Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic
Abstract
1. Introduction
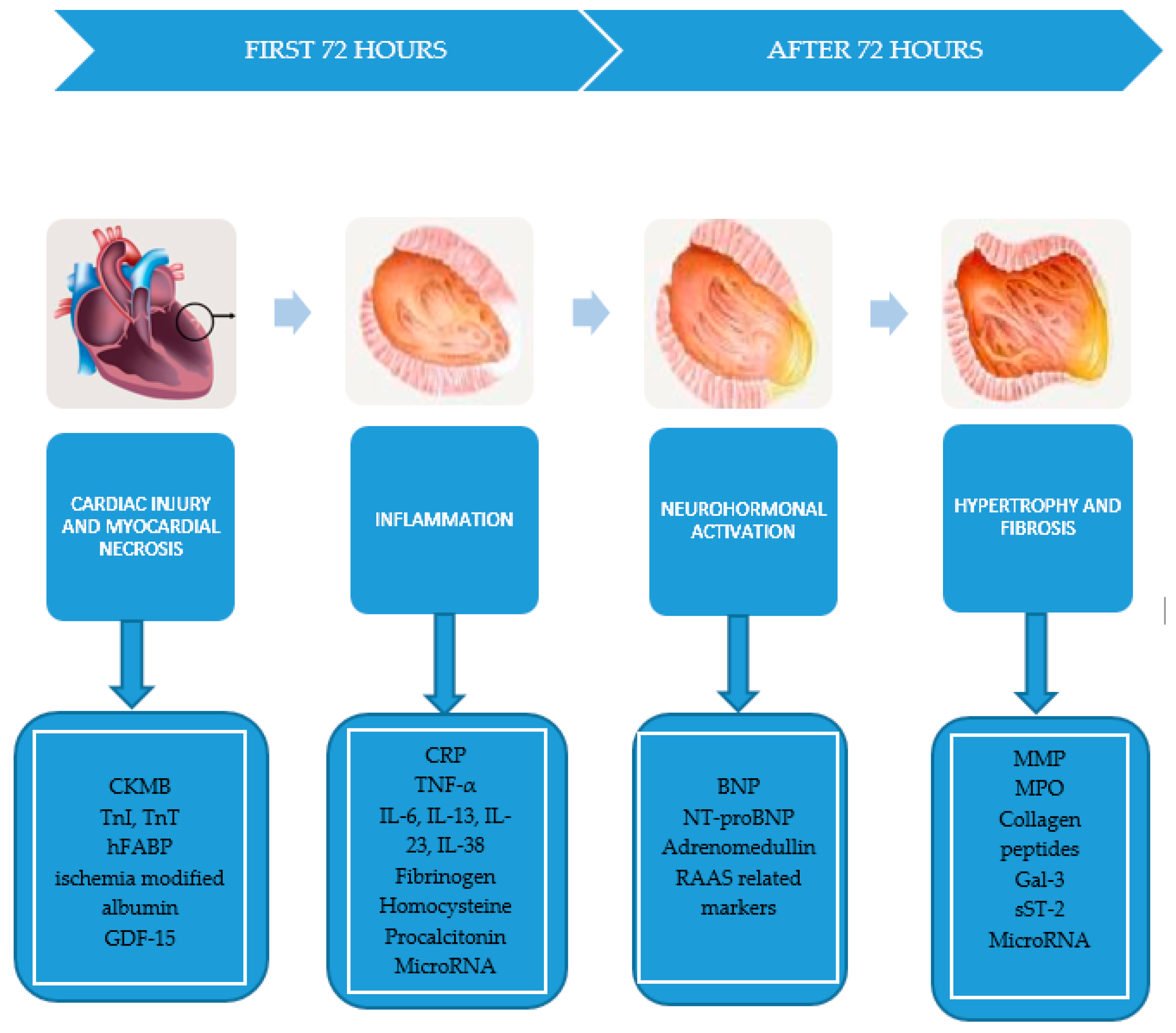
2. Ventricular Remodeling—Pathophysiology
- Myocardial necrosis: creatine kinase–myocardial band (CK-MB), troponin I and T (TnI, TnT), myoglobin, heart fatty acids binding protein (hFABP), ischemia modified albumin, GDF-15.
- Neurohormonal activation: N-terminal-pro type B natriuretic peptide (NT-proBNP), type B natriuretic peptide (BNP), adrenomedullin, renin–angiotensin–aldosterone system (RAAS)-related biomarkers.
- Inflammatory reaction closely related to the release of C-reactive protein (CRP), tumor necrosis factor α (TNF-α), interleukins 6, 13, 23, and 38 (IL-6, IL-13, IL-23, IL-38), homocysteine, procalcitonin.
- Hypertrophy and fibrosis involving MMP, collagen propeptidases, galectin-3 (Gal-3), soluble ST-2 (sST-2) [5].
