Mechanisms and Alterations of Cardiac Ion Channels Leading to Disease: Role of Ankyrin-B in Cardiac Function
Abstract
1. Introduction: Ankyrin Proteins
2. Ankyrin-B Isoforms
3. Ankyrin-B Structure and Binding Partners
4. Ankyrin-B Variants in Cardiovascular Disease
5. Future Implications
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, V.; Stenbuck, P.J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J. Biol. Chem. 1979, 254, 2533–2541. [Google Scholar] [PubMed]
- Bennett, V.; Davis, J.; Fowler, W.E. Immunoreactive forms of erythrocyte spectrin and ankyrin in brain. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 1982, 299, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.; Davis, J. Spectrin and ankyrin in brain. Cell Motil. 1983, 3, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.Q.; Bennett, V. Brain ankyrin. Purification of a 72,000 Mr spectrin-binding domain. J. Biol. Chem. 1984, 259, 1874–1881. [Google Scholar]
- Moon, R.T.; Ngai, J.; Wold, B.J.; Lazarides, E. Tissue-specific expression of distinct spectrin and ankyrin transcripts in erythroid and nonerythroid cells. J. Cell Biol. 1985, 100, 152–160. [Google Scholar] [CrossRef]
- Nelson, W.J.; Lazarides, E. Posttranslational control of membrane-skeleton (ankyrin and alpha beta-spectrin) assembly in early myogenesis. J. Cell Biol. 1985, 100, 1726–1735. [Google Scholar] [CrossRef]
- Otto, E.; Kunimoto, M.; McLaughlin, T.; Bennett, V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J. Cell Biol. 1991, 114, 241–253. [Google Scholar] [CrossRef]
- Davis, J.Q.; Bennett, V. Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J. Biol. Chem. 1984, 259, 13550–13559. [Google Scholar]
- Lambert, S.; Yu, H.; Prchal, J.T.; Lawler, J.; Ruff, P.; Speicher, D.; Cheung, M.C.; Kan, Y.W.; Palek, J. cDNA sequence for human erythrocyte ankyrin. Proc. Natl. Acad. Sci. USA 1990, 87, 1730–1734. [Google Scholar] [CrossRef]
- Ho, T.S.-Y.; Zollinger, D.R.; Chang, K.-J.; Xu, M.; Cooper, E.C.; Stankewich, M.C.; Bennett, V.; Rasband, M.N. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat. Neurosci. 2014, 17, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, E.; Szentesi, P.; Al-Gaadi, D.; Oláh, T.; Dienes, B.; Sztretye, M.; Rossi, D.; Sorrentino, V.; Csernoch, L. Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice. Int. J. Mol. Sci. 2019, 20, 3361. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Mohler, P.J. Coordinating electrical activity of the heart: Ankyrin polypeptides in human cardiac disease. Expert Opin. Targets 2011, 15, 789–801. [Google Scholar] [CrossRef]
- Cunha, S.R.; Mohler, P.J. Ankyrin-based cellular pathways for cardiac ion channel and transporter targeting and regulation. Semin. Cell Dev. Biol. 2011, 22, 166–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makara, M.A.; Curran, J.; Little, S.C.; Musa, H.; Polina, I.; Smith, S.A.; Wright, P.J.; Unudurthi, S.D.; Snyder, J.; Bennett, V.; et al. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ. Res. 2014, 115, 929–938. [Google Scholar] [CrossRef]
