Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation
Abstract
1. Introduction
Aim
- -
- We studied whether chronic stress restraint could increase CCR5/RANTES chemokine as well as IL-6 levels in the hippocampus/prefrontal cortex (PFC) of rats subjected to 21 days of restraint; we evaluate if these chemokines play a role in fear memory consolidation in this CFC paradigm (1 mA, Experiment 1). These beta chemokines were measured 24 h after CFC training.
- -
- We evaluated whether CCR5/RANTES chemokine levels are differentially regulated at 12 and 24 h post-training in CFC-trained rats with a footshock intensity of 1 mA (Experiment 2).
- -
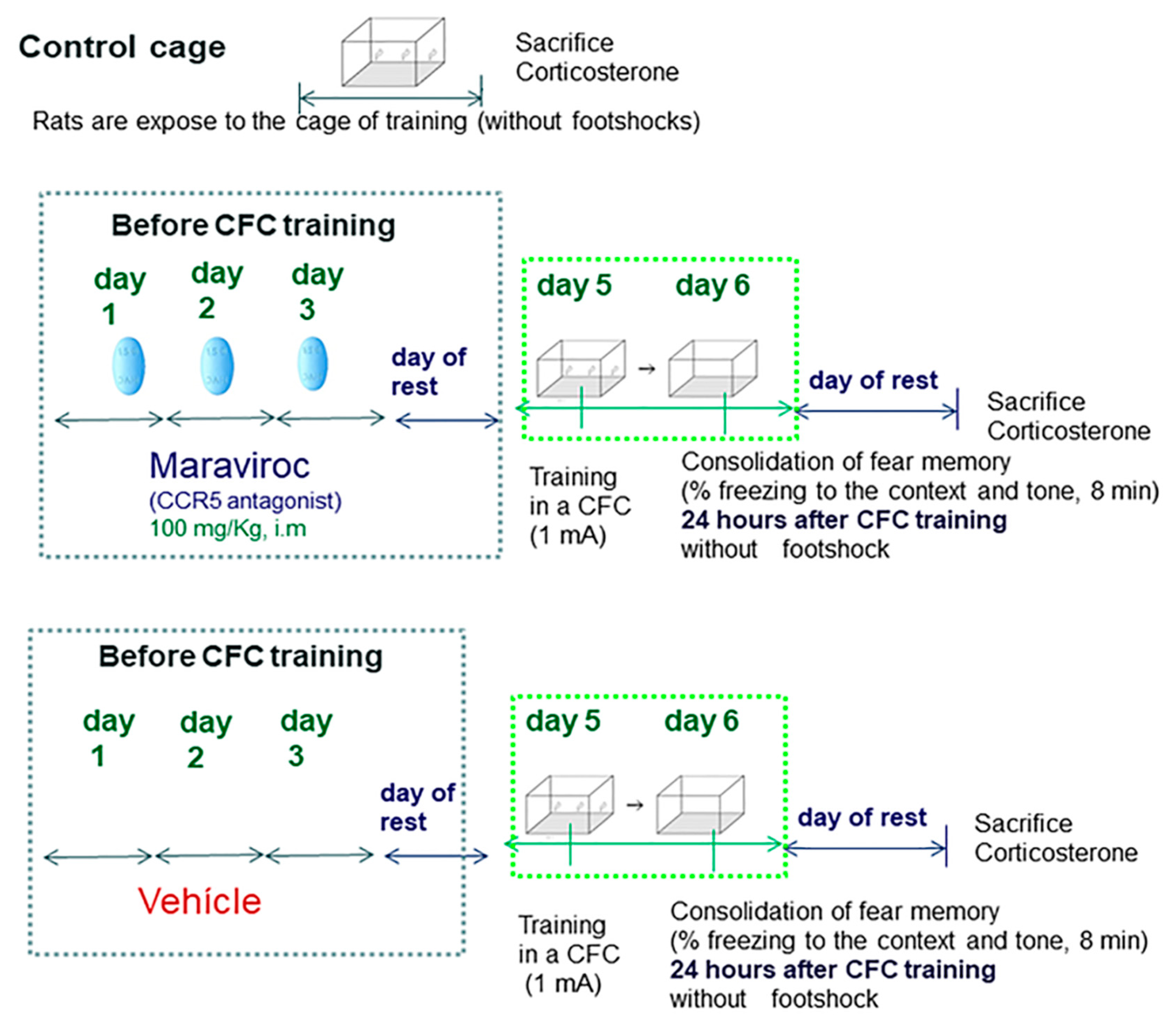
- Once we confirmed elevated CCR5/RANTES levels at 12 and 24 h after CFC training (1 mA), we evaluated whether maraviroc (a CCR5 blocker, i.m injection: 100 mg/Kg) treatment administered for 3 consecutive days before CFC fear training could affect RANTES/IL-6 and corticosterone levels and also impairs fear memory consolidation 24 h after the CFC session (Experiment 3).