3. Biomarkers
3.1. Biomarkers of Cardiac Injury and Myocardial Necrosis
3.1.1. Creatine Kinase MB
3.1.2. Troponin
3.1.3. Myoglobin
3.1.4. Ischemia Modified Albumin
3.1.5. hFABP (Heart-Type Fatty Acid Binding Protein)
3.1.6. GDF-15
3.2. Biomarkers of Neurohormonal Activity
3.2.1. Natriuretic Peptides
3.2.2. Adrenomedullin
3.2.3. Renin–Angiotensin–Aldosterone System-Related Biomarkers
3.3. Inflammatory Biomarkers
3.3.1. C-Reactive Protein
3.3.2. Other Inflammatory Markers
3.4. Biomarkers of Myocardial Fibrosis
3.4.1. Myeloperoxidase
3.4.2. Metalloproteinases
3.4.3. Collagen Peptides
3.4.4. Galectin-3
3.4.5. ST2
3.5. New Generation Biomarkers
MicroRNA
4. Multi Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Antman, E.M.; Braunwald, E. ST-Elevation Myocardial Infarction: Pathology, Pathophysiology, and Clinical Features. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 9th ed.; Zipes, D.P., Libby, P., Bonow, R.O., Braunwald, E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 1207–1230. [Google Scholar]
- Patel, K.V.; Mauricio, R.; Grodin, J.L.; Ayers, C.; Fonarow, G.C.; Berry, J.D.; Pandey, A. Identifying a low-flow phenotype in heart failure with preserved ejection fraction: A secondary analysis of the RELAX trial. ESC Heart Fail. 2019, 6, 613–620. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Tourki, B.; Jadapalli, J.K. Lipoxygenase drives lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism 2019, 96, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.S.J.; Ferrari, V.A. Prevention of Left Ventricular Remodeling After Myocardial Infarction. Circulation 2000, 101, 2981–2988. [Google Scholar] [PubMed]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Barrabes, J.A. Comments on the 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-segment Elevation. Rev. Española de Cardiol. (Engl. Ed.) 2015, 68, 1061–1067. [Google Scholar] [CrossRef]
- Berezin, A.E. Endogenous vascular repair system in cardiovascular disease: The role of endothelial progenitor cells. Australas. Med. J. 2019, 12, 42–48. [Google Scholar] [CrossRef]
- Berezin, A.E. Epigenetics in heart failure phenotypes. BBA Clin. 2016, 6, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 14. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse cardiac remodeling after acute myocardial infarction: Old and new biomarkers. Dis. Markers 2020, 2020, 21. [Google Scholar]
- Libby, P. The vascular biology of atherosclerosis. In Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 9th ed.; Zipes, D.P., Libby, P., Bonow, R.O., Braunwald, E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 1087–1110. [Google Scholar]
- Peksiene, D.Z.; Portacenko, J. Left Ventricular Remodeling after Acute Myocardial Infarction and Biomarkers. J. Cardiovasc. Dis. Diagn. 2017, 5, 5. [Google Scholar] [CrossRef]
- Anzai, T. Post-Infarction Inflammation and Left Ventricular Remodeling. Circ. J. 2013, 77, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sgueglia, G.A.; D’Errico, F.; Gioffrè, G.; De Santis, A.; Summaria, F.; Piccioni, F.; Gaspardone, A. Angiographic and clinical performance of polymer-free biolimus-eluting stent in patients with ST-segment elevation acute myocardial infarction in a metropolitan public hospital: The BESAMI MUCHO study. Catheter. Cardiovasc. Interv. 2018, 91, 851–858. [Google Scholar] [PubMed]
- Scarsini, R.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Ferreira, V.M.; Ribichini, F.; Channon, K.M.; et al. Incremental Value of Coronary Microcirculation Resistive Reserve Ratio in Predicting the Extent of Myocardial Infarction in Patients with STEMI. Insights from the Oxford Acute Myocardial Infarction (OxAMI) Study. Cardiovasc. Revascularization Med. 2019, 20, 1148–1155. [Google Scholar] [CrossRef]
- Long, B.; Li, N.; Xu, X.-X.; Li, X.-X.; Xu, X.-J.; Guo, D.; Zhang, D.; Wu, Z.-H.; Zhang, S.-Y. Long noncoding RNA FTX regulates cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2. Biochem. Biophys. Res. Commun. 2018, 495, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Meng, X.; Mei, L.; Hu, J.; Zhao, C.; Chen, W. The Long Non-Coding RNA SNHG1 Attenuates Cell Apoptosis by Regulating miR-195 and BCL2-Like Protein 2 in Human Cardiomyocytes. Cell. Physiol. Biochem. 2018, 50, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H.; Azzazy, H.M. Biochemical markers of the acute coronary syndromes. Clin. Chem. 1998, 44, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Sharma, N. Biomarkers in Acute Myocardial Infarction. J. Clin. Exp. Cardiol. 2012, 3, 222. [Google Scholar] [CrossRef]
- Bloomberg, D.J.; Kimber, W.D.; Burke, M.D. Cretin kinase isoenzymes. Predictive value in the early diagnosis of acute myocardial infarction. Am. J. Med. 1975, 59, 464–469. [Google Scholar]
- Ishikawa, Y.; Saffitz, J.E.; Mealman, T.L. Reversible myocardial ischemic injury is not associated with increased cretine kinase activity in plasma. Clin. Chem. 1997, 43, 467–475. [Google Scholar]
- Jeffe, A.S. Biochemical detection of acute myocardial infarction. In Acute Myocardial Infarction; Gersh, B., Rahimtoola, S., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 1991; pp. 110–127. [Google Scholar]
- Wu, A.H.; Wang, X.M.; Gornet, T.G.; Ordonez-Llanos, J. Cretine kinase MB isoforms in patients with skeletal muscle injury: Ramifications for early detection of acute myocardial infarction. Clin. Chem. 1992, 38, 2396–2400. [Google Scholar]
- Fioretti, P.; Sclavo, M.; Brower, R.W.; Simoons, M.L.; Hugenholtz, P.G. Prognosis of patients with different peak serum creatine kinase levels after first myocardial infarction. Eur. Heart J. 1985, 6, 473–478. [Google Scholar] [CrossRef]
