The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements
Abstract
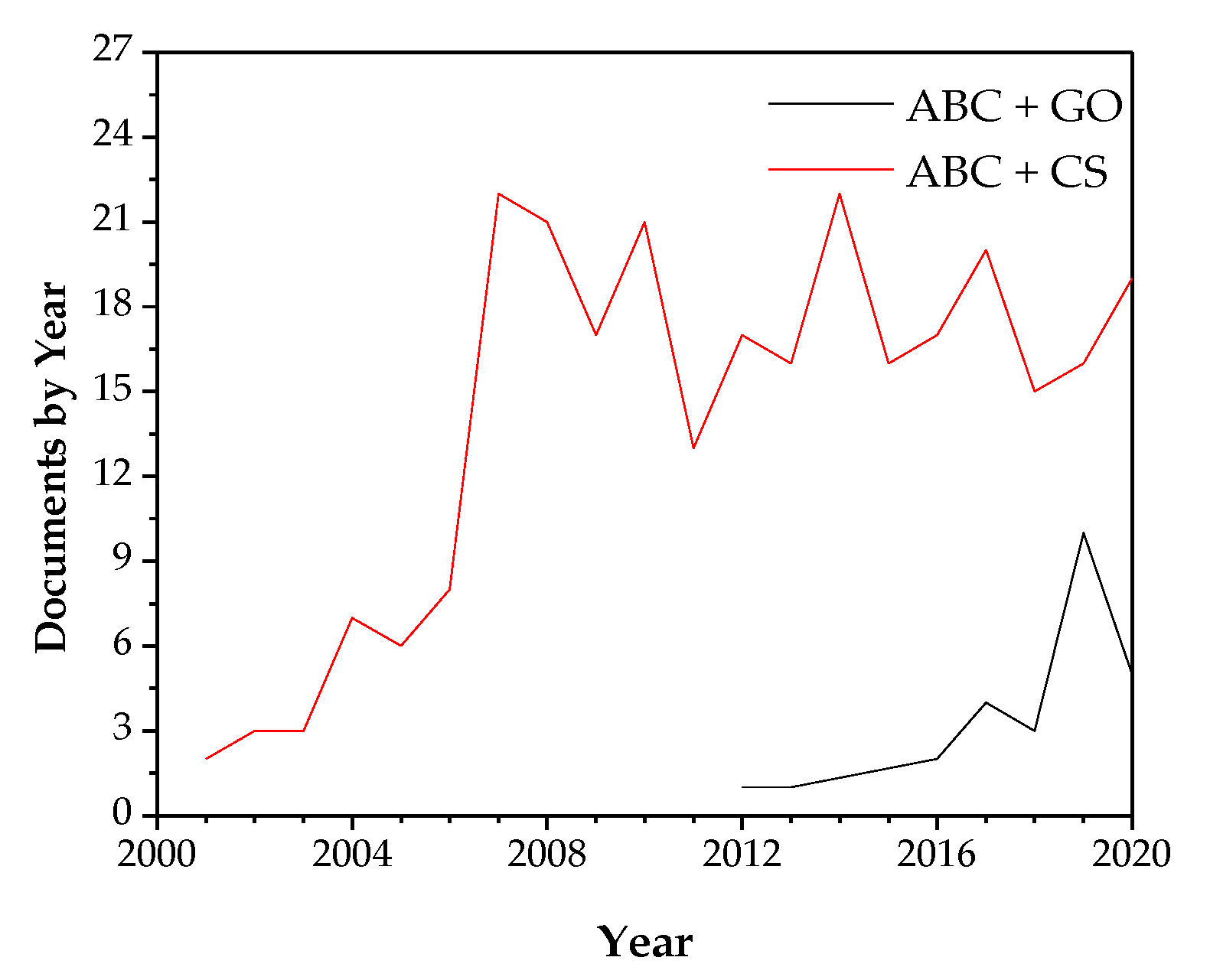
1. Introduction
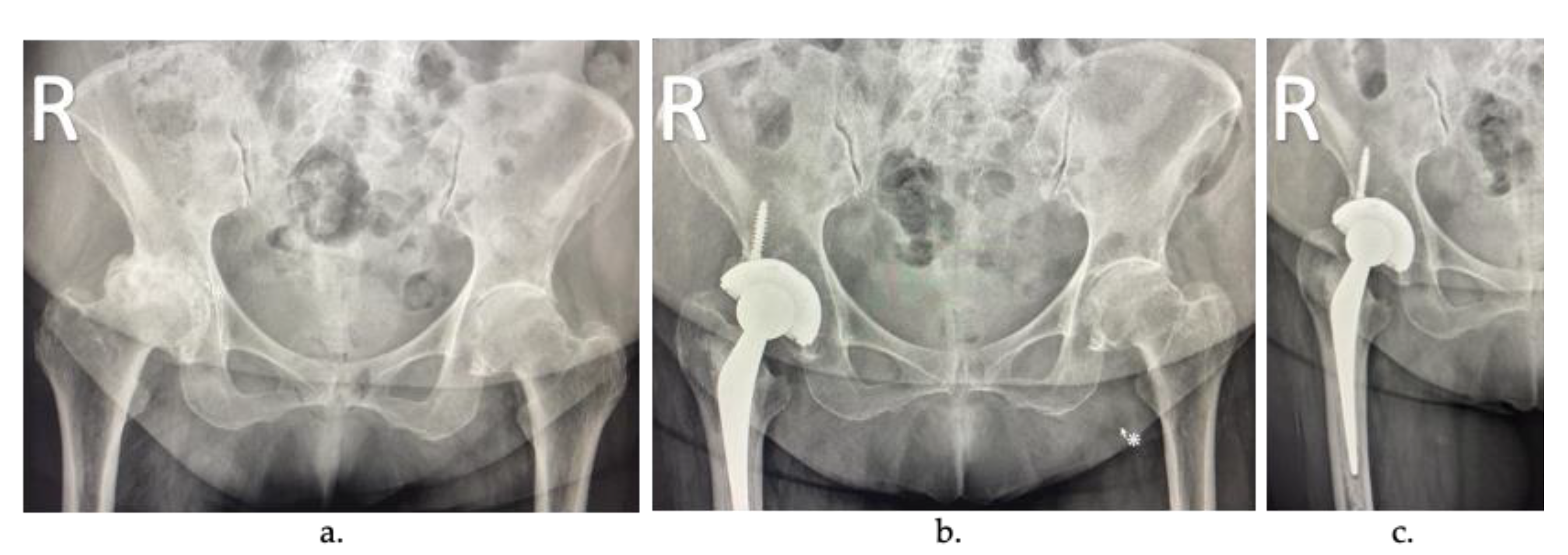
2. Arthroplasty
3. Acrylic Bone Cements (ABC)
- Release of unreacted residual monomer or MMA, which generates chemical necrosis of the bone.