2. Material and Methods
2.1. Animals
2.2. Behavioral Methods
2.2.1. Chronic Stress Restraint Procedure
2.2.2. Contextual Fear Learning Paradigm: A Behavioral Paradigm of PSTD
2.3. Biochemical Methods
2.3.1. Synaptosomes Isolation (Hippocampus and Prefrontal Cortex)
2.3.2. ELISA CCR5/RANTES Protein Levels (Crude Synaptosomes)
2.3.3. Western Blot for CCR5 Detection in Crude Synaptosomes
2.3.4. Corticosterone Levels
3. Results
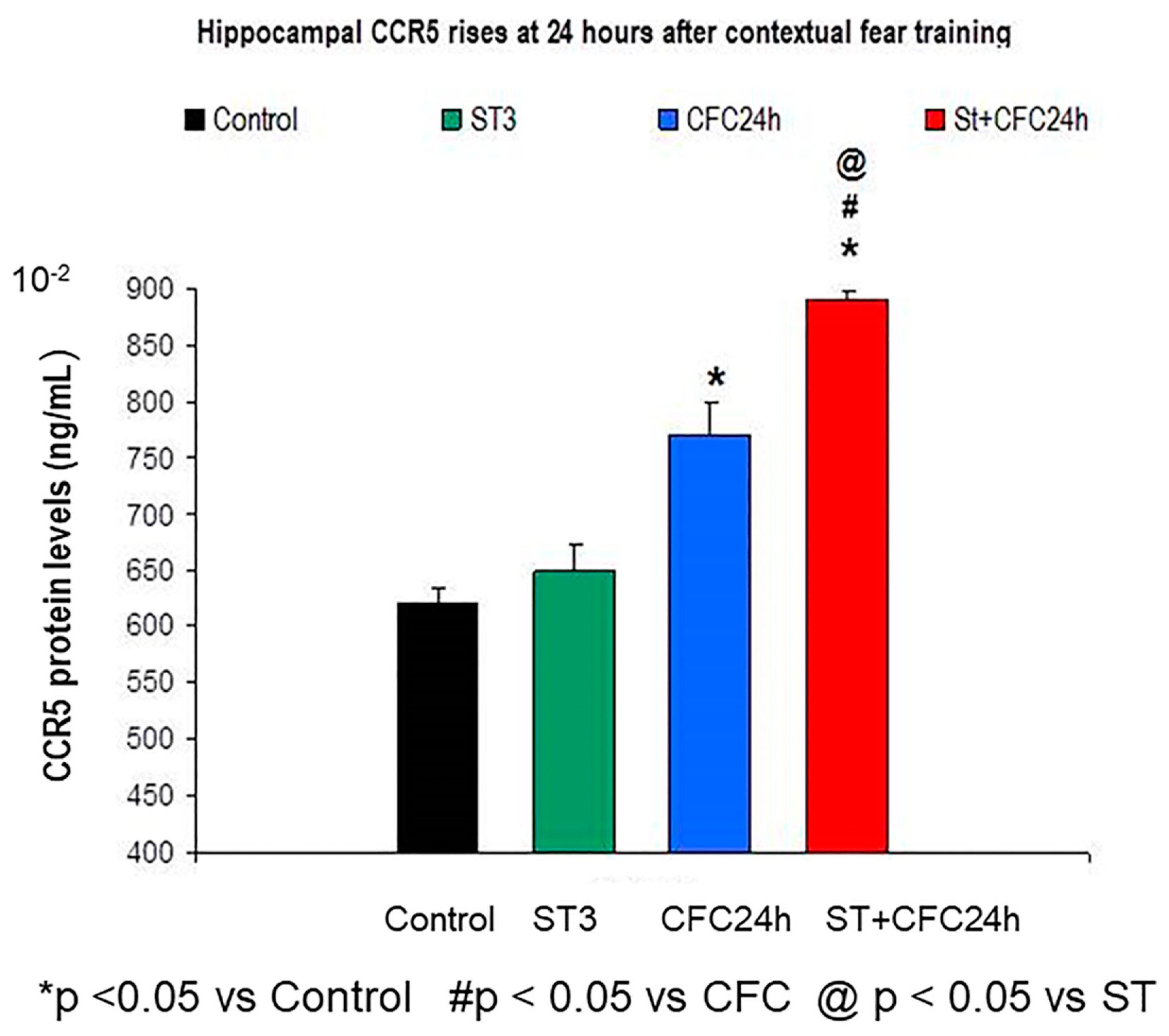
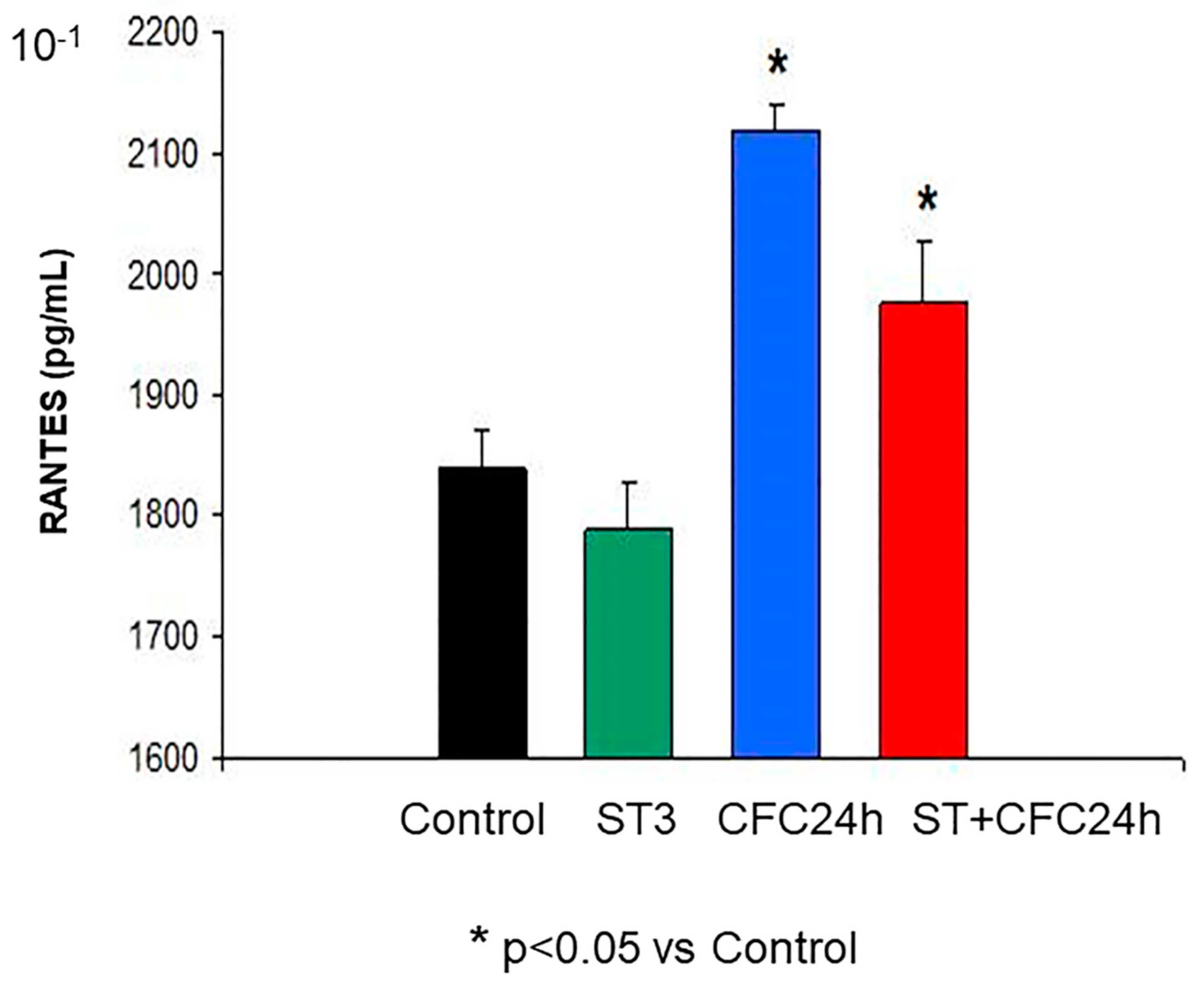
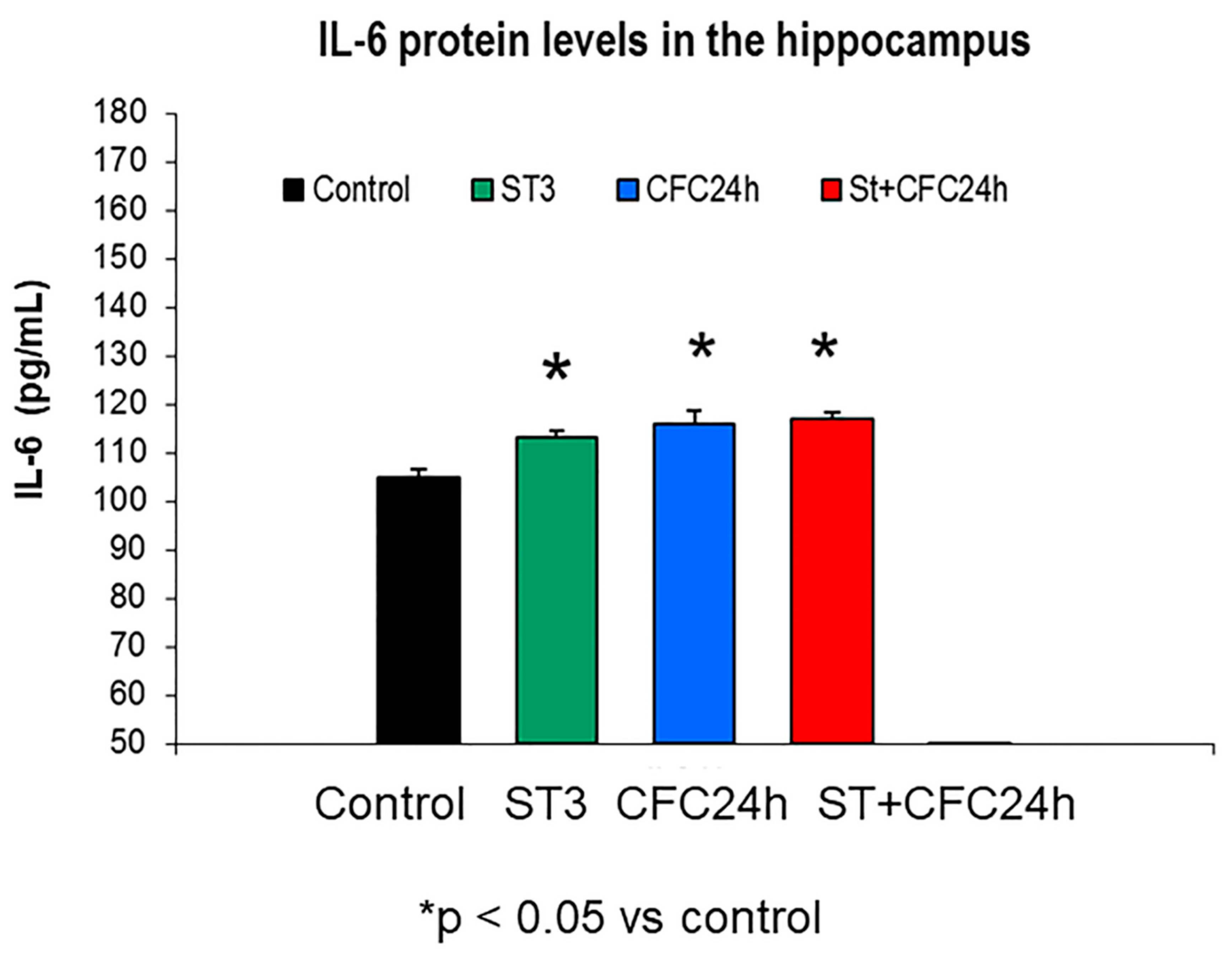
3.1. Experiment 1: Effect of Chronic Stress Restraint and Fear Learning in CCR5/RANTES Levels (Hippocampus/Prefrontal Cortex)
3.1.1. Hippocampus: Increased Hippocampal CCR5 Levels in Chronically Stressed Rats 24 Hours after Contextual Fear Conditioning Training (24 Hours Post-testing)
3.1.2. RANTES (Hippocampus)
3.1.3. Increased IL-6 Levels by Fear Learning in the Hippocampus of Chronically Stressed Rats 24 Hours after CFC Training with 1 mA
3.1.4. Prefrontal Cortex: Chronic Stress and/or Contextual Fear Conditioning (CFC) did not Affect CCR5/RANTES Protein Levels in the Prefrontal Cortex 24 h after the CFC Session
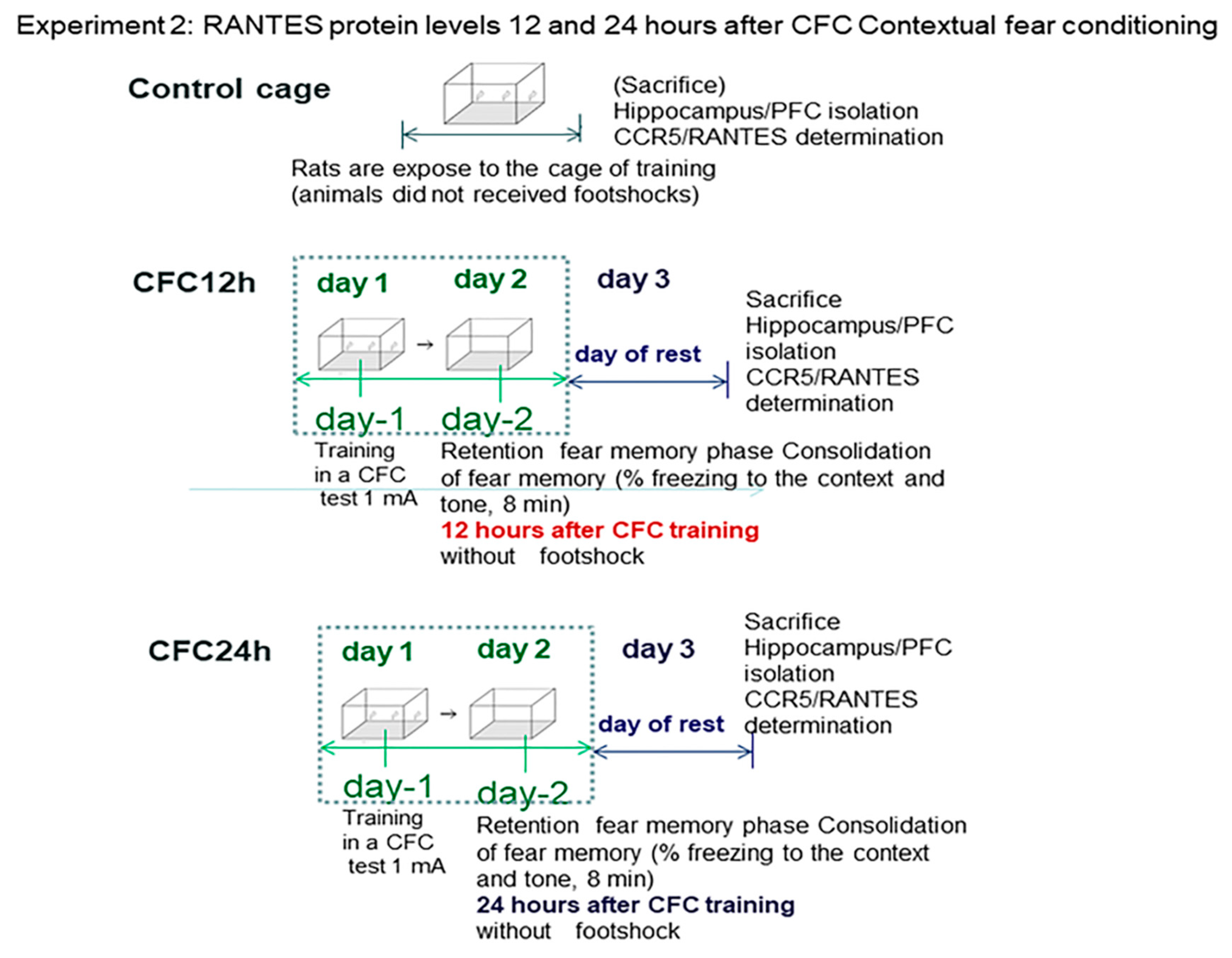
3.2. Experiment 2: Effect of Fear Learning in RANTES Levels (12 and 24 h Post-testing)
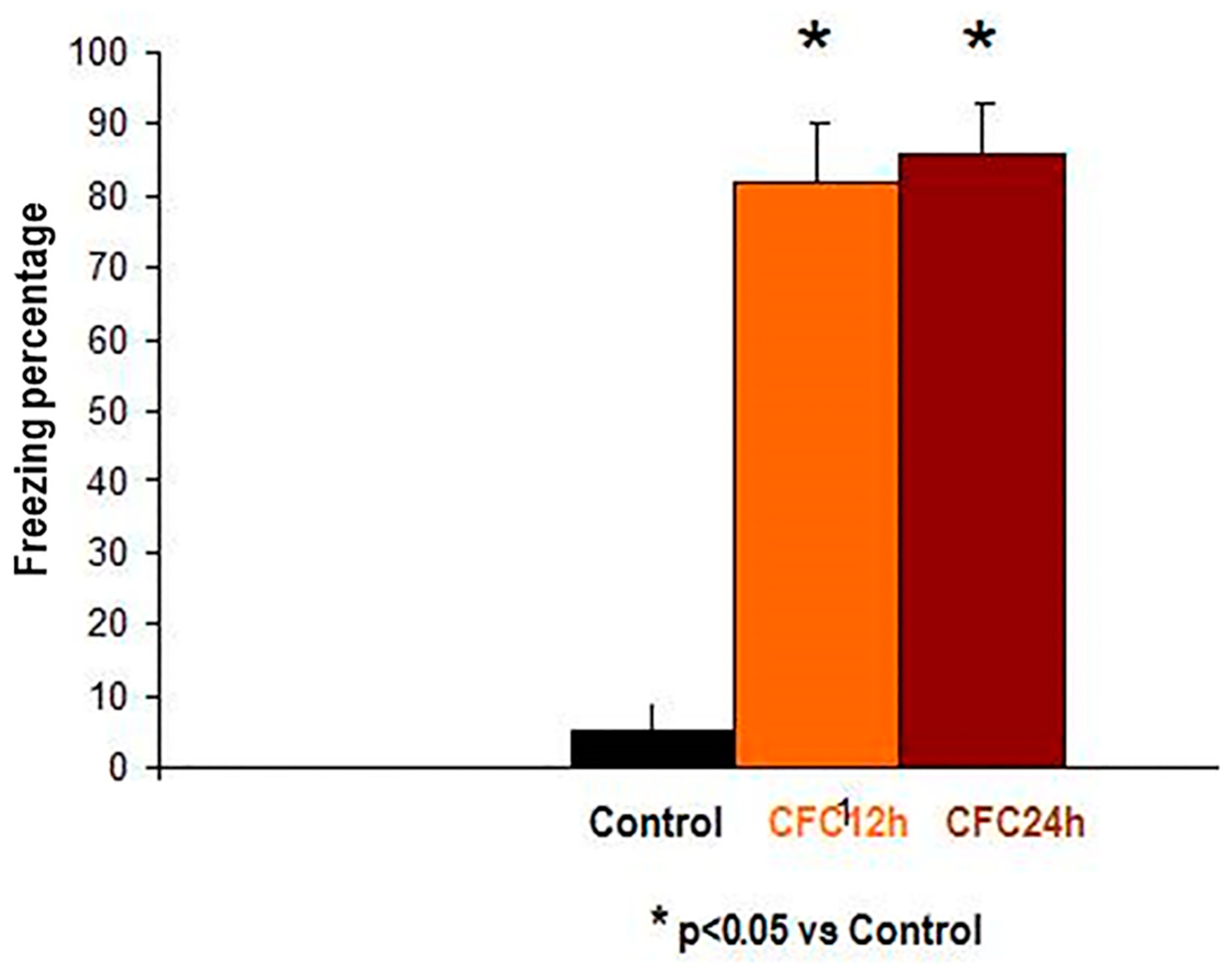
3.2.1. Increased Corticosterone and Enhanced Freezing Percentage to the Context at 12 and 24 h after the CFC Session with 1 mA Footshocks
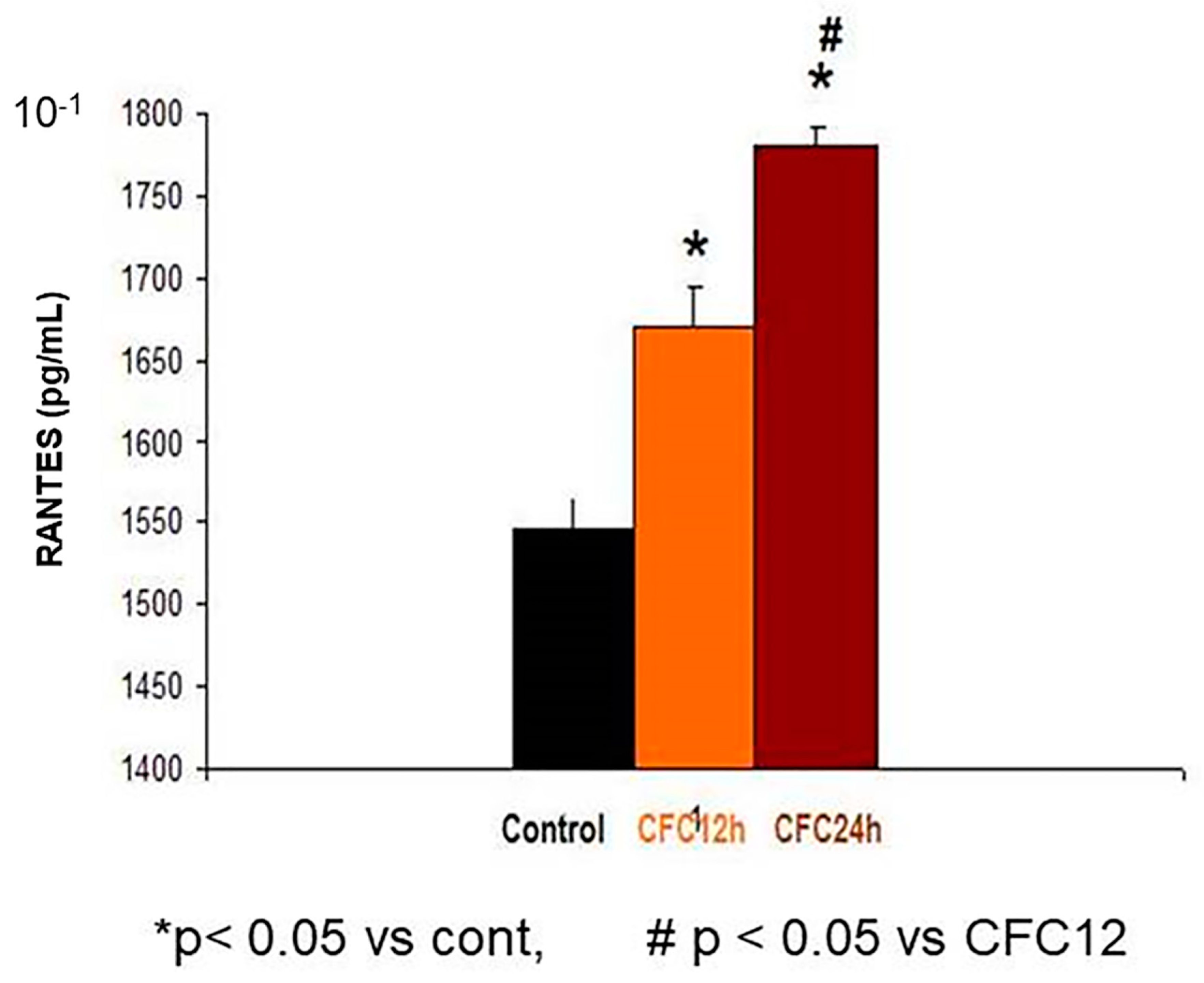
3.2.2. Increased RANTES Protein Levels in the Hippocampus 12 and 24 Hours after CFC Training with 1 mA of Electric Footshocks
3.3. Experiment 3: Effect of CCR5 Blockade by Maraviroc in Corticosterone and Freezing Levels (24 Hours after CFC Training Session)
3.3.1. Maraviroc i.m Injections for 3 Consecutive Days before CFC Training (1 mA Footshocks) Increased Corticosterone Levels and Enhanced Freezing to the Context at 24 Hours after the CFC Session as Compared to Vehicle CFC-Treated rats (1 mA)
