A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wild Type (WT) and Mutant RNA Transcription
2.3. Oocyte Preparation and Injection
2.4. Two-Electrode Voltage Clamp Electrophysiology
2.5. Substrate and Agonist Concentration Responses
2.6. Stop-Flow Recording
2.7. Data Analysis
3. Results
3.1. Glycine-Gated GlyR Peak Currents and Stopped-Flow Currents Are Reduced in Co-expressed Oocytes
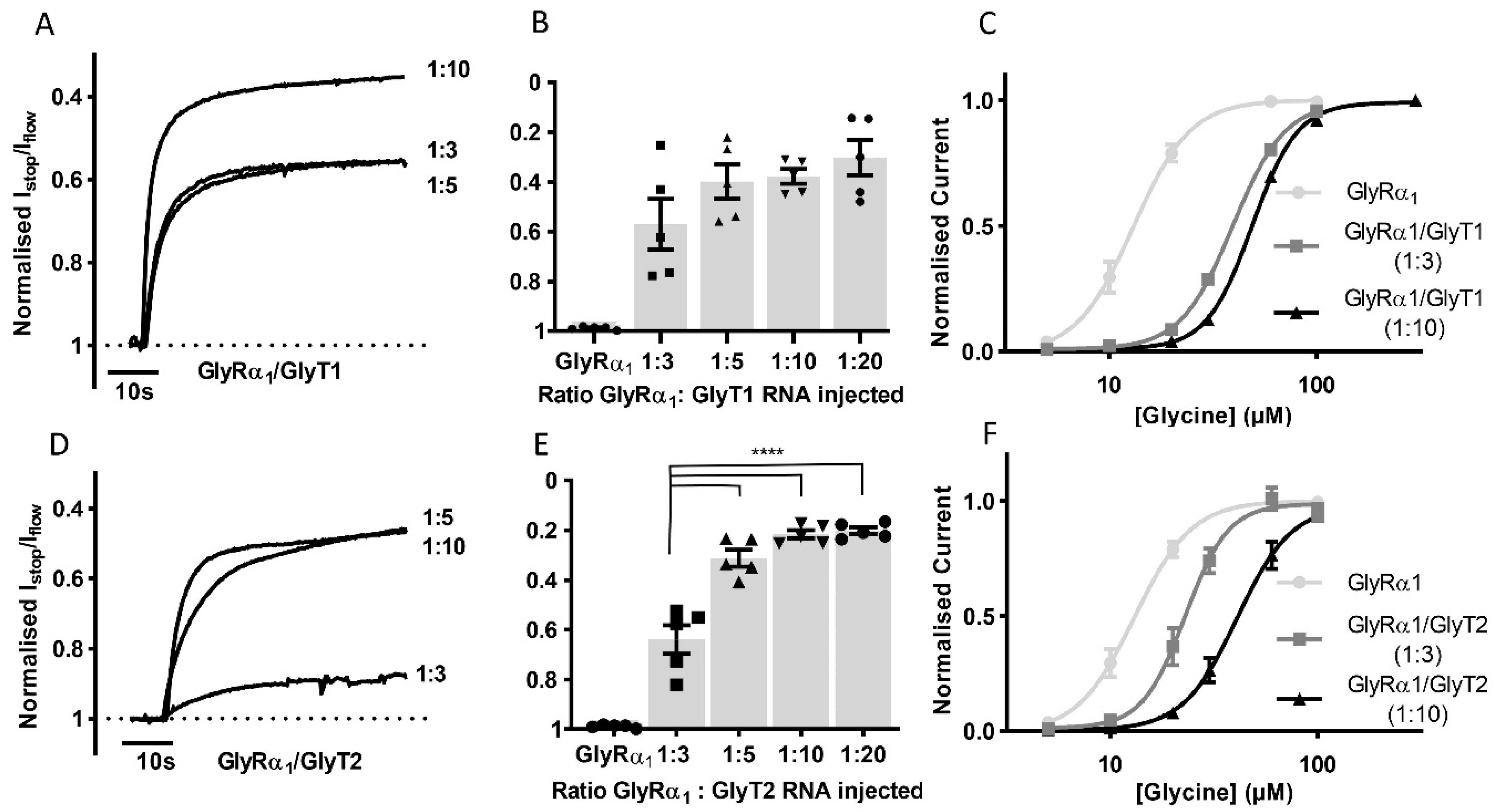
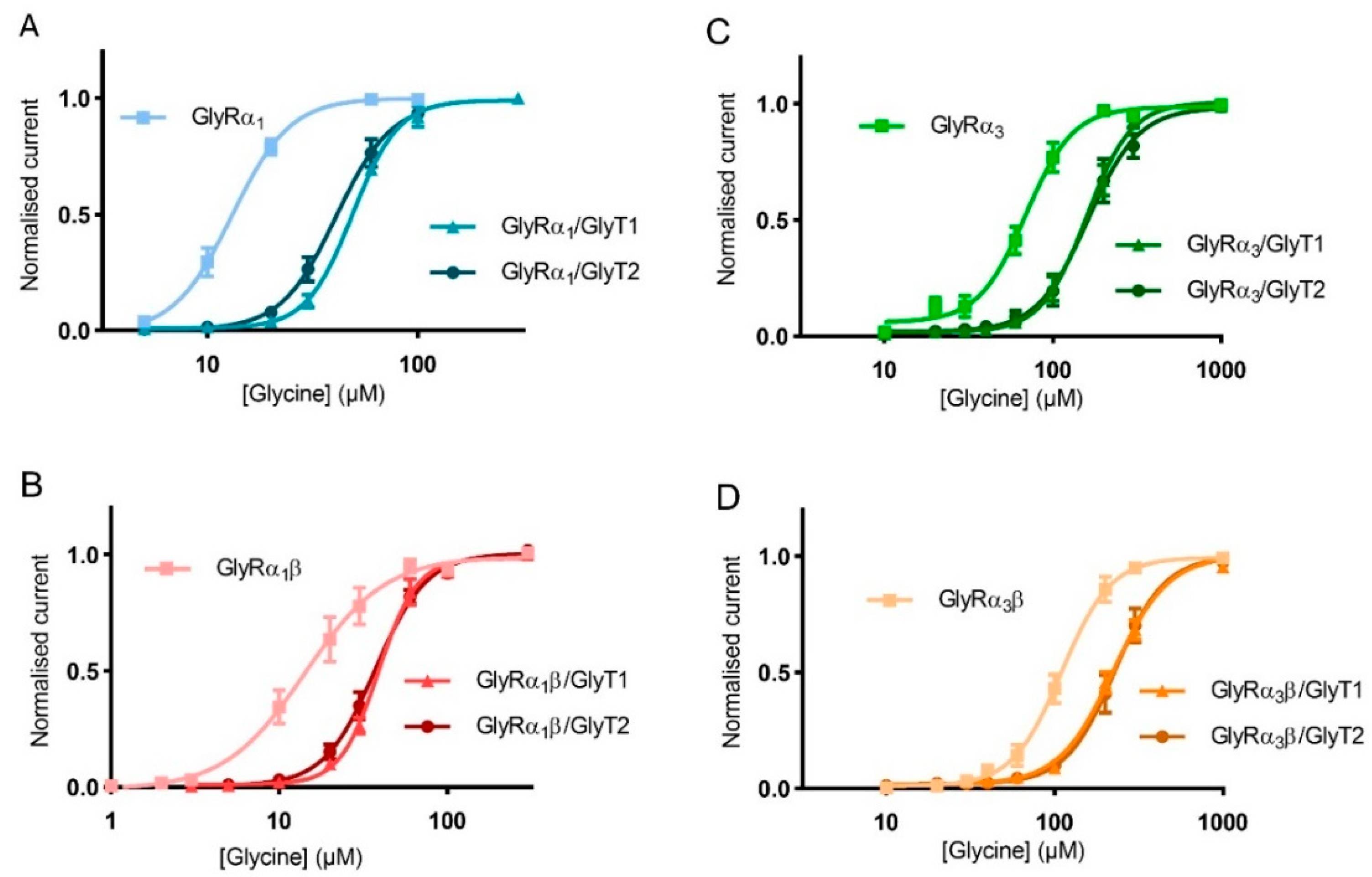
3.2. Stop-Flow and Fast-Flow Reduction of Glycine Gated Currents Are Concentration Dependent
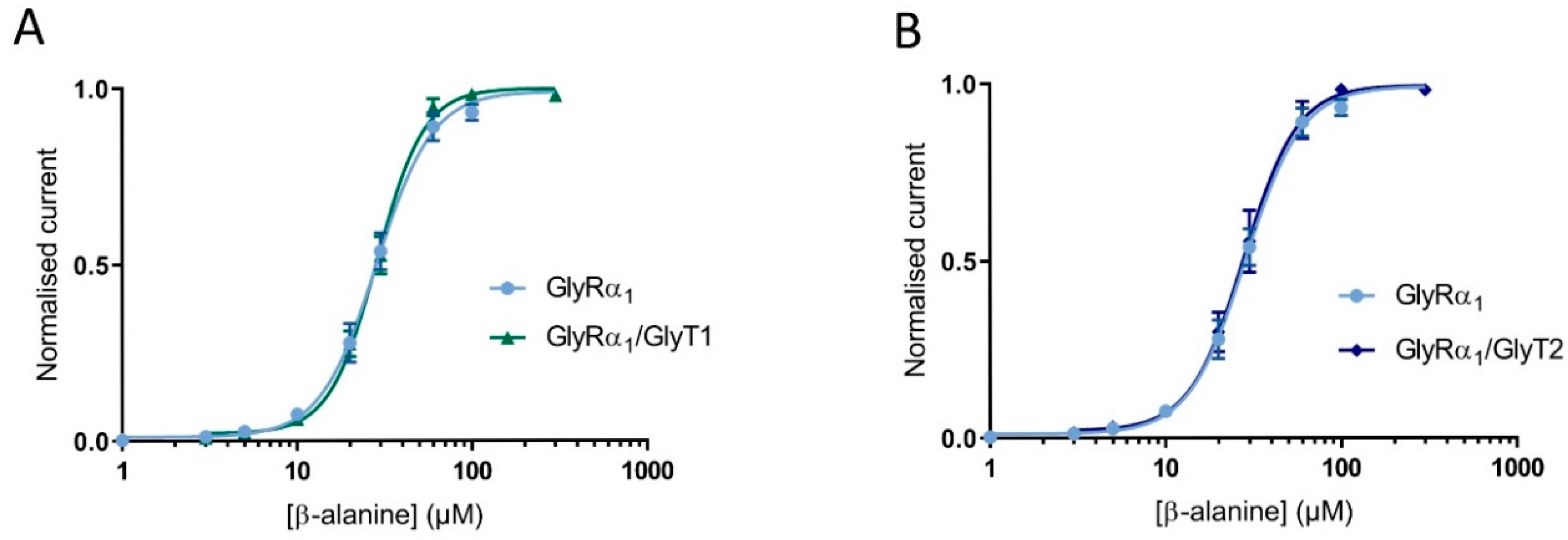
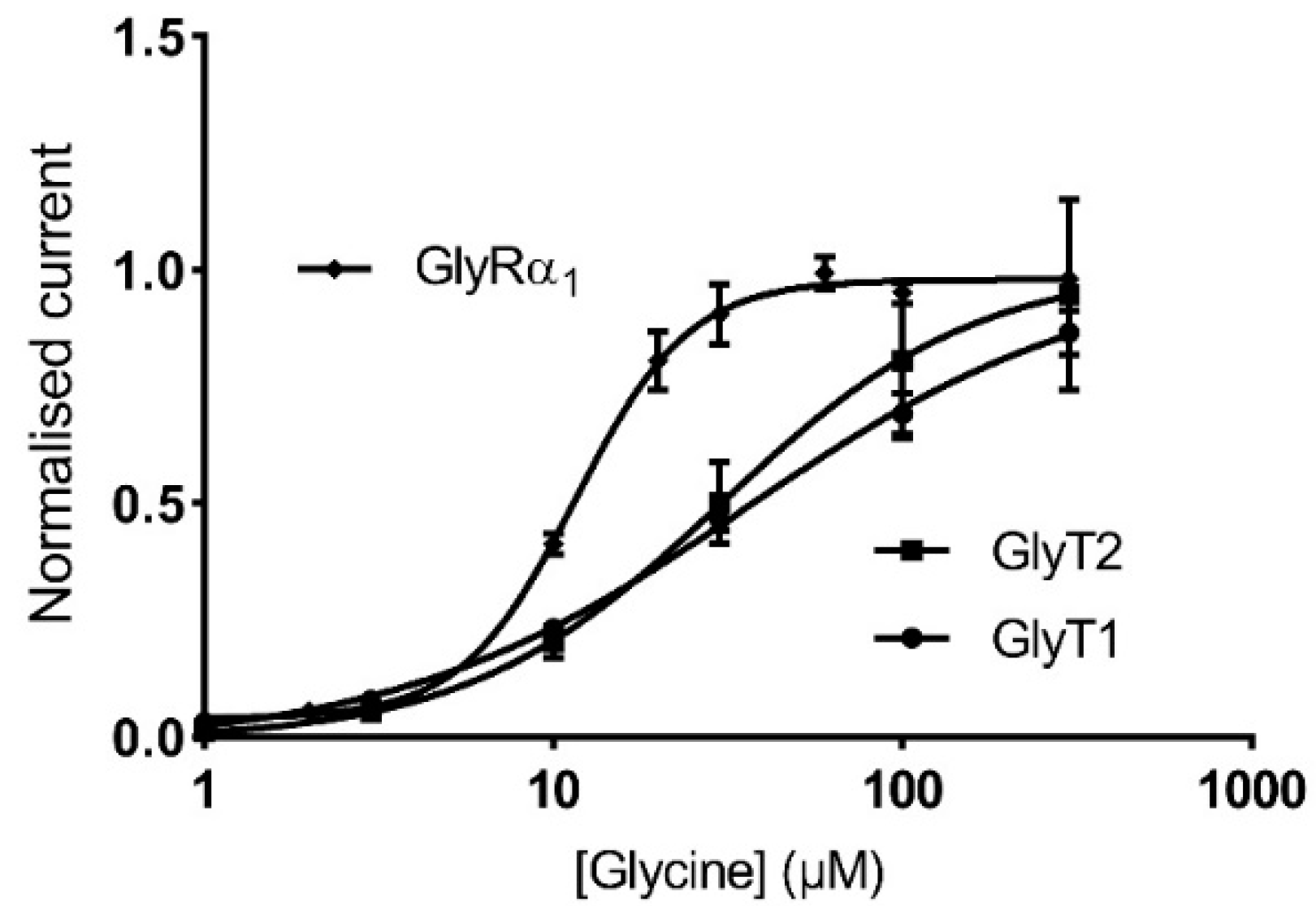
3.3. Glycine Transporter Density Affects Degree of GlyR Modulation and Is Dependent on the Presence of a GlyT Transportable Substrate
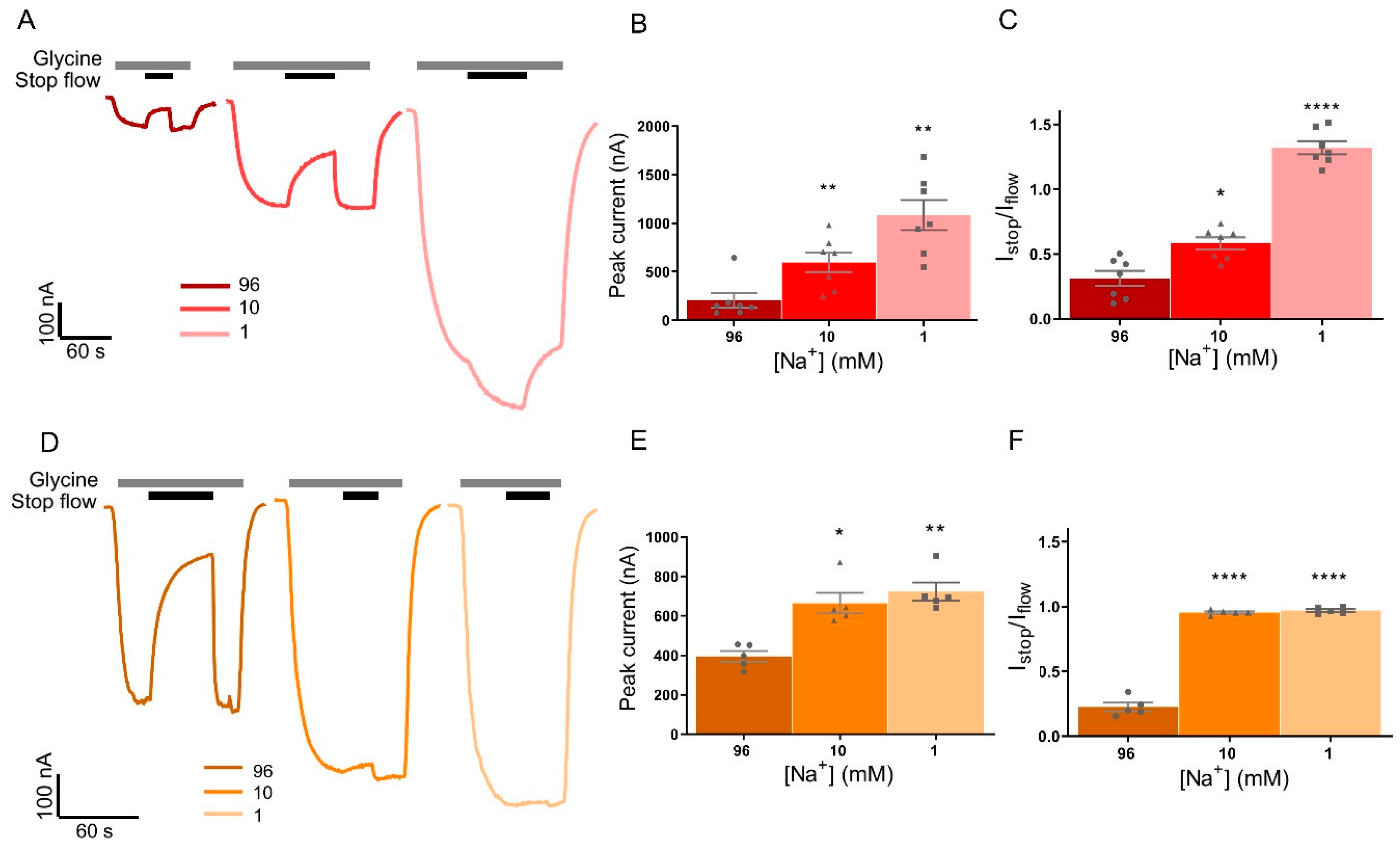
3.4. Stop-Flow and Fast-Flow Reduction of Current in GlyRα1 Is Reliant on Na+ Dependent GlyT Driving Force
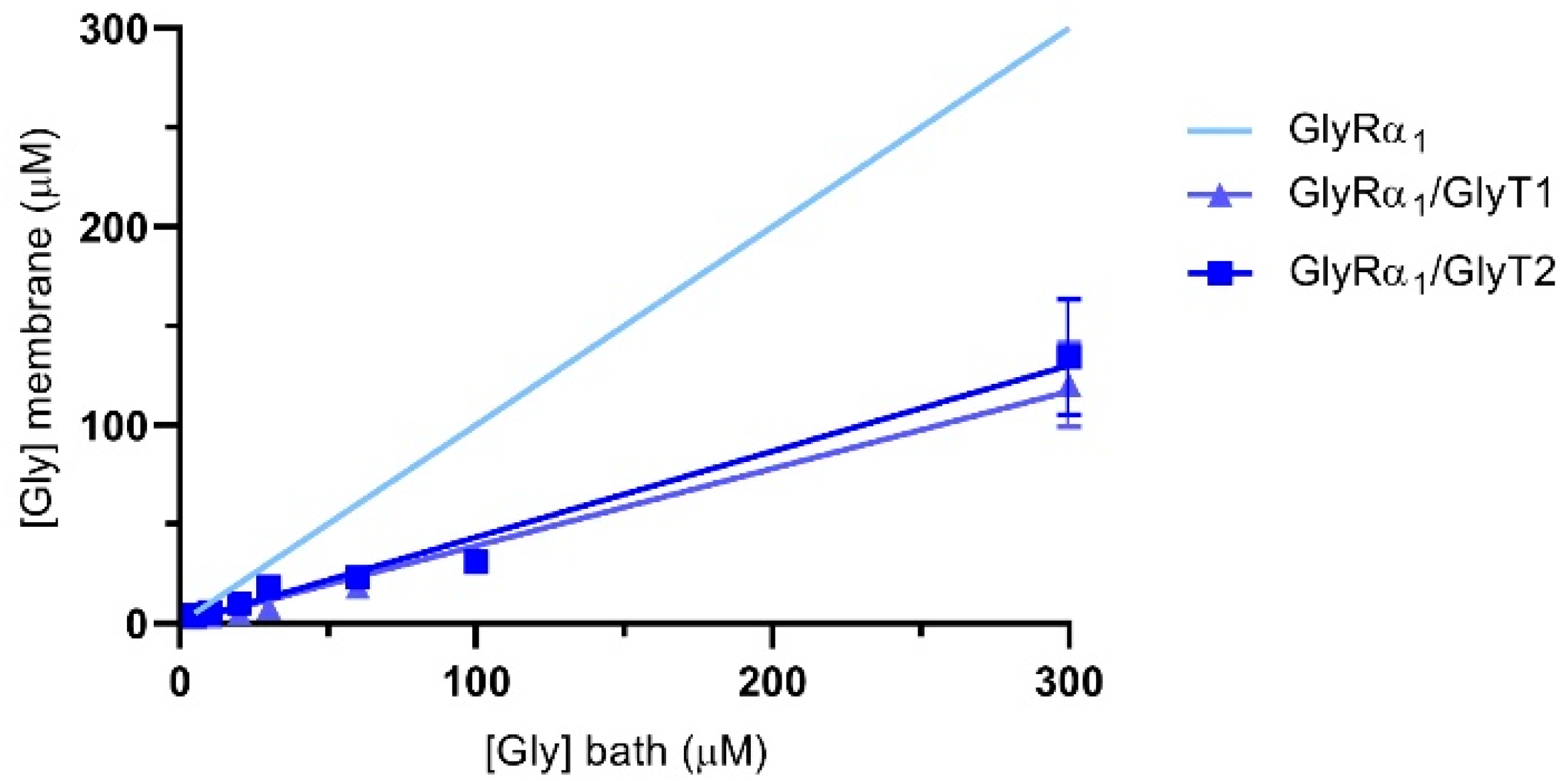
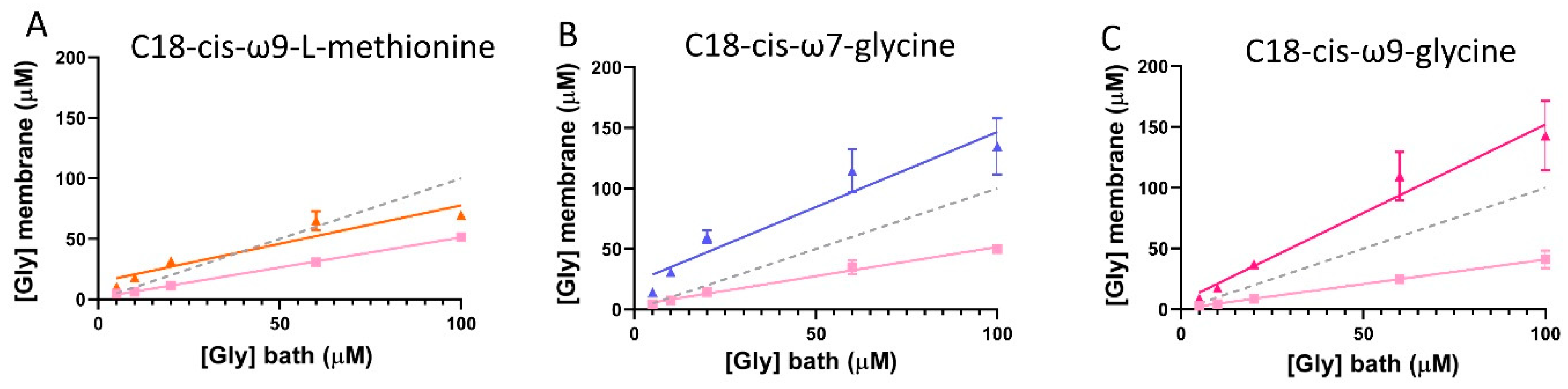
3.5. Estimation of Glycine Sensed at the Membrane by GlyRs in Cells Co-Expressing GlyTs
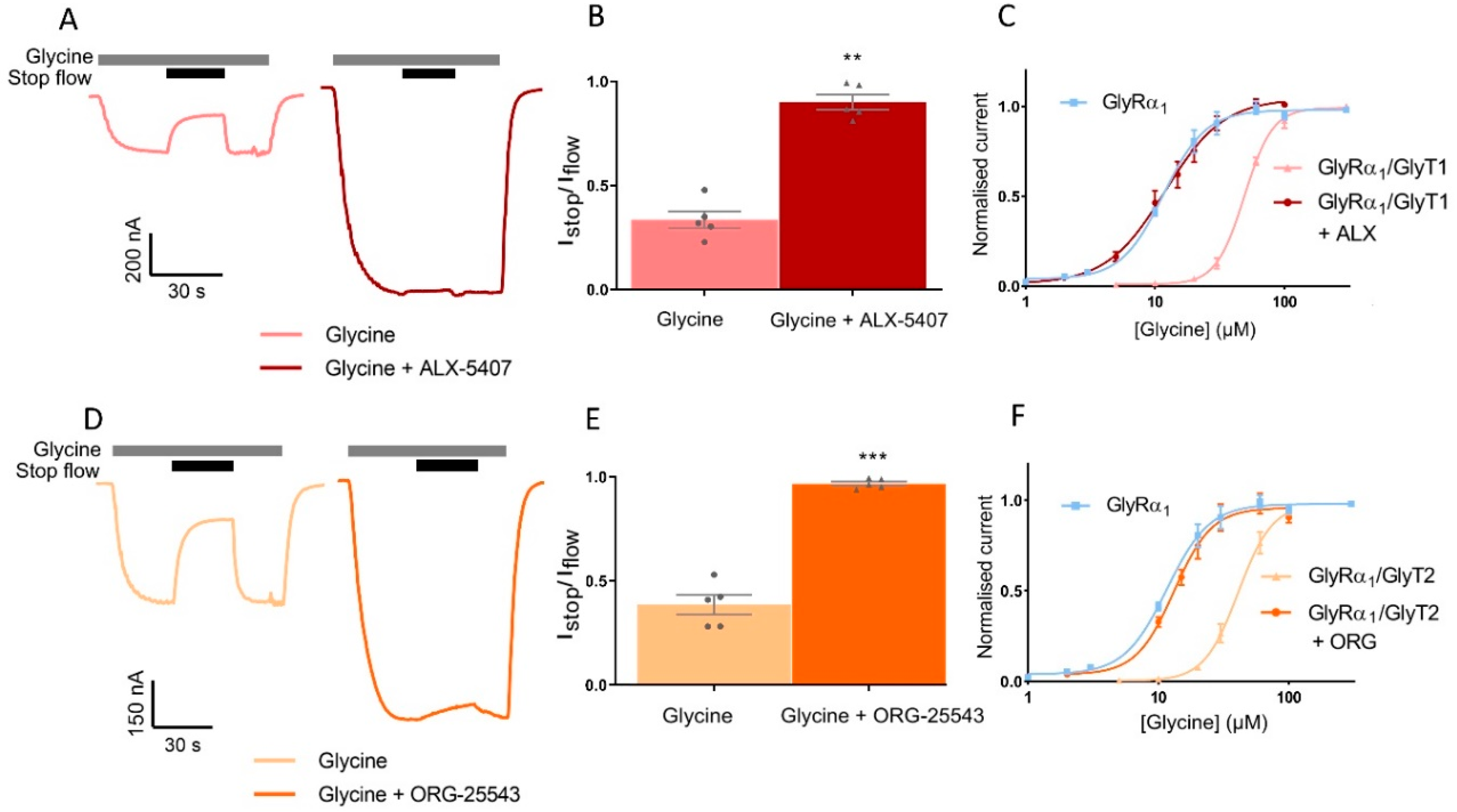
3.6. Pharmacological Inhibition of GlyTs Reverses Stop-Flow and Fast-Flow Changes to GlyR Activation Profiles
3.7. Co-Expression of GlyTs with GlyRs Changes Hill Co-Efficient Values
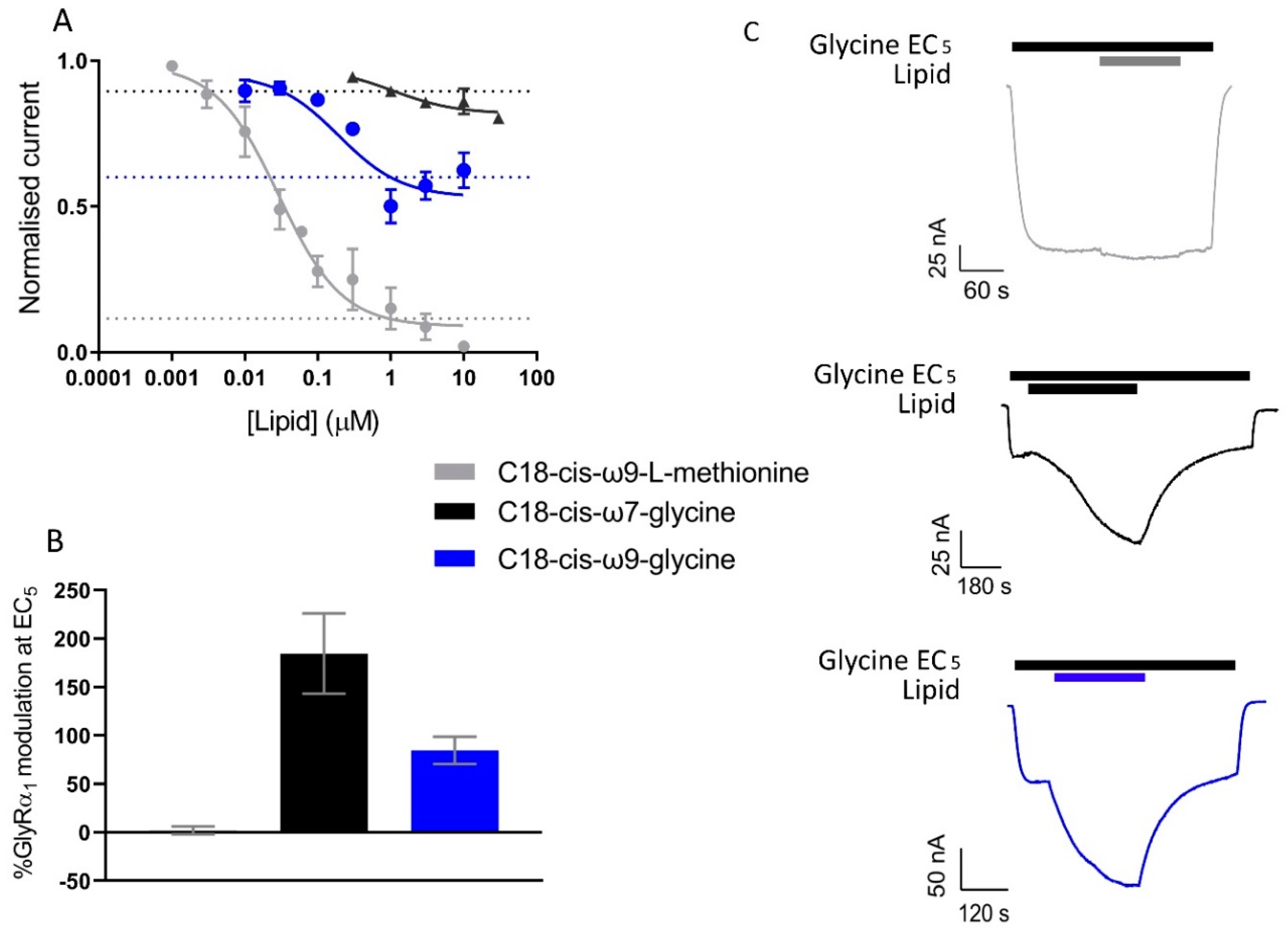
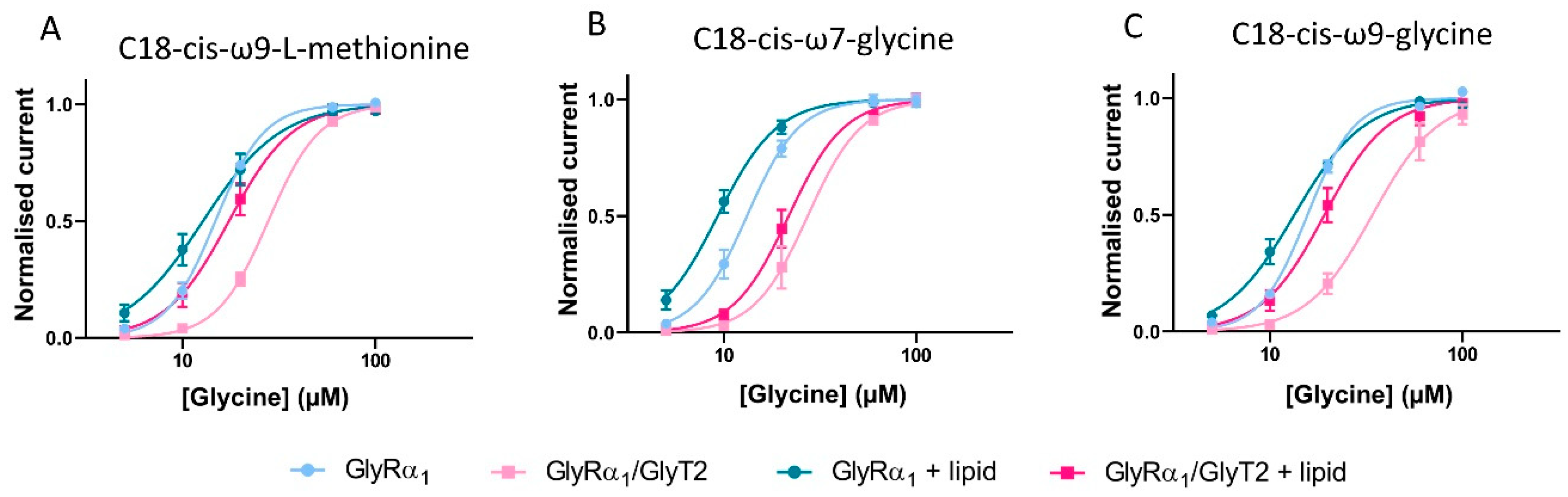
3.8. Novel, Bioactive Lipid Modulators of GlyRα1 and GlyT2
3.9. Estimation of the Apparent Glycine Concentration Sensed at the Membrane by GlyRα1 in GlyRα1/GlyT2 Co-Expressed Cells, in the Presence of Lipid Modulators
4. Discussion
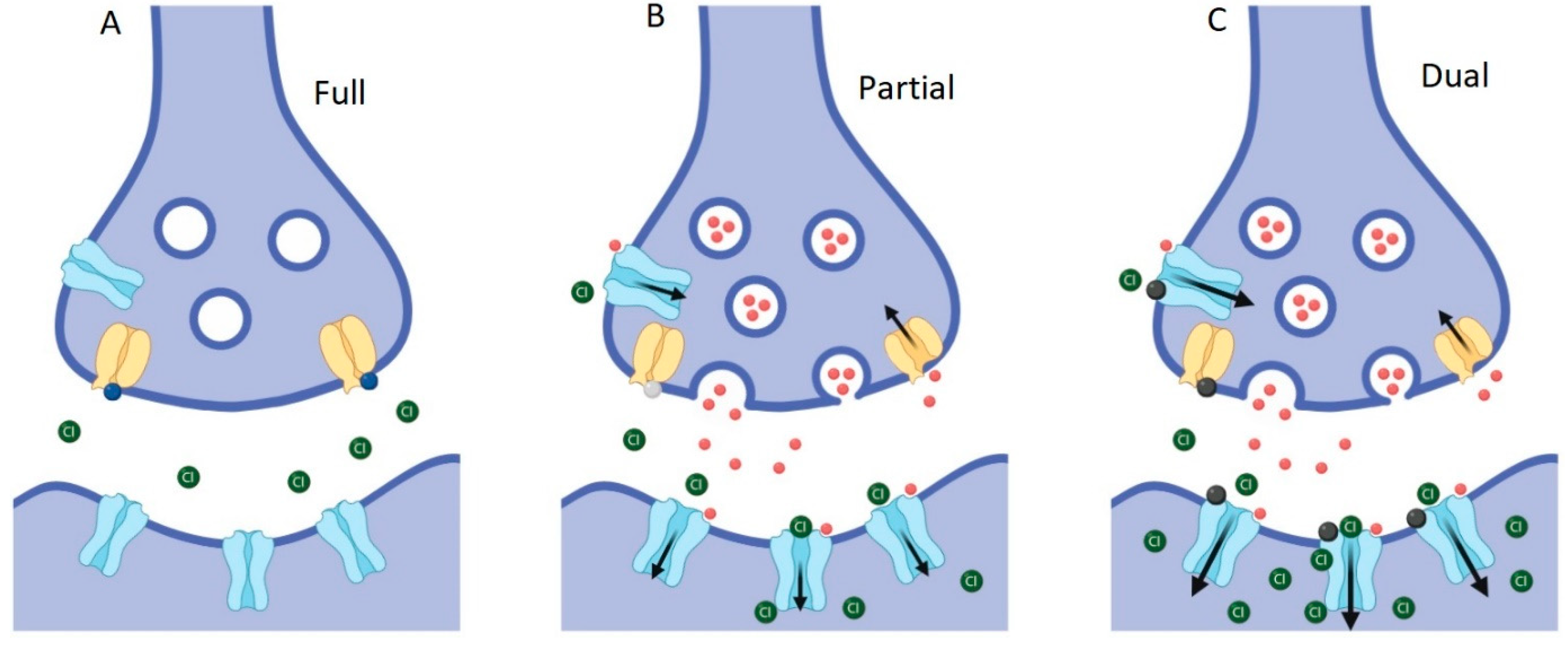
4.1. The Impact of GlyTs on GlyR Function
4.2. Pharmacological Modulation of the GlyR/GlyT Co-Expression System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793. [Google Scholar] [CrossRef] [PubMed]
- Durisic, N.; Godin, A.G.; Wever, C.M.; Heyes, C.D.; Lakadamyali, M.; Dent, J.A. Stoichiometry of the Human Glycine Receptor Revealed by Direct Subunit Counting. J. Neurosci. 2012, 32, 12915–12920. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.J.; Supplisson, S. Neuronal and Glial Glycine Transporters Have Different Stoichiometries. Neuron 2000, 25, 373–383. [Google Scholar] [CrossRef]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Supplisson, S.; Roux, M.J. Why glycine transporters have different stoichiometries. FEBS Lett. 2002, 529, 93–101. [Google Scholar] [CrossRef]
