Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture
Abstract
1. Introduction
2. Discussion
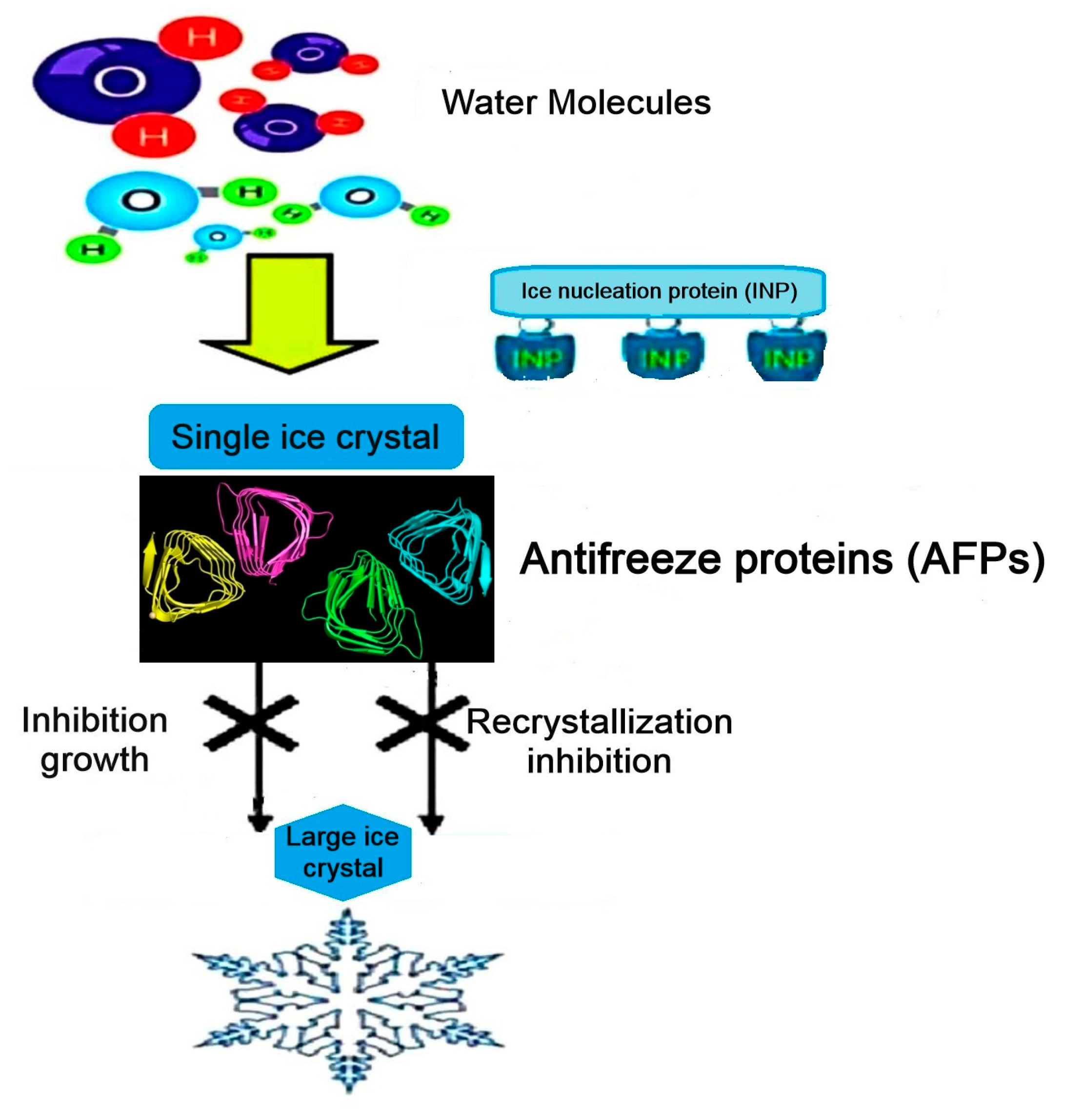
2.1. Properties and Characterizations of AFPs
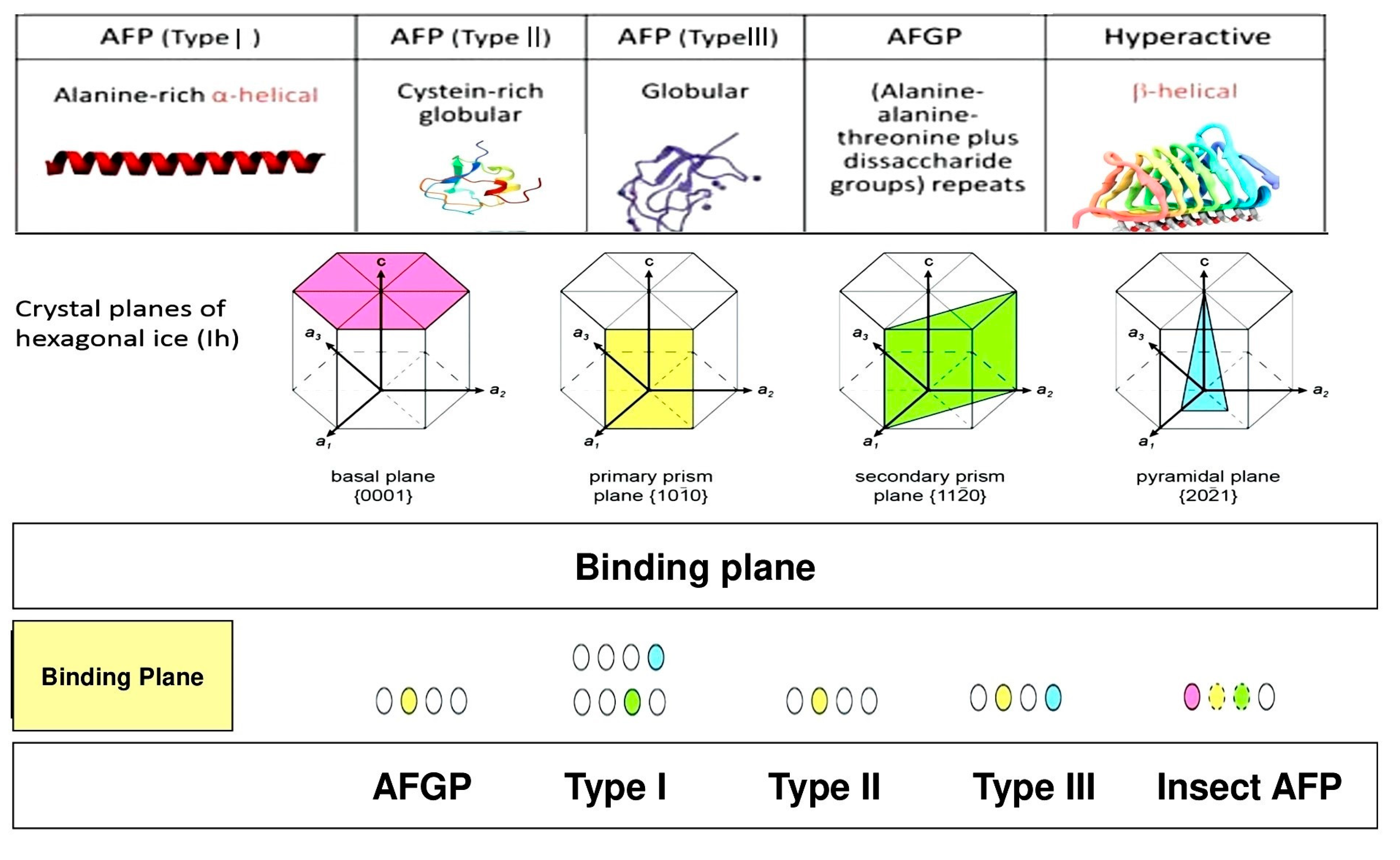
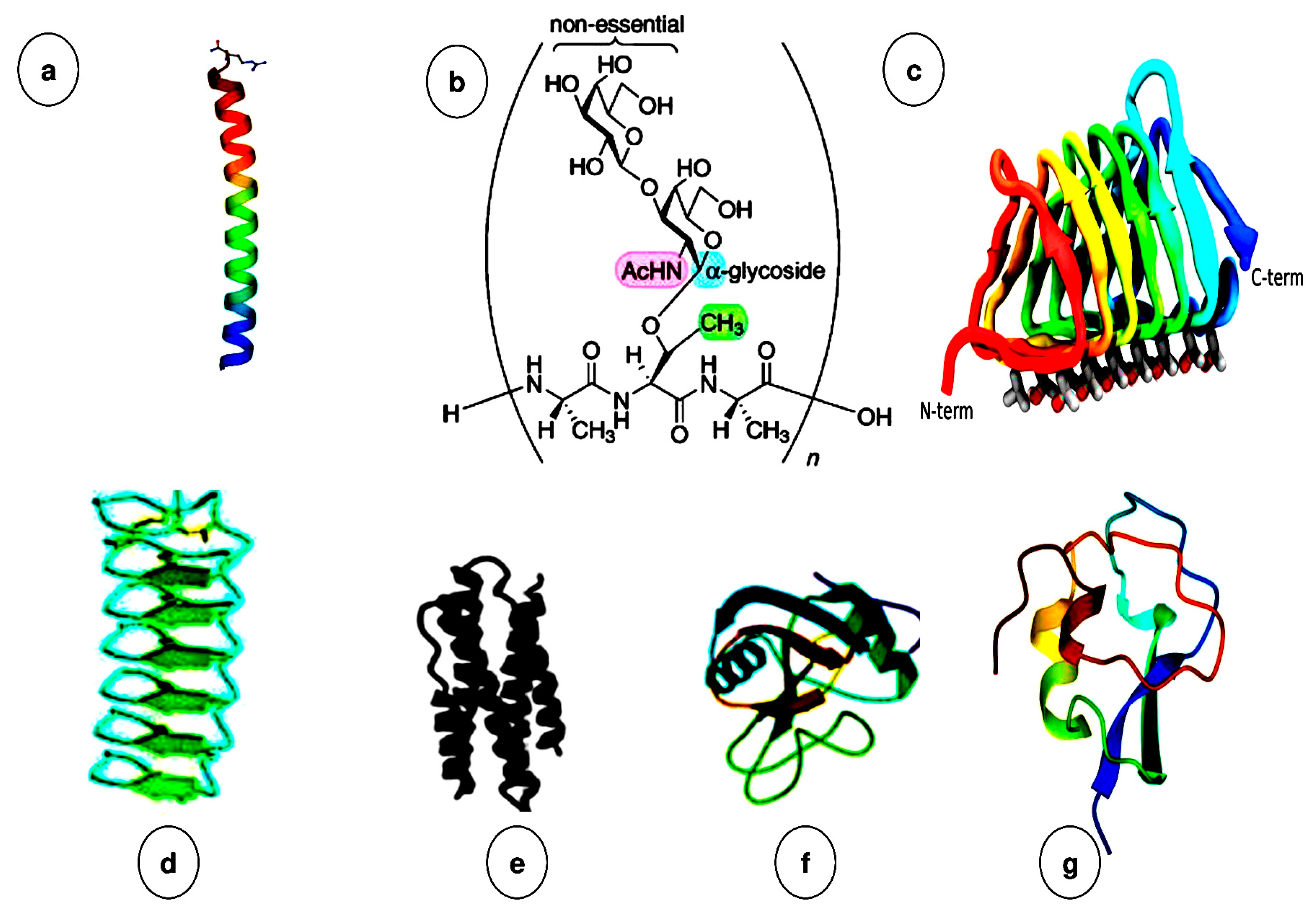
2.2. Origin, Classifications, and Diverse Structures of Antifreeze Proteins (AFPs)
2.3. Applications of Antifreeze Proteins
2.3.1. Industrial Applications of AFPs
Frozen Food
Ice Cream Products
Frozen, Chilled Fish and Meat
Frozen Dough
Creating Freeze-Tolerant Gels and High-Porosity Ceramics
Creating Anti-Ice Surfaces via AFPs on Aluminum
2.3.2. Medical Applications of AFPs
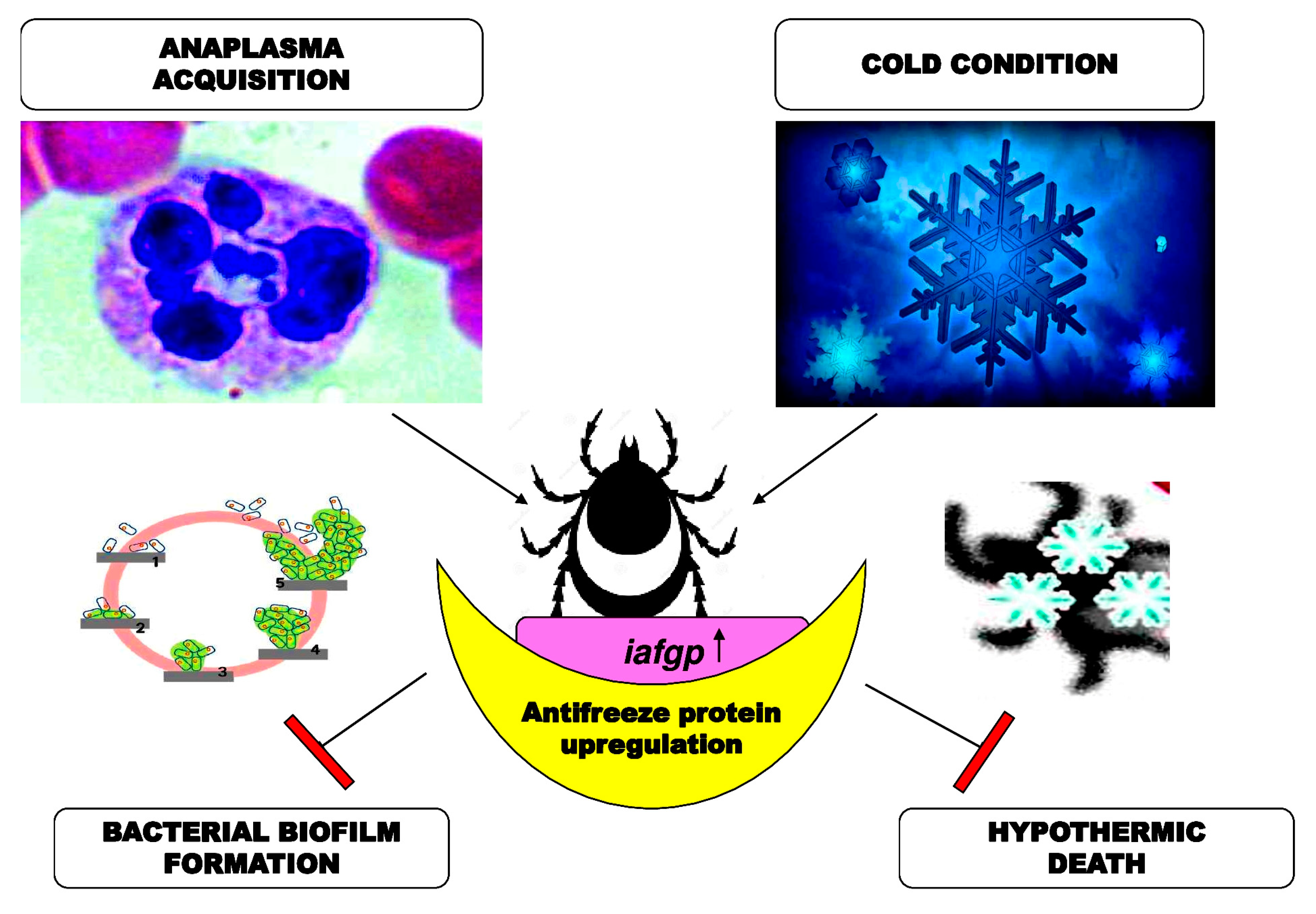
Anti-Infective (Anti-Virulence) Properties of AFP
Cryomedicine (Cryopresevation/Cryosurgery)
Cell and Tissue Cryopreservation
Organ Transplantation
2.3.3. Agricultural Applications
Effects of AFPs in Frost Protecting Crop Plants
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, C.A.; Semrau, J.; Chiriac, D.; Litschko, M.; Campbell, R.L.; Langelaan, D.N.; Smith, S.P.; Davies, P.L.; Allingham, J.S. Peptide backbone circularization enhances antifreeze protein thermo stability, Protein science: A publication of the. Protein Soc. 2017, 26, 1932–1941. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.K.; Lee, J.H.; Murugan, R.N.; Lee, S.G. Antifreeze peptides and glycopeptides, and their derivatives: Potential uses in biotechnology. Mar. Drugs 2013, 11, 2013–2041. [Google Scholar] [CrossRef] [PubMed]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 13, 4808–4823. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Quon, J.C.; Perez, A.F.; Ba, Y. Mechanisms of antifreeze proteins investigated via the site-directed spin labeling technique. Eur. Biophys. J. 2018, 47, 611–630. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H. Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant. Mar. Drugs. 2017, 15, 27. [Google Scholar] [CrossRef]
- Balcerzak, A.K.; Capicciotti, C.J.; Briard, J.G.; Ben, R.N. Designing ice recrystallization inhibitors: From antifreeze (glyco) proteins to small molecules. R. Soc. Chem. Adv. 2014, 4, 42682–42696. [Google Scholar] [CrossRef]
- Laezza, A.; Casillo, S.; Cosconati, C.I.; Biggs, A.; Fabozzi, L.; Paduano, A.; Iadonisi, E.; Novellino, M.I.; Gibson, A.; Corsaro, M.M.; et al. Decoration of chondroitin polysaccharide with threonine: Synthesis, conformational study, and Ice-Recrystallization Inhibition activity. Biomacromolecules 2017, 18, 2267–2276. [Google Scholar] [CrossRef]
- Kristiansen, E.; Zachariassen, K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology 2005, 51, 262–280. [Google Scholar] [CrossRef]
- Xiang, H.; Yang, X.; Ke, L.; Hu, Y. The properties, biotechnologies and applications of antifreeze proteins. Biol. Macromol. 2020, 153, 661–675. [Google Scholar] [CrossRef]
- Celik, Y.; Drori, R.; Pertaya-Braun, N.; Altan, A.; Barton, T. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc. Natl. Acad. Sci. USA 2013, 110, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H. Characterization of functions of biological materials having controlling ability against ice crystal growth. Mater. Sci. 2012. [Google Scholar] [CrossRef]