- Contraction of the cement during polymerization.
- A significant difference between the cement’s stiffness and the adjacent bone generates an inappropriate load transfer [27].
- Interaction of the cement particles with the surrounding tissues, which produces the inflammatory responses of the periprosthetic tissue and increased bone destruction.
- Lack of osseointegration due to its inert nature [14].
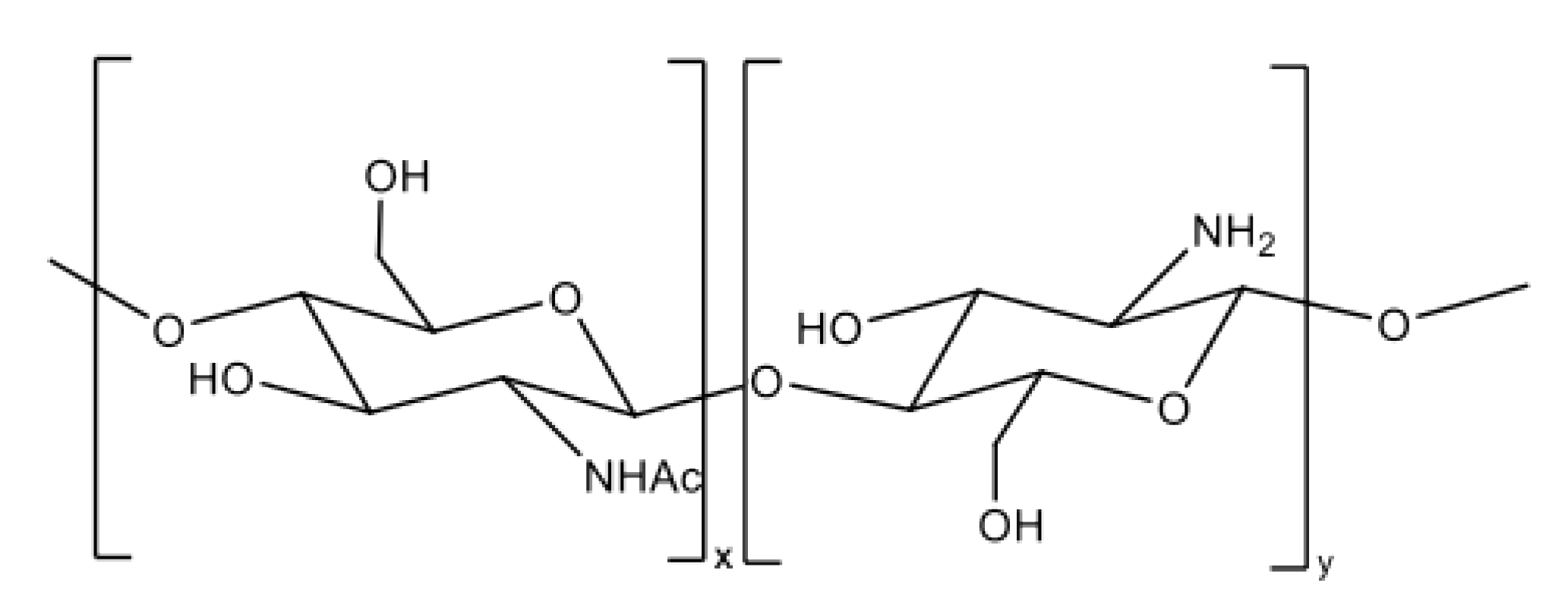
4. Chitosan (CS)
5. Graphene Oxide (GO)
6. Bioactivity
- Stimulate cell differentiation and proliferation
- Stimulate gene and tissue regeneration
- Release bioactive molecules actively and effectively for restoring and repairing the impaired functionality of the organs.
