Abstract
High arsenic (As) levels in food and drinking water, or under some occupational conditions, can precipitate chronic toxicity and in some cases cancer. Millions of people are exposed to unacceptable amounts of As through drinking water and food. Highly exposed individuals may develop acute, subacute, or chronic signs of poisoning, characterized by skin lesions, cardiovascular symptoms, and in some cases, multi-organ failure. Inorganic arsenite(III) and organic arsenicals with the general formula R-As2+ are bound tightly to thiol groups, particularly to vicinal dithiols such as dihydrolipoic acid (DHLA), which together with some seleno-enzymes constitute vulnerable targets for the toxic action of As. In addition, R-As2+-compounds have even higher affinity to selenol groups, e.g., in thioredoxin reductase that also possesses a thiol group vicinal to the selenol. Inhibition of this and other ROS scavenging seleno-enzymes explain the oxidative stress associated with arsenic poisoning. The development of chelating agents, such as the dithiols BAL (dimercaptopropanol), DMPS (dimercapto-propanesulfonate) and DMSA (dimercaptosuccinic acid), took advantage of the fact that As had high affinity towards vicinal dithiols. Primary prevention by reducing exposure of the millions of people exposed to unacceptable As levels should be the prioritized strategy. However, in acute and subacute and even some cases with chronic As poisonings chelation treatment with therapeutic dithiols, in particular DMPS appears promising as regards alleviation of symptoms. In acute cases, initial treatment with BAL combined with DMPS should be considered.
1. Introduction
The element arsenic (As) belongs to Group 15 in the periodic system. Additionally, it is chemically classified as a metalloid. It occurs in many minerals in the Earth’s crust, often together with other metals and sulfur. In recent years China, together with Russia and Morocco, have been the top producers of As [1]. However, nowadays most As refinement operations have been phased out in the USA and Europe, due to environmental concerns.
Until recently, several compounds of As were used as pesticides and herbicides [2]. Due to its antifungal properties, chromated copper arsenate was used to treat and preserve wood. However, the use of this As compound in consumer products was banned in 2004 in the USA as well as in the European Union due to mounting evidence of As toxicity [3]. Spraying of fruit trees with methylated arsenates, as well as with other arsenicals, have also been extensively used until recently, due to their insecticidal properties. However, the use of arsenicals in agricultural activities has been phasing out in the Western world from about 2013. Nowadays, the US Agency for Toxic Substances and Disease Registry places As as number one on their Priority List of Hazardous Substances [4]. The International Agency for Research on Cancer (IARC) classifies arsenic (As) and inorganic arsenic (iAs) compounds in Group 1, carcinogenic to humans [5].
In their hazard assessment, The Joint FAO/WHO Expert Committee on Food Additives (JECFA) [6] modeled dose-response data from recent studies on lung and urinary tract cancer and established a benchmark dose for 0.5% elevated lung cancer incidence above background, with 95% confidence limit, for a daily dose between 3.0 and 5.0 µg As/kg body weight (mainly iAs). They selected the lowest dose of 3.0 µg As/kg body weight per day as a risk assessment reference point. In different countries in Europe and Asia as well as in the USA, it has been reported that mean dietary iAs exposure ranges from 0.1 to 3.0 µg As/kg body weight per day, illustrating that As in food and drinking water may constitute a public health concern [7]. In 2014, the WHO held an advisory conference to confirm limits for rice of 200–300 µg/kg [8].
In 2006, the US Environmental Protection Agency (EPA) set 10 µg/L as the maximum allowed concentration of As in drinking water [9]. The Occupational Safety and Health Administration has set a permissible exposure limit (PEL) as a time-weighted average (TWA) of 0.01 mg/m3. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.002 mg As/m3 for 15-min constant exposure [10].
The biochemistry of As is similar to other elements found in Group 15 of the periodic table, in particular phosphorous, and to some extent also nitrogen. Thus, arsenate (AsO43−), the dominating As species in seawater, occurs together with phosphate (PO43−), and these two compounds are indistinguishable to marine algae, explaining their high AsO43− uptake. During changing conditions of salinity, aquatic organisms synthesize the nitrogen-containing compound glycine betaine to maintain the osmotic balance. However, due to structural mimicry As can replace nitrogen in this synthesis [11], which may explain the high arsenobetaine concentrations in the marine nutrition chain.
The abundant occurrence of organoarsenicals, in nature as in the laboratory, is a result of the high electronegativity of As, viz. 2.18 on the Pauling scale, which is a little lower than that of carbon (2.55), sulfur (2.58) and selenium (2.55). Consequently, As has a high tendency, compared with other metals, to be involved in covalent bonding to carbon, as well as to sulfur and selenium [12].
Before the developments of modern antibiotics and cytostatic agents, different organic as well as iAs compounds were used as pharmaceuticals, for instance, Salvarsan (arsphenamine) for syphilis [13] and arsenic trioxide for cancer [14]. In treatment of psoriasis, the As-containing Fowler’s solution was recommended, but later it was found in a dose-related manner to increase the risk of skin cancer [15], Still. As(III) trioxide is approved as a treatment in cases of acute promyelocytic leukemia, but only when conventional regimens are not effective [16].
Arsenic has four oxidation states, i.e., −3, 0, +3, and +5. Under aerobic conditions, the latter is the dominant oxidation state [17]. This review focuses on the +3 and +5 oxidation states of As. Today both of these series of compounds have worldwide toxicological relevance, which motivated to elaborate the present overview. In the present work we aim to give a narrative review of environmental sources of As exposure, health hazards, molecular targets of toxicity and therapeutic measures in acute and chronic As toxicity.
Literature published after the year 2000 has been searched in Pubmed, Medline and Google scholar by making use of the search keyword As combined with a second keyword, either environment, toxicity, targets or therapeutics. In addition, references to some earlier papers are included to illustrate historical perspectives. The article is focused on inorganic arsenic compounds (iAs), and the symbol As refers to the element arsenic not further specified.
2. Sources of Exposure
2.1. Arsenic in Drinking Water
Worldwide an estimated 200 million people are exposed to As in drinking water above the WHO recommended guideline of 10 µg/L [18]. The majority lives in southern Asia, but also populations living in other areas are affected. Arsenic in ground water and drinking water occurs as iAs, but is in chemical analyses usually determined as total As.
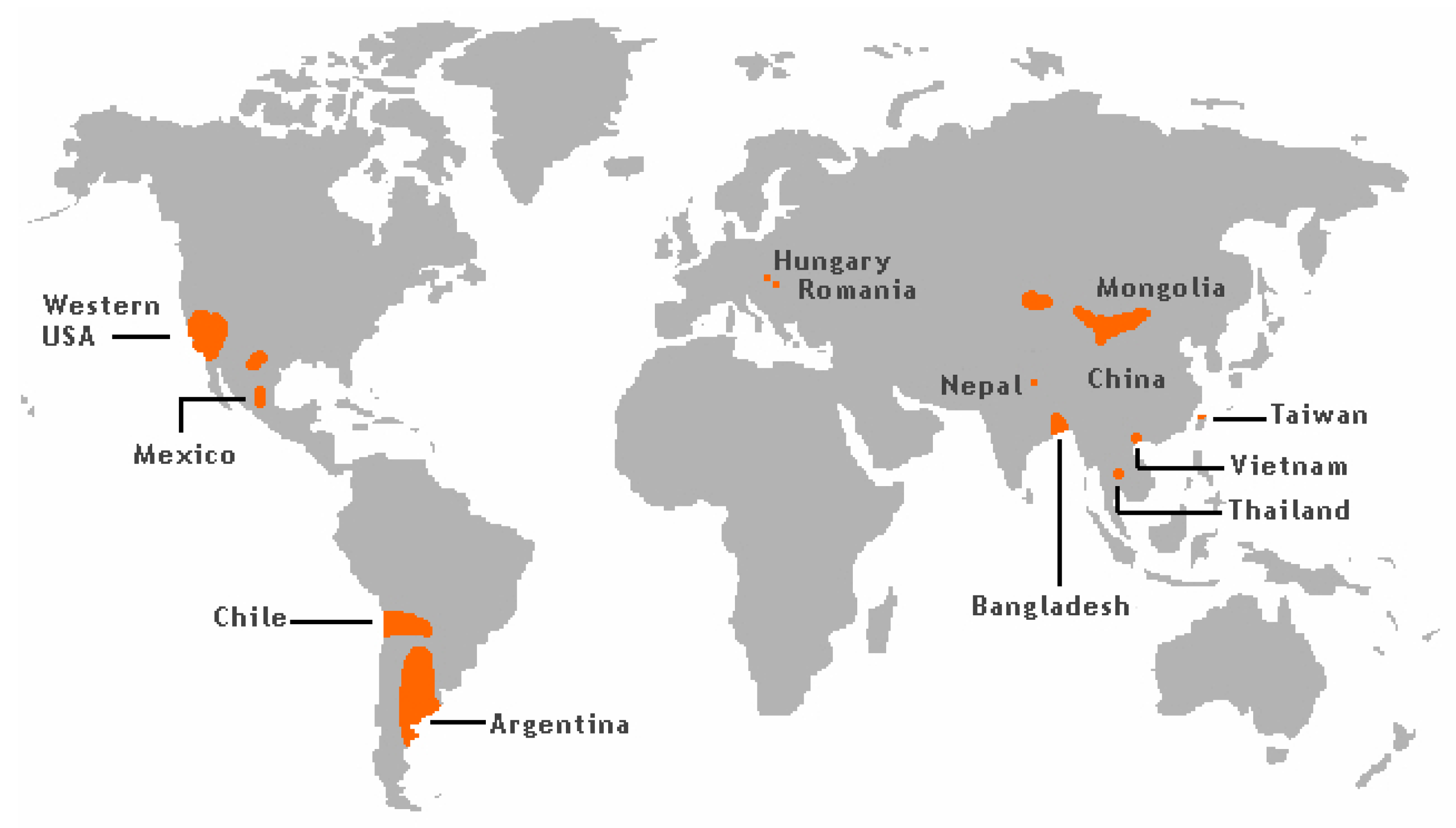
The groundwater levels of As has caused significant exposure and frequent As poisoning, as is reported i.a. from West Bengal and Bangladesh (Figure 1) [19].
