] [CrossRef]
- Barnett, M.E.; Baran, T.M.; Foster, T.H.; Wojtovich, A.P. Quantification of light-induced miniSOG superoxide production using the selective marker, 2-hydroxyethidium. Free. Radic. Boil. Med. 2018, 116, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Lafaye, C.; Signor, L.; Aumonier, S.; Flors, C.; Shu, X.; Nonell, S.; Gotthard, G.; Royant, A. Tailing miniSOG: Structural bases of the complex photophysics of a flavin-binding singlet oxygen photosensitizing protein. Sci. Rep. 2019, 9, 2428. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, G.; Shramova, E.; Shilova, O.; Ryabova, A.; Deyev, S. Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol. B Boil. 2018, 188, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.M.; Jensen, R.L.; Breitenbach, T.; Etzerodt, M.; Ogilby, P.R. Oxygen-Dependent photochemistry and photophysics of “MiniSOG,” a protein-encased flavin. Photochem. Photobiol. 2013, 89, 1116–1126. [Google Scholar] [CrossRef]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Boil. 2011, 9, e1001041. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Berlanga, M.L.; Yang, M.A.; Terada, M.; Hu, J.; Bushong, E.A.; Hwang, M.; Masliah, E.; George, J.M.; Ellisman, M.H. Mapping the subcellular distribution of synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: Implications for Parkinson’s disease pathogenesis. J. Neurosci. 2013, 33, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Nguyen, P.; Hu, J.; Ellisman, M.H.; Sosinsky, G.E. Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while Pannexin1 channels traffic to the plasma membrane. Front. Cell. Neurosci. 2015, 8, 468. [Google Scholar] [CrossRef]
- Boassa, D.; Lemieux, S.P.; Lev-Ram, V.; Hu, J.; Xiong, Q.; Phan, S.; Mackey, M.; Ramachandra, R.; Peace, R.E.; Adams, S.R.; et al. Split-miniSOG for spatially detecting intracellular protein-protein interactions by correlated light and electron microscopy. Cell Chem. Boil. 2019, 26, 1407–1416.e5. [Google Scholar] [CrossRef]
- Sastri, M.; Darshi, M.; Mackey, M.; Ramachandra, R.; Ju, S.; Phan, S.; Adams, S.; Stein, K.; Douglas, C.R.; Kim, J.J.; et al. Sub-mitochondrial localization of the genetic-tagged mitochondrial intermembrane space-bridging components Mic19, Mic60 and Sam50. J. Cell Sci. 2017, 130, 3248–3260. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J.; Han, Z.Q.; Li, Z.Y. Modulating protein activity and cellular function by methionine residue oxidation. Amino Acids 2012, 43, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.; Blüggel, M.; Meyer, H.E.; Kühne, R.; Ter Laak, A.M.; Kojro, E.; Fahrenholz, F. Direct identification of the agonist binding site in the human brain cholecystokininB receptor†. Biochemistry 1999, 38, 6043–6055. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Escrieut, C.; Galès, C.; Fehrentz, J.-A.; Escherich, A.; Wank, S.A.; Martinez, J.; Moroder, L.; Maigret, B.; Bouisson, M.; et al. Evidence for a direct interaction between the penultimate aspartic acid of cholecystokinin and histidine 207, located in the second extracellular loop of the cholecystokinin B receptor. J. Boil. Chem. 1999, 274, 23191–23197. [Google Scholar] [CrossRef] [PubMed]
- Galès, C.; Sanchez, D.; Poirot, M.; Pyronnet, S.; Buscail, L.; Cussac, D.; Pradayrol, L.; Fourmy, D.; Silvente-Poirot, S. High tumorigenic potential of a constitutively active mutant of the cholecystokinin 2 receptor. Oncogene 2003, 22, 6081–6089. [Google Scholar] [CrossRef]
- Langer, I.; Tikhonova, I.G.; Travers, M.-A.; Archer-Lahlou, E.; Escrieut, C.; Maigret, B.; Fourmy, D. Evidence that interspecies polymorphism in the human and rat cholecystokinin receptor-2 affects structure of the binding site for the endogenous agonist cholecystokinin. J. Boil. Chem. 2005, 280, 22198–22204. [Google Scholar] [CrossRef]
- Foucaud, M.; Archer-Lahlou, E.; Marco, E.; Tikhonova, I.G.; Maigret, B.; Escrieut, C.; Langer, I.; Fourmy, D. Insights into the binding and activation sites of the receptors for cholecystokinin and gastrin. Regul. Pept. 2008, 145, 17–23. [Google Scholar] [CrossRef]