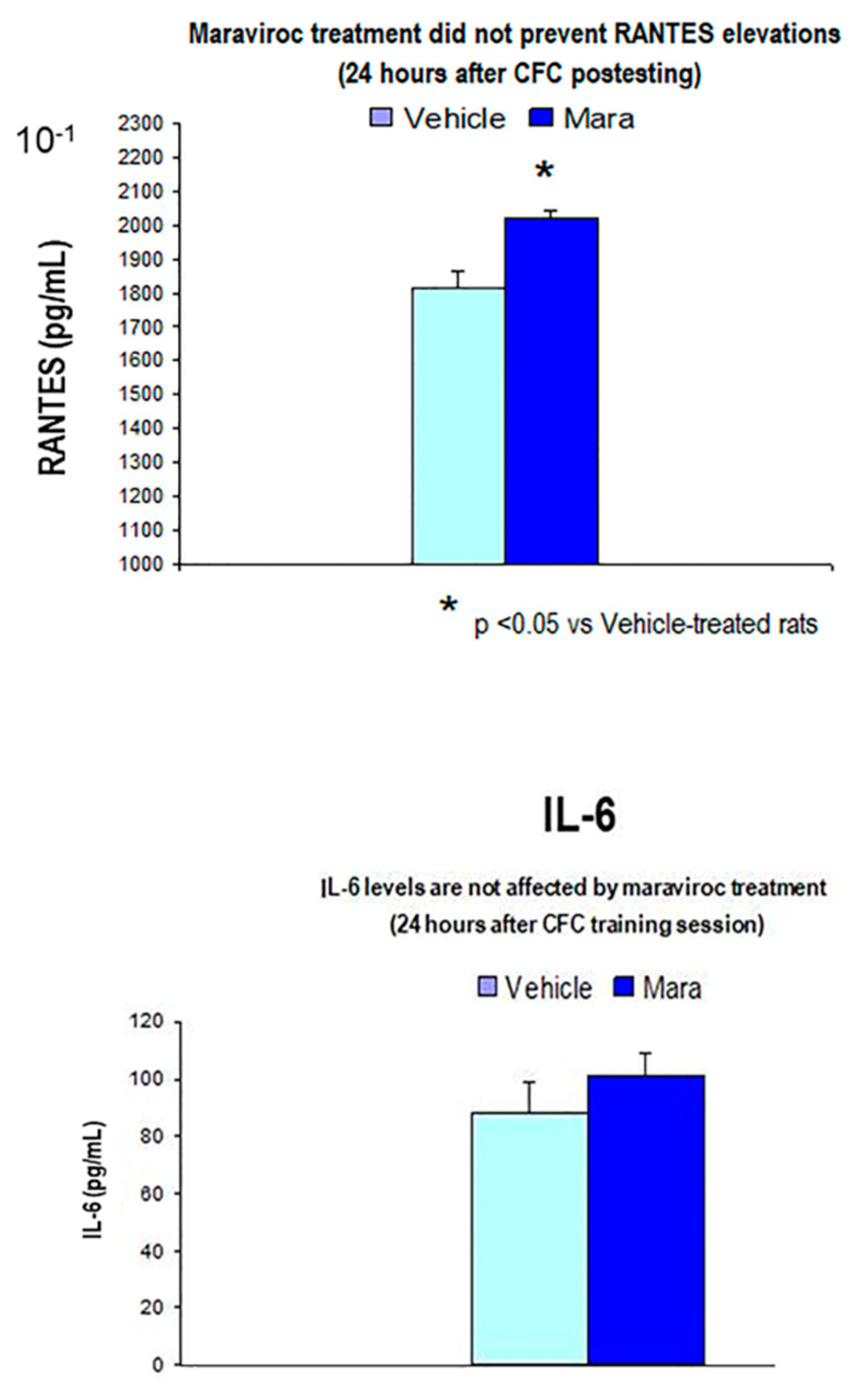
3.3.2. Maraviroc i.m Injections for 3 Consecutive Days before CFC Training (1 mA Footshocks) did not Prevented RANTES Overexpression as Compared to Vehicle CFC-Treated Rats (1 mA)
3.3.3. Maraviroc i.m Injections for 3 Consecutive Days before CFC Training (1 mA Footshocks) did not Affect IL-6 (24 Hours Post-testing) Protein Levels as Compared to Vehicle-CFC Treated Rats (1 mA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Black, P.H. Central nervous system-immune system interactions: Psychoneuroendocrinology of stress and its immune consequences. Antimicrob. Agents Chemother. 1994, 38, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhabhar, F.S.; McEwen, B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav. Immun. 1997, 11, 286–306. [Google Scholar]
- Flügge, G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci. Biobehav. Rev. 1998, 23, 295–300. [Google Scholar]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J.; Gidron, Y.; Matar, M.A.; Belkin, D.; Loewenthal, U.; Kozlovsky, N.; Kaplan, Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry 2006, 15, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Radley, J.J.; Rocher, A.B.; Rodriguez, A.; Ehlenberger, D.B.; Dammann, M.; McEwen, B.S.; Morrison, J.H.; Wearne, S.L.; Hof, P.R. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008, 1, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef]
- Schubert, I.; Ahlbrand, R.; Winter, A.; Vollmer, L.; Lewkowich, I.; Sah, R. Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1- deficient mice. Brain Behav Immun 2018, 68, 34–43, Epub 2017. [Google Scholar] [CrossRef]
- Kleen, J.K.; Sitomer, M.T.; Killeen, P.R.; Conrad, C.D. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 2006, 120, 842–851. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54 (Suppl. 1), 20–23. [Google Scholar] [CrossRef]