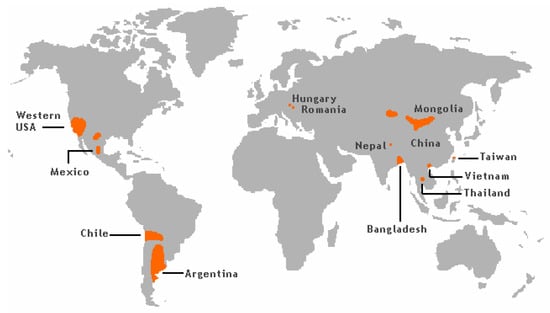
Figure 1.
Arsenic risk areas around the world. From [20], Wikimedia Commons, the free media repository.
More than 50 million people in these regions drink groundwater containing As levels above 50 µg/L [21]. It is known that As occurring in sediments in some areas of the world can be released into the groundwater. Southeast Asian districts and countries, including Cambodia, Vietnam, China, and Taiwan have groundwater with high As content due to their geological environments [22].
In the USA, groundwater in the southwest regions frequently contains significant amounts of As (Figure 1). Thus, it is estimated that the As values in about 16% of wells in New Mexico exceed 10 µg/L, most of this As contamination being focused in the Middle Rio Grande Basin [23,24]. In Arizona, values in the range 10–210 µg/L have been reported for well water [25].
High levels of As in ground and surface water related to mining activities have been reported from Peru [26]. In a district in Northern Chile the As content in drinking water exceeded 100 µg/L until 1979 when an As-removal plant was built. In fact, after 2004 levels of As in drinking water in the same region had dropped to about 10 µg/L [27]. At different sampling sites in Northern Argentina, the measured concentrations of As in drinking water were as high as about 2000 µg As/L [28]. These high levels in Chile and Argentina are explained by geological characteristics related to the Andes mountain range.
Recent studies conducted in Serbia, showed substantially elevated levels of As when compared with the limit of 10 µg/L. Determined levels of As in investigated water samples from the public water supply system of the Northern region of Serbia were more than ten-fold higher than the recommendation and even reached levels above 300 µg/L [29]. These findings can be explained by geological characteristics of the Northern region of Serbia belonging to the Pannonian Basin (Hungary, Romania, Croatia and Serbia) known to contain elevated naturally occurring As. It has been estimated that nearly 500,000 people are exposed to levels above the recommended limit, making this region the largest affected area in Europe [30].
2.2. Arsenic in Food
For individuals who are neither occupationally exposed to As nor via drinking water, the most important source of As is food [31]. Among food of earthbound origin, some cereals, such as rice have As concentrations which are sometimes as high as 0.4 mg/kg dry weight [6]. The main As species in rice and other plants irrigated with As-containing water or grown on As rich soil is iAs [7,32].
In contrast to terrestrial organisms that mainly contain inorganic As, most of As in marine organisms are organoarsenicals. In particular, in organisms from the upper part of the marine food chain, arsenobetaine is the prevalent arsenical compound [33]. However, in marine algae, arsenosugars occur as the most significant arsenicals [34], and these are prevalent also in shellfish [33]. Arsenolipids, also synthesized in algae in place of phosphate, occur in the lipid phase of marine organisms and are present in various types of seafood, including cod liver oil and tuna [35,36]. Marine fish, as well as other kinds of marine seafood, contain As concentrations up to 100 mg As/kg [32]. While in marine organisms arsenobetaine is a more common arsenical [33], fresh-water organisms also contain As as arsenobetaine, but in much lower concentrations. Lower-ranking organisms contain a larger fraction bound to sugars and in various other low molecular weight compounds including sulfur-containing molecules.
2.3. Occupational Exposure
Occupational exposure to As compounds is another important source of exposure. Occupational exposure in some cases can even lead to As poisoning, especially in industries that are using inorganic As, or in workers using other toxic arsenicals. These include wood preservation, vineyard spraying, nonferrous metal alloys, glass production, and production of electronic semiconductors [37]. Inorganic As (iAs) is also emitted from the smelter industries, such as As or copper smelters, resulting in both occupational and environmental exposure associated with increased lung cancer risk among workers and residents in the vicinity of a smelter [38,39].
3. Health Effects of Arsenic Exposure
Acute arsenic poisoning is associated initially with nausea, vomiting, abdominal pain, and severe diarrhea [40]. Encephalopathy and peripheral neuropathy may occur. Paresthesia in the limbs is a frequent symptom of iAs exposure, which in some cases may develop into widespread polyneuropathy [41].
Prolonged iAs exposure affects the skin, particularly with localization at skin folds. This can give rise to changes in pigmentation, hyperkeratosis and over time, development of skin cancer, e.g., in the hands. Upon such exposure As is a well-documented human carcinogen affecting numerous organs [5]. Significantly increased risks of lung, urinary tract as well as skin cancer are reported at levels in drinking water around and above 50 µg/L [42]. Analyses of exposure to (As) in many epidemiological studies suggest that there might be a small elevation of the risk to develop bladder cancer when the As levels in drinking water are as low as 10 µg/L [43], although a recent meta-analysis revealed no increase in risk at this level [44]. JECFA noted, however, that in epidemiological studies in that low dose-range the risk probably would be too low to be detected, due to interferences from confounding factors like cigarette smoking. In Northern Chile, as in Southeast Asian districts, epidemiological evidence has shown a dose-dependent association between chronic As exposure and the different cancer forms [45], An increased risk of bladder as well as lung cancer persisted for at least three decades after a high exposure had ended [46].
Other sensitive endpoints of prolonged exposure are peripheral neuropathy, cardiovascular disease, and in exposed children neurobehavioral effects [47]. Some research suggests that exposure to environmental As might be a risk factor in children for the development of autism spectrum disorder. However, these findings are inconclusive [48,49]. In experimental studies exposure to As disturbs the neurotransmitter metabolism. Arsenic disrupts the glutamate-induced release of gliotransmitters, causing changes in the neuronal function [50]. In addition, iAs exposure may impair the transport of glutamate [51]. In rats, iAs exposure significantly impacted brain cholinergic receptors [52]. Furthermore, in cells, the interplay between dopamine and iAs increased the neurotoxic effects on the dopaminergic neurons [53].
Arsenic exposure can also precipitate type 2 diabetes mellitus in susceptible cases [54]. Long-term iAs exposure induces increased oxidative stress, which may explain deteriorations in structures and functions of the cardiovascular system. Furthermore, by increasing the tendency of platelet aggregation, As can aggravate atherosclerosis. Endothelial nitric oxide synthase is inactivated by As, which causes reduced nitric oxide production in the vascular bed [55]. Arsenic exposures may also upregulate the expression of interleukin-1, tumor necrosis factor-α, vascular endothelial growth factor, and vascular cell adhesion molecule, and thereby causing endothelial dysfunction and aggravating cardiovascular pathology [56]. Furthermore, epidemiological observation studies in humans showed an association between As exposure and impaired reproductive function in males [57].
4. The Metabolism and Mechanisms of Toxicity
Arsenic undergoes in vivo several complex metabolic conversions. Both As and its metabolites interact with extra- and intracellular macromolecules, in particular, those containing vicinal thiols, but the mechanism of action might vary with respect to chemical form of As [7,58]. Methylation of iAs has been considered as a part of a detoxification pathway for many years. However, newer research indicates that intermediary metabolites, in particular, monomethylarsonous acid (MMA-III), but also dimethylarsonous acid (DMA-III), are reactive and toxic [59]. Although MMA-III and DMA-III are usually quickly oxidized to the less toxic pentavalent species, the intermediates in the iAs methylation pathways can cause toxic effects, including DNA-damage [7]. Indeed, a higher primary iAs methylation activity has in epidemiological studies been associated with an increased risk of skin lesions including skin cancer [6].