- Savonitto, S.; Granger, S.B.; Ardissino, D.; Gardner, L.; Cavallini, C.; Galvani, M.; Ottani, F.; White, H.D.; Armstrong, P.W.; Ohman, E.M.; et al. The prognostic value of cretine kinase elevations extends across the whole spectrum of acute coronary syndromes. J. Am. Coll. Cardiol. 2002, 39, 22–29. [Google Scholar]
- Glezer, M.G.; Syrkin, A.L.; Gitel, E.P.; Sulimov, V.A.; Persiianov-Dubrov, I.V. Acute coronary syndrome without elevation of the ST segment: Prognostic significance of determining the levels of troponin I and CPK-MBmass. Ter Arkh 2002, 74, 26–30. [Google Scholar]
- Szymanski, F.M.; Grabowski, M.; Filipiak, K.J.; Karpiński, G.; Hrynkiewicz, A.; Stolarz, P.; Oręziak, A.; Rudowski, R.; Opolski, G. Prognostic implications of myocardial necrosis triad markers’ concentration measured at admission in patients with suspected acute coronary syndrome. Am. J. Emerg. Med. 2007, 25, 65–68. [Google Scholar] [CrossRef]
- Carvalho, G.; Rassi, S. The Prognostic Value of CK-MB in Acute Myocardial Infarction in Developing Countries: A Descriptive Study. Angiol. Open Access 2016, 4, 3. [Google Scholar] [CrossRef]
- Cavallini, C.; Savonitto, S.; Violini, R.; Arraiz, G.; Plebani, M.; Olivari, Z.; Rubartelli, P.; Battaglia, S.; Niccoli, L.; Steffenino, G.; et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention: Results of the CK-MB and PCI study. Eur. Heart J. 2005, 26, 1494–1498. [Google Scholar] [CrossRef]
- Abdelmeguid, A.E.; Topol, E.J.; Whitlow, P.L.; Sapp, S.K.; Ellis, S.G. Significance of mild transient release of creatinine kinase-MB fraction after percutaneous coronary intervention. Circulation 1996, 94, 1528–1536. [Google Scholar]
- Kong, T.Q.; Davidson, C.J.; Meyers, S.N.; Tauke, J.T.; Parker, M.A.; Bonow, R.O. Prognostic implication of creatine kinase elevation following elective coronary artery interventions. JAMA 1997, 277, 461–466. [Google Scholar]
- Akkerhuis, K.M.; Alexander, J.H.; Tardiff, B.E.; Boersma, E.; Harrington, R.A.; Lincoff, A.M.; Simmons, M.L. Minor myocardial damage and prognosis. Are spontaneous and percutaneous coronary intervention- related events different? Circulation 2002, 105, 554–556. [Google Scholar]
- Ioannidis, J.P.; Karvouni, E.; Katritsis, D.G. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2003, 42, 1406–1411. [Google Scholar] [CrossRef][Green Version]
- Roe, M.T.; Mahaffey, K.; Kilaru, R.; Alexander, J.; Akkerhuis, K.; Simoons, M.; Harrington, R.; Tardiff, B.; Granger, C.; Ohman, E.; et al. Creatine kinase-MB elevation after percutaneous coronary intervention predicts adverse outcomes in patients with acute coronary syndromes. Eur. Heart J. 2004, 25, 313–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kini, A.; Marmur, J.D.; Kini, S.; Dangas, G.; Cocke, T.P.; Wallenstein, S.; Brown, E.; Ambrose, J.A.; Sharma, S.K. Creatine kinase-MB elevation after coronary intervention correlates with diffuse atherosclerosis, and low-to-medium level elevation has a benign clinical course. J. Am. Coll. Cardiol. 1999, 34, 663–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baim, D.S.; Cutlip, D.E.; Sharma, S.; Ho, K.K.L.; Fortuna, R.; Schreiber, T.L.; Feldman, R.L.; Shani, J.; Senerchia, C.; Zhang, Y.; et al. Final Results of the Balloon vs Optimal Atherectomy Trial (BOAT). Circulation 1998, 97, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kornowski, R.; Leon, M.B. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: A device-specific analysis of 7147 patients. Circulation 2001, 104, 642–647. [Google Scholar]
- Brener, S.; Ellis, S.; Schneider, J.; Topol, E.J. Frequency and long-term impact of myonecrosis after coronary stenting. Eur. Heart J. 2002, 23, 869–876. [Google Scholar] [CrossRef]
- Yee, K.C.; Mukherjee, D.; Smith, D.E.; Kline-Rogers, E.M.; Fang, J.; Mehta, R.H.; Almanaseer, Y.; Akhras, E.; Cooper, J.V.; Eagle, K.A. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am. J. Cardiol. 2003, 92, 1442–1444. [Google Scholar] [CrossRef]
- Danne, O.; Lueders, C.; Storm, C.; Frei, U.; Möckel, M. Whole blood choline and plasma choline in acute coronary syndromes: Prognostic and pathophysiological implications. Clin. Chim. Acta 2007, 383, 103–109. [Google Scholar] [CrossRef]
- Rosenblat, J.; Zhang, A.; Fear, T. Biomarkers of myocardial infarction: Past, present and future. UWOMJ 2012, 81, 23–26. [Google Scholar]
- Dubois-Deruy, E.; Richard, V.; Mulder, P.; Lamblin, N.; Drobecq, H.; Henry, J.-P.; Amouyel, P.; Thuillez, C.; Bauters, C.; Pinet, F. Decreased Serine207 phosphorylation of troponin T as a biomarker for left ventricular remodelling after myocardial infarction. Eur. Heart J. 2010, 32, 115–123. [Google Scholar] [CrossRef]
- Yan, A.T.; Yan, R.T.; Tan, M.; Chow, C.-M.; Fitchett, D.; Stanton, E.; Langer, A.; Goodman, S.G. Troponin is more useful than creatine kinase in predicting one-year mortality among acute coronary syndrome patients. Eur. Heart J. 2004, 25, 2006–2012. [Google Scholar] [CrossRef][Green Version]
- Kazmi, K.A.; Bakr, A.; Perwaiz Iqbal, S.; Perwaiz Iqbal, M. Admission cretinkinase as a prognostic marker in acute myocardial infaction. J. Pak. Med. Assoc. 2009, 59, 819–822. [Google Scholar] [PubMed]
- Matetzky, S.; Sharir, T.; Domingo, M.; Noc, M.; Chyu, K.-Y.; Kaul, S.; Eigler, N.; Shah, P.K.; Cercek, B. Elevated troponin I level on admission is associated with adverse outcome of primary angioplasty in acute myocardial infarction. Circulation 2000, 102, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Rajappa, M.; Sharma, A. Biomarkers of Cardiac Injury: An Update. Angiology 2005, 56, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Melanson, S.F.; Lewandrowski, E.L.; Januzzi, J.L.; Lewandrowski, K.B. Reevaluation of myoglobin for acute chest pain evaluation: Would false positive results on “first-draw” specimens lead to increased hospital admissions? Am. J. Clin. Pathol. 2004, 121, 804–808. [Google Scholar]