- Magariños, A.M.; Verdugo, J.M.; McEwen, B.S. Chronic stress alters synaptic terminal structure in hippocampus. PNAS 1997, 9, 14002–14008. [Google Scholar]
- Rostène, W.; Kitabgi, P.; Parsadaniantz, S.M. Chemokines: A new class of neuromodulator? Nat. Rev. Neurosci. 2007, 8, 895–903. [Google Scholar]
- Guyon, A.; Nahon, J.L. Multiple actions of the chemokine stromal cell-derived factor-1 alpha on neuronal activity. J. Mol. Endocrinol. 2007, 38, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Mettling, C.; Portalès, P.; Rouzier, R.; Clot, J.; Reynes, J.; Corbeau, P. The chemokine CCL5 regulates the in vivo cell surface expression of its receptor, CCR5. AIDS 2008, 30, 430–432. [Google Scholar] [CrossRef]
- Anders, H.J.; Vielhauer, V.; Frink, M.; Linde, Y.; Cohen, C.D.; Blattner, S.M.; Kretzler, M.; Strutz, F.; Mack, M.; Gröne, H.J.; et al. Chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J. Clin. Investig. 2002, 109, 251–259. [Google Scholar] [CrossRef]
- Wells, T.N.; Power, C.A.; Shaw, J.P.; Proudfoot, A.E. Chemokine blockers—Therapeutics in the making? Trends Pharmacol. Sci. 2006, 27, 41–47. [Google Scholar] [CrossRef]
- Galasso, J.M.; Harrison, J.K.; Silverstein, F.S. Excitotoxic brain injury stimulates expression of the chemokine receptor CCR5 in neonatal rats. Am. J. Pathol. 1998, 153, 1631–1640. [Google Scholar] [CrossRef][Green Version]
- Maung, R.; Hoefer, M.M.; Sánchez, A.B.; Sejbuk, N.E.; Medders, K.E.; Desai, M.K.; Catalan, I.C.; Donwling, C.C.; de Rozieres, C.M.; Garden, G.A.; et al. CCR5 knockout mice prevents neuronal injury and behavioural impairment induced in a transgenic mice model by a CXCR4-using HIV-1 glycoprotein. J. Inmunol. 2004, 15, 1895–1910. [Google Scholar]
- Glass, W.G.; Hickey, M.J.; Hardison, J.L.; Liu, M.T.; Manning, J.E.; Lane, T.E. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 2004, 1, 4018–4025. [Google Scholar] [CrossRef]