Research has identified a monomethyl arsonic acid reductase (MMA-V reductase) that function as a catalyst when As(V), DMA(V), and MMA(V) are reduced to the more toxic trivalent species [60], presumably through reactions depending on the presence of reduced glutathione [61]. Recently, in a study in mice, it has been found that oral DMA-V in significant amounts is reduced to DMA-III in the intestine and liver as first-pass effects, and bonded extensively to tissues and red blood cells [62].
Both the peripheral and the central nervous systems may be affected by As neurotoxicity [63]. In particular, As exposure significantly affects the glial component of the central nervous system [64]. The neurotoxic effects caused by As exposure seems to be oxidative stress-mediated [65]. Mitochondria is the major target for As neurotoxicity [66]. Arsenic inhibits the complexes I, II, and IV of the electron transport chain, which elevate mitochondrial production of reactive oxygen species. In turn, this mitochondrial disturbance may lead to microglial cell apoptosis [67]. Microglia is more sensitive to As (III) toxicity than to As (V) [68]. Brain morphology in rodents may be significantly affected by As exposure, which results in neuronal degeneration, gliosis, and disruption of the blood-brain barrier [69]. Arsenic may impair neurite outgrowth [70]. Chronic As exposure in rats causes neuronal apoptosis in the hippocampus, and cognitive impairments, like spatial memory impairment [71,72,73]. In mice exposed to As, As-induced alteration in the metabolic pathway of arachidonic acid may be a factor in the mediation of neuronal damage and inflammatory response [74]. In astrocytes, sub-toxic doses of monomethylarsonous acid increase the pro-inflammatory cytokines gene expression significantly [75]. Neuronal apoptosis is induced both by dimethylarsinic acid and iAs [76]. In particular, one class of arsenolipids, arsenohydrocarbons, is toxic to human neurons in vitro and is able to cross an in vitro brain barrier model [77,78,79]. Hence, this class of compounds might have a potential for neurodevelopmental toxicity.
The acute toxicity of As is ascribed to the strong affinity of As (III) for SH-groups. It is now well known that endogenous thiols can be occupied by As compounds, such as in some cofactors (i.a. lipoic acid) or cysteine residues that are essential constituents in the active sites of crucial enzymes. Thus, early studies disclosed that As (III)-compounds block the function of the sulfur-containing pyruvate dehydrogenase enzyme complex, which is crucial for the conversion of pyruvate to acetyl-CoA that enters the citric acid cycle. This vulnerable enzyme complex is essential for mitochondrial functions [80]. Experimentally, some of the mitochondrial effects appear to be reversed by administration of the cofactor α-lipoic acid [81]. Additionally, As (V) competes with phosphate, thus further inhibiting or blocking the mitochondrial respiration and the ATP synthesis. These metabolic interferences may lead to death from multi-organ failure.
It has been documented that As(III)-compounds inhibit cytosolic SH-enzymes such as glutathione reductase [82]. Other important targets are selenol groups in, e.g., the seleno-enzymes glutathione peroxidase and thioredoxin reductase, the latter also containing a thiol group vicinal to the selenol group [16,82]. Through these interactions, As appears to impair scavenging of intracellular hydrogen peroxide (H2O2) production, which can react further to highly reactive and short-lived hydroxyl radicals.
Usually As-III-compounds are considered to represent the most toxic and carcinogenic species, and mechanisms of actions including chromosomal effects are discussed in a recent review [83]. However, it should be noted that DMA-V can induce bladder cancer in rats and lung tumors in mice [6]. Research has linked long-term As exposure to epigenetic changes, which might also cause heritable gene expression changes [84]. These changes include histone modification, RNA interference, and DNA methylation [85]. For instance, excessive levels of As cause critical DNA hypermethylation via tumor protein p53, which increase the carcinogenesis risk. Other important mechanisms of carcinogenicity are inhibition of various DNA repair systems as well as interference with redox regulation and ROS production [86,87,88,89]. The organic arsenical arsenobetaine, the dominating species in seafood, is excreted unchanged and considered to be of low toxicity and is not classified as carcinogenic. The possible toxicities of arsenosugars and arsenolipids are insufficiently studied, but these species may split off DMA in the intestine [90] where, as mentioned above, it might be reduced to the bioactive trivalent DMA-III.
5. Treatment of Arsenic Poisoning
The classic antidote against acute arsenic poisoning, BAL (British anti-Lewite, dimercaptopropanol), was originally developed as a war gas antidote for Lewisite (dichlorovinyl arsine). BAL is a dithiol compound, which competes successfully with endogenous SH-groups, e.g., with the sulfur groups of the pyruvate dehydrogenase cofactor α-lipoate for As in cases of arsenical poisonings, thereby enhancing As elimination from the body.
However, due to the rather high toxicity of BAL, and the necessity of frequent and inconvenient intramuscular administrations [91,92], the clinical use of this drug is currently restricted to only the initial treatment for few days after acute As intoxications [93]. Water-soluble and less toxic derivatives of BAL have been developed for therapeutic use, viz. DMSA (dimercaptosuccinic acid) and DMPS (dimercaptopropane sulfonate) which was in regular clinical use in China [94] and the former Soviet Union [95] early after 1950. Several decades had to pass after their original introduction [96,97] before Western clinicians fully realized their value. Today, treatment of acute and also some severe cases of chronic As poisoning makes use of DMPS as chelating antidote [98]. In acute life-threatening poisonings, it is recommended to support vital functions and perform chelation therapy as fast as possible. The therapy implies BAL i.m. in doses calculated as 5 mg/kg and a maximum of 300 mg i.m. four times daily during the first days, combined with DMPS i.v. to provide a more efficient therapeutic approach than obtained with monotherapy in the initial stage [98,99]. It is reasonable to presume that BAL can act as a shuttling agent for intra- to the extracellular transfer of As in emergency cases, the extracellular metal is then picked up by circulating DMPS with the formed As-DMPS-chelate being rapidly excreted via urine. Successful treatment with DMPS has been described in different case reports [100,101]. After the initial high-dosed therapy, the chelation regimen is usually switched to oral monotherapy with DMPS. In acute cases of massive arsenic trioxide poisoning supplementation of the chelator combination with gastric rinsing and forced alkaline diuresis, has been recommended [102]. If renal failure occurs, hemodialysis should be initiated and combined with chelation. The present recommendations have been precipitated from a recent extensive review of the available database of case reports and animal experiments on chelation therapy in As poisonings [103].
The efficacy of DMPS in chronic As toxicity was observed in a placebo-controlled study of patients exposed to As-contaminated drinking water. DMPS was given orally to 11 patients for three weeks with inter-current chelator-free weeks, and ten patients received a placebo. Significant improvement in symptoms of neuropathy and lung symptoms was found in the DMPS group compared to the placebo group [104]. In contrast, only negligible improvement has been observed after monotherapy with DMSA [104,105]. To the knowledge of the authors, combinations of BAL or DMPS with lipoic acid (LA) or its reduced form (DHLA) have yet not been tried clinically in such poisonings. With regard to the carcinogenic effect of prolonged As exposure, it has been hypothesized that an adequate or supra-nutritional status of selenium may exert a protective action [106]. This hypothesis is supported by some epidemiological and experimental studies [107,108], but further research is needed.
6. Chemical Features of BAL, DMSA, and DMPS and Their As(III)-Chelates
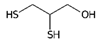
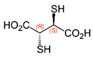
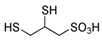
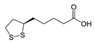
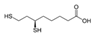
Many of the toxic compounds and metabolites of inorganic and organic arsenicals can be described by the general formula, R-As2+, which explains that vicinal dithiols constitute particularly vulnerable targets. Vicinal dithiols such as DHLA, BAL, DMPS, and DMSA are also the most efficient chelating antidotes.
6.1. Protonation Constants
We report the acidic properties of the chelating agents DHLA, BAL, DMSA, and DMPS, to obtain insight into the chemical form and behavior of the parent molecules in vivo, i.e., in blood with pH 7.4 and urine with pH usually about 6. The protonation constants are of particular importance since they determine the biological properties of a drug, such as its solubility, absorption, cell penetration, and bioavailability. Furthermore, protonation constants are of primary importance also in determining the speciation of the complexes formed with trivalent arsenic. Table 1 reports selected protonation constants of these ligands (those concerning the SH-groups are marked in red) together with their structure, the used acronyms, the formulae, and the molecular weights. It appears that the protonation constants (log K1) of the SH-groups of BAL, DMSA, DMPS and DHLA are in the range 10.6–12, while log K2 is about two units lower, ranging from 8.6 to 9.9, the differences among the various ligands depending on the charge of the molecule and on the distance between the mercapto groups.

Table 1.
Protonation constants of BAL, DMSA, DMPS, lipoic acid (LA), and dihydrolipoic acid (DHLA).
Lipoic acid is also included in Table 1 since this enzymatic cofactor is considered a primary target of the As (III) toxicity when the metalloid blocks the pyruvate dehydrogenase complex.
6.2. Chemical Stabilities of the As-Chelates
Available literature reports only few stoichiometric data on the complex formation equilibria between the thiol chelating agents and As (III) compounds. However, a number of papers describe the principal features of complexes studied with different techniques, briefly summarized in the following.