- Newby, L.K.; Storrow, A.B.; Gibler, W.B.; Garvey, J.L.; Tucker, J.F.; Kaplan, A.L.; Schreiber, D.H.; Tuttle, R.H.; McNulty, S.E.; Ohman, E.M. Bedside multimarker testing for risk stratification in chest pain units: The chest pain evaluation by creatine kinase-MB, myoglobin, and troponin I (CHECKMATE) study. Circulation 2001, 103, 1832–1837. [Google Scholar] [CrossRef]
- Mccord, J.; Nowak, R.M.; Hudson, M.P.; McCullough, P.A.; Tomlanovich, M.C.; Jacobsen, G.; Tokarski, G.; Khoury, N.; Weaver, W. The prognostic significance of serial myoglobin, troponin I, and creatine kinase–MB measurements in patients evaluated in the emergency department for acute coronary syndrome. Ann. Emerg. Med. 2003, 42, 343–350. [Google Scholar] [CrossRef]
- Kontos, M.C.; Garg, R.; Anderson, F.P.; Roberts, C.S.; Ornato, J.P.; Tatum, J.L.; Jesse, R.L. Ability of myoglobin to predict mortality in patients admitted for exclusion of myocardial infarction. Am. J. Emerg. Med. 2007, 25, 873–879. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, Y.; Zhang, L.; Xu, W.; Zhou, X. Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgrad. Med. J. 2019, 95, 210–216. [Google Scholar] [CrossRef]
- Mehta, M.D.; Marwah, S.A.; Ghosh, S.; Shah, H.N.; Trivedi, A.P.; Haridas, N. A synergistic role of ischemia modified albumin and high-sensitivity troponin T in the early diagnosis of acute coronary syndrome. J. Fam. Med. Prim. Care 2015, 4, 570–575. [Google Scholar] [CrossRef]
- Manini, A.F.; Ilgen, J.; E Noble, V.; Bamberg, F.; Koenig, W.; Bohan, J.S.; Hoffmann, U. Derivation and validation of a sensitive IMA cutpoint to predict cardiac events in patients with chest pain. Emerg. Med. J. 2009, 26, 791–796. [Google Scholar] [CrossRef]
- Chan, D.C.S.; Ng, L.L. Biomarkers in acute myocardial infarction. BMC Med. 2010, 8, 34. [Google Scholar] [CrossRef]
- Haltern, G.; Peiniger, S.; Bufe, A.; Reiss, G.; Gülker, H.; Scheffold, T. Comparison of Usefulness of Heart-Type Fatty Acid Binding Protein Versus Cardiac Troponin T for Diagnosis of Acute Myocardial Infarction. Am. J. Cardiol. 2010, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mad, P.; Domanovits, H.; Fazelnia, C.; Stiassny, K.; Russmüller, G.; Cseh, A.; Sodeck, G.; Binder, T.; Christ, G.; Szekeres, T.; et al. Human heart-type fatty-acid-binding protein as a point-of-care test in the early diagnosis of acute myocardial infarction. QJM 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Ryzgar, O.; Blige, A.K.; Bugra, Z. The use of human heart-type fatty-acid binding proteinas an early diagnostic biochemical marker of myocardial necrosis in patients with acute coronary syndrome, and its comparison with troponin T and creatine kinase-myocardial band. Heart Vessel. 2006, 21, 309–314. [Google Scholar]
- Jolly, S.S.; Shenkman, H.; Brieger, D.; Fox, K.A.A.; Yan, A.T.; Eagle, K.A.; Steg, P.G.; Lim, K.-D.; Quill, A.L.; Goodman, S.G.; et al. Quantitative troponin and death, cardiogenic shock, cardiac arrest and new heart failure in patients with non-ST-segment elevation acute coronary syndromes (NSTE ACS): Insights from the Global Registry of Acute Coronary Events. Heart 2010, 97, 197–202. [Google Scholar] [CrossRef]
- Erlikh, A.D.; Katrukha, A.G.; Trifonov, I.R.; Bereznikova, A.V.; A Gratsianskiĭ, N. Prognostic significance of heart fatty acid binding protein in patients with non-ST elevation acute coronary syndrome: Results of follow-up for twelve months. Kardiologiia 2005, 45, 13–21. [Google Scholar]
- Jones, J.D.; Chew, P.G.; Dobson, R.; Wootton, A.; Ashrafi, R.; Khand, A. The Prognostic Value of Heart Type Fatty Acid Binding Protein in Patients with Suspected Acute Coronary Syndrome: A Systematic Review. Curr. Cardiol. Rev. 2017, 13, 189–198. [Google Scholar] [CrossRef]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Schaub, N.; Reichlin, T.; Twerenbold, R.; Reiter, M.; Steuer, S.; Bassetti, S.; Stelzig, C.; Wolf, C.; Winkler, K.; Haaf, P.; et al. Growth Differentiation Factor-15 in the Early Diagnosis and Risk Stratification of Patients with Acute Chest Pain. Clin. Chem. 2012, 58, 441–449. [Google Scholar] [CrossRef]
- Kempf, T.; Björklund, E.; Olofsson, S.; Lindahl, B.; Allhoff, T.; Peter, T.; Tongers, J.; Wollert, K.C.; Wallentin, L. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur. Heart J. 2007, 28, 2858–2865. [Google Scholar] [CrossRef]
- De Lemos, J.A.; Morrow, D.A. Brain Natriuretic Peptide Measurement in Acute Coronary Syndromes. Circulation 2002, 106, 2868–2870. [Google Scholar] [CrossRef] [PubMed]
- Möckel, M.; Danne, O.; Muller, R.; Vollert, J.O.; Müller, C.; Lueders, C.; Stork, T.; Frei, U.; Koenig, W.; Dietz, R.; et al. Development of an optimized multimarker strategy for early risk assessment of patients with acute coronary syndromes. Clin. Chim. Acta 2008, 393, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.; De Lemos, J.; Sabatine, M. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J. Am. Cardiol. 2003, 41, 1264–1272. [Google Scholar]
- Maisel, A.; Krishnawswamy, P.; Nowak, R. Rapid measurement of B-Type natriuretic peptide in the mergency diagnosis of heart failure. N. Engl. J. Med. 2002, 11, 55. [Google Scholar]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.; Bergmann, A.; Gouya, G.; Elhenicky, M.; Pacher, R. Comparison of copeptin B-type natriuretic peptide and amino-terminal pro-B-Type natriuretic peptide in patients with Chronic Heart Failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [PubMed]
