Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ionic Liquids Synthesis
2.3. Cell Culture
2.4. MTT Assay
2.5. Solubility Studies
2.6. Flow Cytometric Analysis of DNA Cell Cycle
2.7. Production of the IL-Nanoparticles Hybrid System
2.8. Particle Size, Polydispersity Index and Zeta Potential Analysis
2.9. Association Efficiency and Loading Capacity of Rutin
2.10. In Vitro Release Study
2.11. Statistical Analyses
3. Results
3.1. Synthesis of ILs
3.2. Cell Viability of Renal Cells
3.2.1. Effect of Rutin on the Viability of Renal Cells
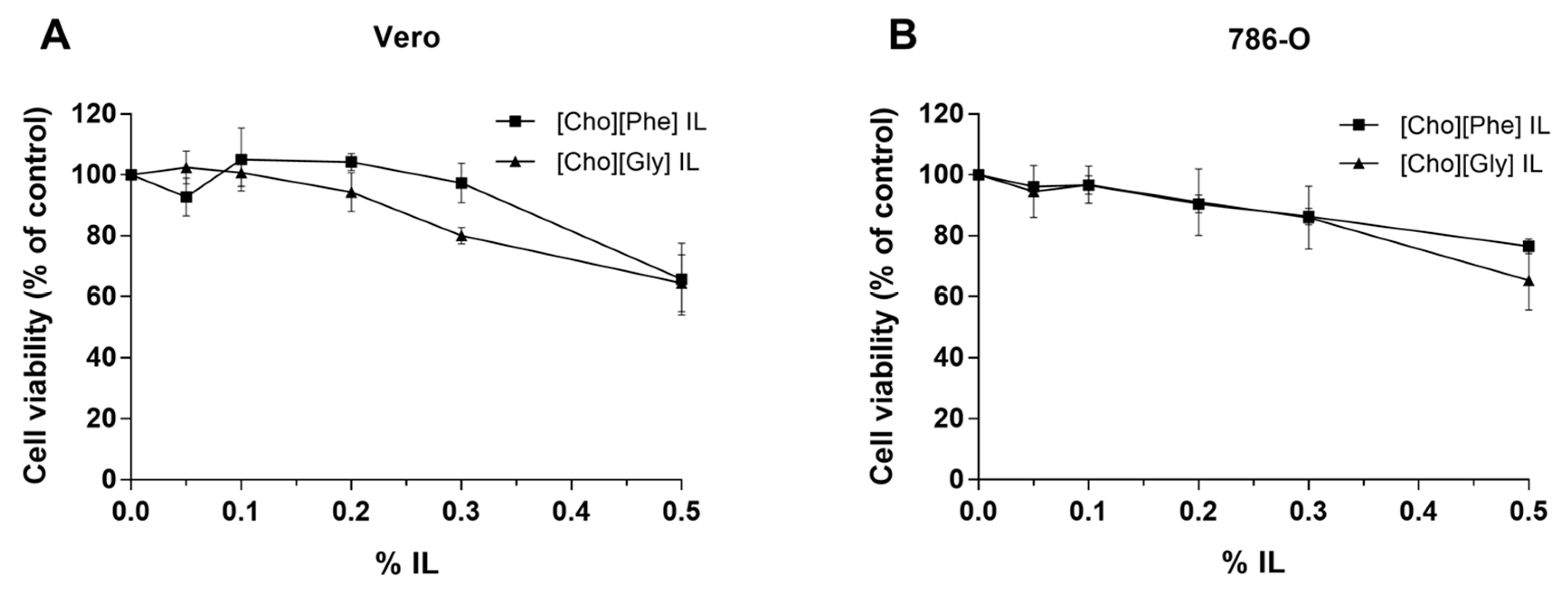
3.2.2. Effect of the Two Choline-amino Acid ILs on the Viability of Renal Cells
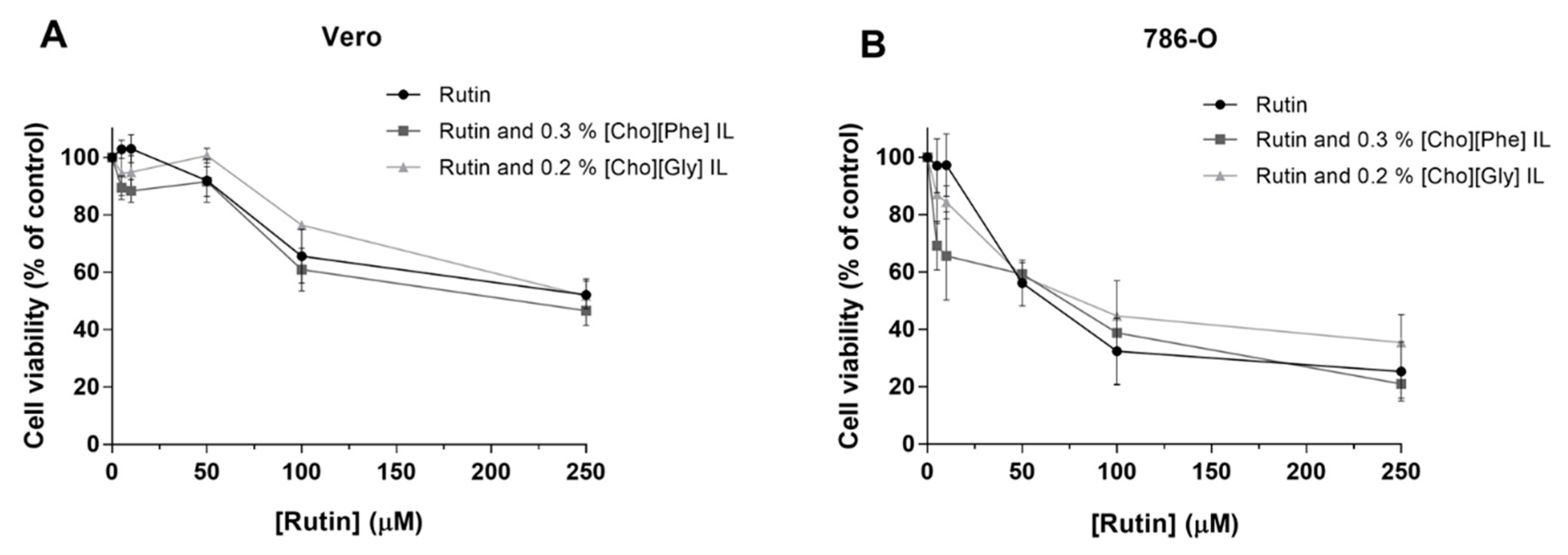
3.2.3. Impact of the Co-treatment of Rutin with ILs on the Viability of Renal Cells
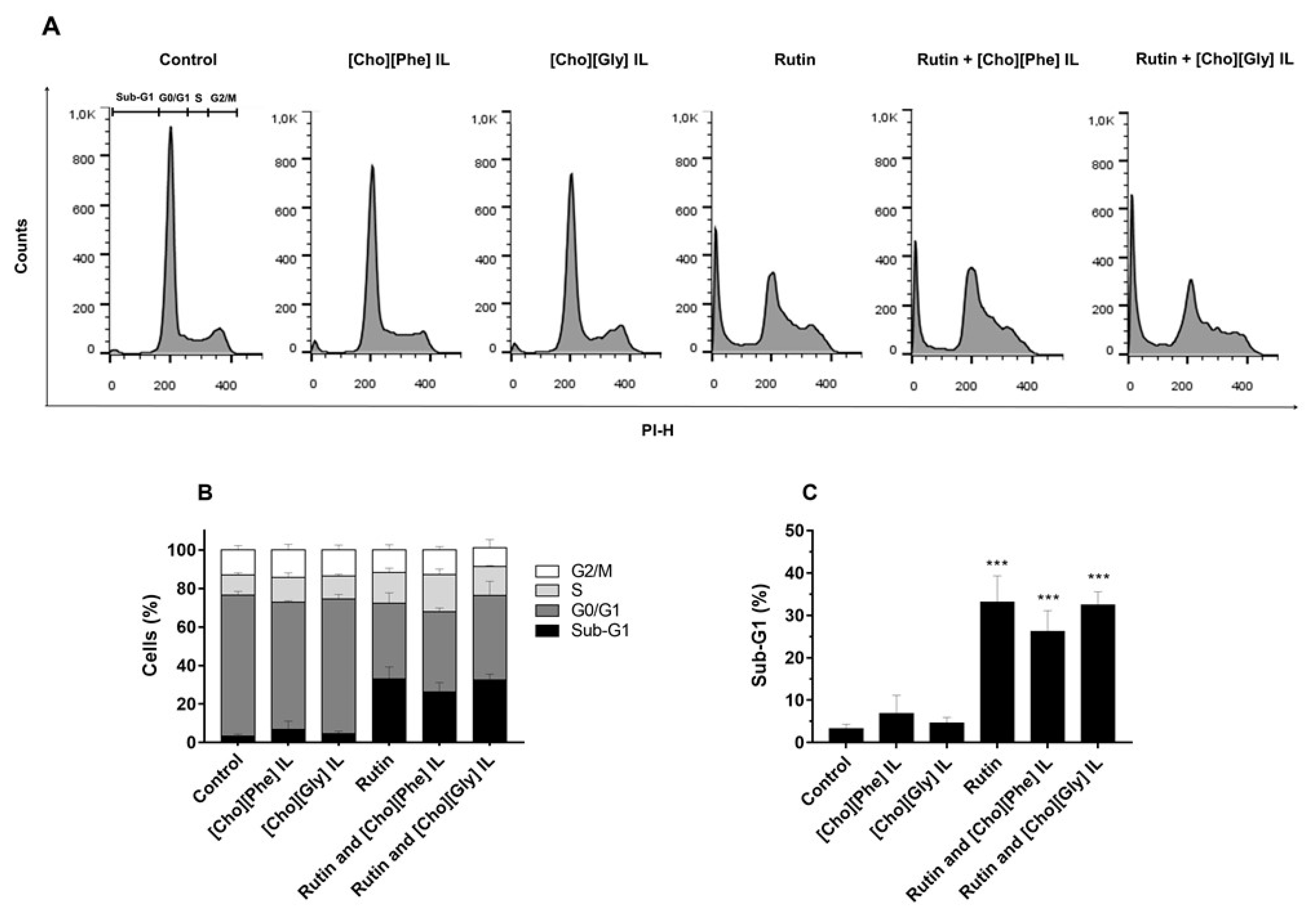
3.3. Cell Cycle Distribution of 786-O Cells Treated with Rutin Individually and in Combination with ILs
3.4. Solubility of Rutin in the Presence of Choline-amino Acid ILs
3.5. Ionic Liquids-Nanoparticles Hybrid Systems Containing Rutin
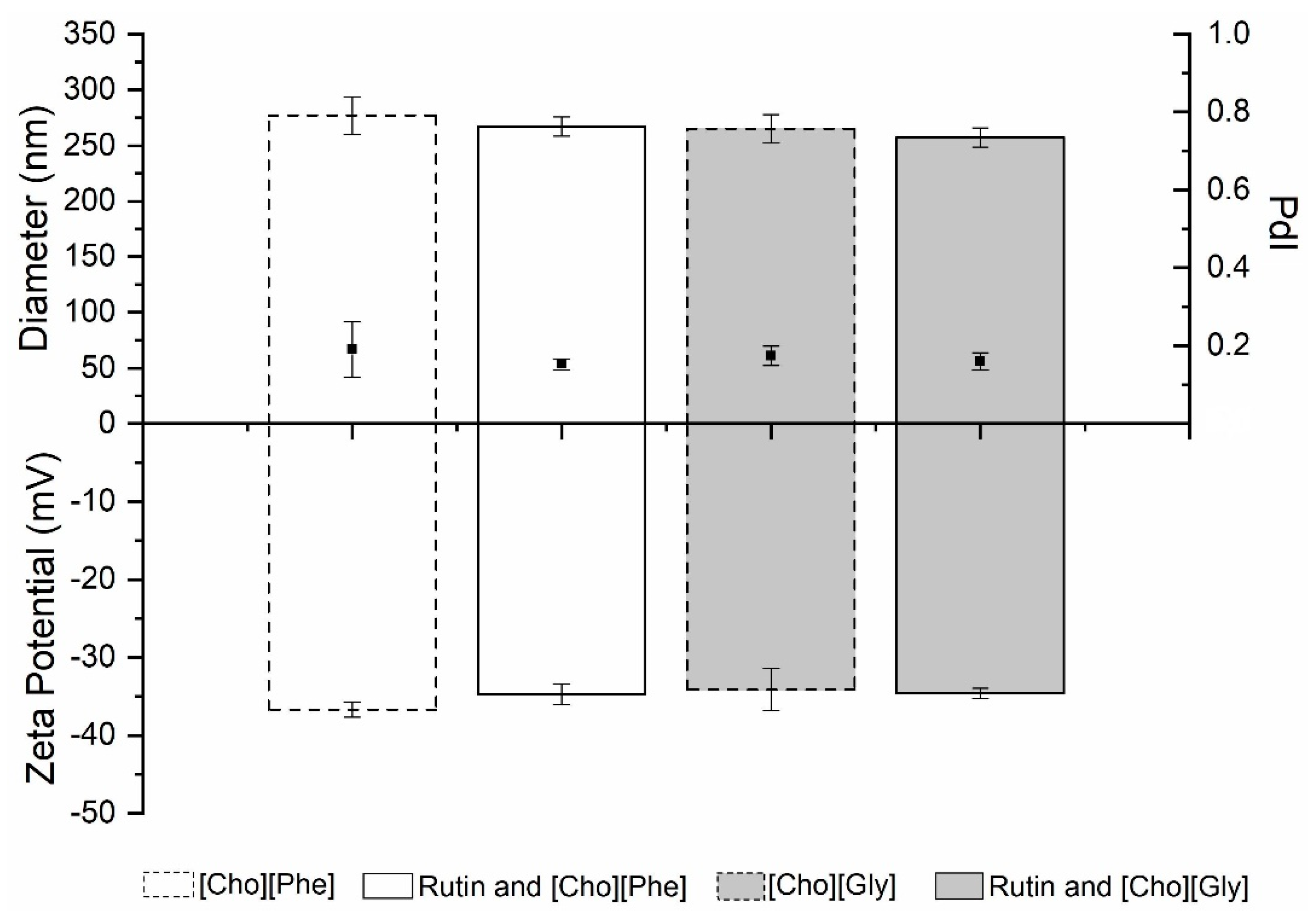
3.5.1. Particle Size, Polydispersity Index and Zeta Potential Analysis
3.5.2. Association Efficiency (AE) and Loading Capacity (LC) of Rutin
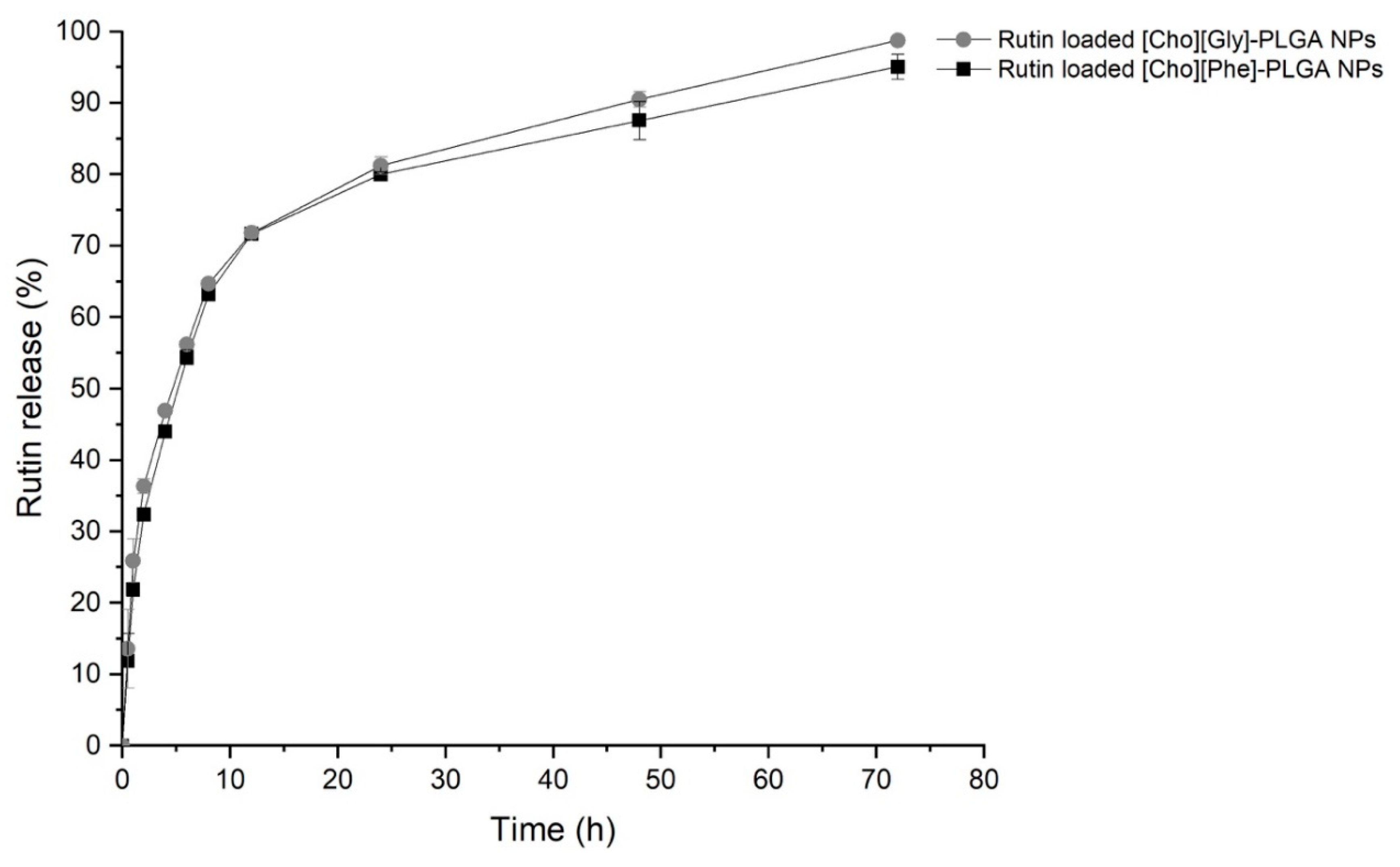