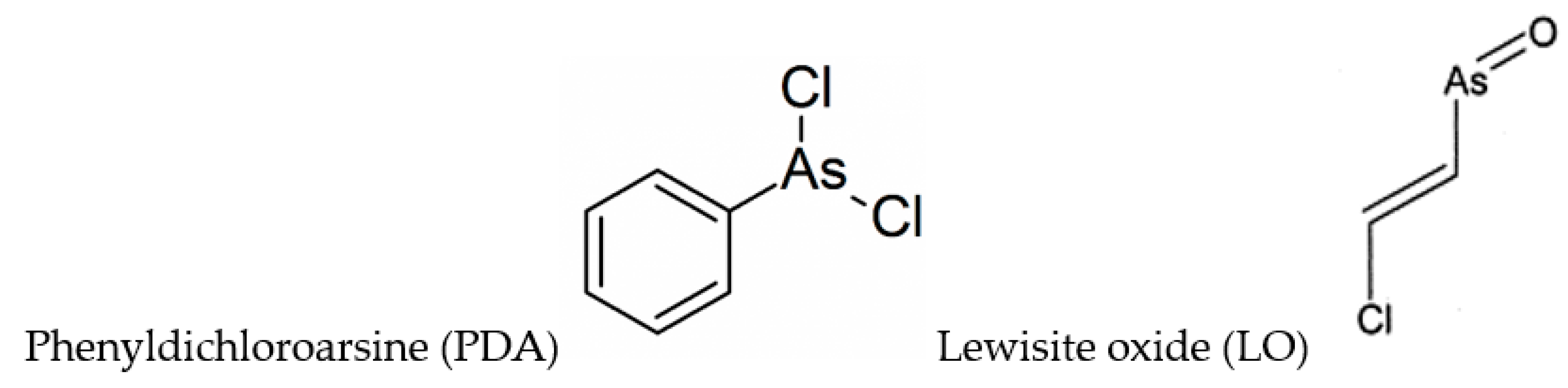
A paper by Dill et al. [113] reports one- and two-dimensional (homonuclear and heteronuclear) NMR studies on BAL and its phenyldichloroarsine (PDA) adduct, where PDA is a Lewisite analog (Figure 2). PDA appears to react with BAL such that in the complex, the hydroxymethyl group is anti to the phenyl ring, and to a less extent a syn structure was observed. A successive paper by the same authors [114] took into consideration the structures of DMSA and DMPS adducts with PDA, and Lewisite (trans-2-chlorovinylarsine) oxide (LO) (Figure 2).
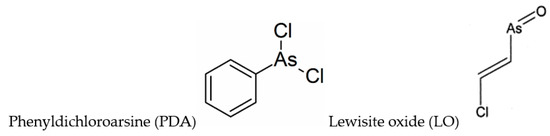
Figure 2.
Chemical formulae of phenyldichloroarsine (PDA) and Lewisite oxide (LO).
The 1:1 adducts were synthesized and characterized by one- and two-dimensional NMR spectroscopy. All the formed complexes were five-membered heteroatomic ring systems (chelates), with different solubility properties (Figure S1). The results indicated that the functional groups of the chelating agent influenced stereochemical aspects of adduct formation [114].
Adams et al. presented an X-Ray and spectroscopic investigation on the interaction of several arylarsenic dichlorides with BAL and DMSA [115]. In the single crystal of the complex between CH3C6H4AsCl2 and BAL the arsenic was found in a distorted-square-pyramidal coordination geometry (Table S1), with one of the sulfur atoms on each of the chelating ligands considerably further away from the arsenic than the other. The five-membered ring presents with an envelope configuration where the hydroxymethyl group is located on a hinge of the envelope anti to the phenyl group of the arsenic. Although no single crystals of the DMSA adducts were suitable for X-ray diffraction, the IR spectra of the complexes clearly showed both the OH groups of the ligand as well as its C=O stretch, and the stretches due to substituted phenyl groups. The 13C NMR spectrum was also highly indicative of arsenic coordination.
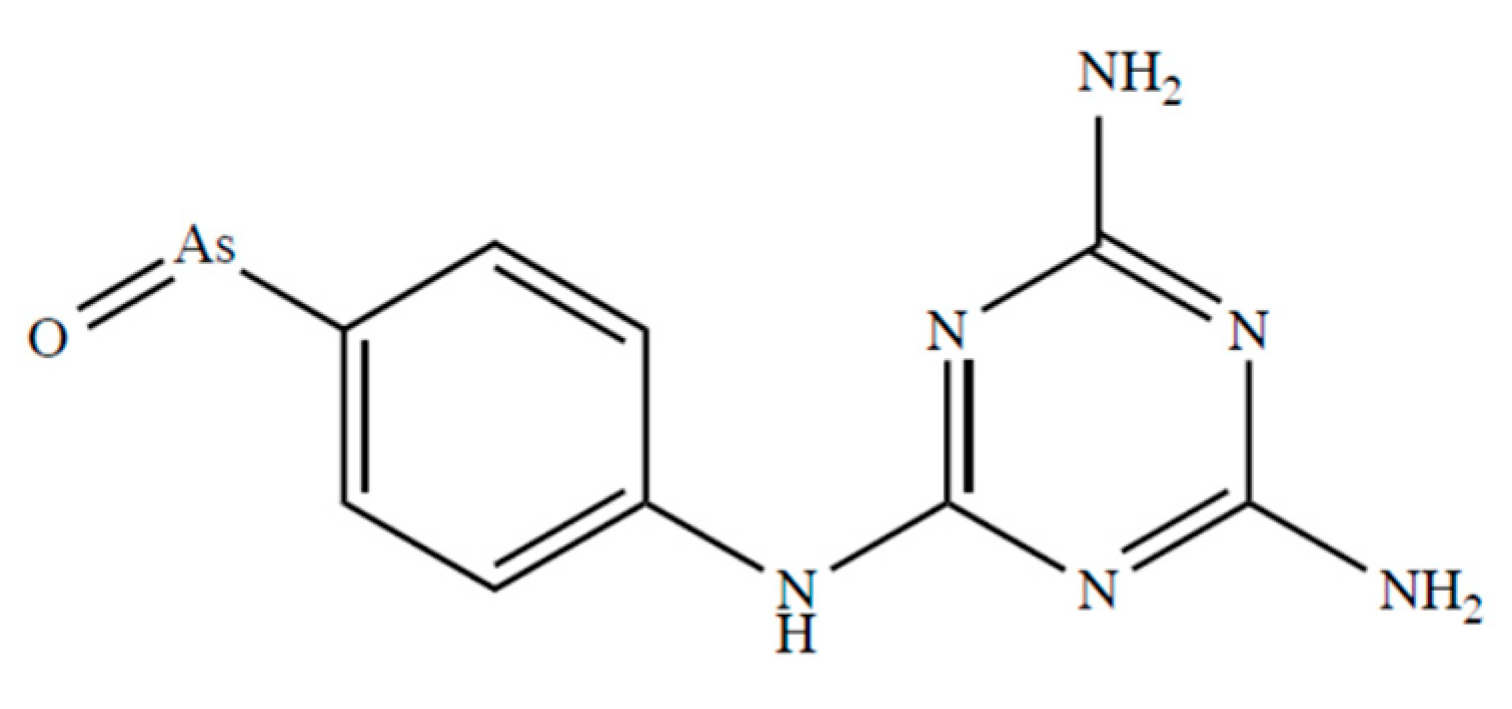
Fairlamb et al. [116] report that D,L-dihydrolipoamide and DHLA form stable complexes with melarsen oxide (Figure 3) with complex formation constants of 5.47 × 109 and 4.51 × 109 M−1, respectively.
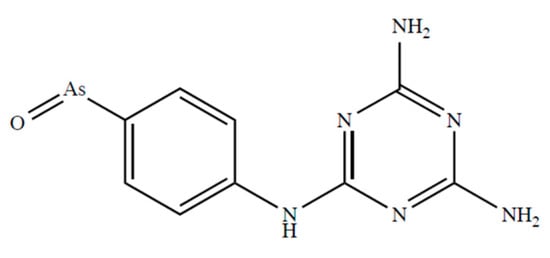
Figure 3.
Chemical formulae of melarsen oxide.