- Hayward, J.A.; Haymet, A.D.J. The Ice/Water Interface: Molecular Dynamics Simulations of the Basal, Prism, {2021}, and {2110} Interfaces of Ice Ih. Chem. Phys. 2001, 114, 3713–3726. [Google Scholar] [CrossRef]
- Olijve, L.L.; Meister, K.; DeVries, A.L.; Duman, L.G.; Guo, S.; Bakker, H.J.; Voets, L.I. Blocking rapid ice crystal growth through non-basal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef]
- Kuiper, M.J.; Lankin, C.; Gauthier, S.Y.; Walker, V.K.; Davies, P.L. Purification of antifreeze proteins by adsorption to ice. Biochem. Biophys. Res. Commun. 2003, 300, 645–648. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Chen, H.L.; Wang, H.; Qian, X. Extraction, purification and identification of antifreeze proteins from cold acclimated malting barley (Hordeum vulgare L.). Food Chem. 2015, 175, 74–81. [Google Scholar] [CrossRef]
- Mao, M.G.; Chen, Y.; Liu, R.T.; Lu, H.Q.; Gu, J. Transcriptome from Pacific cod liver reveals types of apolipoproteins and expression analysis of AFP-IV, structural analogue with mammalian ApoA-I, Comparative biochemistry and physiology. Part D Genom. Proteom. 2018, 28, 204–212. [Google Scholar] [CrossRef]
- Sharma, B.; Sahoo, D.; Deswal, R. Single-step purification and characterization of antifreeze proteins from leaf and berry of a freeze-tolerant shrub sea buckthorn (Hippophae rhamnoides). Sep. Sci. 2018, 41, 3938–3945. [Google Scholar] [CrossRef]
- Cheng, J.; Hanada, Y.; Miura, A.; Tsuda, S.; Kondo, H. Hydrophobic ice-binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochem. J. 2016. [Google Scholar] [CrossRef]
- Basu, K.; Garnham, C.P.; Nishimiya, Y.; Tsuda, S.; Braslavsky, I. Determining the ice-binding planes of antifreeze proteins by fluorescence-based ice plane affinity. Vis. Exp. 2014, 83, e51185. [Google Scholar] [CrossRef]
- Knight, C.A.; Cheng, C.C.; DeVries, A.L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys, J. 1991, 59, 409–418. [Google Scholar] [CrossRef]
- Haleva, L.; Celik, Y.; Bar-Dolev, M.; Pertaya-Braun, N.; Kaner, A.; Davies, P.L. Micro fluidic cold finger device for the investigation of ice-binding proteins. Biophys J. 2016, 111, 1143–1150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Y.; Yang, Z.; Tan, H.; Liu, R.; Chen, G.; Jia, Z. Analysis of ice-binding sites in fish type II antifreeze protein by quantum mechanics. Biophys J. 2002, 83, 2202–2210. [Google Scholar] [CrossRef]
- Loewen, M.C.; Gronwald, W.; Sönnichsen, F.D.; Sykes, B.D.; Davies, P.L. The ice-binding site of sea raven antifreeze protein is distinct from the carbohydrate-binding site of the homologous C-type lectin. Biochem. J. 1998, 37, 17745–17753. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Hallett, J.; DeVries, A.L. Solute effects on ice recrystallization: An assessment technique. Cryobiology 1988, 21, 55–60. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Marshall, C.B.; Gilbert, J.A.; Davies, P.L. A facile method for determining ice recrystallization inhibition by antifreeze proteins. BBRC J. 2003, 311, 1041–1046. [Google Scholar] [CrossRef]