- Aubrey, K.R.; Rossi, F.M.; Ruivo, R.; Alboni, S.; Bellenchi, G.C.; Le Goff, A.; Gasnier, B.; Supplisson, S. The Transporters GlyT2 and VIAAT Cooperate to Determine the Vesicular Glycinergic Phenotype. J. Neurosci. 2007, 27, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, F.; Aubrey, K.R.; Supplisson, S. The Glycine Transporter GlyT2 Controls the Dynamics of Synaptic Vesicle Refilling in Inhibitory Spinal Cord Neurons. J. Neurosci. 2008, 28, 9755–9768. [Google Scholar] [CrossRef]
- Bergeron, R.; Meyer, T.M.; Coyle, J.T.; Greene, R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. USA 1998, 95, 15730–15734. [Google Scholar] [CrossRef]
- Berger, A.J.; Dieudonné, S.; Ascher, P. Glycine Uptake Governs Glycine Site Occupancy at NMDA Receptors of Excitatory Synapses. J. Neurophysiol. 1998, 80, 3336–3340. [Google Scholar] [CrossRef]
- Gabernet, L.; Pauly-Evers, M.; Schwerdel, C.; Lentz, M.; Bluethmann, H.; Vogt, K.; Alberati, D.; Möhler, H.; Boison, D. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci. Lett. 2004, 373, 79–84. [Google Scholar] [CrossRef]
- Ahmadi, S.; Muth-Selbach, U.; Lauterbach, A.; Lipfert, P.; Neuhuber, W.L.; Zeilhofer, H.U. Facilitation of Spinal NMDA Receptor Currents by Spillover of Synaptically Released Glycine. Science 2003, 300, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Korcsmáros, T.; Szalay, M.S.; Böde, C.; A Kovács, I.; Csermely, P. How to design multi-target drugs. Expert Opin. Drug Discov. 2007, 2, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Agoston, V.; Pongor, S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, S.N.; Rawling, T.; Mohammadi, S.; Shimmon, S.; Frangos, Z.J.; Sarker, S.; Yousuf, A.; Vetter, I.; Ryan, R.M.; Christie, M.J.; et al. Development of an N-Acyl Amino Acid That Selectively Inhibits the Glycine Transporter 2 To Produce Analgesia in a Rat Model of Chronic Pain. J. Med. Chem. 2019, 62, 2466–2484. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, S.N.; A Wilson, K.; Schumann-Gillett, A.; Frangos, Z.J.; Shimmon, S.; Rawling, T.; Ryan, R.M.; O’Mara, M.L.; Vandenberg, R.J. Identification of an allosteric binding site on the human glycine transporter, GlyT2, for bioactive lipid analgesics. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, S.N.; Carland, J.E.; Shimmon, S.; Ryan, R.; Rawling, T.; Vandenberg, R.J. Synthesis and Characterization of Novel Acyl-Glycine Inhibitors of GlyT2. ACS Chem. Neurosci. 2017, 8, 1949–1959. [Google Scholar] [CrossRef]