3.5.3. In Vitro Release Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nabi, S.; Kessler, E.R.; Bernard, B.; Flaig, T.W.; Lam, E.T. Renal cell carcinoma : A review of biology and pathophysiology. F1000Research 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.-J.; Chen, S.-C.; Yen, M.-C.; Wu, Y.-H.; Hung, C.-H.; Kuo, P.-L. 6-Shogaol Suppresses 2-Amino-1-Methyl-6- Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients 2019, 11, 2306. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Li, J.; Wang, J.; Chang, C.; Wu, C.; Chen, W.; Kuan, Y.; Liao, S.; Lu, H.; Chen, C. Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells. Mol. Sci. 2019, 20, 2792. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liu, Z.; Liu, J.; Wang, H.; Huang, L.; Lin, T.; Liu, J.; Wei, Q.; Zeng, H.A.O.; He, G.U.; et al. Expression and epigenetic regulatory mechanism of BNIP3 in clear cell renal cell carcinoma. Int. J. Oncol. 2019, 54, 348–360. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, P.-N.; Hsu, L.-S.; Kuo, D.Y.K.; Chu, S.-C.; Hsieh, Y.-S.H. Inhibition of the invasion and migration of renal carcinoma 786-o-si3 cells in vitro and in vivo by Koelreuteria formosana extract. Mol. Med. Rep. 2014, 3334–3342. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Batinic-Haberle, I.; Castro, M.; Oliveira, N.G.; Fernandes, A.S. The SOD Mimic MnTnHex-2-PyP 5 + Reduces the Viability and Migration of 786-O Human Renal Cancer Cells. Antioxidants 2019, 8, 490. [Google Scholar] [CrossRef]