- Leistner, D.M.; Klotsche, J.; Pieper, L.; Palm, S.; Stalla, G.K.; Lehnert, H.; Silber, S.; März, W.; Wittchen, H.-U.; Zeiher, A.M. Prognostic value of NT-pro-BNP and hs-CRP for risk stratification in primary care: Results from the population-based DETECT study. Clin. Res. Cardiol. 2013, 102, 259–268. [Google Scholar] [CrossRef]
- Niu, J.; Ma, Z.; Xie, C.; Zhang, Z. Association of plasma B-type natriuretic peptide concentration with myocardial infarct size in patients with acute myocardial infarction. Genet. Mol. Res. 2014, 13, 6177–6183. [Google Scholar] [CrossRef]
- Drewniak, W.; Szybka, W.; Bielecki, D.; Malinowski, M.; Kotlarska, J.; Krol-Jaskulska, A.; Popielarz-Grygalewicz, A.; Konwicka, A.; Dąbrowski, M. Prognostic Significance of NT-proBNP Levels in Patients over 65 Presenting Acute Myocardial Infarction Treated Invasively or Conservatively. BioMed Res. Int. 2015, 2015, 782026. [Google Scholar] [CrossRef]
- Islam, M.N.; Alam, M.F.; Debnath, R.C.; Aditya, G.P.; Ali, M.H.; Hossain, M.A.; Siddique, S.R. Correlation between Troponin-I and B-Type Natriuretic Peptide Level in Acute Myocardial Infarction Patients with Heart Failure. Mymensingh Med. J. 2016, 25, 226–231. [Google Scholar]
- Reesukumal, K.; Pratumvinit, B. B-type natriuretic peptide not TIMI risk score predicts death after acute coronary syndrome. Clin. Lab. 2012, 58, 1017–1022. [Google Scholar]
- Khan, S.Q.; Quinn, P.; Davies, J.E.; Ng, L.L. N-terminal pro-B-type natriuretic peptide is better than TIMI risk score at predicting death after acute myocardial infarction. Heart 2008, 94, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.A.; Baxter, G.F. Adrenomedullin: Regulator of systemic and cardiac homeostasis in acute myocardial infarction. Pharmacol. Ther. 2005, 105, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Narayan, H.K.; Ng, L.L. Prognostic Significance of Adrenomedullin in Patients with Heart Failure and with Myocardial Infarction. Am. J. Cardiol. 2015, 115, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Masoudi, F.A.; Spertus, J.A.; Wang, Q.; Murugiah, K.; Spatz, E.S.; Li, J.; Li, X.; Ross, J.S.; Krumholz, H.M.; et al. Patterns of Use of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Among Patients with Acute Myocardial Infarction in China From 2001 to 2011: China PEACE-Retrospective AMI Study. J. Am. Heart Assoc. 2015, 4, e001343. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Bakris, G.; Ruilope, L.M.; Dicarlo, L.; Mukherjee, R. On behalf of the EPHESUS Investigators Serum Potassium and Clinical Outcomes in the Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008, 118, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.S.; Gona, P.; Levy, D.; Aragam, J.; Larson, M.G.; Tofler, G.H.; Lieb, W.; Wang, T.J.; Benjamin, E.J.; Vasan, R.S. Relations of Biomarkers Representing Distinct Biological Pathways to Left Ventricular Geometry. Circulation 2008, 118, 2252–2258. [Google Scholar] [CrossRef]
- Nagai, T.; Anzai, T.; Kaneko, H.; Mano, Y.; Anzai, A.; Maekawa, Y.; Takahashi, T.; Meguro, T.; Yoshikawa, T.; Fukuda, K. C-Reactive Protein Overexpression Exacerbates Pressure Overload–Induced Cardiac Remodeling Through Enhanced Inflammatory Response. Hypertension 2011, 57, 208–215. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.Y.; Huang, X.R.; Wu, Y.; Chung, A.C.; Wu, E.X.; Szalai, A.J.; Wong, B.C.; Lau, C.-P.; Lan, H.Y. C-Reactive Protein Promotes Cardiac Fibrosis and Inflammation in Angiotensin II–Induced Hypertensive Cardiac Disease. Hypertension 2010, 55, 953–960. [Google Scholar] [CrossRef]
- Wang, J.; Tang, B.; Liu, X.; Wu, X.; Wang, H.; Xu, D.; Guo, Y. Increased monomeric CRP levels in acute myocardial infarction: A possible new and specific biomarker for diagnosis and severity assessment of disease. Atherosclerosis 2015, 239, 343–349. [Google Scholar] [CrossRef]
- Ørn, S.; Manhenke, C.; Ueland, T.; Damås, J.K.; Mollnes, T.E.; Edvardsen, T.; Aukrust, P.; Dickstein, K. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. Eur. Heart J. 2009, 30, 1180–1186. [Google Scholar] [CrossRef]
- Schoos, M.M.; Munthe-Fog, L.; Skjoedt, M.-O.; Ripa, R.S.; Lønborg, J.; Kastrup, J.; Kelbæk, H.; Laursen, P.N.; Garred, P. Association between lectin complement pathway initiators, C-reactive protein and left ventricular remodeling in myocardial infarction—A magnetic resonance study. Mol. Immunol. 2013, 54, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Puljak, L.; Lukin, A.; Novak, K.; Polić, S. Prognostic value of low and moderately elevated C-reactive protein in acute coronary syndrome: A 2-year follow-up study. Med. Sci. Monit. 2013, 19, 777–786. [Google Scholar] [CrossRef]
- He, L.-P.; Tang, X.-Y.; Ling, W.-H.; Chen, W.-Q.; Chen, Y.-M. Early C-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: A meta-analysis of longitudinal studies. Heart 2010, 96, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bodi, V.; Sanchis, J.; Llácer, A.; Facila, L.; Núñez, J.; Pellicer, M.; Bertomeu, V.; Ruiz, V.; Chorro, F.J. Prognostic markers of non-ST elevation acute coronary syndromes. Rev. Española de Cardiol. 2003, 56, 857. [Google Scholar]
- Fertin, M.; Hennache, B.; Hamon, M.; Ennezat, P.V.; Biausque, F.; Elkohen, M.; Nugue, O.; Tricot, O.; Lamblin, N.; Pinet, F.; et al. Usefulness of Serial Assessment of B-Type Natriuretic Peptide, Troponin I, and C-Reactive Protein to Predict Left Ventricular Remodeling After Acute Myocardial Infarction (from the REVE-2 Study). Am. J. Cardiol. 2010, 106, 1410–1416. [Google Scholar] [CrossRef]