- Bruno, V.; Copani, A.; Besong, G.; Scoto, G.; Nicoletti, F. Neuroprotective activity of chemokines against N-methyl-D-aspartate or beta-amyloid-induced toxicity in cult ure. Eur. J. Pharmacol. 2000, 7, 117–121. [Google Scholar] [CrossRef]
- Di Filippo, M.; Sarchielli, P.; Picconi, B.; Calabresi, P. Neuroinflammation and synaptic plasticity: Theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 2008, 29, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Kasiyanov, A.; Fujii, N.; Tamamura, H.; Xiong, H. Modulation of network-driven, GABA-mediated giant depolarizing potentials by SDF-1 alpha in the developing hippocampus. Dev. Neurosci. 2008, 30, 285–292. [Google Scholar] [CrossRef]
- Ahmed, F.; Tessarollo, L.; Thiele, C.; Mocchetti, I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geppert, A.M. Constitutive patterns of RANTES, MCP-1 and MIP-1 alpha expression at the mRNA and protein level during postnatal development of the rat brain. Folia Neuropathol. 2003, 41, 79–88. [Google Scholar] [PubMed]
- Fanselow, M.S. Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 2000, 1, 73–81. [Google Scholar] [CrossRef]
- Sandi, C.; Merino, J.J.; Cordero, M.I.; Kruyt, N.D.; Murphy, K.J.; Regan, C.M. Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol. Psychiatry 2003, 15, 599–607. [Google Scholar] [CrossRef]
- Merino, J.J.; Cordero, M.I.; Sandi, C. Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. Eur. J. Neurosci. 2000, 12, 3283–3290. [Google Scholar] [CrossRef]
- Vieweg, W.V.; Julius, D.A.; Fernandez, A.; Beatty-Brooks, M.; Hettema, J.M.; Pandurangi, A.K. Posttraumatic stress disorder: Clinical features, pathophysiology, and treatment. Am. J. Med. 2006, 19, 383–390. [Google Scholar] [CrossRef]
- Mikics, E.; Baranyi, J.; Haller, J. Rats exposed to traumatic stress bury unfamiliar objects—A novel measure of hyper-vigilance in PTSD models? Physiol. Behav. 2008, 9, 341–348. [Google Scholar] [CrossRef]