6.1. Bioactivity in Acrylic Bone Cements
6.2. Bioactivity in Acrylic Bone Cements Loaded with Chitosan
6.3. Bioactivity in Acrylic Bone Cements Loaded with Graphene Oxide
7. Antibacterial Properties
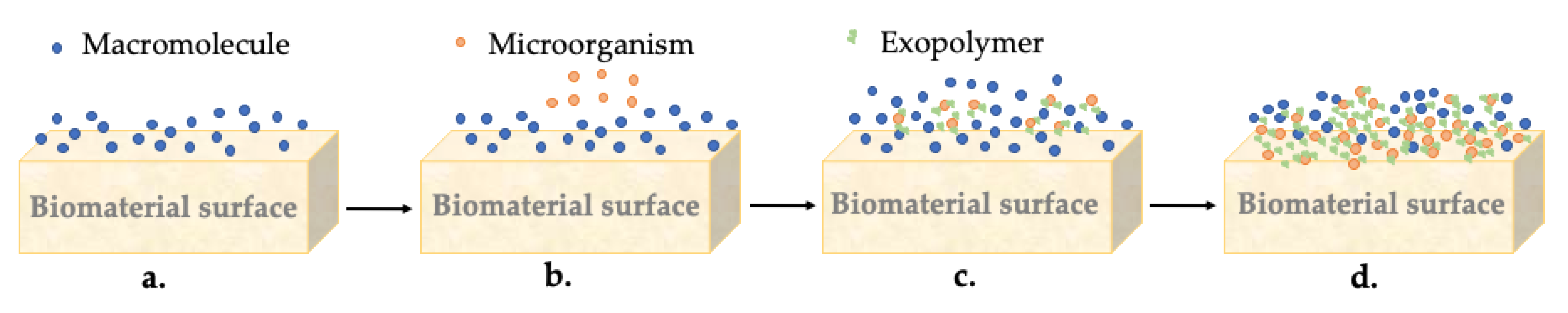
7.1. Formation of the Biofilm
7.2. Antimicrobial Properties in Acrylic Bone Cements
7.3. Antibacterial Mechanism of Chitosan
Antibacterial Effect of Chitosan in Acrylic Bone Cements
7.4. Antibacterial Mechanism of Graphene Oxide
Antibacterial Effect of GO on ABCs
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitt, D.R.; Killen, C.; Murphy, M.; Perry, M.; Romano, J.; Brown, N. The Impact of Antibiotic-Loaded Bone Cement on Antibiotic Resistance in Periprosthetic Knee Infections. Clin. Orthop. Surg. 2020, 12, 318–323. [Google Scholar] [CrossRef]
- Cole, K.A.; Funk, G.A.; Rahaman, M.N.; McIff, T.E. Mechanical and degradation properties of poly(methyl methacrylate) cement/borate bioactive glass composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, R.; O’Donnell, P.W.; Puleo, D.A. Systems for local, sustained release of zoledronic acid as a potential treatment for metastatic bone disease. Mater. Sci. Eng. C 2021, 118, 111395. [Google Scholar] [CrossRef]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Liu, H.; Gu, Z.; Zhang, Y.; Yu, S. A novel and convenient method to evaluate bone cement distribution following percutaneous vertebral augmentation. Sci. Rep. 2020, 10, 16320. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Pandit, H.; Van Duren, B.H. Chapter 3. Knee replacements. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 48–73. ISBN 78-1-84569-517-0. [Google Scholar]
- Johnston, R.C. Acrylic bone cement: Clinical development and current status in North America. Orthop. Clin. N. Am. 2005, 36, 75–84. [Google Scholar] [CrossRef]
- Dunne, N.; Ormsby, R.; Mitchell, C.A. Chapter 8. Carbon Nanotubes in Acrylic Bone Cement. In Biologically Responsive Biomaterials for Tissue Engineering; Antoniac, I., Ed.; Springer: New York, NY, USA, 2013; Volume 1, pp. 173–199. ISBN 978-1-4614-4327-8. [Google Scholar]
- Deb, S.; Koller, G. Chapter 8. Acrylic bone cement: Genesis and evolution. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 167–182. ISBN 978-1-84569-517-0. [Google Scholar]
- Sporer, S.M.; Paprosky, W.G. Biologic fixation and bone ingrowth. Orthop. Clin. N. Am. 2005, 36, 105–111. [Google Scholar] [CrossRef]
- Hendriks, J.G.E.; Van Horn, J.R.; Van Der Mei, H.C.; Busscher, H.J. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials 2004, 25, 545–556. [Google Scholar] [CrossRef]
- Lissarrague, M.H.; Fascio, M.L.; Goyanes, S.; D’Accorso, N.B. Acrylic Bone Cements: The Role of Nanotechnology in Mechanical Properties. J. Biomed. Nanotechnol. 2014, 10, 3536–3557. [Google Scholar] [CrossRef] [PubMed]
- Franco-Marquès, E.; Méndez, J.A.; Gironès, J.; Ginebra, M.P.; Pèlach, M.A. Evaluation of the influence of the addition of biodegradable polymer matrices in the formulation of self-curing polymer systems for biomedical purposes. Acta Biomater. 2009, 5, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Endogan, T.; Kiziltay, A.; Kose, G.T.; Comunoglu, N.; Beyzadeoglu, T.; Hasirci, N. Acrylic bone cements: Effects of the poly(methyl methacrylate) powder size and chitosan addition on their properties. J. Appl. Polym. Sci. 2014, 131, 39662. [Google Scholar] [CrossRef]
- Deb, S.; Koller, G. Chapter 14 Antibiotic-loaded bone cements. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; Volume 1, pp. 311–331. ISBN 978-1-84569-376-3. [Google Scholar]
- Madigan, S.; Towler, M.R.; Lewis, G. Optimisation of the composition of an acrylic bone cement: Application to relative amounts of the initiator and the activator/co-initiator in Surgical Simplex®P. J. Mater. Sci. Mater. Med. 2006, 17, 307–311. [Google Scholar] [CrossRef]
- Kühn, K.-D. Bone Cements; Springer: Berlin/Heidelberg, Germany, 2000; ISBN 9783642641152. [Google Scholar]
- International Standard ISO 5833: Implants for Surgery-Acrylic Resin Cements; International Standards Organization: Geneva, Switzerland, 2002; pp. 1–22.