- Stubbs, C.; Wilkins, L.E.; Fayter, A.E.R.; Walker, M.; Gibson, M.I. Multivalent presentation of ice recrystallization inhibiting polymers on nano-particles retains activity. Am. Chem. Soc. 2018, 35, 7347–7353. [Google Scholar] [CrossRef]
- Yu, S.-H.; Yang, P.; Sun, T.; Qi, Q.; Wang, X.-Q.; Chen, X.M.; Feng, Y. Transcriptomic and proteomic analyses on the super cooling ability and mining of antifreeze proteins of the Chinese white wax scale insect. Insect Sci. 2016, 23, 430–437. [Google Scholar] [CrossRef]
- Carrasco, M.A.; Buechler, S.A.; Arnold, R.J.; Sformo, T.; Barnes, B.M. Investigating the deep super cooling ability of an Alaskan beetle Cucujus clavipes puniceus, via high throughput proteomics. Proteome. J. 2012, 75, 1220–1234. [Google Scholar] [CrossRef]
- Kumar, M.; Saldana, H.; Kumar, R.; Bhattacharyya, H.; Souza, N. In-Silco Analysis of Fish Antifreeze Proteins and their Physiochemical Characterization. Pure App. Biosci. 2018, 1, 1392–1398. [Google Scholar] [CrossRef]
- Wellig, S.; Hamm, P. Salvation layer of antifreeze proteins analyzed with a Markov state model. Phys. Chem. J. 2018, 49, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Doxey, A.C.; Yaish, M.W.; Griffith, M.; McConkey, B.J. Ordered surface carbons distinguish antifreeze proteins and their ice-binding regions. Nat. Biotechnol. 2006, 24, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, K.K.; Chou, K.C.; Martinetz, T.; Moller, S.; Suganthan, P.N. AFP-Pred: A random forest approach for predicting antifreeze proteins from sequence-derived properties. Theor. Biol. 2011, 270, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hui, M.; Liu, Z. iAFP-Ense: An ensemble classifier for identifying antifreeze protein by incorporating Grey model and PSSM into PseAAC. Membr. Biol. 2016, 249, 845–854. [Google Scholar] [CrossRef]
- He, X.; Han, K.; Hu, J.; Yan, H.; Yang, J.Y.; Shen, H.B.; Yu, D.J. Target Freeze: Identifying antifreeze proteins via a combination of weights using sequence evolutionary information and pseudo amino acid composition. Membr. Biol. 2015, 248, 1005–1014. [Google Scholar] [CrossRef]
- Nadu, T. Evaluation of in-silico protein secondary structure prediction methods by employing statistical techniques. Biomed. Biotechnol. Res. J. 2017, 5, 29–36. [Google Scholar] [CrossRef]
- Bhattachary, M.; Hota, A.; Kar, A.; Chini, D.S.; Malick, R.C. In-silico Structural and Functional modeling of Antifreeze protein (AFP) sequences of Ocean pout (Zoarces americanus, Bloch & Schneider 1801). Genet. Eng. Biotechnol. 2018, 16, 721–730. [Google Scholar] [CrossRef]
- Musharaf, M.D. Fish antifreeze proteins: Computational analysis and physicochemical characterization. Int. Curr. Pharm. 2012, 1, 18–26. [Google Scholar] [CrossRef]
- Idrees, S.; Nadeem, S.; Kanwal, S.; Ehsan, B.; Yousaf, A.; Nadeem, S.; Rajoka, M.I. In silico sequence analysis, homology modeling and function annotation of Ocimum basilicum hypothetical protein G1CT28_OCIBA. Bioautomation 2012, 16, 111–118. [Google Scholar]
- Dolev, B.; Braslavsky, M.; Davies, P.L. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef]