- Gallagher, C.I.; Sheipouri, D.; Shimmon, S.; Rawling, T.; Vandenberg, R.J. Identification of N-acyl amino acids that are positive allosteric modulators of glycine receptors. Biochem. Pharmacol. 2020, 180, 114117. [Google Scholar] [CrossRef]
- Sun, W.; Shchepakin, D.; Kalachev, L.V.; Kavanaugh, M.P. Glutamate transporter control of ambient glutamate levels. Neurochem. Int. 2014, 73, 146–151. [Google Scholar] [CrossRef]
- Vandenberg, R.J.; Ryan, R.M.; Carland, J.E.; Imlach, W.L.; Christie, M.J. Glycine transport inhibitors for the treatment of pain. Trends Pharmacol. Sci. 2014, 35, 423–430. [Google Scholar] [CrossRef]
- Dohi, T.; Morita, K.; Kitayama, T.; Motoyama, N.; Morioka, N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol. Ther. 2009, 123, 54–79. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Acuña, M.A.; Gingras, J.; Yévenes, G.E. Glycine receptors and glycine transporters: Targets for novel analgesics? Cell. Mol. Life Sci. 2018, 75, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.A.; Kristiansen, U. Functional characterisation of the human α1 glycine receptor in a fluorescence-based membrane potential assay. Biochem. Pharmacol. 2004, 67, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Guastella, J.; Brecha, N.; Weigmann, C.; Lester, H.A.; Davidson, N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc. Natl. Acad. Sci. USA 1992, 89, 7189–7193. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kingsmore, S.F.; Han, H.; Yang-Feng, T.L.; Godinot, N.; Seldin, M.F.; Caron, M.G.; Giros, B. Cloning of the human glycine transporter type 1: Molecular and pharmacological characterization of novel isoform variants and chromosomal localization of the gene in the human and mouse genomes. Mol. Pharmacol. 1994, 45, 608–617. [Google Scholar] [PubMed]
- Smith, K.E.; Borden, A.L.; Hartig, P.R.; A Branchek, T.; Weinshank, R.L. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 1992, 8, 927–935. [Google Scholar] [CrossRef]
- Aubrey, K.R.; Vandenberg, R.J.; Clements, J.D. Dynamics of Forward and Reverse Transport by the Glial Glycine Transporter, Glyt1b. Biophys. J. 2005, 89, 1657–1668. [Google Scholar] [CrossRef]
- Supplisson, S.; Bergman, C. Control of NMDA Receptor Activation by a Glycine Transporter Co-Expressed in Xenopus Oocytes. J. Neurosci. 1997, 17, 4580–4590. [Google Scholar] [CrossRef]
