Brassica juncea L. (Mustard) Extract Silver NanoParticles and Knocking off Oxidative Stress, ProInflammatory Cytokine and Reverse DNA Genotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Authentication and Preparation of the Plant Extracts
2.3. LC/MS Ytentative analysis for ethanolic Extract of B. juncea L.
2.4. Synthesis of Mustard Silver Nanoparticles
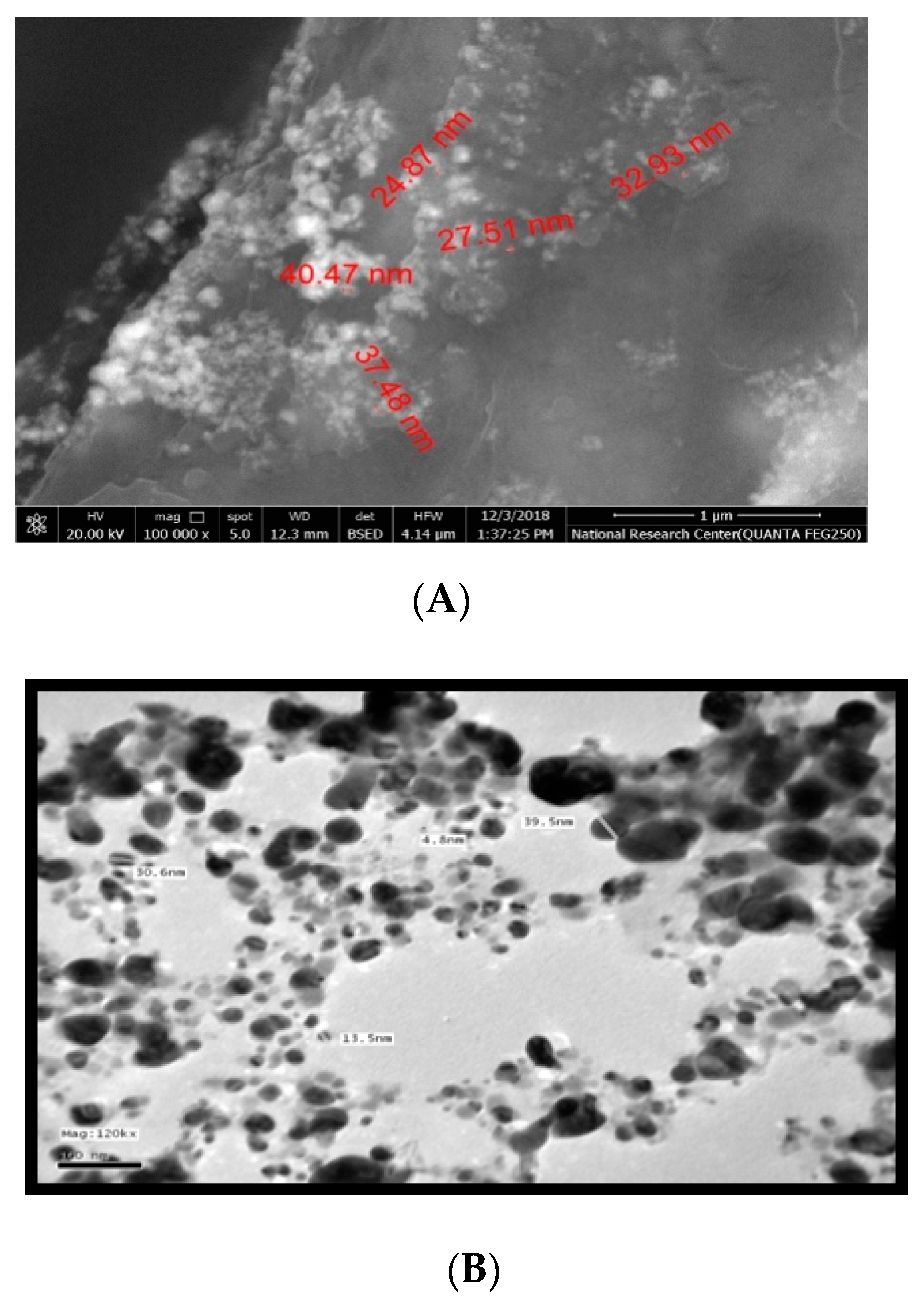
2.5. Characterization of Synthesized Silver Nanoparticles
2.6. Animals
Experimental protocol
- Group (G 1): Normal healthy cont. (3% Tween-80) and water, respectively
- Group (G 2): Intoxicated group was treated intraperitoneal (i.p.) with 350 mg/kg bw of freshly prepared TAA in a single shot [3], 5% glucose solution was added to the drinking water to avoid dehydration 24 h post-injection.
- Groups (G3, G4): were given orally; the standard plant extract and its nano forms (NMs) in a dose of I mL [100 mg/kg] for two weeks as a benchmark/control group of plant extract [12].
- Prophylactic groups (G5, G6): administered a dosage of 1 mL of standard mustard extract and/or its nanoform for two weeks prior to i.p. TAA insulting 350 mg/kg bw in one shot, by then continued with the same dosage of 1 mL of both extracts for two weeks.
- Treatment groups (G7, G8): were given orally; the standard plant extract and its nanoforms in a dose of 1 mL as a treatment for two weeks after (i.p.) single injection with the same assigned dose of TAA. Twenty-four hours following the last drug administration, blood samples were withdrawn from the retro-orbital plexus of the rats under light ether anesthesia. Then, rats were sacrificed by cervical dislocation under the same anesthesia for the collection of liver samples. A weighed part of the liver of each animal was rapidly dissected out, washed and homogenized using phosphate-buffered saline (PBS, 50 mM potassium phosphate, pH 7.5) at 4°C to produce a 20% homogenate. Liver homogenates were kept at −80°C until analysis. Another part of liver tissue was kept in 10% formalin-saline for histopathological and immunohistopathological examinations.