- Kalomiris, E.L.; Bourguignon, L.Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J. Cell Biol. 1988, 106, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Veshnock, P.J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 1987, 328, 533–536. [Google Scholar] [CrossRef]
- Nicolas, V.; Le Van Kim, C.; Gane, P.; Birkenmeier, C.; Cartron, J.-P.; Colin, Y.; Mouro-Chanteloup, I. Rh-RhAG/Ankyrin-R, a New Interaction Site between the Membrane Bilayer and the Red Cell Skeleton, Is Impaired by Rhnull-associated Mutation. J. Biol. Chem. 2003, 278, 25526–25533. [Google Scholar] [CrossRef]
- Skogestad, J.; Aronsen, J.M.; Tovsrud, N.; Wanichawan, P.; Hougen, K.; Stokke, M.K.; Carlson, C.R.; Sjaastad, I.; Sejersted, O.M.; Swift, F. Coupling of the Na+/K+-ATPase to Ankyrin B controls Na+/Ca2+ exchanger activity in cardiomyocytes. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Wolf, R.M.; Glynn, P.; Hashemi, S.; Zarei, K.; Mitchell, C.C.; Anderson, M.E.; Mohler, P.J.; Hund, T.J. Atrial fibrillation and sinus node dysfunction in human ankyrin-B syndrome: A computational analysis. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1253–H1266. [Google Scholar] [CrossRef]
- Smith, S.A.; Sturm, A.C.; Curran, J.; Kline, C.F.; Little, S.C.; Bonilla, I.M.; Long, V.P.; Makara, M.; Polina, I.; Hughes, L.D.; et al. Dysfunction in the βII spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation 2015, 131, 695–708. [Google Scholar] [CrossRef]
- Kordeli, E.; Lambert, S.; Bennett, V. Ankyrin: A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of ranvier. J. Biol. Chem. 1995, 270, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.M.; Bennett, V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001, 155, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Sato, P.Y.; Coombs, W.; Lin, X.; Nekrasova, O.; Green, K.J.; Isom, L.L.; Taffet, S.M.; Delmar, M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ. Res. 2011, 109, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Yamankurt, G.; Luo, J.; Subramaniam, J.; Hashmi, S.S.; Hu, H.; Cunha, S.R. Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovasc. Res. 2015, 107, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, M.; Otto, E.; Bennett, V. A new 440-kD isoform is the major ankyrin in neonatal rat brain. J. Cell Biol. 1991, 115, 1319–1331. [Google Scholar] [CrossRef]
- Perrotta, S.; Gallagher, P.G.; Mohandas, N. Hereditary spherocytosis. Lancet (Lond. Engl.) 2008, 372, 1411–1426. [Google Scholar] [CrossRef]
- Roberts, J.D.; Murphy, N.P.; Hamilton, R.M.; Lubbers, E.R.; James, C.A.; Kline, C.F.; Gollob, M.H.; Krahn, A.D.; Sturm, A.C.; Musa, H.; et al. Ankyrin-B dysfunction predisposes to arrhythmogenic cardiomyopathy and is amenable to therapy. J. Clin. Investig. 2019, 129, 3171–3184. [Google Scholar] [CrossRef]