- Miller, L.J.; Gao, F. Structural basis of cholecystokinin receptor binding and regulation. Pharmacol. Ther. 2008, 119, 83–95. [Google Scholar] [CrossRef]
- Ritler, A.; Shoshan, M.S.; Deupi, X.; Wilhelm, P.; Schibli, R.; Wennemers, H.; Behe, M. Elucidating the structure–activity relationship of the pentaglutamic acid sequence of minigastrin with cholecystokinin receptor subtype 2. Bioconjugate Chem. 2019, 30, 657–666. [Google Scholar] [CrossRef]
- Gigoux, V.; Escrieut, C.; Fehrentz, J.-A.; Poirot, S.; Maigret, B.; Moroder, L.; Gully, D.; Martinez, J.; Vaysse, N.; Fourmy, D. Arginine 336 and asparagine 333 of the human cholecystokinin-A receptor binding site interact with the penultimate aspartic acid and the C-terminal amide of cholecystokinin. J. Boil. Chem. 1999, 274, 20457–20464. [Google Scholar] [CrossRef] [PubMed]
- Escrieut, C.; Gigoux, V.; Archer, E.; Verrier, S.; Maigret, B.; Behrendt, R.; Moroder, L.; Bignon, E.; Silvente-Poirot, S.; Pradayrol, L.; et al. The biologically crucial C terminus of cholecystokinin and the non-peptide agonist SR-146,131 share a common binding site in the human CCK1 receptor. Evidence for a crucial role of Met-121 in the activation process. J. Biol. Chem. 2002, 277, 7546–7555. [Google Scholar] [CrossRef] [PubMed]
- Archer-Lahlou, E.; Tikhonova, I.; Escrieut, C.; Dufresne, M.; Seva, C.; Pradayrol, L.; Moroder, L.; Maigret, B.; Fourmy, D. Modeled structure of a G-protein-coupled receptor: The cholecystokinin-1 receptor. J. Med. Chem. 2005, 48, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Magnan, R.; Escrieut, C.; Gigoux, V.; De, K.; Clerc, P.; Niu, F.; Azema, J.; Masri, B.; Cordomí, A.; Baltas, M.; et al. Distinct CCK-2 receptor conformations associated with β-Arrestin-2 recruitment or phospholipase-C activation revealed by a biased antagonist. J. Am. Chem. Soc. 2013, 135, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- McCrink, K.A.; Maning, J.; Vu, A.; Jafferjee, M.; Marrero, C.; Brill, A.; Bathgate-Siryk, A.; Dabul, S.; Koch, W.J.; Lymperopoulos, A. β-Arrestin2 improves post-myocardial infarction heart failure via sarco(endo)plasmic reticulum Ca2+-ATPase-dependent positive inotropy in cardiomyocytes. Hypertension 2017, 70, 972–981. [Google Scholar] [CrossRef]
- Ding, W.Q.; Kuntz, S.M.; Miller, L.J. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: Role of reduced cellular U2AF35 and a suboptimal 3’-splicing site leading to retention of the fourth intron. Cancer Res. 2002, 62, 947–952. [Google Scholar]
- Cheng, Z.-J.; Harikumar, K.G.; Ding, W.-Q.; Holicky, E.L.; Miller, L.J. Analysis of the cellular and molecular mechanisms of trophic action of a misspliced form of the type B cholecystokinin receptor present in colon and pancreatic cancer. Cancer Lett. 2005, 222, 95–105. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Rui, X.-L.; Hellmich, H.L.; Fleming, R.Y.D.; Evers, B.M.; Townsend, C.M. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J. Biol. Chem. 2000, 275, 32122–32128. [Google Scholar] [CrossRef]
- Baier, J.; Maisch, T.; Maier, M.; Engel, E.; Landthaler, M.; Bäumler, W. Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys. J. 2006, 91, 1452–1459. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.Z.; Cui, Z.J. Permanent Photodynamic Activation of the Cholecystokinin 2 Receptor. Biomolecules 2020, 10, 236. https://doi.org/10.3390/biom10020236
Tang WZ, Cui ZJ. Permanent Photodynamic Activation of the Cholecystokinin 2 Receptor. Biomolecules. 2020; 10(2):236. https://doi.org/10.3390/biom10020236
Chicago/Turabian StyleTang, Wen Zhu, and Zong Jie Cui. 2020. "Permanent Photodynamic Activation of the Cholecystokinin 2 Receptor" Biomolecules 10, no. 2: 236. https://doi.org/10.3390/biom10020236
APA StyleTang, W. Z., & Cui, Z. J. (2020). Permanent Photodynamic Activation of the Cholecystokinin 2 Receptor. Biomolecules, 10(2), 236. https://doi.org/10.3390/biom10020236