2.7. Biochemical Analysis
2.8. Histopathological Studies
Histopathological and Immunohistochemical Examinations
2.9. Statistical Analysis
3. Results
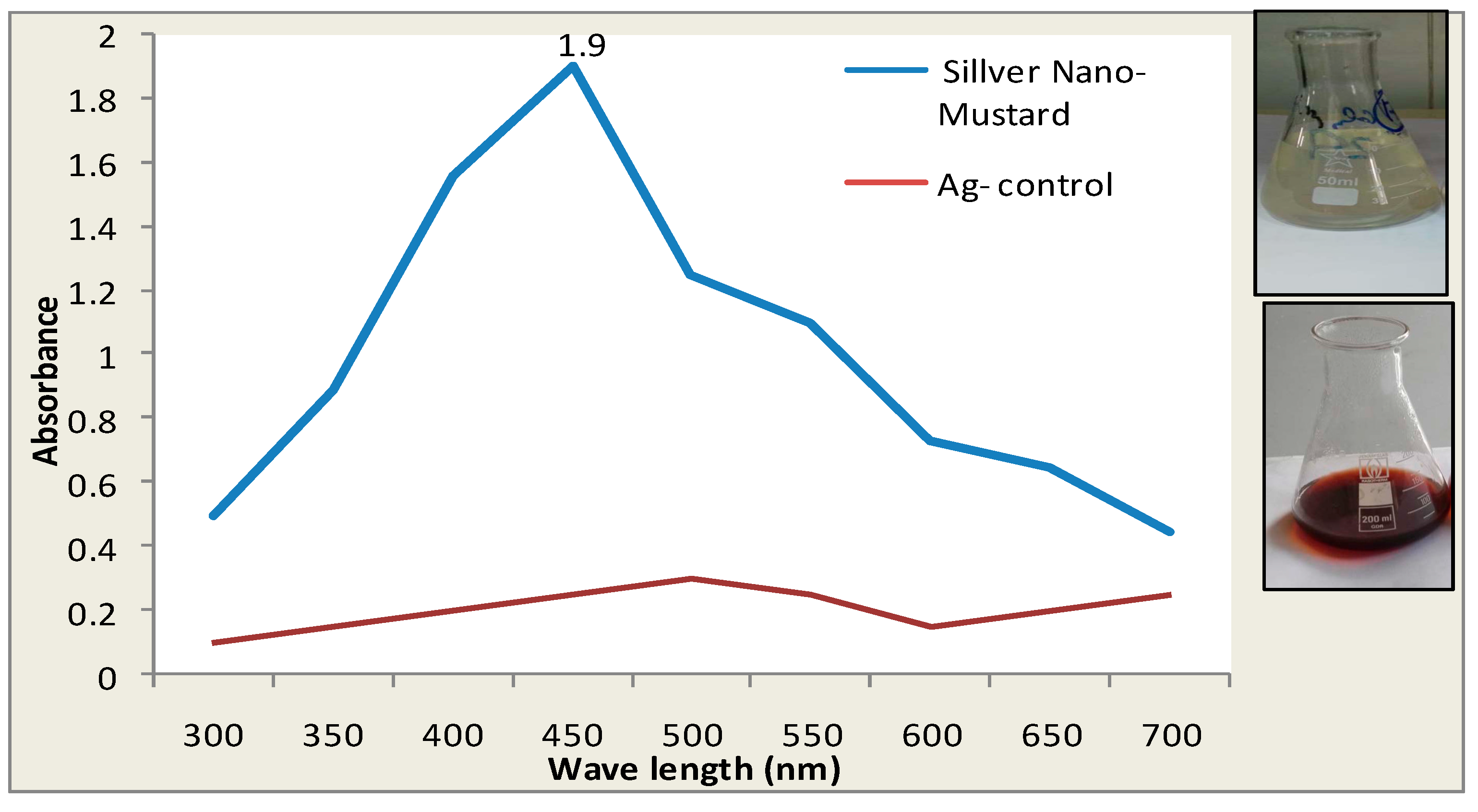
3.1. The Visual Observation of Color Changes of Silver Nano Mustard (B. juncea) Extract
3.2. Effect of Brassica juncea L. (Mustard) Extract and Its Nanoforms on Liver Functions
3.3. Effect of Brassica juncea L. (Mustard) Extract in Their Normal and Its Nanoforms on Lipid Profile Parameters
3.4. Effect of Brassica juncea L. (Mustard) Extract in Their Normal and Its Nanoforms on Oxidative Stress Markers and Inflammatory Cytokines
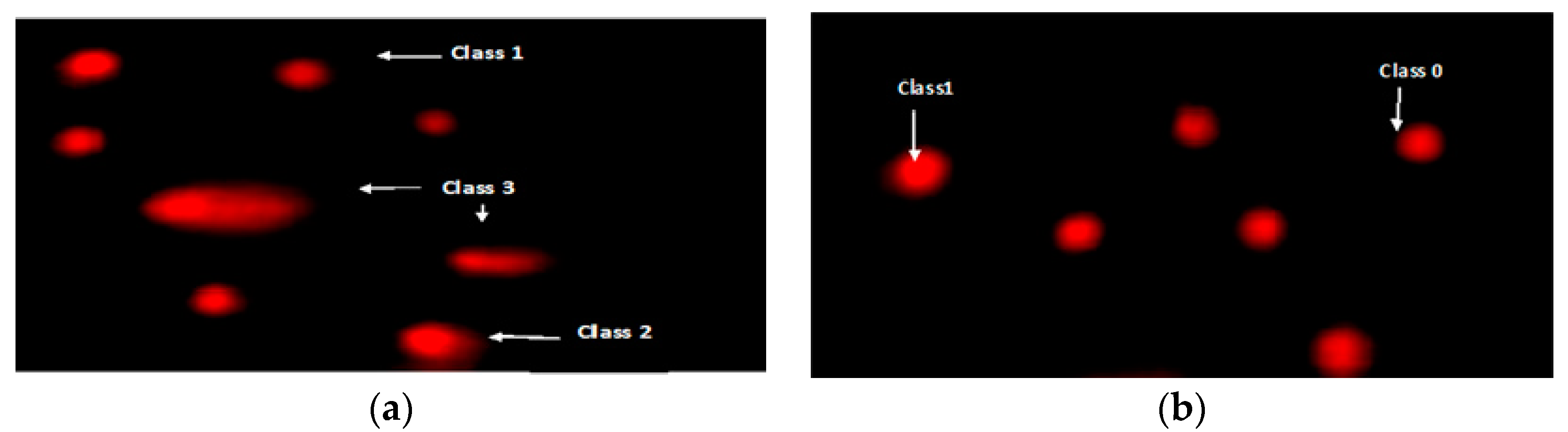
3.5. Effects of Brassica juncea L. (Mustard) Extract and Its Nanoparticles on DNA Degradation
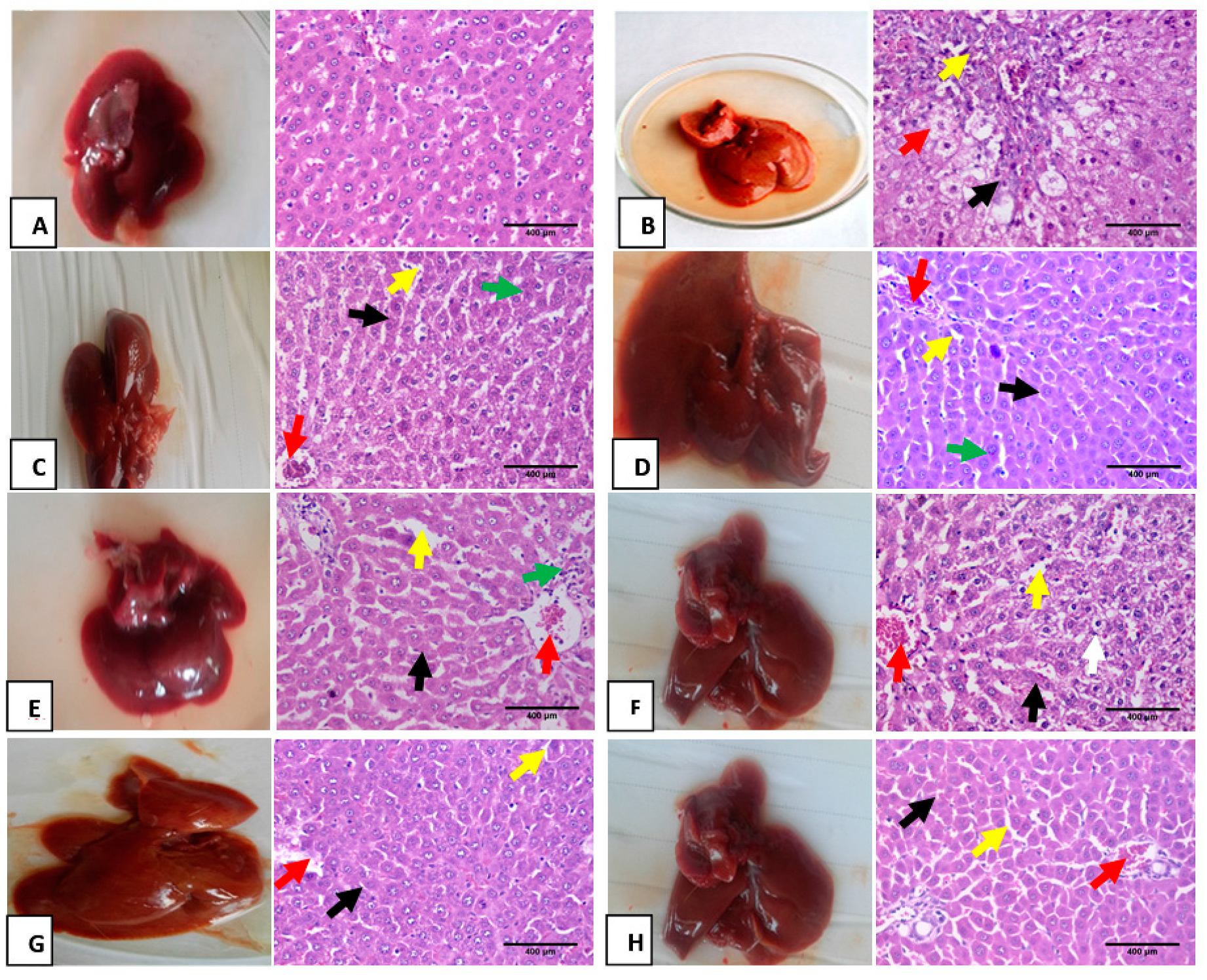
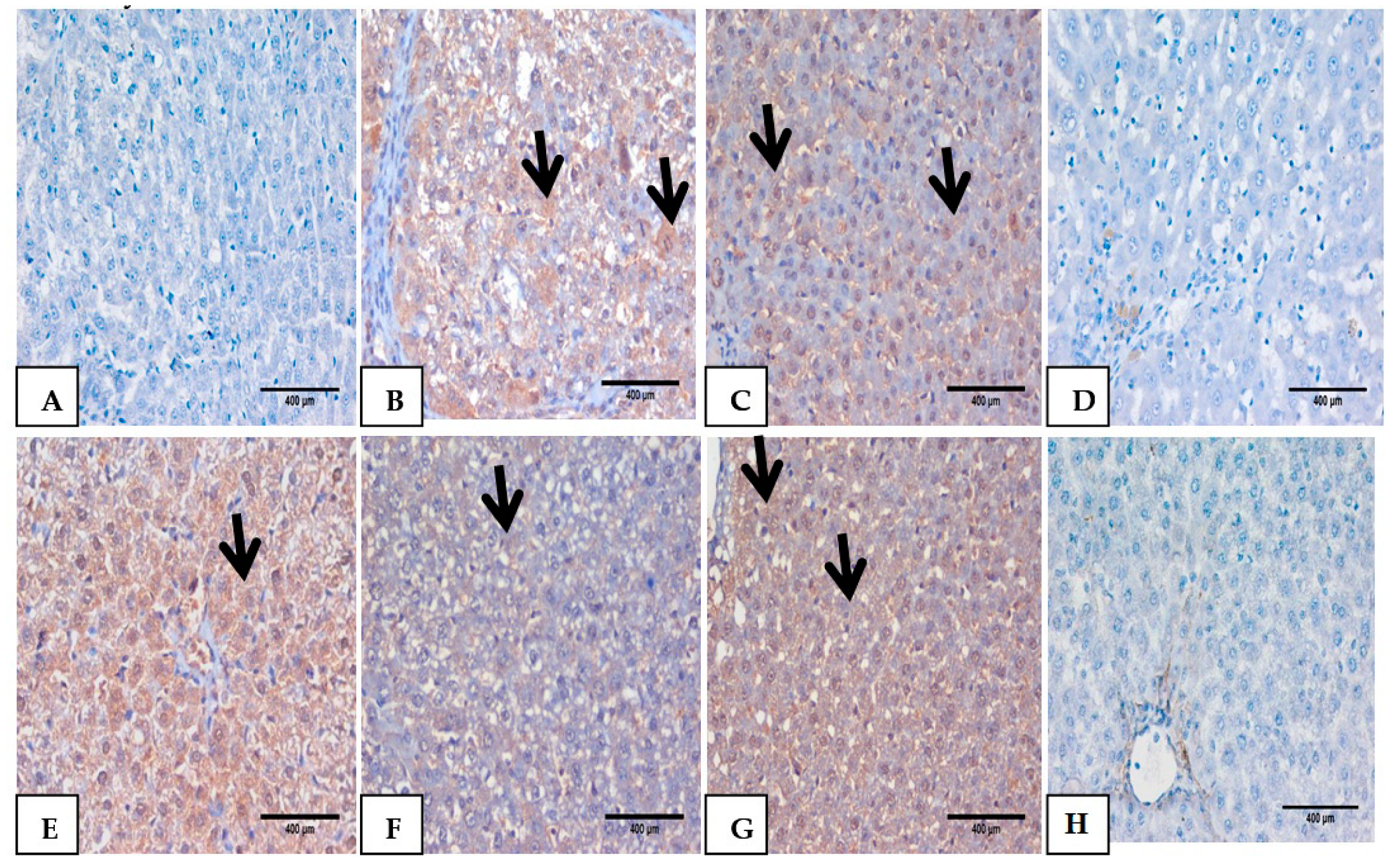
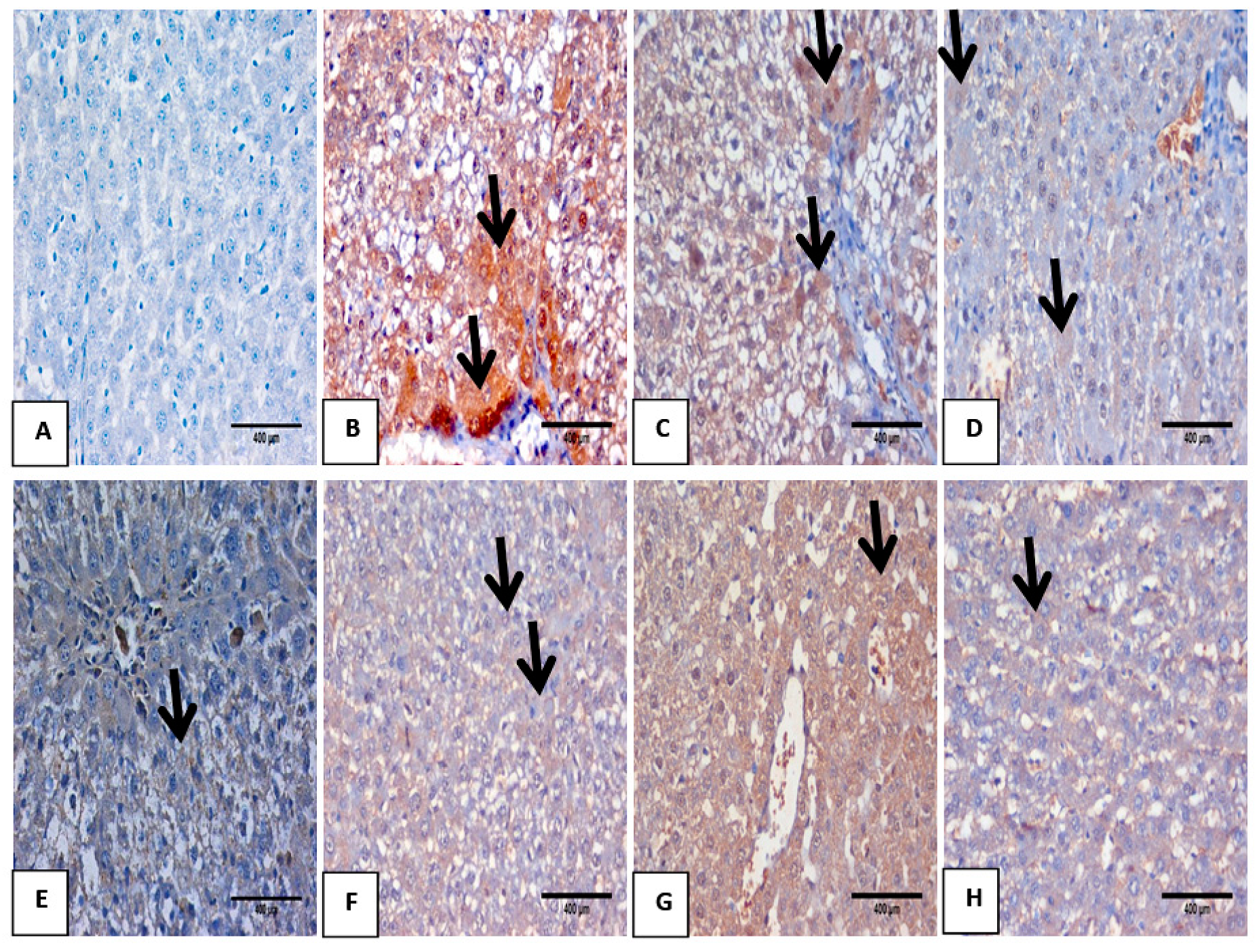
3.6. Hematoxylin and Eosin Pathological Examination
3.7. Impact of Mustard Extract and Its Nanoform on the Inflammatory Markers through Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Real, M.; Barnhill, M.S.; Higley, C.; Rosenberg, J.; Lewis, J.H. Drug-induced liver injury: Highlights of the recent literature. Drug Saf. 2019, 42, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.K.; Jena, G.B. Glibenclamide protects against thioacetamide-induced hepatic damage i Wistar rat: Investigation on NLRP3, MMP-2, and stellate cell activation. Naunyn Schmiedeberg Arch. Pharmacol. 2018, 391, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Koblihová, E.; Mrazova, I.; Vernerova, Z.; Ryska, M. Acute liver failure induced by thioacetamide: Selection of optimal dosage in Wistar and Lewis rats. Physiol. Res. 2014, 63, 491–503. [Google Scholar] [CrossRef]
- De Minicis, S.; Candelaresi, C.; Agostinelli, L.; Taffetani, S.; Saccomanno, S.; Rychlicki, C.; Trozzi, L.; Marzioni, M.; Benedetti, A.; Svegliati-Baroni, G. Endoplasmic Reticulum stress induces hepatic stellate cell apoptosis and contributes to fibrosis resolution. Liver Int. 2012, 32, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Crosas-Molist, E.; Fabregat, I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015, 6, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Khan, M.Q.; Hussain, N.; Habib, T. Antibacterial and antifungal potential of leaves and twigs of Viscum album L. J. Med. Plants Res. 2011, 5, 5545–5549. [Google Scholar]