- Jia, Z.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- Chakraborty, S.; Biman, J. An unusual tale of structural evolution, hydration and function. Proc. Indian Natn Sci. Acad. 2018, 1, 169–187. [Google Scholar] [CrossRef]
- Mahatabuddin, S.; Hanada, Y.; Nishimiya, Y.; Miura, A. Concentration-dependent oligomerization of an alpha-helical antifreeze polypeptide makes it hyperactive. Sci. Rep. 2017, 7, 42501. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Kondo, H.; Takamichi, M.; Sugimoto, H.; Suzuki, M.; Miura, A.; Tsuda, S. Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from long snout poacher, Brachyopsis rostratus. Mol. Biol. 2008, 382, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, H.; Goff, H.D. Ice structuring proteins from plants: Mechanism of action and food application. Food Res. 2012, 46, 425–436. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, R.; Zhu, H.; Peng, C.; Chen, L. The cold resistant activities of multi-domain type III antifreeze protein from Antarctic eelpout Lycodrchths deaborni revealed by transgenic tobaccos. Aquac. Fishes 2019, 1–6. [Google Scholar] [CrossRef]
- Sönnichsen, F.D.; DeLuca, C.I.; Davies, P.L.; Sykes, B.D.; Sönnichsen, F.D.; DeLuca, C.I.; Davies, P.L.; Sykes, B.D. Refined solution structure of type III antifreeze protein: Hydrophobic groups may be involved in the energetics of the protein–ice interaction. Structure 1996, 4, 1325–1337. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Gora, J.; Latajka, R.; Norbert, S. Antifreeze glycopeptides: From structure and activity structure to current approaches in chemical synthesis. Amino Acids 2017, 49, 209–222. [Google Scholar] [CrossRef]
- Duman, J.C. Animal ice-binding (Antifreeze proteins) proteins and glycopeptides. Exp. Biol. 2015, 218, 1846–1855. [Google Scholar] [CrossRef]
- Ye, Q.; Eves, R.; Campbell, R.L.; Davies, P.L. Crystal structure of an insect antifreeze proteins reveals ordered waters on the ice-binding surface. Biochemical 2020, 17, 3271–3286. [Google Scholar] [CrossRef]
- Bryon, A.; Wybouw, N.; Dermauw, W. Genome wide gene-expression analysis of facultative reproductive diapauses in two-spotted spider mite. BMC Genom. 2013, 14, 815. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.; Toprak, U.; Dageri, A. Identification of a putative antifreeze protein gene that is highly expressed during preparation foe winter in the sunn pests. Insect Physiol. 2014, 68, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hon, W.; Griffith, M.; Chong, P.; Yang, D.S.C. Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol. Bethesda 1994, 104, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Provesi, J.G.; Volentim, P.A.; Arisi, A.C.M. Antifreeze proteins in naturally cold acclimated leaves of Drimys angustifolia, Senecio icoglossus, and Eucalyptus ssp. Food Technol. 2016, 19, e2016110. [Google Scholar] [CrossRef][Green Version]
- Munoz, P.A.; Marquez, S.L.; Gonzalez, F.D. Structure and application of antifreeze proteins from Antarctic bacteria. Microb. Cell Factories 2017, 16, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ustun, N.S.; Turhan, S. Antifreeze proteins: Characteristics function mechanisms of action, sources and application to foods. Food Process. Preserv. 2015, 39, 3189–3197. [Google Scholar] [CrossRef]