- Gola, H.; Engler, H.; Sommershof, A.; Adenauer, H.; Kolassa, S.; Schedlowski, M.; Groettrup, M.; Elbert, T.; Kolassa, I.T. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 2013, 13, 40. [Google Scholar] [CrossRef]
- Zhou, D.; Kusnecov, A.W.; Shurin, M.R.; DePaoli, M.; Rabin, B.S. Exposure to physical and psychological stressors elevates plasma interleukin 6: Relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 1993, 13, 32523–32530. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.A.; Kuziel, W.A.; Rivest, S.; Owens, T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 2003, 27, 7922–7930. [Google Scholar] [CrossRef]
- Merino, J.J.; Bellver-Landete, V.; Oset-Gasque, M.J.; Cubelos, B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: Focus in CNS repair. J. Cell Physiol. 2015, 230, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Muñetón-Gómez, V.; Alvárez, M.I.; Toledano-Díaz, A. Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer’s Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD? Curr. Alzheimer. Res. 2016, 13, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.H. Chemokine receptors in the central nervous system: Role in brain inflammation and neurodegenerative diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Lu, M.; Grove, E.A.; Miller, R.J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. PNAS 2002, 99, 7090–7095. [Google Scholar] [CrossRef]
- Arakawa, Y.; Bito, H.; Furuyashiki, T.; Tsuji, T.; Takemoto-Kimura, S.; Kimura, K.; Nozaki, K.; Hashimoto, N.; Narumiya, S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 2003, 161, 381–391. [Google Scholar] [CrossRef]
- Merino, J.J.; Garcimartín, A.; López-Oliva, M.E.; Benedí, J.; González, M.P. The impact of CXCR4 blockade on the survival of rat brain cortical neurons. Int. J. Mol. Sci. 2016, 17, 2005. [Google Scholar] [CrossRef]