- Madigan, S.; Towler, M.R.; Lewis, G. Influence of two changes in the composition of an acrylic bone cement on some of its properties: The case of Surgical Simplex® P. J. Mater. Sci. 2006, 41, 5758–5759. [Google Scholar] [CrossRef]
- Sharma, R.; Kapusetti, G.; Bhong, S.Y.; Roy, P.; Singh, S.K.; Singh, S.; Balavigneswaran, C.K.; Mahato, K.K.; Ray, B.; Maiti, P.; et al. Osteoconductive Amine-Functionalized Graphene-Poly(methyl methacrylate) Bone Cement Composite with Controlled Exothermic Polymerization. Bioconjug. Chem. 2017, 28, 2254–2265. [Google Scholar] [CrossRef]
- Hasenwinkel, J.M.; Lautenschlager, E.P.; Wixson, R.L.; Gilbert, J.L. A novel high-viscosity, two-solution acrylic bone cement: Effect of chemical composition on properties. J. Biomed. Mater. Res. 1999, 47, 36–45. [Google Scholar] [CrossRef]
- Toksvig-Larsen, S.; Franzen, H.; Ryd, L. Cement interface temperature in hip arthroplasty. Acta Orthop. Scand. 1991, 62, 102–105. [Google Scholar] [CrossRef]
- Feith, R. Side-effects of acrylic cement. Acta Orthop. Scand. 1975, 46, 3–136. [Google Scholar]
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. Vol. 2007, 89-B, 851–857. [Google Scholar] [CrossRef]
- Boesel, L.F.; Cachinho, S.C.P.; Fernandes, M.H.V.; Reis, R.L. The in vitro bioactivity of two novel hydrophilic, partially degradable bone cements. Acta Biomater. 2007, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Fernández, M.; Méndez, J.A.; Vázquez, B.; San Román, J.; Ginebra, M.P.; Gil, F.J.; Manero, J.M.; Planell, J.A. Acrylic-phosphate glasses composites as self-curing controlled delivery systems of antibiotics. J. Mater. Sci. Mater. Med. 2002, 13, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.P.; Ferreira, B.L.; Gomes, P.S.; Correia, R.N.; Fernandes, M.H.; Fernandes, M.H. V Silicate and borate glasses as composite fillers: A bioactivity and biocompatibility study. J. Mater. Sci. Mater. Med. 2011, 22, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, S.; Nakamura, T.; Kokubo, T.; Kitamura, Y. A new bioactive bone cement: Effect of glass bead filler content on mechanical and biological properties. J. Biomed. Mater. Res. 2001, 54, 491–500. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Kong, L.; Li, Y.; Wu, Z.; Lei, W. Porous Surface Modified Bioactive Bone Cement for Enhanced Bone Bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.Z.; Bou-Akl, T.H.; Blowytsky, O.; Walters, H.L.; Matthew, H.W.T. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Mon. Chem. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Ikada, Y. Biological Materials. In Integrated Biomaterials Science; Barbucci, R., Ed.; Springer: New York, NY, USA, 2002; pp. 1–23. [Google Scholar]
- Wekwejt, M.; Michalska-Sionkowska, M.; Bartmański, M.; Nadolska, M.; Łukowicz, K.; Pałubicka, A.; Osyczka, A.M.; Zieliński, A. Influence of several biodegradable components added to pure and nanosilver-doped PMMA bone cements on its biological and mechanical properties. Mater. Sci. Eng. C 2020, 117, 111286. [Google Scholar] [CrossRef]
- Wang, M.; Sa, Y.; Li, P.; Guo, Y.; Du, Y.; Deng, H.; Jiang, T.; Wang, Y. A versatile and injectable poly(methyl methacrylate) cement functionalized with quaternized chitosan-glycerophosphate/nanosized hydroxyapatite hydrogels. Mater. Sci. Eng. C 2018, 90, 264–272. [Google Scholar] [CrossRef]
- Gao, W. Synthesis, Structure, and Characterizations. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Gao, W., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 1–28. [Google Scholar]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide–A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, L.; Qian, S.; Qian, W.; Liu, X. Why does nitrogen-doped graphene oxide lose the antibacterial activity? J. Mater. Sci. Technol. 2021, 62, 44–51. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.N.; Arsalani, N. Nano graphene oxide: A novel carrier for oral delivery of flavonoids. Colloids Surf. B Biointerfaces 2014, 123, 331–338. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Wang, C.; Mallela, J.; Garapati, U.S.; Ravi, S.; Chinnasamy, V.; Girard, Y.; Howell, M.; Mohapatra, S. A chitosan-modified graphene nanogel for noninvasive controlled drug release. Nanomedicine 2013, 9, 903–911. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef]
- Girish, C.M.; Sasidharan, A.; Gowd, G.S.; Nair, S.; Koyakutty, M. Confocal raman imaging study showing macrophage mediated biodegradation of graphene in vivo. Adv. Healthc. Mater. 2013, 2, 1489–1500. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Kurapati, R.; Bonachera, F.; Russier, J.; Sureshbabu, A.R.; Ménard-Moyon, C.; Kostarelos, K.; Bianco, A. Covalent chemical functionalization enhances the biodegradation of graphene oxide. 2D Mater. 2018, 5, 015020. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized graphene oxide as a vehicle for targeted drug delivery and bioimaging applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; Maulucci, G.; De Spirito, M.; Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; et al. The future development of bacteria fighting medical devices: The role of graphene oxide The future development of bacteria fighting medical devices: The role of graphene oxide. Expert Rev. Med. Devices 2016, 13, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Lu, N.; Li, Z. Chapter 5. Graphene Oxide: Theoretical Perspectives. In Quantum Simulations of Materials and Biological Systems; Zeng, J., Zhang, R.-Q., Treutlein, H.R., Eds.; Springer: New York, NY, USA, 2012; pp. 69–84. ISBN 978-94-007-4947-4. [Google Scholar]