- Parikh, H.; Pandita, N.; Khanna, A. Phytoextract of Indian mustard seeds acts by suppressing the generation of ROS against acetaminophen-induced hepatotoxicity in HepG2 cells. Pharm. Biol. 2015, 53, 975–984. [Google Scholar] [CrossRef]
- Manohar, P.R.; Pushpan, R.; Rohini, S. MustardanditsusesinAyurveda. Indian J. Tradit. Know. 2009, 8, 400–404. [Google Scholar]
- Kumar, V.; Thakur, A.K.; Barothia, N.D.; Chatterjee, S.S. Therapeutic potentials of Brassica juncea: An overview. Tang 2011, 1, e2. [Google Scholar] [CrossRef]
- Walia, A.; Malan, R.; Saini, S.; Saini, V.; Gupta, S. Hepatoprotective effects from the leaf extracts of Brassica juncea in CCl4 induced rat model. Pharm. Sin. 2011, 2, 274–285. [Google Scholar]
- Azubuike, N.C.; Okwuosa, C.N.; Maduakor, U.C.; Onwukwe, O.S.; Onyemelukwe, A.O.; Ogu, C.O.; Akande, A. Effects of methanolic extract of Brassica juncea seeds on biochemical parameters and histological integrity of the heart and liver of albino rats. Int. J. Morphol. 2019, 37, 237–240. [Google Scholar] [CrossRef]
- El-Hagrassy, A.M.; Osman, A.F.; Mhafouz Eskander, D.; Nassar, M.I. Chemical Constituents and Cytotoxic Evaluation of Abelmoschusesculentus L. Leaves Grown in Egypt. J. Chem. Pharm. 2019, 11, 1–13. [Google Scholar]
- El-Hagrassy, A.M.; Elkhateeb, A.; Sameh, H.R.; El-Sayed, S.A.-H.; Marzouk, M.M. LC–ESI-MS profile, anti-oxidant activity and cytotoxic screening of Oligomerislinifolia (Vahl) Macbr. (Resedaceae). J. Appl. Pharm. Sci. 2017, 7, 43–47. [Google Scholar]
- Marzouk, M.M.; Sameh, H.R.; Elkhateeb, A.; El-Shabrawy, M.; El-Sayed, S.A.-H.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar]
- Hassan, S.A.; Hammam, O.; Hussein, S.O.; Aziz, W.M. The Conquering influence of the Nano extracts of pomegranate peels and pistachio leaves in amelioration of acute liver failure. bioRxiv 2020. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Arunachalam, K.D. Memecylonedule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Method Enzym. 1978, 52, 302–310. [Google Scholar]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Moshage, H.; Kok, B.; Huizenga, J.R.; Jansen, P.L. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin. Chem. 1995, 41, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Masoomi, K.M.; Jafari, S.M.; Zaree, M.A.; Jafari, S.A.; Khatibi, S.R. Effect of acute toxicity of cadmium in mice kidney cells. Iran. J. Toxicol. 2012, 6, 691–698. [Google Scholar]