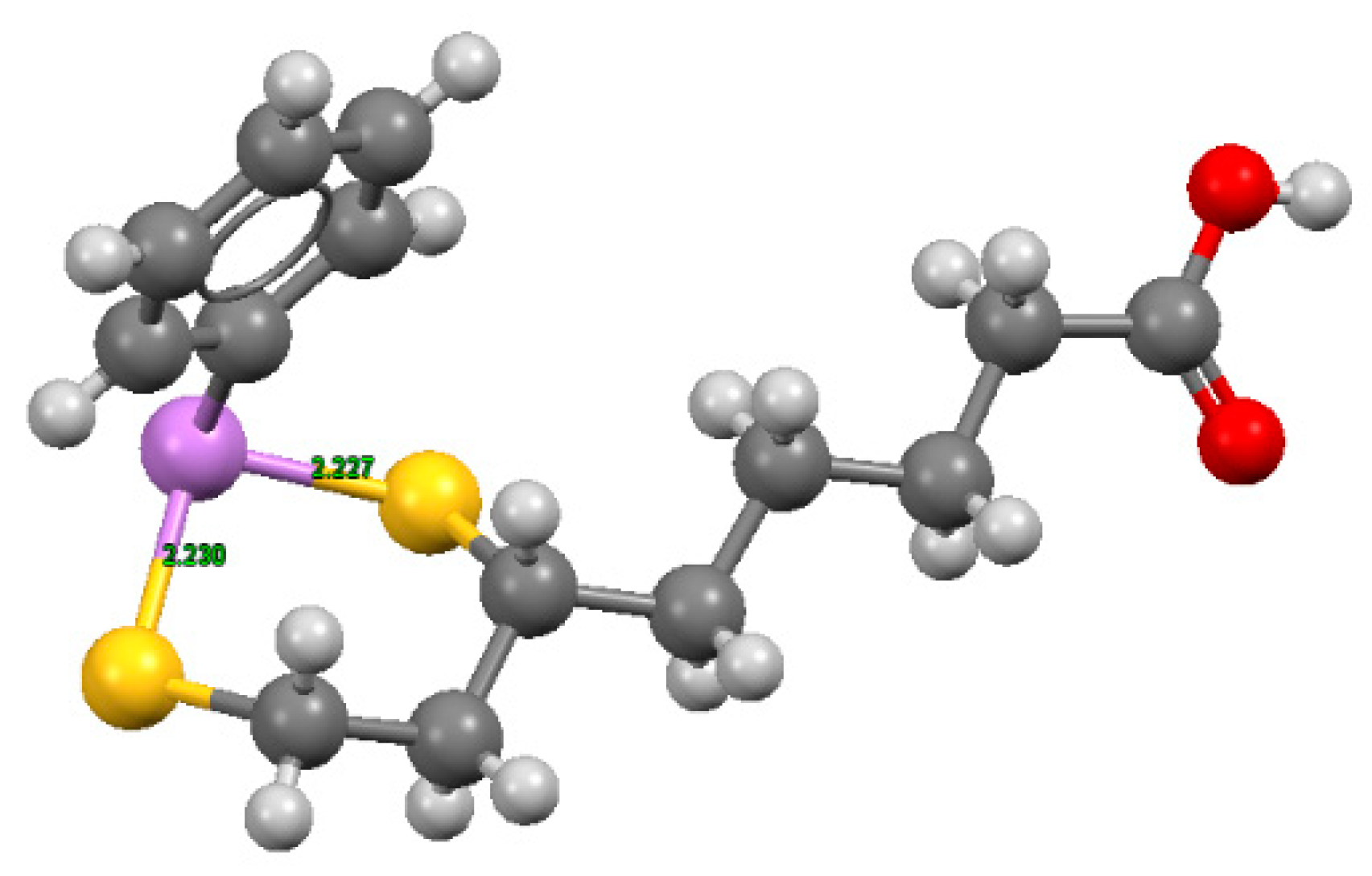
These complexes, characterized by six-membered cyclic dithioarsenite rings, are 10-fold less stable than the complexes with five-membered rings found with BAL (79.30 × 109 M−1) and DMSA (45.00 × 109 M−1) by Fairlamb et al. [117]. The solid-state structure of the complex between PhAs (III) and DHLA shown in Figure 4, was reported by von Döllen and Strasdeit [118].
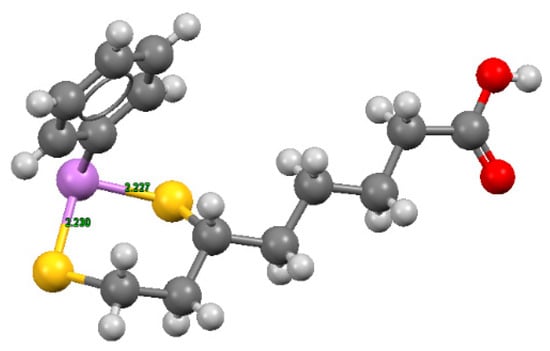
Figure 4.
Structures of phenylarsenic with DHLA, where hydrogen is shown in light grey, carbon in grey, sulfur in yellow, oxygen in red, and As (III) in violet. The coordinates were obtained from the Cambridge Structural Database (NIDKAM), and the image was created using Mercury3.5.
Spuches et al. [119] used near-UV absorption spectroscopy and isothermal titration calorimetry to quantify the stability and thermodynamics of formation of arsenite and monomethylarsenite (MMA) complexes with DMSA, dithiothreitol (DTT), and DHLA. They found that MMA forms chelates with the dithiols somewhat more stable than those of arsenite.
Cavanillas et al. [120] made use of a new methodology that combines voltammetry, ITC, ESI-MS, and several chemometric tools to the study of As(III) complexes with DMSA and DMPS, obtaining estimates of the stoichiometries of the complexes formed (ML2, with the appearance of only minor amounts of ML-species), and approximately similar As(III)-complex formation constants for the two agents (log β2 9.2 and 9.8 for DMSA and DMPS, respectively).
Harper and Bayse [121] used density functional theory (DFT) and solvent-assisted proton exchange (SAPE) to model the chelation of the As in Lewisite by the vicinal thiols of BAL. They concluded, “the low barriers for lewisite detoxification by BAL and the greater stability of the chelation complexes of small dithiols are consistent with the rapid reversal of toxicity demonstrated in previously reported animal models”.
7. Discussion and Conclusions
Humans are exposed to As compounds from many sources, including occupational and environmental sources, which include drinking water and food items. Exposure may range from acutely toxic to various levels of long-term or life-long exposure. High As levels in food or drinking water, or under some occupational conditions, can in some cases precipitate chronic poisoning and in some cases cancer. Individuals subjected to high or long-term exposure may develop acute, subacute, or chronic signs of poisoning, characterized by skin lesions, cardiovascular diseases, and/or neurological symptoms. Pentavalent organic As found in marine species appears to be less toxic than trivalent inorganic and methylated species.
The reviewed analytical results support the hypotheses that the endogenous vicinal dithiol DHLA, and the therapeutic agents BAL and DMPS, have a particularly high affinity for arsenical compounds. The relative affinity of selenol groups for arsenicals may be even higher, but determinations of the chemical stabilities of these chelates are sparse or missing.
Apparently, both inorganic arsenite (III) and R-As2+-compounds are bound tightly to thiol groups, which constitute vulnerable targets for the toxic action of As. Selenol groups, e.g., in seleno-enzymes and particularly in conjunction with thiol groups as in thioredoxin reductase, are anticipated to have an even higher affinity to R-As2+-compounds, explaining the oxidative stress associated with arsenic toxicity. The strong general affinity to thiols and selenols may explain the many targets of arsenicals and a wide range of adverse health effects. Among therapeutic agents, the most efficient chelators are the dithiols BAL and DMPS, both of which with particularly high affinity to R-As2+-compound.
Whereas primary prevention aiming to reduce environmental exposure to toxic inorganic As and organic As from drinking water and food for the many millions of people with unacceptable exposure to As should be the prioritized approach, therapeutic intervention may be needed in cases of acute, subacute, and even chronic As poisonings. Here chelation with DMPS, which can be administered orally or intravenously, appears promising as regards alleviation of symptoms. In severe acute cases, a combination of DMPS intravenously with BAL intramuscularly can be used in the first days of the treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/2/235/s1, Figure S1. Molecular formulae of the 1:1 adducts proposed in reference [114] based on NMR results formed between A) trans-2-chlorovinylarsine oxide or lewisite oxide (LO) and DHLA; B) LO and DMSA; C) LO and DMPS; D) PDA and DMSA; E) PDA and DMPS, Table S1. Structures of As(III) with different ligands bearing mercapto groups, where hydrogen is shown in light grey, carbon in grey, nitrogen in blue, sulfur in yellow, oxygen in red, and As(III) in violet. The coordinates were obtained from the Cambridge Structural Database, and the image was created using Mercury3.5.
Funding
This research was partially funded by Innlandet Hospital Trust, Norway. VMN thanks Regione Autonoma della Sardegna for the financial support of the project RASSR79857.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sackett, P.D. Elemental cycles in the Anthropocene: Mining aboveground. In Geological Society of America Special Papers; Geological Society of America: Boulder, CO, USA, 2016; Volume 520, pp. 99–116. ISBN 978-0-8137-2520-8. [Google Scholar]
- Rahman, F.A.; Allan, D.L.; Rosen, C.J.; Sadowsky, M.J. Arsenic availability from chromated copper arsenate (CCA)-treated wood. J. Environ. Qual. 2004, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Substance Priority List. ATSDR. Available online: https://www.atsdr.cdc.gov/SPL/ (accessed on 24 September 2019).
- Smoke, T.; Smoking, I. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2004; Volume 100C, ISBN 978-92-832-1320-8. [Google Scholar]
- Benford, D.J.; Alexander, J.; Baines, J.; Bellinger, D.C.; Carrington, C.; Devesa i Pérez, V.A.; Duxbury, J.; Fawell, J.; Hailemariam, K.; Montoro, R.; et al. ARSENIC. In Safety Evaluation of Certain Contaminants in Food; FAO and WHO: Geneva, Switzerland, 2011; ISBN 978-92-4-166063-1. [Google Scholar]
- Molin, M.; Ulven, S.M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology–Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Arsenic in Your Food. Available online: https://www.consumerreports.org/cro/magazine/2012/11/arsenic-in-your-food/index.htm. (accessed on 22 November 2019).