- Hamzic-Mehmedbasic, A. Inflammatory Cytokines as Risk Factors for Mortality After Acute Cardiac Events. Med. Arch. 2016, 70, 252–255. [Google Scholar] [CrossRef]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-Sensitivity C-Reactive Protein and Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef]
- Puri, R.; Nissen, S.E.; Shao, M.; Uno, K.; Kataoka, Y.; Kapadia, S.R.; Tuzcu, E.M.; Nicholls, S.J. Impact of Baseline Lipoprotein and C-Reactive Protein Levels on Coronary Atheroma Regression Following High-Intensity Statin Therapy. Am. J. Cardiol. 2014, 114, 1465–1472. [Google Scholar] [CrossRef]
- Mueller, C. Biomarkers and acute coronary syndromes: An update. Eur. Heart J. 2014, 35, 552–556. [Google Scholar] [CrossRef]
- Duffy, J.R.; Salerno, M. New Blood Test to Measure Heart Attack Risk. J. Cardiovasc. Nurs. 2004, 19, 425–429. [Google Scholar] [CrossRef]
- De Servi, S.; Mariani, M.; Mariani, G.; Mazzone, A. C-reactive protein increase in unstable coronary disease cause or effect? J. Am. Cardiol. 2005, 456, 1496–1502. [Google Scholar]
- Bodi, V.; Sanchis, J.; Lopez Llereu, M.P.; Losada, A.; Nunez, J.; Pellicer, M.; Bertomeu, V.; Chorro, F.J.; Llacer, A. Usefullness of comprehensive cardiovascular magnetic resonance imaging assessment for predicting recovery of left ventricular wall motion in the setting of myocardial stunning. J. Am. Cardiol. 2005, 46, 1747–1752. [Google Scholar]
- García-Salas, J.M.; Tello-Montoliu, A.; Manzano-Fernández, S.; Casas-Pina, T.; López-Cuenca, A.; Pérez-Berbel, P.; Puche-Morenilla, C.; Martínez-Hernández, P.; Valdés, M.; Marín, F. Interleukin-6 as a predictor of cardiovascular events in troponin-negative non-ST elevation acute coronary syndrome patients. Int. J. Clin. Pract. 2014, 68, 294–303. [Google Scholar] [CrossRef]
- Kubková, L.; Spinar, J.; Goldbergová, M.P.; Jarkovský, J.; Pařenica, J. Inflammatory response and C-reactive protein value in patient with acute coronary syndrome. Vnitrni Lek. 2013, 59, 981–988. [Google Scholar]
- Cherneva, Z.V.; Denchev, S.V.; Gospodinova, M.V.; Cakova, A.; Cherneva, R.V. Inflammatory cytokines at admission-independent prognostic markers in patients with acute coronary syndrome and hyperglycemia. Acute Card Care 2012, 14, 13–19. [Google Scholar]
- Hofmann, U.; Knorr, S.; Vogel, B.; Weirather, J.; Frey, A.; Ertl, G.; Frantz, S. Interleukin-13 Deficiency Aggravates Healing and Remodeling in Male Mice After Experimental Myocardial Infarction. Circ. Heart Fail. 2014, 7, 822–830. [Google Scholar] [CrossRef]
- Savvatis, K.; Pappritz, K.; Becher, P.M.; Lindner, D.; Zietsch, C.; Volk, H.-D.; Westermann, D.; Schultheiss, H.-P.; Tschöpe, C. Interleukin-23 Deficiency Leads to Impaired Wound Healing and Adverse Prognosis After Myocardial Infarction. Circ. Heart Fail. 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, Y.; Zhong, Y.; Yu, K.; Xu, W.; Zhu, R.; Sun, H.; Ding, Y.; Wang, Y.; Zeng, Q. Interleukin-38 alleviates cardiac remodelling after myocardial infarction. J. Cell. Mol. Med. 2020, 24, 371–384. [Google Scholar] [CrossRef]
- Toss, H.; Lindahl, B.; Siegbahn, A.; Wallentin, L. Prognostic Influence of Increased Fibrinogen and C-Reactive Protein Levels in Unstable Coronary Artery Disease. Circulation 1997, 96, 4204–4210. [Google Scholar] [CrossRef]
- Sanchis, J.; Bodi, V.; Navarro, A.; Llacer, A.; Nunez, J.; Blasco, M.; Mainar, L.; Monmeneu, J.V.; Insa, L.; Ferrero, J.A.; et al. Factores prognosticos en la angina inestable con cambios dinamicos del electrocardiograma. Valor del fibrinogeno. Rev. Esp. Cardiol. 2002, 55, 921–927. [Google Scholar]
- Bozkurt, E.; Erol, M.K.; Keleş, S.; Acikel, M.; Yilmaz, M.; Gurlertop, Y.; Yilmaz, M. Relation of plasma homocysteine levels to intracoronary thrombus in unstable angina pectoris and in non–Q-wave acute myocardial infarction. Am. J. Cardiol. 2002, 90, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, N.; Venetsanou, K.; Patsilinakos, S.; Voudris, V.; Antonatos, D.; Kelesidis, K.; Baltopoulos, G.; Maniatis, P.; Cokkinos, D.V. Procalcitonin in acute myocardial infarction. Acute Card. Care 2008, 10, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ataoğlu, H.E.; Yilmaz, F.; Uzunhasan, I.; Çetin, F.; Temiz, L.; Döventaş, Y.E.; Kaya, A.; Yenigün, M. Procalcitonin: A Novel Cardiac Marker with Prognostic Value in Acute Coronary Syndrome. J. Int. Med. Res. 2010, 38, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Khan, S.Q.; Dhillon, O.; Quinn, P.; Struck, J.; Squire, I.B.; Davies, J.E.; Ng, L.L. Procalcitonin as a prognostic marker in patients with acute myocardial infarction. Biomarkers 2010, 15, 325–331. [Google Scholar] [CrossRef]
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Hazen, S.L. Nitric Oxide Is a Physiological Substrate for Mammalian Peroxidases. J. Biol. Chem. 2000, 275, 37524–37532. [Google Scholar] [CrossRef]
- Vasilyev, N.; Williams, T.; Brennan, M.-L.; Unzek, S.; Zhou, X.; Heinecke, J.W.; Spitz, D.R.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase-Generated Oxidants Modulate Left Ventricular Remodeling but Not Infarct Size After Myocardial Infarction. Circulation 2005, 112, 2812–2820. [Google Scholar] [CrossRef]
- Askari, A.T.; Brennan, M.-L.; Zhou, X.; Drinko, J.; Morehead, A.; Thomas, J.D.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase and Plasminogen Activator Inhibitor 1 Play a Central Role in Ventricular Remodeling after Myocardial Infarction. J. Exp. Med. 2003, 197, 615–624. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Firedrichs, K.; Lange, K.; Gesenberg, J.; Remane, L.; Kerkenpas, C.; Krause, J.; Schneider, J.; Ravekes, T.; Maass, M.; et al. Myeloperoxidase mediates postischemic arrhytmogenic ventricular remodeling. Circ. Res. 2017, 121, 56–70. [Google Scholar]