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to histopathological evaluation. In Drug Safety Evaluation; Humana Press: Totowa, NJ, USA, 2011; pp. 69–82. [Google Scholar]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and applications of noble metal nanoparticles: A review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Mali, R.G. Cleomeviscosa (wild mustard): A review on ethnobotany, phytochemistry, and pharmacology. Pharm. Biol. 2010, 48, 105–112. [Google Scholar] [CrossRef]
- Melrose, J. The Glucosinolates: A SulphurGlucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Muzaffar, S. Phytochemical Composition and Antioxidant Potential of Brassica. In Brassica Germplasm: Breeding and Utilization; Intech Open: London, UK, 2018. [Google Scholar]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant activity of extracts from Acacia confusa bark and heart wood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Simon-Giavarotti, K.A.; Giavarotti, L.; Gomes, L.F.; Lima, A.F.; Veridiano, A.M.; Garcia, E.; Mora, A.O.A.; Fernández, V.; Videla, L.A.; Junqueira, V.B. Enhancement of lindane-induced liver oxidative stress and hepatotoxicity by thyroid hormone is reduced by gadolinium chloride. Free Radic. Res. 2002, 36, 1033–1039. [Google Scholar] [CrossRef]
- Yang, H.Y.; Kim, K.S.; Lee, Y.H.; Park, J.H.; Kim, J.H.; Lee, S.Y.; Kim, Y.M.; Kim, I.S.; Kacew, S.; Lee, B.M.; et al. Dendropanaxmorbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019, 15, 800. [Google Scholar] [CrossRef]
- Khaled, H. Possible hepatoprotective Effects of Mustard Seed Extract against Paracetamol-Induced Liver Injury in Male Albino Rat. Catrina Int. J. Environ. Sci. 2018, 17, 85–90. [Google Scholar]
- Le, B.; Anh, P.T.; Yang, S.H. Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives. Foods 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; Du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, H.A.; Lee, J. The effects of Brassica Juncea, L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr. Res. Pract. 2018, 12, 298–306. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Jangi, S.; Otterbein, L.; Robson, S. The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int. J. Biochem. Cell Biol. 2013, 45, 2843–2851. [Google Scholar] [CrossRef]
- Horsfall, L.J.; Nazareth, I.; Pereira, S.P.; Petersen, I. Gilbert’s syndrome and the risk of death: A population-based cohort study. J. Gastroenterol. Hepatol. 2013, 28, 1643–1647. [Google Scholar] [CrossRef]