- Makara, M.A.; Curran, J.; Lubbers, E.R.; Murphy, N.P.; Little, S.C.; Musa, H.; Smith, S.A.; Unudurthi, S.D.; Rajaram, M.V.S.; Janssen, P.M.L.; et al. Novel Mechanistic Roles for Ankyrin-G in Cardiac Remodeling and Heart Failure. Jacc Basic Transl. Sci. 2018, 3, 675–689. [Google Scholar] [CrossRef]
- Iqbal, Z.; Vandeweyer, G.; van der Voet, M.; Waryah, A.M.; Zahoor, M.Y.; Besseling, J.A.; Roca, L.T.; Vulto-van Silfhout, A.T.; Nijhof, B.; Kramer, J.M.; et al. Homozygous and heterozygous disruptions of ANK3: At the crossroads of neurodevelopmental and psychiatric disorders. Hum. Mol. Genet. 2013, 22, 1960–1970. [Google Scholar] [CrossRef]
- Bennett, V.; Stenbuck, P.J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature 1979, 280, 468–473. [Google Scholar] [CrossRef]
- Narla, J.; Mohandas, N. Red cell membrane disorders. Int. J. Lab. Hematol. 2017, 39, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Satchwell, T.J.; Bell, A.J.; Hawley, B.R.; Pellegrin, S.; Mordue, K.E.; van Deursen, C.T.B.M.; Braak, N.H.-T.; Huls, G.; Leers, M.P.G.; Overwater, E.; et al. Severe Ankyrin-R deficiency results in impaired surface retention and lysosomal degradation of RhAG in human erythroblasts. Haematologica 2016, 101, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.; Baines, A.J. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001, 81, 1353–1392. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Mohler, P.J. Alternative paradigms for ion channelopathies: Disorders of ion channel membrane trafficking and posttranslational modification. Annu. Rev. Physiol. 2015, 77, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.M.; Kim, N.; Jones, S.L.; Tseng, W.C.; Svitkina, T.M.; Yin, H.H.; Bennett, V. Giant ankyrin-G: A critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Camors, E.; Mohler, P.J.; Bers, D.M.; Despa, S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J. Mol. Cell Cardiol. 2012, 52, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Kashef, F.; Li, J.; Wright, P.; Snyder, J.; Suliman, F.; Kilic, A.; Higgins, R.S.D.; Anderson, M.E.; Binkley, P.F.; Hund, T.J.; et al. Ankyrin-B protein in heart failure: Identification of a new component of metazoan cardioprotection. J. Biol. Chem. 2012, 287, 30268–30281. [Google Scholar] [CrossRef]
- He, M.; Tseng, W.-C.; Bennett, V. A single divergent exon inhibits ankyrin-B association with the plasma membrane. J. Biol. Chem. 2013, 288, 14769–14779. [Google Scholar] [CrossRef]
- Yang, R.; Walder-Christensen, K.K.; Kim, N.; Wu, D.; Lorenzo, D.N.; Badea, A.; Jiang, Y.-H.; Yin, H.H.; Wetsel, W.C.; Bennett, V. ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc. Natl. Acad. Sci. USA 2019, 116, 15262–15271. [Google Scholar] [CrossRef]
- Koenig, S.N.; Mohler, P.J. The evolving role of ankyrin-B in cardiovascular disease. Heart Rhythm 2017, 14, 1884–1889. [Google Scholar] [CrossRef]