- Atkinson, B.N.; Bell, S.C.; De Vivo, M.; Kowalski, L.R.; Lechner, S.M.; Ognyanov, V.I.; Tham, C.-S.; Tsai, C.; Jia, J.; Ashton, D.; et al. ALX 5407: A Potent, Selective Inhibitor of the hGlyT1 Glycine Transporter. Mol. Pharmacol. 2001, 60, 1414–1420. [Google Scholar] [CrossRef]
- Aubrey, K.R.; Vandenberg, R.J. N [3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br. J. Pharmacol. 2001, 134, 1429–1436. [Google Scholar] [CrossRef]
- Caulfield, W.L.; Collie, I.T.; Dickins, R.S.; Epemolu, O.; McGuire, R.; Hill, D.R.; McVey, G.; Morphy, J.; Rankovic, Z.; Sundaram, H. The First Potent and Selective Inhibitors of the Glycine Transporter Type 2. J. Med. Chem. 2001, 44, 2679–2682. [Google Scholar] [CrossRef] [PubMed]
- Meur, A.M.-L.; Ghisdal, P.; Mullier, B.; De Ron, P.; Downey, P.; Van Der Perren, C.; Declercq, V.; Cornelis, S.; Famelart, M.; Van Asperen, J.; et al. Reversible inhibition of the glycine transporter GlyT2 circumvents acute toxicity while preserving efficacy in the treatment of pain. Br. J. Pharmacol. 2013, 170, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lü, W.; Wu, S.; Cheng, Y.; Gouaux, E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 2015, 526, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ivica, J.; Lape, R.; Jazbec, V.; Yu, J.; Zhu, H.; Gouaux, E.; Gold, M.G.; Sivilotti, L.G. The intracellular domain of homomeric glycine receptors modulates agonist efficacy. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Jan, D.D.S.; David-Watine, B.; Korn, H.; Bregestovski, P. Activation of human alpha1 and alpha2 homomeric glycine receptors by taurine and GABA. J. Physiol. 2001, 535, 741–755. [Google Scholar] [CrossRef]
- Grudzinska, J.; Schemm, R.; Haeger, S.; Nicke, A.; Schmalzing, G.; Betz, H.; Laube, B. The β Subunit Determines the Ligand Binding Properties of Synaptic Glycine Receptors. Neuron 2005, 45, 727–739. [Google Scholar] [CrossRef]
- Bormann, J.; Rundström, N.; Betz, H.; Langosch, D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1994, 13, 1493. [Google Scholar] [CrossRef]
- Legendre, P.; Muller, E.; Badiu, C.I.; Meier, J.C.; Vannier, C.; Triller, A. Desensitization of homomeric alpha1 glycine receptor increases with receptor density. Mol. Pharmacol. 2002, 62, 817–827. [Google Scholar] [CrossRef]