- Boonsupthip, W.; Lee, T.C. Application of antifreeze protein for food preservation: Effect of type III antifreeze protein for preservation of gel-forming of frozen and chilled actomyosin. Food Sci. 2003, 68, 1804–1809. [Google Scholar] [CrossRef]
- Payne, S.R.; Young, O.A. Effects of pre-slaughter administration of antifreeze proteins on frozen meat quality. Meat Sci. 1995, 41, 147–155. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Liu, W.; Wang, L.; Qian, H. Extraction of carrot (Daucus carota) antifreeze proteins and evaluation of their effects on frozen white salted noodles. Food Bioprocess Technol. 2014, 7, 842–852. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Wang, L.; Gao, H.; Guo, X.N. Effect of carrot antifreeze proteins on texture properties and frozen dough and volatile compounds of crumb. Food Sci. Technol. 2007, 41, 1029–1036. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Wang, L. Effect of carrot (Daucus carota) antifreeze proteins on the fermentation capacity of frozen dough. Food Res. Int. 2007, 40, 763–769. [Google Scholar] [CrossRef]
- Panadero, J.; Randez, F.; Prieto, J.A. Heterologous expression of type I antifreeze peptide GS-5 in Baker’s yeast increases freeze tolerance and provides enhanced gas production in frozen dough. Agric. Food Chem. 2005, 53, 9966–9970. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-N.; Huang, W.; Jia, C.; Kim, Y.; Liu, H. Evaluation of water holding capacity and bread making properties for frozen dough containing ice structuring proteins from winter wheat. Cereal Sci. 2009, 49, 250–253. [Google Scholar] [CrossRef]
- Liu, M.; Liang, Y.; Zhang, H.; Wu, G.; Wang, L.; Qian, H. Production of a recombinant carrot antifreeze protein by Pichia pastoris GS115 and its cryoprotective effects on frozen dough properties and bread quality. Food Sci. Technol. 2018, 96, 543–550. [Google Scholar] [CrossRef]
- Sheikh, M.; Tsuda, S. Application of antifreeze proteins: Practical use of the quality products from Japanese fishes. Adv. Exp. Med. Biol. 2018, 13, 321–337. [Google Scholar] [CrossRef]
- Fukushima, M.; Tsuda, S.; Yoshizawa, Y. Fabrication of highly porous alumina prepared by gelatin freezing route with antifreeze protein. Am. Ceram. Soc. 2013, 96, 1029–1031. [Google Scholar] [CrossRef]
- Gwak, Y.; Park, J.I.; Kim, M.; Kim, H.S.; Kwon, M.J.; Oh, S.J.; Kim, Y.P.; Jin, E.S. Creating anti-icing surfaces via the direct immobilization of antifreeze proteins on aluminum. Nat. Sci. Rep. 2015, 5, 1038–1046. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.Y.; Zhao, M.; Yan, W.; Chien, C.W.; Chen, M.; Yang, H.; Machiyama, H.; Lin, S. Characteristics and possible origin of native aluminum in cold seep sediments from the northeastern South China Sea. Appl. Sci. Comput. 2011, 40, 363–370. [Google Scholar] [CrossRef]
- Gwak, I.G.; sic Jung, W.; Kim, H.J.; Kang, S.H.; Jin, E. Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar. Biotechnol. 2010, 12, 630–639. [Google Scholar] [CrossRef]