- US EPA. Arsenic Rule Compliance Success Stories. Available online: https://www.epa.gov/dwreginfo/arsenic-rule-compliance-success-stories (accessed on 26 September 2019).
- CDC Template Package 4. Available online: https://www.cdc.gov/index.htm (accessed on 26 September 2019).
- European Food and Safety Authority. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Aaseth, J.; Skaug, M.A.; Cao, Y.; Andersen, O. Chelation in metal intoxication—Principles and paradigms. J. Trace Elem. Med. Biol. 2015, 31, 260–266. [Google Scholar] [CrossRef]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Emadi, A.; Gore, S.D. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010, 24, 191–199. [Google Scholar] [CrossRef]
- Farber, E.M. History of the treatment of psoriasis. J. Am. Acad. Dermatol. 1992, 27, 640–645. [Google Scholar] [CrossRef]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef]
- Gomez-Caminero, A.; Howe, P.D.; Hughes, M.; Kenyon, E.; Lewis, D.R.; Moore, M.; Aitio, A.; Becking, G.C.; Ng, J.; Safety, I.P.; et al. Arsenic and Arsenic Compounds; World Health Organization: Geneva, Switzerland, 2001; ISBN 978-92-4-157224-8. [Google Scholar]
- Arsenic, Fact Sheet No 372. Geneva: World Health Organization; 2012. Available online: http://www.who.int/mediacentre/factsheets/fs372/en/ (accessed on 11 January 2020).
- Chakraborti, D.; Rahman, M.M.; Mukherjee, A.; Alauddin, M.; Hassan, M.; Dutta, R.N.; Pati, S.; Mukherjee, S.C.; Roy, S.; Quamruzzman, Q.; et al. Groundwater arsenic contamination in Bangladesh-21 Years of research. J. Trace Elem. Med. Biol. 2015, 31, 237–248. [Google Scholar] [CrossRef]
- Arsenic Contamination Areas. Available online: https://commons.wikimedia.org/wiki/File:Arsenic_contamination_areas.png (accessed on 4 February 2020).
- Mondal, P.; Majumder, C.B.; Mohanty, B. Laboratory based approaches for arsenic remediation from contaminated water: Recent developments. J. Hazard. Mater. 2006, 137, 464–479. [Google Scholar] [CrossRef]
- Rahman, M.M.; Naidu, R.; Bhattacharya, P. Arsenic contamination in groundwater in the Southeast Asia region. Environ. Geochem. Health 2009, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, L.M.; Plummer, L.N. Occurrence of arsenic in ground water of the Middle Rio Grande Basin, central New Mexico. In Arsenic in Ground Water; Welch, A.H., Stollenwerk, K.G., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 295–327. ISBN 978-1-4020-7317-5. [Google Scholar]
- Scanlon, B.R.; Nicot, J.P.; Reedy, R.C.; Tachovsky, J.A.; Nance, S.H.; Smyth, R.C.; Keese, K.; Ashburn, R.E.; Christian, L. Evaluation of Arsenic Contamination in Texas; Prepared for Texas Commission on Environmental Quality Austin Texas; The University of Texas at Austin: Austin, TX, USA, 2005; p. 177. [Google Scholar]
- Foust, R.D.; Mohapatra, P.; Compton-O’Brien, A.-M.; Reifel, J. Groundwater arsenic in the Verde Valley in central Arizona, USA. Appl. Geochem. 2004, 19, 251–255. [Google Scholar] [CrossRef]
- George, C.M.; Sima, L.; Arias, M.H.J.; Mihalic, J.; Cabrera, L.Z.; Danz, D.; Checkley, W.; Gilman, R.H. Arsenic exposure in drinking water: An unrecognized health threat in Peru. Bull. World Health Organ. 2014, 92, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.D.; Pino, P.; Montesinos, N.; Atalah, E.; Amigo, H.; Loomis, D. Exposure to inorganic arsenic in drinking water and total urinary arsenic concentration in a Chilean population. Environ. Res. 2005, 98, 151–159. [Google Scholar] [CrossRef]
- Concha, G.; Nermell, B.; Vahter, M.V. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ. Health Perspect. 1998, 106, 355–359. [Google Scholar] [CrossRef]
- Stanisavljev, B.; Bulat, Z.; Buha, A.; Matović, V. Arsenic in drinking water in Northern region of Serbia. E3S Web Conf. 2013, 1, 24006. [Google Scholar] [CrossRef]
- Rowland, H.A.L.; Omoregie, E.O.; Millot, R.; Jimenez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef]
- Edmonds, J.S.; Francesconi, K.A. Methylated arsenic from marine fauna. Nature 1977, 265, 436. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-M.; Ye, J.; Raber, G.; Francesconi, K.A.; Li, G.; Gao, H.; Yan, Y.; Rensing, C.; Zhu, Y.-G. Arsenic Methyltransferase is Involved in Arsenosugar Biosynthesis by Providing DMA. Environ. Sci. Technol. 2017, 51, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Taleshi, M.S.; Edmonds, J.S.; Goessler, W.; Ruiz-Chancho, M.J.; Raber, G.; Jensen, K.B.; Francesconi, K.A. Arsenic-Containing Lipids Are Natural Constituents of Sashimi Tuna. Environ. Sci. Technol. 2010, 44, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Glabonjat, R.A.; Ehgartner, J.; Duncan, E.G.; Raber, G.; Jensen, K.B.; Krikowa, F.; Maher, W.A.; Francesconi, K.A. Arsenolipid biosynthesis by the unicellular alga Dunaliella tertiolecta is influenced by As/P ratio in culture experiments. Metallomics 2018, 10, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Román, M.D.; Niclis, C.; Aballay, L.R.; Lantieri, M.J.; Díaz, M.D.P.; Muñoz, S.E. Do Exposure to Arsenic, Occupation and Diet Have Synergistic Effects on Prostate Cancer Risk? Asian Pac. J. Cancer Prev. 2018, 19, 1495–1501. [Google Scholar] [PubMed]
- Pershagen, G. Lung cancer mortality among men living near an arsenic-emitting smelter. Am. J. Epidemiol. 1985, 122, 684–694. [Google Scholar] [CrossRef]
- De Gregori, I.; Fuentes, E.; Rojas, M.; Pinochet, H.; Potin-Gautier, M. Monitoring of copper, arsenic and antimony levels in agricultural soils impacted and non-impacted by mining activities, from three regions in Chile. J. Environ. Monitor. 2003, 5, 287–295. [Google Scholar] [CrossRef]
- Ratnaike, R. Acute and chronic arsenic toxicity. Postgrad Med J 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Tseng, H.-P.; Wang, Y.-H.; Wu, M.-M.; The, H.-W.; Chiou, H.-Y.; Chen, C.-J. Association between chronic exposure to arsenic and slow nerve conduction velocity among adolescents in Taiwan. J. Health Popul. Nutr. 2006, 24, 182–189. [Google Scholar]
- National Research Council. Arsenic in Drinking Water; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Mink, P.J.; Alexander, D.D.; Barraj, L.M.; Kelsh, M.A.; Tsuji, J.S. Low-level arsenic exposure in drinking water and bladder cancer: A review and meta-analysis. Regul. Toxicol. Pharmacol. 2008, 52, 299–310. [Google Scholar] [CrossRef]
- Boffetta, P.; Borron, C. Low-Level Exposure to Arsenic in Drinking Water and Risk of Lung and Bladder Cancer: A Systematic Review and Dose-Response Meta-Analysis. Dose-Response 2019, 17, 1559325819863634. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; González, C.; Milosavjlevic, V.; Marshall, G.; Sancha, A.M.; Smith, A.H. Lung Cancer and Arsenic Concentrations in Drinking Water in Chile. Epidemiology 2000, 11, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.M.; Ferreccio, C.; Romo, J.A.; Yuan, Y.; Cortes, S.; Marshall, G.; Moore, L.E.; Balmes, J.R.; Liaw, J.; Golden, T.; et al. Drinking Water Arsenic in Northern Chile: High Cancer Risks 40 Years after Exposure Cessation. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Tippairote, T.; Rahaman, M.D.S.; Aaseth, J. Developmental toxicity of arsenic: A drift from the classical dose–response relationship. Arch. Toxicol. 2019, 94, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Bjørklund, G.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Hair toxic and essential trace elements in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Skalny, A.V.; Rahman, M.M.; Dadar, M.; Yassa, H.A.; Aaseth, J.; Chirumbolo, S.; Skalnaya, M.G.; Tinkov, A.A. Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ. Res. 2018, 166, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, F.; Liao, Y.; Jin, Y.; Sun, G. Arsenic exposure and glutamate-induced gliotransmitter release from astrocytes. Neural. Regen. Res. 2012, 7, 2439–2445. [Google Scholar]