- Lu, J.; Fan, S.; Zou, G.; Hou, Y.; Pan, T.; Guo, W.; Yao, L.; Du, F.; Homanics, G.E.; Liu, D.; et al. Involvement of glycine receptor α1 subunits in cannabinoid-induced analgesia. Neuropharmacology 2018, 133, 224–232. [Google Scholar] [CrossRef]
- Xiong, W.; Cheng, K.; Cui, T.; Godlewski, G.; Rice, K.C.; Xu, Y.; Zhang, L. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat. Chem. Biol. 2011, 7, 296–303. [Google Scholar] [CrossRef]
- Barry, P.H.; Diamond, J.M. Effects of unstirred layers on membrane phenomena. Physiol. Rev. 1984, 64, 763–872. [Google Scholar] [CrossRef] [PubMed]
- Fornés, A.; Núñez, E.; Alonso-Torres, P.; Aragón, C.; López-Corcuera, B. Trafficking properties and activity regulation of the neuronal glycine transporter GLYT2 by protein kinase C. Biochem. J. 2008, 412, 495–506. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Sanz, J.; Núñez, E.; Villarejo-López, L.; Pérez-Hernandez, D.; Rodriguez-Fraticelli, A.; López-Corcuera, B.; Vazquez, J.; Aragón, C. Na+/K+-ATPase Is a New Interacting Partner for the Neuronal Glycine Transporter GlyT2 That Downregulates Its Expression In Vitro and In Vivo. J. Neurosci. 2013, 33, 14269–14281. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Sanz, J.; Zafra, F.; López-Corcuera, B.; Aragón, C. Endocytosis of the Neuronal Glycine Transporter GLYT2: Role of Membrane Rafts and Protein Kinase C-Dependent Ubiquitination. Traffic 2011, 12, 1850–1867. [Google Scholar] [CrossRef] [PubMed]
- Villarejo-López, L.; Jiménez, E.; Bartolomé-Martín, D.; Zafra, F.; Lapunzina, P.; Aragón, C.; López-Corcuera, B. P2X receptors up-regulate the cell-surface expression of the neuronal glycine transporter GlyT2. Neuropharmacology 2017, 125, 99–116. [Google Scholar] [CrossRef]
- Eulenburg, V.; Becker, K.; Gomeza, J.; Schmitt, B.; Becker, C.-M.; Betz, H. Mutations within the human GLYT2 (SLC6A5) gene associated with hyperekplexia. Biochem. Biophys. Res. Commun. 2006, 348, 400–405. [Google Scholar] [CrossRef]
- Akagi, H.; Hirai, K.; Hishinuma, F. Functional properties of strychnine-sensitive glycine receptors expressed in Xenopus oocytes injected with a single mRNA. Neurosci. Res. 1991, 11, 28–40. [Google Scholar] [CrossRef]
- Tang, B.; Lummis, S.C. Multiple regions in the extracellular domain of the glycine receptor determine receptor activity. J. Biol. Chem. 2018, 293, 13889–13896. [Google Scholar] [CrossRef]