- Gwak, Y.; Jung, W.; Lee, Y.; Kim, J.S.; Kim, C.G.; Ju, J.H.; Song, C.; Hyun, J.K.; Jin, E. An intracellular antifreeze protein from an Antarctic microalga that responds to various environmental stresses. FASEB J. 2014, 28, 4924–4935. [Google Scholar] [CrossRef]
- Kreilgaard, L.; Frokjaer, S.; Flink, J.M.; Randolph, T.W.; Carpenter, J.F. Effects of Additives on the Stability of Recombinant Human Factor XIII during Freeze-Drying and Storage in the Dried Solid. Arch. Biochem. Biophys. 1998, 360, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, J.K.; Bhat, R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nagayama, M.; Gohara, K. Trehalose solution viscosity at low temperatures measured by dynamic light scattering method: Trehalose depresses molecular transportation for ice crystal growth. J. of Cryst. Growth 2009, 311, 4747–4752. [Google Scholar] [CrossRef]
- Fikrig, E.; Eeising, M.; Abraham, N.; Neelakanta, G. Anti-Infective Properties of Antifreeze Protein. U.S. Patent 10792332B2, 6 October 2020. [Google Scholar]
- Heisig, M.; Agaisse, H.; Fikrig, E. Anti-virulence properties of an antifreeze protein. Cell Press 2014, 9, 417–424. [Google Scholar] [CrossRef]
- Yarely, M.; Ramos, L. Biology of cell survival in the cold: The basis for bioservation of tissues and organs. Adv. Bio-Preserv. 2010, 96, 15–62. [Google Scholar]
- Abraham, N.M.; Liu, L.; Jutras, B.L.; Murfin, K.; Acar, A.; Yarovinsky, T.O.; Sutton, E.; Heisig, M.; Jacobs-Wagner, C. A tick anti virulence protein potentiates antibiotics against Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.K.; Youm, H.W.; Kim, H.J.; Lee, J.R. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLOS ONE 2015, 10, 1371–1385. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajta, G.; Kato, O.; Labo, S. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. BioMed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Kratochvílová, I.; Kopečná, O.; Bačíková, A.; Pagáčová, E.; Falková, I.; Elliott, S.E. Changes in cryopreserved cell nuclei serve as indicators of processes during freezing and thawing. Langmuir 2019, 35, 7496–7508. [Google Scholar] [CrossRef]
- Yang, J.; Pan, C.; Zhang, J.; Sui, X.; Zhu, Y.; Wen, C.; Zhang, L. Exploring the potential of biocompatible osmoprotectants as highly efficient cryoprotectants. Am. Chem. Sci. Appl. Mater. 2017, 9, 42516–42524. [Google Scholar] [CrossRef]
- Carpenter, F.J.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Physiology 1992, 89, 8953–8957. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D.J. ‘Antifreeze’ glycoprotein from polar fish. Biochemical 2003, 270, 1381–1392. [Google Scholar]
- Wang, J.H. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology 2000, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Youm, H.W.; Lee, H.J.; Suh, C.H.S. Effect of antifreeze protein on ovarian tissue cryopreservation and transplantation. Yonsei Med. J. 2015, 56, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yuan, B.; Kwon, J.W.; Ahn, M.; Cui, X.S.; Bang, J.K.; Kim, N.H. Effect of antifreeze glycoprotein 8 supplementation during vitrification on the developmental competence of bovine oocytes. Theriogenology 2016, 86, 485–494.e1. [Google Scholar] [CrossRef]