- Cristina Marcarini, J.; Ferreira Tsuboy, M.S.; Cabral Luiz, R.; Regina Ribeiro, L.; Beatriz Hoffmann-Campo, C.; Ségio Mantovani, M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic Interaction of Rutin and Silibinin on Human Colon Cancer Cell Line. Arch. Med. Res. 2018. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phyther. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Martínez-García, D.; Pérez-Hernández, M.; Korrodi-Gregório, L.; Quesada, R.; Ramos, R.; Baixeras, N.; Pérez-Tomás, R.; Soto-Cerrato, V. The Natural-Based Antitumor Compound T21 Decreases Survivin Levels through Potent STAT3 Inhibition in Lung Cancer Models. Biomolecules 2019, 9, 361. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids : New Frontier for Immuno-Regulation and Breast Cancer Control. antioxidants 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-rad, J.; Ozleyen, A.; Tumer, T.B.; Adetunji, C.O.; Omari, N.E.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, B.A.; Adineh, M.; Hajiali, F.; Nassiri-asl, M. Treatment with Rutin-A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases. J. Pharmacopuncture 2017, 52–56. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant : Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bispo, A.; Coelho, P.L.C.; Oliveira, M.d.N.; Oliveira, J.L.; Amparo, J.A.O.; Costa da Silva, K.; Soares, J.R.P.; Pitanga, B.P.S.; Souza, C.d.S.; Lopes, G.P.d.F.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2019. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Koja, E.; Ohata, S.; Maruyama, Y.; Suzuki, H.; Shimosaka, M.; Taguchi, G. Identification and characterization of a rhamnosyltransferase involved in rutin biosynthesis in fagopyrum esculentum (common buckwheat). Biosci. Biotechnol. Biochem. 