- García Martínez, V. Estudio de la Estabilidad Del Óxido de Grafeno Con el Tiempo. Master’s Thesis, Universidad de Oviedo, Oviedo, Spain, 2013. [Google Scholar]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Le, J.; Zhongqun, L.; Zhaoyan, W.; Yijun, S.; Yingjin, W.; Yaojie, W.; Yanan, J.; Zhanrong, J.; Chunyang, M.; Fangli, G.; et al. Development of methods for detecting the fate of mesenchymal stem cells regulated by bone bioactive materials. Bioact. Mater. 2021, 6, 613–626. [Google Scholar] [CrossRef]
- Miao, Q.; Yang, S.; Ding, H.; Liu, J. Controlled degradation of chitosan-coated strontium-doped calcium sulfate hemihydrate composite cement promotes bone defect repair in osteoporosis rats. Biomed. Mater. 2020, 15, 55039. [Google Scholar] [CrossRef]
- Zhao, X. Introduction to bioactive materials in medicine. In Bioactive Materials in Medicine; Zhao, X., Courtney, J.M., Qian, H., Eds.; Woodhead Publishing: Washington, DC, USA, 2011; pp. 1–13. ISBN 978-1-84569-624-5. [Google Scholar]
- Jones, J.R. Scaffolds for tissue engineering. In Biomaterials, Artificial Organs and Tissue Engineering; Hench, L.L., Jones, J., Eds.; Woodhead Publishing: Washington, DC, USA, 2005; pp. 201–214. ISBN 978-1-85573-737-2. [Google Scholar]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive Ceramics: Physical Chemistry. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 187–221. ISBN 978-0-08-055294-1. [Google Scholar]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. In vitro evaluation of a new polymethylmethacrylate cement reinforced with hydroxyapatite. J. Mater. Sci. Mater. Med. 1999, 10, 793–796. [Google Scholar] [CrossRef]
- Espigares, I.; Elvira, C.; Mano, J.F.; Vázquez, B.; San Román, J.; Reis, R.L. New partially degradable and bioactive acrylic bone cements based on starch blends and ceramic fillers. Biomaterials 2002, 23, 1883–1895. [Google Scholar] [CrossRef]
- Lozano, K.; Mina, J.; Zuluaga, F.; Valencia, C.; Valencia, M. Influencia de la incorporación de un co-monómero alcalino e hidroxiapatita en las propiedades de cementos óseos acrílicos. DYNA 2013, 80, 153–162. [Google Scholar]
- Heikkilä, J.T.; Aho, A.J.; Kangasniemi, I.; Yli-Urpo, A. Polymethylmethacrylate composites: Disturbed bone formation at the surface of bioactive glass and hydroxyapatite. Biomaterials 1996, 17, 1755–1760. [Google Scholar] [CrossRef]
- Lopes, P.P.; Ferreira, B.L.; Almeida, N.A.F.; Fredel, M.C.; Fernandes, M.H.V.; Correia, R.N. Preparation and study of in vitro bioactivity of PMMA-co-EHA composites filled with a Ca3(PO4)2-SiO2-MgO glass. Mater. Sci. Eng. C 2008, 28, 572–577. [Google Scholar] [CrossRef]
- Lopes, P.P.; Garcia, M.P.; Fernandes, M.H.; Fernandes, M.H.V. Acrylic formulations containing bioactive and biodegradable fillers to be used as bone cements: Properties and biocompatibility assessment. Mater. Sci. Eng. C 2013, 33, 1289–1299. [Google Scholar] [CrossRef]
- Fini, M.; Giavaresi, G.; Aldini, N.N.; Torricelli, P.; Botter, R.; Beruto, D.; Giardino, R. A bone substitute composed of polymethylmethacrylate and α-tricalcium phosphate: Results in terms of osteoblast function and bone tissue formation. Biomaterials 2002, 23, 4523–4531. [Google Scholar] [CrossRef]
- García-Enriquez, S.; Guadarrama, H.E.R.; Reyes-González, I.; Mendizábal, E.; Jasso-Gastinel, C.F.; García-Enriquez, B.; Rembao-Bojórquez, D.; Pane-Pianese, C. Mechanical performance and in vivo tests of an acrylic bone cement filled with bioactive sepia officinalis cuttlebone. J. Biomater. Sci. Polym. Ed. 2010, 21, 113–125. [Google Scholar] [CrossRef]
- Valencia, M.E.; Mina, J.H.; Zuluaga, F.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Efecto del contenido de BaSO4 y DEAEA sobre las propiedades reológicas de cementos óseos para vertebroplastia. Ing. Compet. 2012, 14, 51–62. [Google Scholar]
- Cervantes-Uc, J.M.; Vázquez-Torres, H.; Cauich-Rodríguez, J.V.; Vázquez-Lasa, B.; del Barrio, J.S.R. Comparative study on the properties of acrylic bone cements prepared with either aliphatic or aromatic functionalized methacrylates. Biomaterials 2005, 26, 4063–4072. [Google Scholar] [CrossRef]
- Elvira, C.; Vazquez, B.; San Román, J.; Levenfeld, B.; Ginebra, P.; Gil, X.; Planell, J.A. Acrylic bone cements incorporating polymeric active components derived from salicylic acid: Curing parameters and properties. J. Mater. Sci. Mater. Med. 1998, 9, 679–685. [Google Scholar] [CrossRef]
- Pascual, B.; Gurruchaga, M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; Goñi, I. Influence of the modification of P/L ratio on a new formulation of acrylic bone cement. Biomaterials 1999, 20, 465–474. [Google Scholar] [CrossRef]
- Gutiérrez-Mejía, a.; Herrera-Kao, W.; Duarte-Aranda, S.; Loría-Bastarrachea, M.I.; Canché-Escamilla, G.; Moscoso-Sánchez, F.J.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Synthesis and characterization of core-shell nanoparticles and their influence on the mechanical behavior of acrylic bone cements. Mater. Sci. Eng. C 2013, 33, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Ido, K.; Matsuda, Y.; Iida, H.; Oka, M.; Kitamura, Y.; Nakamura, T. Evaluation of bioactive bone cement in canine total hip arthroplasty. J. Biomed. Mater. Res. 2000, 49, 273–288. [Google Scholar] [CrossRef]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status. key issues, and future prospects. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 84, 301–319. [Google Scholar] [CrossRef]
- Franco-Marquès, E.; Méndez, J.A.; Gironès, J.; Pèlach, M.A. Thermal and dynamic mechanical characterization of acrylic bone cements modified with biodegradable polymers. J. Appl. Polym. Sci. 2013, 128, 3455–3464. [Google Scholar] [CrossRef]