- El Refaey, M.M.; Mohler, P.J. Ankyrins and Spectrins in Cardiovascular Biology and Disease. Front. Physiol. 2017, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Greenstein, J.L.; Winslow, R.L. Na+ microdomains and sparks: Role in cardiac excitation-contraction coupling and arrhythmias in ankyrin-B deficiency. J. Mol. Cell Cardiol. 2019, 128, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Michaely, P.; Tomchick, D.R.; Machius, M.; Anderson, R.G.W. Crystal structure of a 12 ANK repeat stack from human ankyrinR. Embo. J. 2002, 21, 6387–6396. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C.; Ye, F.; Wei, Z.; Zhang, M. Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proc. Natl. Acad. Sci. USA 2012, 109, 4822–4827. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, C.; Hu, J.; Wan, R.; Yu, J.; Xie, J.; Ma, J.; Guo, L.; Ge, J.; Qiu, Y.; et al. Ankyrin-B Q1283H Variant Linked to Arrhythmias Via Loss of Local Protein Phosphatase 2A Activity Causes Ryanodine Receptor Hyperphosphorylation. Circulation 2018, 138, 2682–2697. [Google Scholar] [CrossRef]
- Qu, F.; Lorenzo, D.N.; King, S.J.; Brooks, R.; Bear, J.E.; Bennett, V. Ankyrin-B is a PI3P effector that promotes polarized α5β1-integrin recycling via recruiting RabGAP1L to early endosomes. Elife 2016, 5, e20417. [Google Scholar] [CrossRef]
- Kontrogianni-Konstantopoulos, A.; Bloch, R.J. Obscurin: A multitasking muscle giant. J. Muscle Res. Cell Motil. 2005, 26, 419–426. [Google Scholar] [CrossRef]
- Hall, T.G.; Bennett, V. Regulatory domains of erythrocyte ankyrin. J. Biol. Chem. 1987, 262, 10537–10545. [Google Scholar]
- Davis, L.H.; Davis, J.Q.; Bennett, V. Ankyrin regulation: An alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J. Biol. Chem. 1992, 267, 18966–18972. [Google Scholar]
- Lopes, L.R.; Syrris, P.; Guttmann, O.P.; O’Mahony, C.; Tang, H.C.; Dalageorgou, C.; Jenkins, S.; Hubank, M.; Monserrat, L.; McKenna, W.J.; et al. Novel genotype-phenotype associations demonstrated by high-throughput sequencing in patients with hypertrophic cardiomyopathy. Heart 2015, 101, 294–301. [Google Scholar] [CrossRef]
- Ichikawa, M.; Aiba, T.; Ohno, S.; Shigemizu, D.; Ozawa, J.; Sonoda, K.; Fukuyama, M.; Itoh, H.; Miyamoto, Y.; Tsunoda, T.; et al. Phenotypic Variability of ANK2 Mutations in Patients With Inherited Primary Arrhythmia Syndromes. Circ. J. 2016, 80, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Robaei, D.; Ford, T.; Ooi, S.-Y. Ankyrin-B Syndrome: A Case of Sinus Node Dysfunction, Atrial Fibrillation and Prolonged QT in a Young Adult. Heartlung Circ. 2015, 24, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Charpentier, F.; Peltier, S.; Foley, P.; Drouin, E.; Bouhour, J.B.; Donnelly, P.; Vergnaud, G.; Bachner, L.; Moisan, J.P. Mapping of a gene for long QT syndrome to chromosome 4q25-27. Am. J. Hum. Genet. 1995, 57, 1114–1122. [Google Scholar] [PubMed]
- Swayne, L.A.; Murphy, N.P.; Asuri, S.; Chen, L.; Xu, X.; McIntosh, S.; Wang, C.; Lancione, P.J.; Roberts, J.D.; Kerr, C.; et al. Novel Variant in the ANK2 Membrane-Binding Domain Is Associated With Ankyrin-B Syndrome and Structural Heart Disease in a First Nations Population With a High Rate of Long QT Syndrome. Circ. Cardiovasc. Genet. 2017, 10, e001537. [Google Scholar] [CrossRef]