- McCracken, L.M.; Trudell, J.R.; Goldstein, B.E.; Harris, R.A.; Mihic, S.J. Zinc enhances ethanol modulation of the α1 glycine receptor. Neuropharmacology 2010, 58, 676–681. [Google Scholar] [CrossRef]
- Tipps, M.E.; Lawshe, J.E.; Ellington, A.D.; Mihic, S.J. Identification of Novel Specific Allosteric Modulators of the Glycine Receptor Using Phage Display. J. Biol. Chem. 2010, 285, 22840–22845. [Google Scholar] [CrossRef]
- Prevost, M.S.; Moraga-Cid, G.; Van Renterghem, C.; Edelstein, S.J.; Changeux, J.-P.; Corringer, P.-J. Intermediate closed state for glycine receptor function revealed by cysteine cross-linking. Proc. Natl. Acad. Sci. USA 2013, 110, 17113–17118. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lummis, S.C. The roles of aromatic residues in the glycine receptor transmembrane domain. BMC Neurosci. 2018, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309. [Google Scholar] [CrossRef]
- Pribilla, I.; Takagi, T.; Langosch, D.; Bormann, J.; Betz, H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1994, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.S.; Beato, M.; Harvey, R.J.; Smart, T.G. Molecular determinants of glycine receptor αβ subunit sensitivities to Zn2+-mediated inhibition. J. Physiol. 2005, 566, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Krampfl, K.; Cetinkaya, C.; Moschref, H.; Grosskreutz, J.; Dengler, R.; Bufler, J. Kinetic analysis of recombinant mammalian alpha(1) and alpha(1)beta glycine receptor channels. Eur. Biophys. J. 2003, 32, 529–536. [Google Scholar] [CrossRef]
- Handford, C.A.; Lynch, J.W.; Baker, E.; Webb, G.C.; Ford, J.H.; Sutherland, G.R.; Schofield, P.R. The human glycine receptor beta subunit: Primary structure, functional characterisation and chromosomal localisation of the human and murine genes. Mol. Brain Res. 1996, 35, 211–219. [Google Scholar] [CrossRef]
- Beato, M. The time course of transmitter at glycinergic synapses onto motoneurons. J. Neurosci. 2008, 28, 7412–7425. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Vandenberg, R.J.; Vaughan, C.L. N-arachidonyl-glycine modulates synaptic transmission in superficial dorsal horn. Br. J. Pharmacol. 2010, 161, 925–935. [Google Scholar] [CrossRef]
- Winters, B.L.; Rawling, T.; Vandenberg, R.J.; Christie, M.J.; Bhola, R.F.; Imlach, W. Activity of novel lipid glycine transporter inhibitors on synaptic signalling in the dorsal horn of the spinal cord. Br. J. Pharmacol. 2018, 175, 2337–2347. [Google Scholar] [CrossRef]