- Abraham, G.A.; Vallo, C.I.; San Román, J.; Cuadrado, T.R. Mechanical characterization of self-curing acrylic cements formulated with poly(methylmethacrylate)/poly(epsilon-caprolactone) beads. J. Biomed. Mater. Res. B. Appl. Biomater. 2004, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kretlow, J.D.; Spicer, P.P.; Tabata, Y.; Demian, N.; Wong, M.E.; Kasper, F.K.; Mikos, A.G. Antibiotic-releasing porous polymethylmethacrylate / gelatin/antibiotic constructs for craniofacial tissue engineering. J. Control. Release 2011, 152, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F.; Reis, R.L. A review on the polymer properties of Hydrophilic, partially Degradable and Bioactive acrylic Cements (HDBC). Prog. Polym. Sci. 2008, 33, 180–190. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Huang, F.-M.; Chang, Y.-C. Mechanisms of cytotoxicity of eugenol in human osteoblastic cells in vitro. Int. Endod. J. 2006, 39, 389–393. [Google Scholar] [CrossRef]
- Bong, S.; Jick, Y.; Lim, T.; Park, S.A.; Hee, I.; Jung, E.; Ae, I.; Shin, J. The characteristics of a hydroxyapatite–chitosan–PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef]
- Girones, J.; Alberto, J.; San, J. Bioresorbable and Nonresorbable Polymers for Bone Tissue Engineering. Curr. Pharm. Des. 2012, 18, 2536–2557. [Google Scholar] [CrossRef]
- Lim, H.N.; Huang, N.M.; Loo, C.H. Facile preparation of graphene-based chitosan films: Enhanced thermal, mechanical and antibacterial properties. J. Non. Cryst. Solids 2012, 358, 525–530. [Google Scholar] [CrossRef]
- Khandaker, M.; Vaughan, M.B.; Morris, T.L.; White, J.J.; Meng, Z. Effect of additive particles on mechanical, thermal, and cell functioning properties of poly ( methyl methacrylate ) cement. Int. J. Nanomed. 2014, 9, 2699–2712. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Brady, A.J.; Buchanan, F.; Newe, C.; Dunne, N.J. Incorporation of chitosan in acrylic bone cement: Effect on antibiotic release, bacterial biofilm formation and mechanical properties. J. Mater. Sci. Mater. Med. 2008, 19, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.; Buchanan, F.; Hill, J.; Newe, C.; Tunney, M.; Brady, A.; Walker, G. In vitro testing of chitosan in gentamicin-loaded bone cement: No antimicrobial effect and reduced mechanical performance. Acta Orthop. 2008, 79, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chang, S.; Kuo, S.M.; Chen, S.H.U.F.E.N. Evaluation of chitosan/β-tricalcium phosphate microspheres as a constituent to PMMA cement. J. Mater. Sci. Mater. Med. 2005, 16, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lagos, S.I.Z.; Salas, J.M.; Zapata, M.E.V.; Hernandez, J.H.M.; Valencia, C.H.; Rojo, L.; Tovar, C.D.G. Influence of the chitosan morphology on the properties of acrylic cements and their biocompatibility. RSC Adv. 2020, 10, 31156–31164. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G. Acrylic Bone Cement Incorporated with Low Chitosan Loadings. Polymers 2020, 12, 1617. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Vázquez-Lasa, B.; San Román, J.; Rojo, L. Osseointegration of Antimicrobial Acrylic Bone Cements Modified with Graphene Oxide and Chitosan. Appl. Sci. 2020, 10, 6528. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Karbasi, S.; Hassanzadeh Tabrizi, S.A.; Ebrahimi-Kahrizsangi, R.; Salehi, H. Evaluation of the effects of chitosan/multiwalled carbon nanotubes composite on physical, mechanical and biological properties of polymethyl methacrylate-based bone cements. Mater. Technol. 2019, 35, 267–280. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon N.Y. 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Graphene and its nanostructure derivatives for use in bone tissue engineering: Recent advances. J. Biomed. Mater. Res. Part A 2016, 104, 1250–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Cruz, S.M.A.; Ramalho, A.; Grácio, J.; Marques, P.A.A.P. Graphene oxide versus functionalized carbon nanotubes as a reinforcing agent in a PMMA/HA bone cement. Nanoscale 2012, 4, 2937–2945. [Google Scholar] [CrossRef]
- Valencia Zapata, M.E.; Ruiz Rojas, L.M.; Mina Hernandez, J.H.; Delgado-Ospina, J.; Grande Tovar, C.D. Acrylic Bone Cements Modified with Graphene Oxide: Mechanical, Physical, and Antibacterial Properties. Polymers 2020, 12, 1773. [Google Scholar] [CrossRef]
- Valencia Zapata, M.E.; Mina Hernandez, J.H.; Grande Tovar, C.D.; Valencia Llano, C.H.; Diaz Escobar, J.A.; Vázquez-Lasa, B.; San Román, J.; Rojo, L.; Rojo, L. Novel Bioactive and Antibacterial Acrylic Bone Cement Nanocomposites Modified with Graphene Oxide and Chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene oxide versus graphene for optimisation of PMMA bone cement for orthopaedic applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef]
- Gonçalves, G.; Portolés, M.-T.; Ramírez-Santillán, C.; Vallet-Regí, M.; Serro, A.P.; Grácio, J.; Marques, P.A.A.P. Evaluation of the in vitro biocompatibility of PMMA/high-load HA/carbon nanostructures bone cement formulations. J. Mater. Sci. Mater. Med. 2013, 24, 2787–2796. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Hamzah, E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J. Mech. Behav. Biomed. Mater. 2018, 82, 257–267. [Google Scholar] [CrossRef]
- Mirza, E.H.; Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Fareedi, F.; Khan, M.M.; Alfayez, M.; Al-Fotawi, R.; Vallittu, P.K.; et al. Characterization of osteogenic cells grown over modified graphene-oxide-biostable polymers. Biomed. Mater. 2019, 14, 65004. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; del Real, J.C.; Dunne, N.J. Graphene oxide and graphene reinforced PMMA bone cements: Evaluation of thermal properties and biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef]