- Valerio, A.; Ferrario, M.; Martinez, F.O.; Locati, M.; Ghisi, V.; Bresciani, L.G.; Mantovani, A.; Spano, P. Gene expression profile activated by the chemokine CCL5/RANTES in human neuronal cells. J. Neurosci. Res. 2004, 1, 371–382. [Google Scholar] [CrossRef]
- Pujol, F.; Kitabgi, P.; Boudin, H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J. Cell Sci. 2005, 118, 1071–1080. [Google Scholar] [CrossRef]
- O’Sullivan, N.C.; McGettigan, P.A.; Sheridan, G.K.; Pickering, M.; Conboy, L.; O’Connor, J.J.; Moynagh, P.N.; Higgins, D.G.; Regan, C.M.; Murphy, K.J. Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning. J. Neurochem. 2007, 101, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Schoner, J.; Heinz, A.; Endress, M.; Gertz, K.; Kronenberg, G. Post-traumatic stress disorder and beyond: And overview of rodent stress models. J. Cell. Mol. Med. 2017, 21, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- D’Antoni, M.L.; Paul, R.H.; Mitchell, B.I.; Kohorm, L.; Fischer, L.; Lefebvre, E.; Seyedkazemi, S.; Nakamoto, B.K.; Walker, M.; Kallianpur, J.K.; et al. Improved cognitive performmance and reduced monocyte activation in virally supressed chronic HIV following dual CCR2 and CCR5 antagonism. J. Acquir. Immune Defic. Syndr. 2018, 79, 108–116. [Google Scholar]
- Baudouin, S.J.; Pujol, F.; Nicot, A.; Kitabgi, P.; Boudin, H. Dendrite-selective redistribution of the chemokine receptor CXCR4 following agonist stimulation. Mol. Cell Neurosci. 2006, 33, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Martin-Blondel, G.; Brassat, D.; Bauer, J.; Lassmann, H.; Liblau, R.S. CCR5 blockade for neuroinflammatory diseases-beyond control of HIV. Nat. Rev. Neurol. 2016, 12, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kiss, D.L.; Longden, J.; Fechner, G.A.; Avery, V.M. The functional antagonist Met-RANTES: A modified agonist that induces differential CCR5 trafficking. Cell. Mol. Biol Lett. 2009, 14, 537–547. [Google Scholar] [CrossRef]