- Cioffi, C.L. Modulation of Glycine-Mediated Spinal Neurotransmission for the Treatment of Chronic Pain. J. Med. Chem. 2018, 61, 2652–2679. [Google Scholar] [CrossRef] [PubMed]
- Succar, R.; A Mitchell, V.; Vaughan, C.L. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol. Pain 2007, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Carland, J.E.; Mansfield, R.; Ryan, R.; Vandenberg, R.J. Oleoyl-L-carnitine inhibits glycine transport by GlyT2. Br. J. Pharmacol. 2013, 168, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.J.; Depner, U.B.; Wässle, H.; Ahmadi, S.; Heindl, C.; Reinold, H.; Smart, T.G.; Schütz, B.; Abo-Salem, O.M.; Zimmer, A.; et al. GlyR 3: An Essential Target for Spinal PGE2-Mediated Inflammatory Pain Sensitization. Science 2004, 304, 884–887. [Google Scholar] [CrossRef] [PubMed]
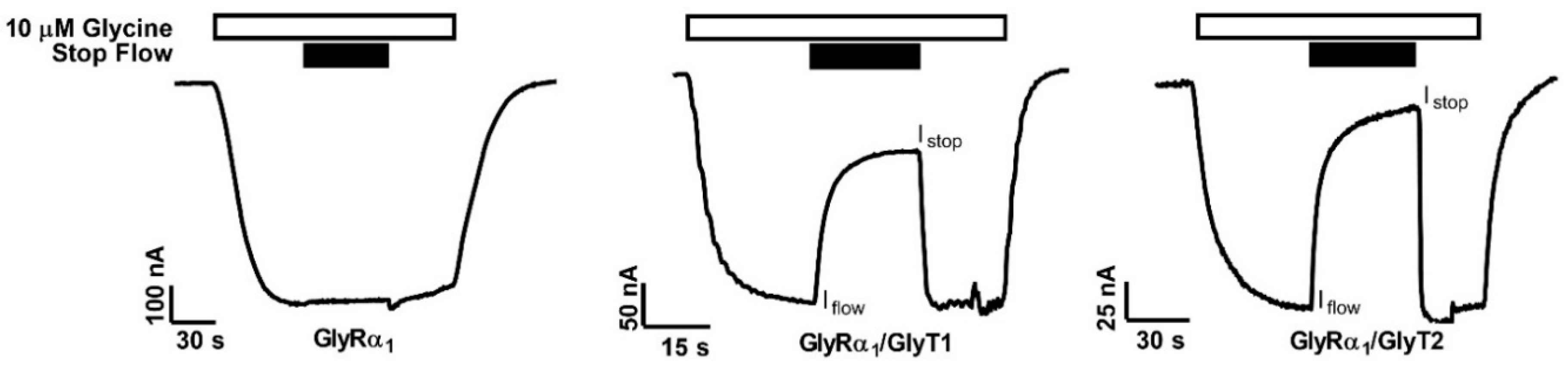

| Istop/Iflow | Peak Current (nA) | |
|---|---|---|
| GlyRα1 | 0.98 ± 0.01 | 740.6 ± 105.9 |
| GlyRα1/GlyT1 | 0.29 ± 0.04 **** | 62.3 ± 7.9 **** |
| GlyRα1/GlyT2 | 0.23 ± 0.02 **** | 150.7 ± 14.2 **** |
| Fast-Flow Glycine EC50 (µM) | 95% CI | nH | Stop-Flow Glycine EC50 (µM) | 95% CI | nH | |
|---|---|---|---|---|---|---|
| GlyRα1 | 13.1 | 12.1–14.4 | 3.2 ± 0.4 | – | – | – |
| GlyRα1/ GlyT1 | 47.3 | 45.0–49.7 | 3.7 ± 0.2 | 88.3 **** | 85.2–91.4 | 4.9 ± 0.4 |
| GlyRα1/ GlyT2 | 30.9 | 27.8–34.6 | 3.0 ± 0.47 | 83.1 **** | 80.0–86.2 | 4.0 ± 0.3 |
| Peak Current (nA) | 96 mM Na+ | 10 mM Na+ | 1 mM Na+ |
|---|---|---|---|
| GlyRα1/GlyT1 | 395.2 ± 27.0 | 664.8 ± 52.6 * | 723.2 ± 46.6 ** |
| GlyRα1/GlyT2 | 204.1 ± 88.6 | 596.4 ± 120.8 ** | 1084 ± 182.6 ** |
| Istop/Iflow | 96 mM Na+ | 10 mM Na+ | 1 mM Na+ |
| GlyRα1/GlyT1 | 0.31 ± 0.06 | 0.58 ± 0.05 * | 1.32 ± 0.06 **** |
| GlyRα1/GlyT2 | 0.23 ± 0.03 | 0.95 ± 0.01 **** | 0.97 ± 0.01 **** |
| Fast-Flow Glycine EC50 (µM) | 95% CI | |
|---|---|---|
| GlyRα1 | 13.2 | 12.9–14.3 |
| GlyRα1/GlyT1 | 48.8 **** | 46.6–51.0 |
| GlyRα1/GlyT2 | 41.6 **** | 38.8–44.7 |
| GlyRα1β | 14.7 | 12.6–16.9 |
| GlyRα1β/GlyT1 | 39.1 **** | 37.0–41.4 |
| GlyRα1β/GlyT2 | 36.8 **** | 34.7–39.0 |
| GlyRα3 | 64.8 | 59.0–70.9 |
| GlyRα3/GlyT1 | 153.9 **** | 143.7–164.6 |
| GlyRα3/GlyT2 | 160.2 **** | 145.7–175.8 |
| GlyRα3β | 109.4 | 102.4–117.1 |
| GlyRα3β/GlyT1 | 219.7 **** | 209.2–230.8 |
| GlyRα3β/GlyT2 | 224.5 **** | 208.6–241.5 |
| Istop/Iflow | Glycine EC50 (μM) | 95% CI | nH | |
|---|---|---|---|---|