- Shen, S.-C.; Ng, W.K.; Dong, Y.-C.; Ng, J.; Tan, R.B.H. Nanostructured material formulated acrylic bone cements with enhanced drug release. Mater. Sci. Eng. C 2016, 58, 233–241. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Magnan, B.; Bondi, M.; Maluta, T.; Samaila, E.; Schirru, L.; Dall’Oca, C. Acrylic bone cement: Current concept review. Musculoskelet. Surg. 2013, 97, 93–100. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Miola, M.; Bistolfi, A.; Valsania, M.C.; Bianco, C.; Fucale, G.; Verné, E. Antibiotic-loaded acrylic bone cements: An in vitro study on the release mechanism and its efficacy. Mater. Sci. Eng. C 2013, 33, 3025–3032. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef]
- Weiser, M.C.; Moucha, C.S. The Current State of Screening and Decolonization for the Prevention of Staphylococcus aureus Surgical Site Infection after Total Hip and Knee Arthroplasty. J. Bone Jt. Surg. 2015, 97, 1449–1458. [Google Scholar] [CrossRef]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current Options and Emerging Biomaterials for Periprosthetic Joint Infection. Curr. Rheumatol. Rep. 2018, 20, 33. [Google Scholar] [CrossRef]
- Jaekel, D.J.; Ong, K.L.; Lau, E.C.; Watson, H.N.; Kurtz, S.M. Chapter 1. Epidemiology of Total Hip and Knee Arthroplasty Infection. In Periprosthetic Joint Infection of the Hip and Knee; Springer, B.D., Parvizi, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–14. ISBN 978-1-4614-7927-7. [Google Scholar]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 2001, 22, 1607–1611. [Google Scholar] [CrossRef]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Kelm, J. Enhancement of antibiotic elution from acrylic bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 467–475. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Uges, D.R.A.; Schenk, W.; Van Horn, J.R.; Van Der Mei, H.C.; Busscher, H.J.; van de Belt, H.; Neut, D.; Uges, D.R.A.; et al. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef]
- Virto, M.R.; Frutos, P.; Torrado, S.; Frutos, G. Gentamicin release from modified acrylic bone cements with lactose and hydroxypropylmethylcellulose. Biomaterials 2003, 24, 79–87. [Google Scholar] [CrossRef]
- Pellegrini, A.V.; Suardi, V. Antibiotics and cement: What I need to know? HIP Int. 2020, 30, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Liu, Y.; Christensen, R.; Raina, D.B.; Tägil, M.; Lidgren, L. Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: A systematic review and meta-analysis. J. Orthop. Transl. 2020, 23, 53–60. [Google Scholar] [CrossRef]
- Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. J. Funct. Biomater. 2020, 11, 25. [Google Scholar] [CrossRef]
- Walker, L.C.; Baker, P.; Holleyman, R.; Deehan, D. Microbial resistance related to antibiotic-loaded bone cement: A historical review. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 3808–3817. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Meyer, C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. BioMed. Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef]
- Frutos, P.; Diez-Peña, E.; Frutos, G.; Barrales-Rienda, J.M. Release of gentamicin sulphate from a modified commercial bone cement. Effect of (2-hydroxyethyl methacrylate) comonomer and poly(N-vinyl-2-pyrrolidone) additive on release mechanism and kinetics. Biomaterials 2002, 23, 3787–3797. [Google Scholar] [CrossRef]
- Letchmanan, K.; Shen, S.-C.; Ng, W.K.; Kingshuk, P.; Shi, Z.; Wang, W.; Tan, R.B.H. Mechanical properties and antibiotic release characteristics of poly(methyl methacrylate)-based bone cement formulated with mesoporous silica nanoparticles. J. Mech. Behav. Biomed. Mater. 2017, 72, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ao, H.; Lin, W.; Tang, T. In Vivo Effect of Quaternized Chitosan-Loaded Polymethylmethacrylate Bone Cement on Methicillin-Resistant Staphylococcus epidermidis Infection of the Tibial Metaphysis in a rabbit model. Antimicrob. Agents Chemother. 2014, 58, 6016–6023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunne, N.; Hill, J.; McAfee, P.; Todd, K.; Kirkpatrick, R.; Tunney, M.; Patrick, S. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: Effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007, 78, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Gandomkarzadeh, M.; Moghimi, H.R.; Mahboubi, A. Evaluation of the Effect of Ciprofloxacin and Vancomycin on Mechanical Properties of PMMA Cement; a Preliminary Study on Molecular Weight. Sci. Rep. 2020, 10, 3981. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Segal, E.; Lipovsky, A.; Gedanken, A.; Banin, E.; Natan, M. New Life for an Old Antibiotic. ACS Appl. Mater. Interfaces 2015, 7, 7324–7333. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Doiron, R.; Disilvio, L.; Punyani, S.; Singh, H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 130–139. [Google Scholar] [CrossRef]
- Punyani, S.; Deb, S.; Singh, H. Contact killing antimicrobial acrylic bone cements: Preparation and characterization. J. Biomater. Sci. Ed. 2007, 18, 131–145. [Google Scholar] [CrossRef]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Cao, L.; Xie, X.; Wang, B.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Zhang, N.; Bai, Y. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J. Dent. 2018, 79, 39–45. [Google Scholar] [CrossRef]
- Cavalu, S.; Simon, V.; Goller, G.; Akin, I. Bioactivity and Antimicrobial Properties of Pmma/Ag2o Acrylic Bone Cement Collagen Coated. Dig. J. Nanomater. Biostruct. 2011, 6, 779–790. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Y.; Zhou, D. Antimcriobial Activity and Mechanism of Chitosan with Different Molecular Weight. Seventh Int. Conf. Meas. Technol. Mechatron. Autom. 2015, 166–169. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Zivanovic, S.; Davis, R.H.; Golden, D.A. Chitosan as an antimicrobial in food products. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 153–181. ISBN 9781782420347. [Google Scholar]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Tan, H.; Guo, S.; Yang, S.; Xu, X.; Tang, T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012, 8, 2166–2174. [Google Scholar] [CrossRef]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D.F. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef]