- Eggers, K.M.; Dellborg, M.; Johnston, N.; Oldgren, J.; Swahn, E.; Venge, P.; Lindahl, B. Myeloperoxidase is not useful for the early assessment of patients with chest pain. Clin. Biochem. 2010, 43, 240–245. [Google Scholar] [CrossRef]
- Hochholzer, W.; Morrow, D.A.; Giugliano, R.P. Novel biomarkers in cardiovascular disease: Update 2010. Am. Heart J. 2010, 160, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.M.; Zahran, F.M.; Kadry, M.; Belal, A.A.M.; Emran, T.M. Role of myeloperoxidase in early diagnosis of acute myocardial infarction in patients admitted with chest pain. J. Immunoass. Immunochem. 2018, 39, 337–347. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Lopez-Haldon, J.E.; Fernandez, M.; Mancha, F.; Sanchez, A.; Rodriguez-Puras, M.J.; Villa, M.; Lopez-Pardo, F.; De La Llera, L.D.; Valle, J.I.; et al. Prognostic value of different serum biomarkers for left ventricular remodelling after ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. BMJ 2012, 48, 15–18. [Google Scholar]
- Mittal, B.; Mishra, A.; Srivastava, A.; Kumar, S.; Garg, N. Matrix Metalloproteinases in Coronary Artery Disease. Int. Rev. Cytol. 2014, 64, 1–72. [Google Scholar] [CrossRef]
- Moore, L.; Fan, D.; Basu, R.; Kandalam, V.; Kassiri, Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail. Rev. 2011, 17, 693–706. [Google Scholar] [CrossRef]
- Nuttall, R.K.; Sampieri, C.L.; Pennington, C.J.; E Gill, S.; A Schultz, G.; Edwards, D.R. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004, 563, 129–134. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Kandalam, V.; Basu, R.; Abraham, T.; Wang, X.; Soloway, P.D.; Jaworski, D.M.; Oudit, G.Y.; Kassiri, Z. TIMP2 Deficiency Accelerates Adverse Post–Myocardial Infarction Remodeling Because of Enhanced MT1-MMP Activity Despite Lack of MMP2 Activation. Circ. Res. 2010, 106, 796–808. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, Y.; McTiernan, C.F.; Pei, W.; Moravec, C.S.; Wang, P.; Rosenblum, W.; Kormos, R.L.; Feldman, A.M. Downregulation of Matrix Metalloproteinases and Reduction in Collagen Damage in the Failing Human Heart After Support With Left Ventricular Assist Devices. Circulation 2001, 104, 1147–1152. [Google Scholar] [CrossRef]
- Heymans, S.; Schroen, B.; Vermeersch, P.; Milting, H.; Gao, F.; Kassner, A.; Gillijns, H.; Herijgers, P.; Flameng, W.; Carmeliet, P.; et al. Increased Cardiac Expression of Tissue Inhibitor of Metalloproteinase-1 and Tissue Inhibitor of Metalloproteinase-2 Is Related to Cardiac Fibrosis and Dysfunction in the Chronic Pressure-Overloaded Human Heart. Circulation 2005, 112, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; Heymans, S. TIMPs and cardiac remodeling: Embracing the MMP-independent-side of the family. J. Mol. Cell. Cardiol. 2010, 48, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Liu, X.; Bai, Y.; Cui, C.; Li, J.; Li, Y.; Hu, S.; Wei, Y.-J. In Vitro Effects of Pirfenidone on Cardiac Fibroblasts: Proliferation, Myofibroblast Differentiation, Migration and Cytokine Secretion. PLoS ONE 2011, 6, e28134. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Kumar, S.G.; Banks, J.; Fortson, W. Co-expression of tissue inhibitor and matrix metalloproteinase in myocardium. J. Mol. Cell. Cardiol. 1995, 27, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.E.; Kandalam, V.; Chakrabarti, S.; Wang, X.; Penninger, J.M.; Davidge, S.T.; Oudit, G.Y.; Kassiri, Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am. J. Physiol. Physiol. 2010, 298, C679–C692. [Google Scholar] [CrossRef]
- Deschamps, A.M.; Spinale, F.G. Pathways of matrix metalloproteinase induction in heart failure: Bioactive molecules and transcriptional regulation. Cardiovasc. Res. 2006, 69, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Boerrigter, G.; Huntley, B.K.; Noser, J.A.; Cataliotti, A.; Costello-Boerrigter, L.C.; Chen, H.H.; Burnett, J.C. Brain Natriuretic Peptide Is Produced in Cardiac Fibroblasts and Induces Matrix Metalloproteinases. Circ. Res. 2002, 91, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Zavadzkas, J.A.; Mukherjee, R.; Rivers, W.T.; Patel, R.K.; Meyer, E.C.; Black, L.E.; McKinney, R.A.; Oelsen, J.M.; Stroud, R.E.; Spinale, F.G. Direct regulation of membrane type 1 matrix metalloproteinase following myocardial infarction causes changes in survival, cardiac function, and remodeling. Am. J. Physiol. Circ. Physiol. 2011, 301, H1656–H1666. [Google Scholar] [CrossRef]
- Spinale, F.G.; Escobar, G.P.; Mukherjee, R.; Zavadzkas, J.A.; Saunders, S.M.; Jeffords, L.B.; Leone, A.M.; Beck, C.; Bouges, S.; Stroud, R.E. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: Effects on myocardial remodeling with aging. Circ. Heart Fail. 2009, 2, 351–360. [Google Scholar] [CrossRef]
- Halade, G.V.; Jin, Y.-F.; Lindsey, M.L. Matrix metalloproteinase (MMP)-9: A proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol. Ther. 2013, 139, 32–40. [Google Scholar] [CrossRef]
- Eschalier, R.; Fertin, M.; Fay, R.; Bauters, C.; Zannad, F.; Pinet, F.; Rossignol, P. Extracellular Matrix Turnover Biomarkers Predict Long-Term Left Ventricular Remodeling After Myocardial Infarction Insights From the REVE-2 Study. Circ. Heart Fail. 2013, 6, 1199–1205. [Google Scholar] [PubMed]