2018, 82, 1790–1802. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-Tumor Effect of Rutin on Human Neuroblastoma Cell Lines through Inducing G2 / M Cell Cycle Arrest and Promoting Apoptosis. Sci. Worl J. 2013. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-mytsyk, I.; Gerçek, Y.C.; Bozta, K.; Yazgan, M.; Fayyaz, S. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Yang, C.Y.; Hsiu, S.L.; Wen, K.C.; Lin, S.P.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D.L. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005, 13, 244–250. [Google Scholar]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; Tânia, S.d.A. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Santos de Almeida, T.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Rosado, C.; Santos, T. Applicability of Ionic Liquids in Topical Drug Delivery Systems : A Mini Review. J. Pharmacol. Clin. Res. 2018, 4. [Google Scholar] [CrossRef]
- Rosado, C.; Caparica, R.; Mineiro, R.; Raposo, M.; Júlio, A.; M. Araújo, M.E.; Fonte, P.; Santos de Almeida, T.; Antunes, C. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018, 15, 96–102. [Google Scholar] [CrossRef]
- Lee, J.Y.; Selfridge, K.M.; Kohn, E.M.; Vaden, T.D.; Caputo, G.A. Effects of Ionic Liquid Alkyl Chain Length on Denaturation of Myoglobin by Anionic, Cationic, and Zwitterionic Detergents. Biomolecules 2019, 9, 264. [Google Scholar] [CrossRef]