- Castro-Coronel, Y.; Del Razo, L.M.; Huerta, M.; Hernandez-Lopez, A.; Ortega, A.; López-Bayghen, E. Arsenite exposure downregulates EAAT1/GLAST transporter expression in glial cells. Toxicol. Sci. 2011, 122, 539–550. [Google Scholar] [CrossRef]
- Chandravanshi, L.P.; Yadav, R.S.; Shukla, R.K.; Singh, A.; Sultana, S.; Pant, A.B.; Parmar, D.; Khanna, V.K. Reversibility of changes in brain cholinergic receptors and acetylcholinesterase activity in rats following early life arsenic exposure. Int. J. Dev. Neurosci. 2014, 34, 60–75. [Google Scholar] [CrossRef]
- Shavali, S.; Sens, D.A. Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2008, 102, 254–261. [Google Scholar] [CrossRef]
- Maull, E.A.; Ahsan, H.; Edwards, J.; Longnecker, M.P.; Navas-Acien, A.; Pi, J.; Silbergeld, E.K.; Styblo, M.; Tseng, C.-H.; Thayer, K.A.; et al. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2012, 120, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Pi, J. Molecular basis for arsenic-Induced alteration in nitric oxide production and oxidative stress: Implication of endothelial dysfunction. Toxicol. Appl. Pharmacol. 2004, 198, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Kaur, J. Arsenic Exposure and Cardiovascular Disorders: An Overview. Cardiovasc. Toxicol. 2009, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lovaković, B.T. Cadmium, arsenic and lead—Elements affecting male reproductive health. Curr. Opin. Toxicol. 2019. [Google Scholar]
- Naranmandura, H.; Xu, S.; Sawata, T.; Hao, W.H.; Liu, H.; Bu, N.; Ogra, Y.; Lou, Y.J.; Suzuki, N. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem. Res. Toxicol. 2011, 24, 1094–1103. [Google Scholar] [CrossRef]
- Bozack, A.K.; Saxena, R.; Gamble, M.V. Nutritional Influences on One-Carbon Metabolism: Effects on Arsenic Methylation and Toxicity. Annu. Rev. Nutr. 2018, 38, 401–429. [Google Scholar] [CrossRef]
- Zakharyan, R.A.; Tsaprailis, G.; Chowdhury, U.K.; Hernandez, A.; Aposhian, H.V. Interactions of sodium selenite, glutathione, arsenic species, and omega class human glutathione transferase. Chem. Res. Toxicol. 2005, 18, 1287–1295. [Google Scholar] [CrossRef]
- Németi, B.; Gregus, Z. Reduction of arsenate to arsenite by human erythrocyte lysate and rat liver cytosol—Characterization of a glutathione—And NAD-dependent arsenate reduction linked to glycolysis. Toxicol. Sci. 2005, 85, 847–858. [Google Scholar] [CrossRef]
- Twaddle, N.C.; Vanlandingham, M.; Beland, F.A.; Doerge, D.R. Metabolism and disposition of arsenic species from controlled dosing with dimethylarsinic acid (DMAV) in adult female CD-1 mice. V. Toxicokinetic studies following oral and intravenous administration. Food Chem. Toxicol. 2019, 130, 22–31. [Google Scholar] [CrossRef]
- Frankel, S.; Concannon, J.; Brusky, K.; Pietrowicz, E.; Giorgianni, S.; Thompson, W.D.; Currie, D.A. Arsenic exposure disrupts neurite growth and complexity in vitro. Neurotoxicology 2009, 30, 529–537. [Google Scholar] [CrossRef]
- Chen, G.; Mao, J.; Zhao, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and the secretion of inflammatory cytokine IL-6 via Akt/NF-κB signaling pathway. Regul. Toxicol. Pharmacol. 2016, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Yao, H.; Zhou, L.; Sun, D.; Wang, J. Neuroglobin involvement in the course of arsenic toxicity in rat cerebellar granule neurons. Biol. Trace Elem. Res. 2013, 155, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Soni, M.; Kumar, V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016, 36, 179–188. [Google Scholar] [CrossRef]
- Kharroubi, W.; Haj Ahmed, S.; Nury, T.; Andreoletti, P.; Sakly, R.; Hammami, M.; Lizard, G. Mitochondrial dysfunction, oxidative stress and apoptotic induction in microglial BV-2 cells treated with sodium arsenate. J. Environ. Sci. 2017, 51, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Weng, S.T.; Tzeng, S.F. Effect of arsenite and arsenate on microglial cell survival. In Arsenic in Geosphere and Human Diseases Arsenic 2010, Proceedings of the Third International Congress on Arsenic in the Environment (As-2010); CRC Press: London, UK, 2010; ISBN 9780415578981. [Google Scholar]
- Selim, S.A.; Selim, A.O.; Askar, E.M. Harmful effects of arsenic on the cerebral cortex of adult male albino rats: Light and electron microscopic studies. Egyptian J. Histol. 2012, 35, 249–258. [Google Scholar] [CrossRef]
- Wang, X.; Meng, D.; Chang, Q.; Pan, J.; Zhang, Z.; Chen, G.; Ke, Z.; Luo, J.; Shi, X. Arsenic Inhibits Neurite Outgrowth by Inhibiting the LKB1–AMPK Signaling Pathway. Environ. Health Perspect. 2010, 118, 627–634. [Google Scholar] [CrossRef]
- Pandey, R.; Rai, V.; Mishra, J.; Mandrah, K.; Kumar Roy, S.; Bandyopadhyay, S. From the Cover: Arsenic Induces Hippocampal Neuronal Apoptosis and Cognitive Impairments via an Up-Regulated BMP2/Smad-Dependent Reduced BDNF/TrkB Signaling in Rats. Toxicol. Sci. 2017, 159, 137–158. [Google Scholar] [CrossRef]
- Chandravanshi, L.P.; Shukla, R.K.; Sultana, S.; Pant, A.B.; Khanna, V.K. Early life arsenic exposure and brain dopaminergic alterations in rats. Int. J. Dev. Neurosci. 2014, 38, 91–104. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Rendón-López, C.R.R.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; Elshenawy, O.H.; El-Sherbeni, A.A.; Abdelrady, M.; El-Kadi, A.O.S. Acute arsenic treatment alters arachidonic acid and its associated metabolite levels in the brain of C57Bl/6 mice. Can. J. Physiol. Pharmacol. 2014, 92, 693–702. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C.; Uresti-Rivera, E.E.; Oliva-González, C.; Torres-Ramos, M.A.; Aguirre-Bañuelos, P.; Gandolfi, A.J. Erratum to: Cortical Astrocytes Acutely Exposed to the Monomethylarsonous Acid (MMAIII) Show Increased Pro-inflammatory Cytokines Gene Expression that is Consistent with APP and BACE-1 Over-expression. Neurochem. Res. 2016, 41, 2573. [Google Scholar] [CrossRef] [PubMed]
- Namgung, U.; Xia, Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol. Appl. Pharmacol. 2001, 174, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Witt, B.; Ebert, F.; Meyer, S.; Francesconi, K.A.; Schwerdtle, T. Assessing neurodevelopmental effects of arsenolipids in pre-differentiated human neurons. Mol. Nutr. Food Res. 2017, 61, 1700199. [Google Scholar] [CrossRef] [PubMed]
- Witt, B.; Meyer, S.; Ebert, F.; Francesconi, K.A.; Schwerdtle, T. Toxicity of two classes of arsenolipids and their water-soluble metabolites in human differentiated neurons. Arch. Toxicol. 2017, 91, 3121–3134. [Google Scholar] [CrossRef]
- Müller, S.M.; Ebert, F.; Raber, G.; Meyer, S.; Bornhorst, J.; Hüwel, S.; Galla, H.-J.; Francesconi, K.A.; Schwerdtle, T. Effects of arsenolipids on in vitro blood-brain barrier model. Arch. Toxicol. 2018, 92, 823–832. [Google Scholar] [CrossRef]
- Peters, R.A.; Stocken, L.A.; Thompson, R.H.S. British Anti-Lewisite (BAL). Nature 1945, 156, 616–619. [Google Scholar] [CrossRef]
- Shila, S.; Subathra, M.; Devi, M.A.; Panneerselvam, C. Arsenic intoxication-induced reduction of glutathione level and of the activity of related enzymes in rat brain regions: Reversal by DL-alpha-lipoic acid. Arch. Toxicol. 2005, 79, 140–146. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Del Razo, L.M.; Limón-Pacheco, J.H.; Giordano, M.; Sánchez-Peña, L.C.; Uribe-Querol, E.; Gutiérrez-Ospina, G.; Gonsebatt, M.E. Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol. Sci. 2005, 84, 157–166. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: London, UK, 2014; pp. 581–624. [Google Scholar]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Chirumbolo, S.; Urbina, M.A.; Uddin, R. Effects of arsenic toxicity beyond epigenetic modifications. Environ. Geochem. Health 2018, 40, 955–965. [Google Scholar] [CrossRef]
- Ebert, F.; Weiss, A.; Bültemeyer, M.; Hamann, I.; Hartwig, A.; Schwerdtle, T. Arsenicals affect base excision repair by several mechanisms. Mutat. Res.-Fund. Mol. M 2011, 715, 32–41. [Google Scholar] [CrossRef]