- Mao, F.; Zhu, X.; Lu, B.; Li, Y. The association between serum bilirubin level and electrochemical skin conductance in Chinese patients with type 2 diabetes. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef]
- Rawat, V.; Bortolussi, G.; Gazzin, S.; Tiribelli, C.; Muro, A.F. Bilirubin-induced oxidative stress leads to DNA damage in the cerebellum of hyperbilirubinemic neonatal mice and activates DNA double-strand break repair pathways in human cells. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Zakaria, S.; Fahmy, A. Can chronic nitric oxide inhibition improve liver and renal dysfunction in bile duct ligated rats? Adv. Pharmacol. Pharm. Sci. 2015, 2015. [Google Scholar] [CrossRef][Green Version]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- López-García, M.P.; Sanz-González, S.M. Peroxynitrite generated from constitutive nitric oxide synthase mediates the early biochemical injury in short-term cultured hepatocytes. FEBS Lett. 2000, 466, 187–191. [Google Scholar] [CrossRef]
- Mannaa, F.A.G.; Abdel-Wahhab, K. Physiological potential of cytokines and liver damages. Hepatoma Res. 2016, 2, 131–143. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Yang, J.; Yang, X.; Qin, S.; Zeng, H.; Wu, X.; Tang, S.; Zeng, W. Alpha-Fetoprotein as a Predictive Marker for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. J. Gastroenterol. Hepatol. 2018, 2018. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A.; Alamro, A.A.; Ganaie, M.A. Amelioration of thioacetamide-induced liver toxicity in Wistar rats by rutin. Int. J. Immunopathol. Pharmacol. 2017, 30, 207–214. [Google Scholar] [CrossRef]
- Ansar, S.; Siddiqi, N.J.; Zargar, S.; Ganaie, M.A.; Abudawood, M. Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistarrats. BMC Complement Altern. Med. 2016, 16, 327. [Google Scholar] [CrossRef]
- Surendran, S.P.; Thomas, R.G.; Moon, M.J.; Jeong, Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997. [Google Scholar] [CrossRef]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophoraapiculata. Int. J. Nanomed. 2019, 14, 3517. [Google Scholar] [CrossRef]
- Duy, N.L.B.; Trang, D.T.D. Preliminary phytochemical, acute oral toxicity and anticonvulsant activity of the seed extract of Brassica juncea. Eur. J. Med. Plants 2016, 14, 1–9. [Google Scholar] [CrossRef]

| Peak No | Rt (min.) | [M] | [M − H]− | Fragments m/z | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 13.3 | 180 | 179 | 135 | Caffeic acid |