- Balk, A.; Holzgrabe, U.; Meinel, L. Pro et contra’ ionic liquid drugs-Challenges and opportunities for pharmaceutical translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Hidalgo, J.M.; Collado-González, M.; Díaz Baños, F.G.; Víllora, G. Assessing chemical toxicity of ionic liquids on Vibrio fischeri: Correlation with structure and composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E. Studies on thermal stability of amino acid ionic liquids. Chemik 2016, 70, 483–484. [Google Scholar]
- Quraish, K.S.; Bustam, M.A.; Krishnan, S.; Aminuddin, N.F.; Azeezah, N.; Ghani, N.A.; Uemura, Y.; Lévêque, J.M. Ionic liquids toxicity on fresh water microalgae, Scenedesmus quadricauda, Chlorella vulgaris & Botryococcus braunii; selection criterion for use in a two-phase partitioning bioreactor (TPPBR). Chemosphere 2017, 642–651. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Júlio, A.; Caparica, R.; Lima, S.A.C.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos de Almeida, T. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.S.; Corvacho, E.; Costa, J.G.; Saraiva, N.; Fernandes, A.S.; Castro, M.; Miranda, J.P.; Oliveira, N.G. The APE1 redox inhibitor E3330 reduces collective cell migration of human breast cancer cells and decreases chemoinvasion and colony formation when combined with docetaxel. Chem. Biol. Drug Des. 2017, 90, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Júlio, A.; Lima, S.A.C.; Reis, S.; De Almeida, T.S.