- Bagis, H.; Aktoprakligil, D.; Mercan, H.O.; Yurdusev, N.; Turgut, G.; Sekmen, S.; Arat, S.; Cetin, S. Stable transmission and transcription of newfoundland ocean pout type III fish antifreeze protein (AFP) gene in transgenic mice and hypothermic storage of transgenic ovary and testis. Mol. Reprod. Dev. 2006, 73, 1404–1411. [Google Scholar] [CrossRef]
- Jo, J.W.; Jee, B.C.; Suh, C.S.; Kim, S.H. The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS ONE 2012, 7, e37043. [Google Scholar] [CrossRef]
- Jo, J.W.; Jee, B.C.; Lee, J.R.; Suh, S. Effect of antifreeze protein supplementation invitrification medium on mouse oocyte developmental competence. Fertil. Steril. 2011, 96, 1239–1245. [Google Scholar] [CrossRef]
- Chaves, D.F.; Campelo, I.S.; Silva, M.; Bhat, M.H.; Teixeira, D.I.A.; Melo, L.M.; Souza-Fabjan, J.M.G.; Mermillod, P.; Freitas, V.J.F. The use of antifreeze protein type III for vitrification of in vitro matured bovine oocytes. Cryobiology 2016, 73, 324–328. [Google Scholar] [CrossRef]
- Zandiyeh, S.; Ebrahimi, F.; Sabbaghian, M. Application of antifreeze protein on sperm cryopreservation. Crimson Publ. 2018, 1, 22–34. [Google Scholar] [CrossRef]
- Zilli, L.; Beirão, J.; Schiavone, R.; Herraez, M.P.; Gnoni, A. Comparative proteome analysis of cryopreserved flagella and head plasma membrane protein from sea bream spermatozoa: Effect of antifreeze protein. PLoS ONE 2016, 9, e99992. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Iqbal, R.; Younis, M.; Ullah, N.; DeVries, A.L. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015, 157, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.U.; Ashiq, M.; Iqbal, R.; Ullah, N.; Akhter, S. Evaluation of antifreeze protein III for cryopreservation of NiliRavi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2014, 148, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shaliutina-Kolešová, A.; Dietrich, M.; Xian, M.; Nian, R. Seminal plasma transferrin effects on cryopreserved common carp Cyprinus carpio sperm and comparison with bovine serum albumin and antifreeze proteins. Anim. Reprod. Sci. 2019, 204, 125–130. [Google Scholar] [CrossRef]
- Martinez-Paramo, S.; Barbosa, V.; Perez-Cerezales, S.; Robles, V.; Herraez, M.P. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology 2009, 58, 128–133. [Google Scholar] [CrossRef]
- Amir, G.; Rubinsky, B.; Kassif, Y.; Horowitz, L.; Smolinsky, A.K.; Lavee, J. Preservation of myocyte structure and mitochondrial integrity in subzero cryopreservation of mammalian hearts for transplantation using antifreeze proteins—An electron microscopy study. Eur. Assoc. Cardio-Thorac. Surg. 2003, 24, 292–296. [Google Scholar] [CrossRef]
- Amir, G.; Horowitz, L.; Rubinsky, B.; Yousif, B.S.; Lavee, J.; Smolinsky, A.K. Sub zero non freezing cryopresevation of rat hearts using antifreeze protein I and antifreeze protein III. Cryobiology 2004, 48, 273–282. [Google Scholar] [CrossRef]