- Huq, A.J.; Pertile, M.D.; Davis, A.M.; Landon, H.; James, P.A.; Kline, C.F.; Vohra, J.; Mohler, P.J.; Delatycki, M.B. A Novel Mechanism for Human Cardiac Ankyrin-B Syndrome due to Reciprocal Chromosomal Translocation. Heart Lung Circ. 2017, 26, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef]
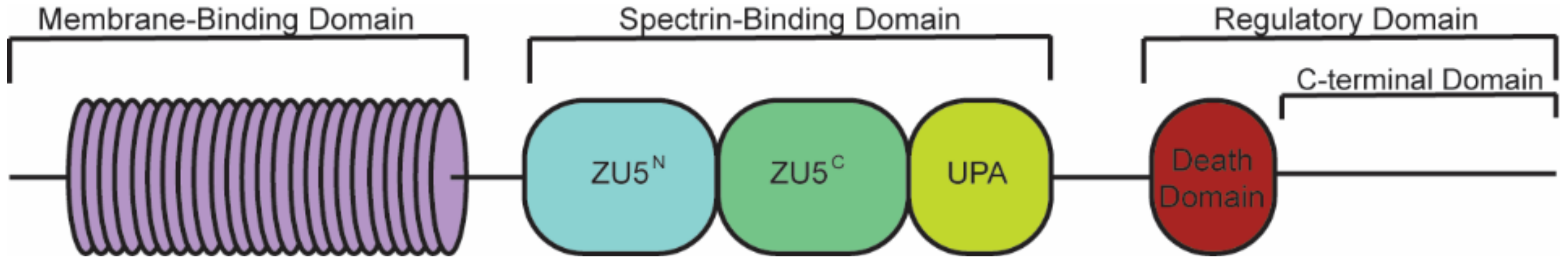
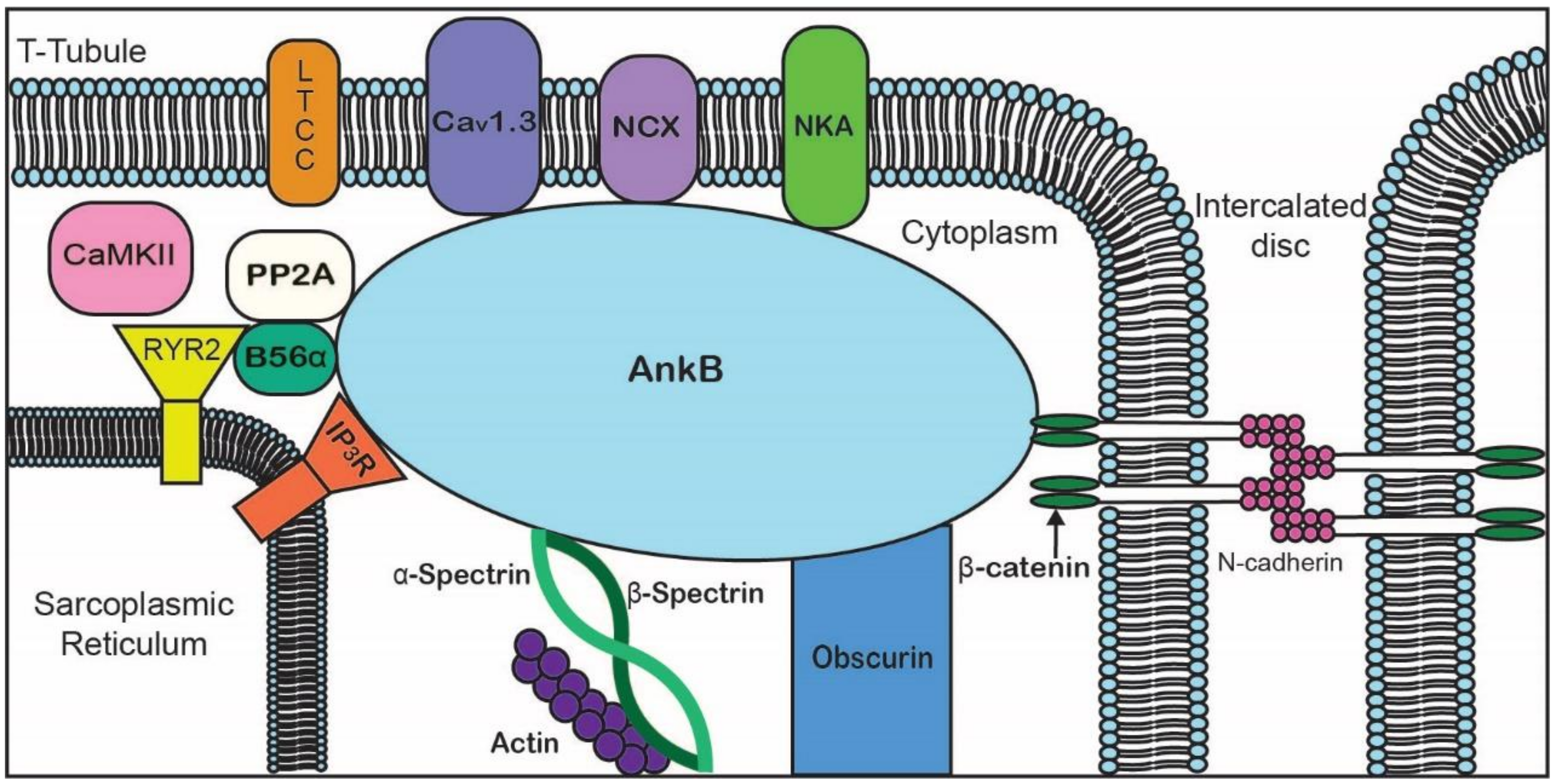
| Ankyrin-R | Ankyrin-B | Ankyrin-G | |
|---|---|---|---|
| Tissue Expression | erythrocytes [1], myelinated axons [10], striated muscle [11] | ubiquitously expressed, cardiomyocytes (T-tubules, SR, plasma membrane) [12], neurons [8] | ubiquitously expressed, neurons (AIS, and nodes of Ranvier) [13], cardiomyocytes (intercalated disc) [14] |
| Examples of Binding Partners | CD44 [15], NKA [16], Rh type A glycoprotein [17], obscurin [11] | PP2A [12,13], NCX [12], NKA [18], Kir6.2 [12,13], CaV1.3 [19], βII-spectrin [20] | NaV1.6, βIV-spectrin, L1CAMs [1,21,22], plakophilin-2 [23] NaV1.5 [14] |
| Isoforms | sAnk1.5, 1.6, 1.7, and 1.9 [11] | AnkB-188 and AnkB-212 [24]. Giant AnkB (440-kD) | Giant AnkG (480-kD) [25] |
| Disease associated with variants | hereditary spherocytosis [26] | Ankyrin B syndrome: SCD, SND, AF, LQTS, VT, bradycardia, syncope [12], ARVC [27] | Brugada syndrome [12], dilated cardiomyopathy [28], cognitive disabilities [29] |
| Membrane-Binding Domain | Spectrin-Binding Domain | Regulatory Domain | |
|---|---|---|---|
| Ion channels | Transporters/Pumps | β-spectrin | HSP40 |
| IP3R | Anion Exchanger | PP2A | Obscurin |
| Cav1.3 | Na/Ca Exchanger | Ankyrin MBD | |
| Kir6.2 | Na/K ATPase | ||
| Structural | Cell adhesion | ||
| Tubulin β-catenin | L1CAMs | ||
| β-dystroglycan | |||
| Dystrophin | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sucharski, H.C.; Dudley, E.K.; Keith, C.B.R.; El Refaey, M.; Koenig, S.N.; Mohler, P.J. Mechanisms and Alterations of Cardiac Ion Channels Leading to Disease: Role of Ankyrin-B in Cardiac Function. Biomolecules 2020, 10, 211. https://doi.org/10.3390/biom10020211
Sucharski HC, Dudley EK, Keith CBR, El Refaey M, Koenig SN, Mohler PJ. Mechanisms and Alterations of Cardiac Ion Channels Leading to Disease: Role of Ankyrin-B in Cardiac Function. Biomolecules. 2020; 10(2):211. https://doi.org/10.3390/biom10020211
Chicago/Turabian StyleSucharski, Holly C., Emma K. Dudley, Caullin B. R. Keith, Mona El Refaey, Sara N. Koenig, and Peter J. Mohler. 2020. "Mechanisms and Alterations of Cardiac Ion Channels Leading to Disease: Role of Ankyrin-B in Cardiac Function" Biomolecules 10, no. 2: 211. https://doi.org/10.3390/biom10020211
APA StyleSucharski, H. C., Dudley, E. K., Keith, C. B. R., El Refaey, M., Koenig, S. N., & Mohler, P. J. (2020). Mechanisms and Alterations of Cardiac Ion Channels Leading to Disease: Role of Ankyrin-B in Cardiac Function. Biomolecules, 10(2), 211. https://doi.org/10.3390/biom10020211