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Bex, A.; Khoo, V.; Gruenvald, V.; Horwich, A. Renal cell carcinoma : ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Sghaier, M.B.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Silva, M.J.; Miranda, J.P.; Castro, M.; Batinic-haberle, I.; Fernandes, A.S.; Nuno, G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2015. [Google Scholar] [CrossRef]
- Hasani, N.A.H.; Amin, I.M.; Kamaludin, R.; Rosdy, N.M.M.N.M.; Ibahim, M.J.; Kadir, S.H.S.A. p53 and CYCLIN B1 MEDIATE APOPTOTIC EFFECTS OF A PIGENIN AND RUTIN IN ERa + - BREAST CANCER MCF-7. J. Teknol. 2018, 1, 133–140. [Google Scholar] [CrossRef]
- Santos, A.G.; Ribeiro, B.D.; Alviano, D.S.; Coelho, M.A.Z. Toxicity of ionic liquids toward microorganisms interesting to the food industry. RSC Adv. 2014, 4, 37157–37163. [Google Scholar] [CrossRef]
- Radošević, K.; Železnjak, J.; Cvjetko Bubalo, M.; Radojčić Redovniković, I.; Slivac, I.; Gaurina Srček, V. Comparative in vitro study of cholinium-based ionic liquids and deep eutectic solvents toward fish cell line. Ecotoxicol. Environ. Saf. 2016, 131, 30–36. [Google Scholar] [CrossRef] [PubMed]
- He, M.H.; Chen, L.; Zheng, T.; Tu, Y.; He, Q.; Fu, H.L.; Lin, J.C.; Zhang, W.; Shu, G.; He, L.; et al. Potential applications of nanotechnology in urological cancer. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Rodrigues de Azevedo, C.; Von Stosch, M.; Costa, M.S.; Ramos, A.M.; Cardoso, M.M.; Danhier, F.; Préat, V.; Oliveira, R. Modeling of the burst release from PLGA micro - and nanoparticles as function of physicochemical parameters and formulation characteristics. Int. J. Pharm. 2017. [Google Scholar] [CrossRef]

| Solvent | Water:IL Ratio (%) | Rutin Concentration (mg/ mL) |
|---|---|---|
| Water | 100:0.00 | 0.19 ± 0.01 |
| Water:[Cho][Phe] | 99.7:0.30 | 2.49 ± 0.12 *** |
| Water:[Cho][Gly] | 99.8:0.20 | 1.73 ± 0.03 *** |
| IL | AE (%) | LC (%) |
|---|---|---|
| [Cho][Phe] | 84.5 ± 0.3 | 1.0 ± 0.1 |
| [Cho][Gly] | 84.7 ± 0.3 | 1.1 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules 2020, 10, 233. https://doi.org/10.3390/biom10020233
Caparica R, Júlio A, Araújo MEM, Baby AR, Fonte P, Costa JG, Santos de Almeida T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules. 2020; 10(2):233. https://doi.org/10.3390/biom10020233
Chicago/Turabian StyleCaparica, Rita, Ana Júlio, Maria Eduarda Machado Araújo, André Rolim Baby, Pedro Fonte, João Guilherme Costa, and Tânia Santos de Almeida. 2020. "Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells" Biomolecules 10, no. 2: 233. https://doi.org/10.3390/biom10020233
APA StyleCaparica, R., Júlio, A., Araújo, M. E. M., Baby, A. R., Fonte, P., Costa, J. G., & Santos de Almeida, T. (2020). Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules, 10(2), 233. https://doi.org/10.3390/biom10020233