- Holcomb, N.; Goswami, M.; Han, S.G.; Scott, T.; D’Orazio, J.; Orren, D.K.; Gairola, C.G.; Mellon, I. Inorganic arsenic inhibits the nucleotide excision repair pathway and reduces the expression of XPC. DNA Repair 2017, 52, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Metal interaction with redox regulation: An integrating concept in metal carcinogenesis? Free Rad. Biol. Med. 2013, 55, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nollen, M.; Ebert, F.; Moser, J.; Mullenders, L.H.F.; Hartwig, A.; Schwerdtle, T. Impact of arsenic on nucleotide excision repair: XPC function, protein level, and gene expression. Mol. Nutr. Food Res. 2009, 53, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.F.; Li, Z.; Sayarath, V.; Palys, T.J.; Morse, K.R.; Scholz-Bright, R.A.; Karagas, M.R. Distinct arsenic metabolites following seaweed consumption in humans. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J. Recent advance in the therapy of metal poisonings with chelating agents. Hum. Toxicol. 1983, 2, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef]
- Andersen, O.; Aaseth, J. Molecular mechanisms of in vivo metal chelation: Implications for clinical treatment of metal intoxications. Environ. Health Perspect. 2002, 110 (Suppl. 5), 887–890. [Google Scholar] [CrossRef]
- Ding, G.S.; Liang, Y.Y. Antidotal effects of dimercaptosuccinic acid. J. Appl. Toxicol. 1991, 11, 7–14. [Google Scholar] [CrossRef]
- Oginski, M. Use of Unitiol for speeding up renal excretion of chlormerodrin 203 Hg. Int. Urol. Nephrol. 1971, 3, 203–208. [Google Scholar] [CrossRef]
- Friedheim, E.A.; Da Silva, J.R.; Martins, A.V. Treatment of schistosomiasis mansoni with antimony-omega, omega-dimercapto-potassium succinate (TWSb). Am. J. Trop. Med. Hyg. 1954, 3, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Frieheim, E.A. Treatment of methyl mercury poisoning in mice with 2,3-dimercaptosuccinic acid and other complexing thiols. Acta Pharmacol. Toxicol. (Copenh) 1978, 42, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Aaseth, J. Guidance for clinical treatment of metal poisonings—Use and misuse of chelating agents. In Chelation Therapy in Treatment of Metal Intoxication; Aaseth, J., Crisponi, G., Andersen, O., Eds.; Elsevier: London, UK, 2016; pp. 313–341. [Google Scholar]
- Aaseth, J.; Ajsuvakova, O.P.; Skalny, A.V.; Skalnaya, M.G.; Tinkov, A.A. Chelator combination as therapeutic strategy in mercury and lead poisonings. Coord. Chem. Rev. 2018, 358, 1–12. [Google Scholar] [CrossRef]
- Moore, D.F.; O’Callaghan, C.A.; Berlyne, G.; Ogg, C.S.; Davies, H.A.; House, I.M.; Henry, J.A. Acute arsenic poisoning: Absence of polyneuropathy after treatment with 2,3-dimercaptopropanesulphonate (DMPS). J. Neurol. Neurosurg. Psychiatry 1994, 57, 1133–1135. [Google Scholar] [CrossRef]
- Wax, P.M.; Thornton, C.A. Recovery from severe arsenic-induced peripheral neuropathy with 2,3-dimercapto-1-propanesulphonic acid. J. Toxicol. Clin. Toxicol. 2000, 38, 777–780. [Google Scholar] [CrossRef]
- Vantroyen, B.; Heilier, J.F.; Meulemans, A.; Michels, A.; Buchet, J.P.; Vanderschueren, S.; Haufroid, V.; Sabbe, M. Survival after a lethal dose of arsenic trioxide. J. Toxicol. Clin. Toxicol. 2004, 42, 889–895. [Google Scholar] [CrossRef]
- Aaseth, J.; Crisponi, G.; Anderson, O. Chelation Therapy in the Treatment of Metal Intoxication; Elsevier: London, UK, 2016; pp. 85–252. [Google Scholar]
- Guha Mazumder, D.N.; De, B.K.; Santra, A.; Ghosh, N.; Das, S.; Lahiri, S.; Das, T. Randomized placebo-controlled trial of 2,3-dimercapto-1-propanesulfonate (DMPS) in therapy of chronic arsenicosis due to drinking arsenic-contaminated water. J. Toxicol. Clin. Toxicol. 2001, 39, 665–674. [Google Scholar] [CrossRef]
- Stenehjem, A.-E.; Vahter, M.; Nermell, B.; Aasen, J.; Lierhagen, S.; Mørland, J.; Jacobsen, D. Slow recovery from severe inorganic arsenic poisoning despite treatment with DMSA (2.3-dimercaptosuccinic acid). Clin. Toxicol. 2007, 45, 424–428. [Google Scholar] [CrossRef]
- Spallholz, J.; Malloryboylan, L.; Rhaman, M. Environmental hypothesis: Is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India? Sci. Tot. Environ. 2004, 323, 21–32. [Google Scholar] [CrossRef]
- Chen, Y.; Hall, M.; Graziano, J.H.; Slavkovich, V.; van Geen, A.; Parvez, F.; Ahsan, H. A Prospective Study of Blood Selenium Levels and the Risk of Arsenic-Related Premalignant Skin Lesions. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 207–213. [Google Scholar] [CrossRef]
- Zeng, H.; Uthus, E.O.; Combs, G.F., Jr. Mechanistic aspects of the interaction between selenium and arsenic. J. Inorg. Biochem. 2005, 99, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Canty, A.J.; Reid, R.S.; Rabenstein, D.L. Nuclear magnetic resonance and potentiometric studies of the complexation of methylmercury(II) by dithiols. Can. J. Chem. 1985, 63, 2430–2436. [Google Scholar] [CrossRef]
- Carla Aragoni, M.; Arca, M.; Crisponi, G.; Cristiani, F.; Isaia, F.; Nurchi, V.M. Characterization of the ionization and spectral properties of mercapto-carboxylic acids Correlation with substituents and structural features. Talanta 1996, 43, 1357–1366. [Google Scholar] [CrossRef]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Buha Djordjevic, A.; Aaseth, J. A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef]
- Bonomi, F.; Pagani, S.; Cariati, F.; Pozzi, A.; Crisponi, G.; Cristiani, F.; Diaz, A.; Zanoni, R. Synthesis and characterization of metal derivatives of dihydrolipoic acid and dihydrolipoamide. Inorganica Chim. Acta 1992, 192, 237–242. [Google Scholar] [CrossRef]
- Dill, K.; Hu, S.; O’Connor, R.J.; McGown, E.L. Preparation, structure, and solution dynamics of phenyldichloroarsine-thio sugar adducts. Carbohydr. Res. 1990, 196, 141–146. [Google Scholar] [CrossRef]
- O’Connor, R.J.; McGown, E.L.; Dill, K.; Hallowell, S.F. Two-dimensional NMR studies of arsenical-sulfhydryl adducts. Magn. Reson. Chem. 1989, 27, 669–675. [Google Scholar] [CrossRef]
- Adams, E.; Jeter, D.; Cordes, A.W.; Kolis, J.W. Chemistry of organometalloid complexes with potential antidotes: Structure of an organoarsenic(III) dithiolate ring. Inorg. Chem. 1990, 29, 1500–1503. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Carter, N.S.; Cunningham, M.; Smith, K. Characterisation of melarsen-resistant Trypanosoma brucei brucei with respect to cross-resistance to other drugs and trypanothione metabolism. Mol. Biochem. Parasit. 1992, 53, 213–222. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Henderson, G.B.; Cerami, A. Trypanothione is the primary target for arsenical drugs against African trypanosomes. Proc. Natl. Acad. Sci. USA 1989, 86, 2607–2611. [Google Scholar] [CrossRef]
- Von Döllen, A.; Strasdeit, H. Models for the Inhibition of Dithiol-Containing Enzymes by Organoarsenic Compounds: Synthetic Routes and the Structure of [PhAs(HlipS2)] (HlipS22− = Reduced Lipoic Acid). Eur. J. Inorg. Chem. 1998, 1998, 61–66. [Google Scholar] [CrossRef]
- Spuches, A.M.; Kruszyna, H.G.; Rich, A.M.; Wilcox, D.E. Thermodynamics of the As(III)−Thiol Interaction: Arsenite and Monomethylarsenite Complexes with Glutathione, Dihydrolipoic Acid, and Other Thiol Ligands. Inorg. Chem. 2005, 44, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Cavanillas, S.; Chekmeneva, E.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Electroanalytical and isothermal calorimetric study of As(III) complexation by the metal poisoning remediators, 2,3-dimercapto-1-propanesulfonate and meso-2,3-dimercaptosuccinic acid. Anal. Chim. Acta 2012, 746, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.K.; Bayse, C.A. Modeling the chelation of As(III) in lewisite by dithiols using density functional theory and solvent-assisted proton exchange. J. Inorg. Biochem. 2015, 153, 60–67. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).