| GlyRα1 | 11.2 | 10.0–12.4 | 2.2 ± 0.2 | |
| GlyRα1/GlyT1 | 0.39 ± 0.05 | 48.8 **** | 46.6–51.0 | 3.8 ± 0.2 *** |
| + ALX-5407 | 0.97 ± 0.01 ** | 11.3 ns | 10.0–12.3 | 2.0 ± 0.2 ns |
| GlyRα1/GlyT2 | 0.33 ± 0.04 | 41.6 **** | 38.8–44.7 | 3.2 ± 0.3 ns |
| + ORG-25543 | 0.90 ± 0.04 *** | 13.2 ns | 11.7–14.6 | 2.6 ± 0.4 ns |
| Lipid | Structure | GlyRα1 Potentiation at EC5 (%) | GlyT2 Activity |
|---|---|---|---|
| C18-cis-ω9-L-methionine |  | 2.2 ± 4.2 (6) | % Inhibition (1 µM) 84.9 ± 0.07 (4) IC50 29.2 nM Max inhibition (%) 91.2 |
| C18-cis-ω7-glycine |  | 184.7 ± 41.3 (5) | % Inhibition (1 µM) 10.5 ± 0.02 (4) IC50 >10 µM Max inhibition (%) – |
| 18-cis-ω9-glycine |  | 84.7 ± 14.0 (8) | % Inhibition (1 µM) 89.6 ± 0.01 (10) IC50 31 nM Max inhibition (%) 95.0 |
| Glycine EC50 (µM) | 95% CI | nH | |
|---|---|---|---|
| GlyRα1 | 14.8 | 14.1–15.6 | 3.4 ± 0.2 |
| + C18-cis-ω9-L-methionine | 12.8 ns | 11.2–14.7 | 2.2 ± 0.3 ns |
| GlyRα1/GlyT2 | 27.9 **** | 26.5–29.3 | 3.3 ± 0.2 |
| + C18-cis-ω9-L-methionine | 17.4 * | 15.7–19.5 | 2.6 ± 0.4 ns |
| GlyRα1 | 13.2 | 12.2–14.3 | 3.2 ± 0.3 |
| + C18-cis-ω7-glycine | 9.3 **** | 8.6–10.0 | 2.8 ± 0.3 ns |
| GlyRα1/GlyT2 | 27.3 **** | 23.6–31.4 | 3.1 ± 0.5 |
| + C18-cis-ω7-glycine | 21.7 **** | 19.7–24.4 | 3.0 ± 0.5 ns |
| GlyRα1 | 15.7 | 15.1–16.4 | 3.5 ± 0.2 |
| + C18-cis-ω9-glycine | 13.4 ns | 12.4–14.5 | 2.4 ± 0.2 ns |
| GlyRα1/GlyT2 | 34.1 **** | 29.6–39.4 | 2.6 ± 0.3 |
| + C18-cis-ω9-glycine | 18.0 * | 16.1–20.2 | 2.7 ± 0.4 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheipouri, D.; Gallagher, C.I.; Shimmon, S.; Rawling, T.; Vandenberg, R.J. A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors. Biomolecules 2020, 10, 1618. https://doi.org/10.3390/biom10121618
Sheipouri D, Gallagher CI, Shimmon S, Rawling T, Vandenberg RJ. A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors. Biomolecules. 2020; 10(12):1618. https://doi.org/10.3390/biom10121618
Chicago/Turabian StyleSheipouri, Diba, Casey I. Gallagher, Susan Shimmon, Tristan Rawling, and Robert J. Vandenberg. 2020. "A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors" Biomolecules 10, no. 12: 1618. https://doi.org/10.3390/biom10121618
APA StyleSheipouri, D., Gallagher, C. I., Shimmon, S., Rawling, T., & Vandenberg, R. J. (2020). A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors. Biomolecules, 10(12), 1618. https://doi.org/10.3390/biom10121618