| 2 | 15.22 | 164 | 163 | 119 | p-Coumaric acid |
| 3 | 16.51 | 224 | 223 | 179 | Sinapic acid |
| 4 | 18.11 | 194 | 193 | 149 | Ferulic acid |
| 5 | 19.21 | 192 | 191 | ----- | Quinic acid |
| 6 | 19.52 | 354 | 353 | 191,179,135 | 3-caffeoylqunic acid |
| 7 | 20.1 | 368 | 367 | 191, 193, 149 | 3-feruloylquinic acid |
| 8 | 21.2 | 356 | 355 | 193, 149 | Ferulic acid hexoside |
| 9 | 21.44 | 342 | 341 | 163, 135 | Caffeic acid hexoside |
| 10 | 22.31 | 386 | 385 | 203, 179 | Sinapic acid hexoside |
| 11 | 23.23 | 280 | 279 | 163, 133, 119 | p-Coumaroyl malic acid |
| 12 | 23.51 | 310 | 309 | 193, 149, 133 | Feruloyl malic acid |
| 13 | 24.12 | 340 | 339 | 223, 179, 133 | Sinapoy lmalic acid |
| 14 | 25.05 | 360 | 359 | ---------- | Rosmarinic acid |
| 15 | 25.55 | 287 | 286 | ---- | Kaempferol |
| 16 | 25.41 | 302 | 301 | ---- | Quercetin |
| 17 | 26.51 | 610 | 609 | 477, 285 | Kaempferol-O-dihexoside |
| 18 | 27.61 | 448 | 447 | 285 | Kaempferol-O-hexoside |
| 19 | 28.52 | 772 | 771 | 609, 285 | Kaempferol-O-cafeoyl dihexoside |
| 20 | 29.50 | 756 | 755 | 609, 447, 285 | Kaempferol-O-p-coumaroyl dihexoside |
| 21 | 29.71 | 802 | 801 | 609, 447, 285 | Kaempferol-O-hydroxyferuloyl dihexoside |
| 22 | 29.81 | 788 | 787 | 625, 463,301 | Quercetin-O-trihexoside |
| 23 | 30.10 | 626 | 625 | 463, 301 | Quercetin-O-dihexoside |
| 24 | 31.21 | 786 | 785 | 609, 447, 285 | Kaempferol-O-feruloyl dihexoside |
| 25 | 32.21 | 610 | 609 | 301 | Quercetin-3-O-rutinoside |
| 26 | 33.32 | 464 | 463 | 301 | Quercetin-O-hexoside |
| 27 | 33.50 | 918 | 917 | 755, 609, 447, 285 | Kaempferol-O-p-coumaroyl trihexoside |
| 28 | 33.80 | 964 | 963 | 801, 625, 301 | Quercetin-O-feruloyl trihexoside |
| Groups Parameters | ALT(U/mL) Mean ± SD | AST(U/mL) Mean ± SD | ALP(U/L) Mean ± SD |
|---|---|---|---|
| Improvement% | Improvement% | Improvement% | |
| Control (-ve) | 53 b ± 7.4 | 56.2 b ± 8.07 | 115.4 d ± 14.70 |
| TAA (+ve) | 124 a ± 10.20 | 156.6 a ± 9.64 | 215.4 a ± 9.36 |
| Control-Mustard (Ms) | 51.4 b ± 8.45 | 61.2 b ± 6.05 | 138.2 c ± 6.18 |
| Control- Ag nano Mustard(NMs) | 62.8 b ± 7.89 | 68.8 b ± 8.28 | 125.4 cd ± 13.65 |
| Prophylactic-Mustard(PMs) | 68 b ± 8.60 (105%) | 66.6 b ± 6.35 (163%) | 171 b ± 12.74 (38%) |
| Prophylactic Ag-nano-Mustard(PNMs) | 62.2 b± 9.20 (116%) | 62.8 b ± 8.05 (168%) | 132.4 cd ± 7.04 (72%) |