- Cardona, A.E.; Sasse, M.E.; Liu, L.; Cardona, S.M.; Mizutani, M.; Savarin, C.; Hu, T.; Ransohoff, R.M. Scavenging roles of chemokine receptors: Chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 2008, 15, 256–263. [Google Scholar] [CrossRef]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimmig, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011, 9, 16241–16250. [Google Scholar] [CrossRef]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef]
- Neuner, F.; Schauer, M.; Karunakara, U.; Klaschik, C.; Robert, C.; Elbert, T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry 2004, 4, 34. [Google Scholar] [CrossRef]
- Birrer, E.; Michael, T. Rumination in PSTD as well as in traumatized and non traumatized depressed patients: A cross-sectional clinical study. Behav. Cogn. Psychother. 2011, 39, 381–397. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, J.J.; Muñetón-Gomez, V.; Muñetón-Gómez, C.; Pérez-Izquierdo, M.Á.; Loscertales, M.; Toledano Gasca, A. Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation. Biomolecules 2020, 10, 212. https://doi.org/10.3390/biom10020212
Merino JJ, Muñetón-Gomez V, Muñetón-Gómez C, Pérez-Izquierdo MÁ, Loscertales M, Toledano Gasca A. Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation. Biomolecules. 2020; 10(2):212. https://doi.org/10.3390/biom10020212
Chicago/Turabian StyleMerino, José Joaquín, Vilma Muñetón-Gomez, César Muñetón-Gómez, María Ángeles Pérez-Izquierdo, María Loscertales, and Adolfo Toledano Gasca. 2020. "Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation" Biomolecules 10, no. 2: 212. https://doi.org/10.3390/biom10020212
APA StyleMerino, J. J., Muñetón-Gomez, V., Muñetón-Gómez, C., Pérez-Izquierdo, M. Á., Loscertales, M., & Toledano Gasca, A. (2020). Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation. Biomolecules, 10(2), 212. https://doi.org/10.3390/biom10020212