- Malini, M.; Thirumavalavan, M.; Yang, W.-Y.; Lee, J.-F.; Annadurai, G. A versatile chitosan/ZnO nanocomposite with enhanced antimicrobial properties. Int. J. Biol. Macromol. 2015, 80, 121–129. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int. J. Biol. Macromol. 2015, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of non-cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Hassabo, A.G.; Nada, A.A.; Ibrahim, H.M.; Abou-Zeid, N.Y. Impregnation of silver nanoparticles into polysaccharide substrates and their properties. Carbohydr. Polym. 2014, 122, 343–350. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Perreault, F.; Fonseca de Faria, A.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Fan, J.; Grande, C.D.; Rodrigues, D.F. Biodegradation of graphene oxide-polymer nanocomposite films in wastewater. Environ. Sci. Nano 2017, 4, 1808–1816. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed]


| Component | Composition (~wt.%) | Function | Structural Formula |
|---|---|---|---|
| Solid Phase | |||
| PMMA | 87.5–89.25 | Polymer | 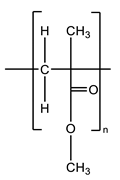 |
| Barium sulfate or zirconium dioxide | 10 | Radiopaque agent | BaSO4 or ZrO2 |
| Benzoyl peroxide | 0.75–2.5 | Polymerization reaction initiator |  |
| Liquid Phase | |||
| MMA | 97.0–97.5 | Monomer |  |
| N,N-Dimethyl-p-toluidine | 2.0–2.5 | Room-temperature polymerization reaction accelerator | 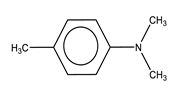 |
| Hydroquinone | 75 ppm | Inhibitor that prevents premature polymerization of MMA |  |
| Materials | Advantages | Disadvantages | References |
|---|---|---|---|
| ABC | Inert behavior | Insufficient adhesion to the bone | [10,14,32] |
| ABC + chitosan | Porous ABCs Improvement in osseointegration Reduction in Tmax reached during polymerization Increasing cellular activity of osteoblasts | Reduction in mechanical properties Increasing residual monomer content | [4,16,45,92,95,96,97,98,99,100,101,102,103] |
| ABC + graphene oxide | Improvement of mechanical properties Reduction Tmax reached during polymerization Good compatibility with bone tissue Accelerates bone formation Noncytotoxic to human osteoblasts, L929 fibroblasts, MG-63, hBMSCs, and MC3-T3 cells. | Increasing residual monomer content Reduction in cell viability against MG-63 cell culture. | [22,101,102,107,108,109,110,111,112,113,114] |
| ABC + chitosan + graphene oxide | Increased osseointegration Increased mechanical properties Increased viability and cell adhesion (MG-63 and human osteoblast) Increased antibacterial activity compared to separate loads | Increase in residual monomer content | [101,102,109] |
| Proposed Mechanism | Definition | References |
|---|---|---|
| Extremely sharp edges | It is the primary mechanism of antimicrobial activity in GO. Damage to the membrane when it comes into contact with the edges. | [59,163] |
| Oxidative stress (through the production of ROS) | It plays a minor role in antimicrobial activity. GO induces reactive oxygen species (ROS), which disrupts the balance in redox processes inside the cell and causes damage to cell components leading to apoptosis in bacteria. | [164] |
| Oxidative stress (independent of ROS production) | GO sheets interrupt a specific microbial process by disturbing or oxidizing a vital cell component or structure without producing ROS. | [50,165] |
| Formation of GO-cell aggregates | GO sheets envelop the bacteria forming aggregates, locally disturbing the cell membrane, and inducing the decrease in the bacterial membrane potential and the leakage of fungal spore electrolytes. | [59,166] |
| Strategies | Studied Bacteria | Advantages | Disadvantages | References |
|---|---|---|---|---|
| ABC + antibiotics | S. Aureus | Releases the antibiotic | Most of the antibiotic is trapped inside the cement Antibiotic-resistant bacteria have been developed | [118,124,125,126,127] |
| ABC + antibiotics + bioactive fillers | --- | A higher percentage of antibiotic is released Improves osseointegration | Reduces mechanical properties Antibiotic-resistant bacteria have been developed | [89,115,126,128,134,136] |
| ABC + chitosan | S. epidermidis S. Aureus E. coli S. Capitis | Presents good antimicrobial activity when the CS is functionalized or in nanosize. | CS without modifications Presents extremely low or no antimicrobial activity in ABCs | [96,97,101,109,120,136,153] |
| ABC + graphene oxide | B. cereus S. Aureus S. enterica E. coli | Shows antimicrobial activity against Gram-positive and Gram-negative bacteria | [101,108,109,114] | |
| ABC + chitosan + graphene oxide | E. coli | Increased antibacterial activity compared to cement with separately added | [101,109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. https://doi.org/10.3390/biom10121616
Zapata MEV, Tovar CDG, Hernandez JHM. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules. 2020; 10(12):1616. https://doi.org/10.3390/biom10121616
Chicago/Turabian StyleZapata, Mayra Eliana Valencia, Carlos David Grande Tovar, and José Herminsul Mina Hernandez. 2020. "The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements" Biomolecules 10, no. 12: 1616. https://doi.org/10.3390/biom10121616
APA StyleZapata, M. E. V., Tovar, C. D. G., & Hernandez, J. H. M. (2020). The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules, 10(12), 1616. https://doi.org/10.3390/biom10121616