| Treated-Mustard(TMs) | 58.2 b ± 8.50 (125%) | 54.6 b ± 7.75 (182%) | 141.2 c ± 5.26 (64%) |
| Treated Ag nano-Mustard(TNMs) | 55.4 b ± 11.09 (130%) | 57.8 b ± 8.07 (177%) | 121.40 cd ± 15.63 (82%) |
| Groups Parameters | Total Cholesterol (mg/dL) Mean± SD | Total Glycerides (mg/dL) Mean± SD | Total Lipids (g/dL) Mean± SD | Total Bilirubin (mg/dL) Mean± SD |
|---|---|---|---|---|
| Improvement% | Improvement% | Improvement% | Improvement% | |
| Control (−ve) | 40.36 d ± 1.8 | 100.6 e ± 12.02 | 0.39 d ± 0.035 | 1.15 b ± 0.095 |
| TAA (+ve) | 90.25 a ± 2.37 | 283.2 a ± 18.99 | 0.92 a ± 0.036 | 2.34 a ± 0.125 |
| Control-Mustard(Ms) | 58.81 b ± 9.45 | 135.8 d ± 12.40 | 0.44 d ± 0.035 | 1.22 b ± 0.09 |
| Control-Ag nano Mustard(NMs) | 44.65 cd ± 3.51 | 103.6 e ± 12.70 | 0.42 d ± 0.025 | 1.17 b ± 0.065 |
| Prophylactic-Mustard(PMs) | 56.4 b ± 3.04 (85%) | 196 b ± 23.60 (87%) | 0.66 b ± 0.050 (67%) | 1.25 b ± 0.11 (95%) |
| Prophylactic-Ag nano-Mustard(PNMs) | 50.2 c ± 3.70 (99%) | 166.8 c ± 16.60 (116%) | 0.59 c ± 0.040 (85%) | 1.36 b ± 0.095 (85%) |
| Treatment-Mustard(TMs) | 60.5 b ± 3.65 (73%) | 193.8 b ± 12.05 (89%) | 0.71 b ± 0.052 (54%) | 1.29 b ± 0.135 (91%) |
| Treatment-Ag nano-Mustard(TNMs) | 57.52 b ± 4.085 (80%) | 173.6 bc ± 16 (109%) | 0.66 b ± 0.070 (67%) | 1.30 b ± 0.11 (90%) |
| Groups Parameters | SOD (Umol/mg Protein) Mean± SD | Glutathione (ug/mg Protein) Mean± SD | Lipid Peroxidation (Umol/mg Protein) Mean± SD | Nitric oxide (mmol/g Tissue) Mean± SD | IL6 (Pg/mL) Mean± SD | TNF-α (Pg/mL) Mean± SD |
|---|---|---|---|---|---|---|
| Improvement% | Improvement% | Improvement% | Improvement% | Improvement% | Improvement% | |
| Control (-ve) | 125.21 a ± 8.60 | 25.90 a ± 3.1 | 3.03 c ± 0.17 | 11.28 d ± 1.70 | 98.87 f ± 2.35 | 62.38 f ± 1.71 |
| TAA (+ve) | 53.42 d ± 7.25 | 10.10 c ± 2.05 | 7.5 a ± 0.47 | 34.96 a ± 2.25 | 236.77 a ± 9.95 | 159.9 a ± 2.14 |
| Control-Mustard (Ms) | 98.45 bc ± 4.03 | 23.17 a ± 2.7 | 4.11 b± 0.51 | 18.36 b ± 1.9 | 125.18 d ± 5.33 | 75.91 e ± 5.46 |
| Control- Ag nano Mustard(NMs) | 92.08 c± 8.60 | 24.98 a ± 1.8 | 4.14 b ± 0.25 | 13.78 cd ± 2.24 | 108.83 e ± 5.18 | 73.48 e ± 4.61 |
| Prophylactic-Mustard(PMs) | 90.63 c ± 9.64 (29%) | 16.72 b ± 1.65 (26%) | 4.55 b ± 0.25 (97%) | 18.90 b ± 2.50 (142%) | 155.54 b ± 4.52 (82%) | 139.86 b ± 5.15 (32%) |
| Prophylactic Ag nano-Mustard(PNMs) | 94.08 c ± 7.35 (32%) | 24.68 a ± 2.9 (56%) | 3.89 b ± 0.30 (119%) | 15.97 bc ± 1.70 (168%) | 141.33 c ± 5.04 (97%) | 127.61 c ± 5.14 (51%) |