- Amir, G.; Rubinsky, B.; Horowitz, L.; Miller, L.; Leor, J.; Kassif, Y.; Mishaly, D.; Smolinsky, A.K.; Lavee, J. Prolonged 24-hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann. Thorac. Surg. 2004, 77, 1648–1655. [Google Scholar] [CrossRef]
- Muldrew, K.; Rewcastle, J.; Donnelly, B.J.; Saliken, J.C.; Liang, S.; Goldie, S.; Olson, M.; Baissalov, R.; Sandison, G. Flounder antifreeze peptides increase the efficacy of cryosurgery. Cryobiology 2001, 42, 182–189. [Google Scholar] [CrossRef]
- Bagis, H.; Akkoc, T.; Tass, A.; Ktoprakligil, D.A. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol. Reprod. Dev. 2008, 74, 608–613. [Google Scholar] [CrossRef]
- Fernanda, P.C.; Joaquin, I.R.; Greather, S.P.; Bravo, L.A. Properties and biotechnological applications of ice-binding proteins in bacteria, Environmental Microbiology. FEMS. J. 2016, 363. [Google Scholar] [CrossRef]
- Jing, T.; Wang, P.; Gao, X. Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography-tendem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction. Anal. Bioanal. Chem. 2009, 393, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.; Attard, E.; Sancelme, M. Ice nucleation activity of bacteria isolated from cloud water. Atoms Environ. 2013, 74, 392–400. [Google Scholar] [CrossRef]
- Cutler, A.J.; Saleem, M.; Kendall, E.; Gusta, L.V.; Georges, F.; Fletcher, G.L. Winter flounder antifreeze protein improves the cold hardiness of plant tissues. Plant Physiol. 1989, 135, 351–354. [Google Scholar] [CrossRef]

| Industrial Applications | Organism Source (Protein Type) | Main Functions |
|---|---|---|
| Food | Fish (type III) | Applied as recombinant protein to improve milk fermentation, especially for the storage of frozen yogurt and ice cream |
| Plant (type III) | Screened for proteolytic and biolytic activities to obtain a heat-stable protein | |
| Fish (type I) | Applied as recombinant protein to use in frozen food such as meat | |
| Medicine | Fish (type I) | Cryopreservation of rat organs |
| Fish (type III) | Cryopreservation of mammalian cells | |
| Fish (type I) | Cryopreservation of red blood cells | |
| Fish (type I) | Cryopreservation for subcutaneous tumor cells | |
| Agriculture | Bacteria | As biofertilizer to increase plant growth at cold temperature |
| AFGP | Inhibition of ice nucleating activity in Erwinia herbicola (a bacterium) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskandari, A.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture. Biomolecules 2020, 10, 1649. https://doi.org/10.3390/biom10121649
Eskandari A, Leow TC, Rahman MBA, Oslan SN. Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture. Biomolecules. 2020; 10(12):1649. https://doi.org/10.3390/biom10121649
Chicago/Turabian StyleEskandari, Azadeh, Thean Chor Leow, Mohd Basyaruddin Abdul Rahman, and Siti Nurbaya Oslan. 2020. "Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture" Biomolecules 10, no. 12: 1649. https://doi.org/10.3390/biom10121649
APA StyleEskandari, A., Leow, T. C., Rahman, M. B. A., & Oslan, S. N. (2020). Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture. Biomolecules, 10(12), 1649. https://doi.org/10.3390/biom10121649