| Treatment-Mustard(TMs) | 105.7 bc ± 9.85 (42%) | 26.28 a ± 2.85 (62%) | 3.90 b ± 0.49 (118%) | 18.35 b ± 2.70 (147%) | 148.05 c ± 4.52 (90%) | 135.65 b ± 3.85 (39%) |
| Treatment Ag nano-Mustard(TNMs) | 111.09 b ±10.85 (46%) | 25.61 a ± 3.9 (60%) | 4.12 b ± 0.52 (112%) | 16.43 bc ± 1.44 (164%) | 120.38 d ± 6.54 (118%) | 122.15 d ± 3.07 (60%) |
| Treatment | No. of Cells | Class ¥ of comet | DNA Damaged Cells (Mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
| Analyzed | Total Comets | 0 | 1 | 2 | 3 | ||
| Control negative | 500 | 34 | 466 | 23 | 11 | 0 | 6.83 ± 0.11 d |
| TAA Control positive | 500 | 124 | 376 | 32 | 44 | 48 | 24.81 ± 0.82 a |
| Ms | 500 | 83 | 417 | 35 | 26 | 22 | 16.62 ± 0.46 b |
| AgNMs | 500 | 52 | 448 | 19 | 18 | 15 | 10.43 ± 0.41 c |
| Prophylactic-mustard PMs | 500 | 79 | 421 | 33 | 25 | 21 | 15.82 ± 0.61 b |
| Prophylactic Ag nano-mustard PNMs | 500 | 59 | 441 | 21 | 23 | 15 | 11.80 ± 0.44 c |
| Treatment TMs | 500 | 67 | 433 | 27 | 23 | 17 | 13.44 ± 0.36 bc |
| Treatment T AgNMs | 500 | 51 | 449 | 17 | 19 | 15 | 10.20 ± 0.28 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.A.; Hagrassi, A.M.E.; Hammam, O.; Soliman, A.M.; Ezzeldin, E.; Aziz, W.M. Brassica juncea L. (Mustard) Extract Silver NanoParticles and Knocking off Oxidative Stress, ProInflammatory Cytokine and Reverse DNA Genotoxicity. Biomolecules 2020, 10, 1650. https://doi.org/10.3390/biom10121650
Hassan SA, Hagrassi AME, Hammam O, Soliman AM, Ezzeldin E, Aziz WM. Brassica juncea L. (Mustard) Extract Silver NanoParticles and Knocking off Oxidative Stress, ProInflammatory Cytokine and Reverse DNA Genotoxicity. Biomolecules. 2020; 10(12):1650. https://doi.org/10.3390/biom10121650
Chicago/Turabian StyleHassan, Sohair Aly, Ali Mohamed El Hagrassi, Olfat Hammam, Abdelmohsen M. Soliman, Essam Ezzeldin, and Wessam Magdi Aziz. 2020. "Brassica juncea L. (Mustard) Extract Silver NanoParticles and Knocking off Oxidative Stress, ProInflammatory Cytokine and Reverse DNA Genotoxicity" Biomolecules 10, no. 12: 1650. https://doi.org/10.3390/biom10121650
APA StyleHassan, S. A., Hagrassi, A. M. E., Hammam, O., Soliman, A. M., Ezzeldin, E., & Aziz, W. M. (2020). Brassica juncea L. (Mustard) Extract Silver NanoParticles and Knocking off Oxidative Stress, ProInflammatory Cytokine and Reverse DNA Genotoxicity. Biomolecules, 10(12), 1650. https://doi.org/10.3390/biom10121650