- Iraqi, W.; Rossignol, P.; Angioi, M.; Fay, R.; Nuée, J.; Ketelslegers, J.-M.; Vincent, J.; Pitt, B.; Zannad, F. Extracellular Cardiac Matrix Biomarkers in Patients with Acute Myocardial Infarction Complicated by Left Ventricular Dysfunction and Heart Failure. Circulation 2009, 119, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Infarct scar: A dynamic tissue. Cardiovasc. Res. 2000, 46, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Mann, D.L.; Entman, M.L.; Spinale, F.G. Extracellular matrix remodeling following myocardial injury. Ann. Med. 2003, 35, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.L.; Banerjee, I.; Baudino, T.A. The extracellular matrix: At the center of it all. J. Mol. Cell. Cardiol. 2010, 48, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Jourdan-LeSaux, C.; Zhang, J.; Lindsey, M.L. Extracellular matrix roles during cardiac repair. Life Sci. 2010, 87, 391–400. [Google Scholar] [CrossRef]
- Kang, Q.; Li, X.; Yang, M.; Fernando, T.; Wan, Z. Galectin-3 in patients with coronary heart disease and atrial fibrillation. Clin. Chim. Acta 2018, 478, 166–170. [Google Scholar] [CrossRef]
- Bivona, G.; Bellia, C.; Sasso, B.L.; Agnello, L.; Scazzone, C.; Novo, G.; Ciaccio, M. Short-term Changes in Gal 3 Circulating Levels After Acute Myocardial Infarction. Arch. Med. Res. 2016, 47, 521–525. [Google Scholar] [CrossRef]
- Gonzalez, G.E.; Cassaglia, P.; Truant, S.N.; Fernández, M.M.; Wilensky, L.; Volberg, V.; Malchiodi, E.L.; Morales, C.; Gelpi, R.J. Galectin-3 is essential for early wound healing and ventricular remodeling after myocardial infarction in mice. Int. J. Cardiol. 2014, 176, 1423–1425. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Sung, P.-H.; Chang, L.-T.; Sun, C.-K.; Yeh, K.-H.; Chung, S.-Y.; Chua, S.; Chen, Y.-L.; Wu, C.-J.; Chang, H.-W.; et al. Value and level of galectin-3 in acute myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Atheroscler. Thromb. 2012, 19, 1073–1082. [Google Scholar] [CrossRef]
- Weir, R.A.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and Cardiac Function in Survivors of Acute Myocardial Infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, G.; Caretta, G.; De Maria, R.; Parolini, M.; Bassi, L.; Testa, S.; Pirelli, S. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart 2014, 103, 1–5. [Google Scholar]
- Andrejic, O.M.; Vucic, R.M.; Pavlovic, M.; McClements, L.; Stokanovic, D.; Jevtovic–Stoimenov, T.; Nikolic, V.N. Association between Galectin-3 levels within central and peripheral venous blood, and adverse left ventricular remodelling after first acute myocardial infarction. Sci. Rep. 2019, 9, 131–145. [Google Scholar] [CrossRef]
- Kohli, P.; Bonaca, M.P.; Kakkar, R.; Kudinova, A.Y.; Scirica, B.M.; Sabatine, M.S.; Murphy, S.A.; Braunwald, E.; Lee, R.T.; Morrow, D.A. Role of ST2 in Non–ST-Elevation Acute Coronary Syndrome in the MERLIN-TIMI 36 Trial. Clin. Chem. 2012, 58, 257–266. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Pavan, C. Prognostic biomarkers in acute coronary syndrome. Ann. Transl. Med. 2016, 4, 258. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.-C.; Mi, Y.-H.; Liu, J. Predicting value of serum soluble ST2 and interleukin-33 for risk stratification and prognosis in patients with acute myocardial infarction. Chin. Med. J. 2013, 126, 3628–3631. [Google Scholar]
- Sun, T.; Dong, Y.-H.; Du, W.; Shi, C.-Y.; Wang, K.; Akram, J.; Wang, J.; Li, P. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int. J. Mol. Sci. 2017, 18, 745. [Google Scholar] [CrossRef]
- Gidlöf, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef]
- Xiao, J.; Shen, B.; Li, J.; Lv, D.; Zhao, Y.; Wang, F.; Xu, J. Serum microRNA-499 and microRNA-208a as biomarkers of acute myocardial infarction. Int. J. Clin. Exp. Med. 2014, 7, 136–141. [Google Scholar]
- Zhu, J.; Yao, K.; Wang, Q.; Guo, J.; Shi, H.; Ma, L. Circulating miRNA-181a as a potential novel biomarker for diagnosis of acute myocardial infarction. Cell Physiol. Biochem. 2016, 40, 1591–1602. [Google Scholar]
- Zhang, Y.; Cheng, J.; Chen, F.; Wu, C.; Zhang, J.; Ren, X.; Pan, Y.; Nie, B.; Li, Q.; Li, Y. Circulating endothelial microparticles and miR-92a in acute myocardial infarction. Biosci. Rep. 2017, 37, 37. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Mosterd, A.; Dekker, M.S.; De Vrey, E.A.; Van Mil, A.; Pasterkamp, G.; Doevendans, P.A.; Hoes, A.W.; Sluijter, J.P.G. Early assessment of acute coronary syndromes in the emergency department: The potential diagnostic value of circulating microRNAs. EMBO Mol. Med. 2012, 4, 1176–1185. [Google Scholar] [CrossRef]
- Schulte, C.; Molz, S.; Appelbaum, S.; Karakas, M.; Ojeda, F.; Lau, D.M.; Hartmann, T.; Lackner, K.J.; Westermann, D.; Schnabel, R.B.; et al. miRNANA-197 and miRNANA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS ONE 2015, 10, e0145930. [Google Scholar]
- He, F.; Lv, P.; Zhao, X.; Wang, X.; Ma, X.; Meng, W.; Meng, X.; Dong, S. Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction. Mol. Cell. Biochem. 2014, 394, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a are Associated with Left Ventricular Remodeling after Acute Myocardial Infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jing, Q. Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol. Sin. 2018, 39, 1110–1119. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Zangrando, J.; Kelly, D.; Razvi, N.; Zhang, L.; Ng, L.L.; Wagner, D.R.; Squire, I.B. MicroRNA-150: A novel marker of left ventricular remodeling after acute myocardial infarction. Circ. Cardiovasc. Genet. 2013, 6, 290–298. [Google Scholar]
- Galeano-Otero, I.; Del Toro, R.; Rasco, A.G.; Díaz, I.; Mayoral-González, I.; Guerrero-Márquez, F.; Gutiérrez-Carretero, E.; Casquero-Domínguez, S.; De La Llera, L.S.D.; Barón-Esquivias, G.; et al. Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2020, 9, 1051. [Google Scholar] [CrossRef]
- Widera, C.; Gupta, S.K.; Lorenzen, J.M.; Bang, C.; Bauersachs, J.; Bethmann, K.; Kempf, T.; Wollert, K.C.; Thum, T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell. Cardiol. 2011, 51, 872–875. [Google Scholar] [CrossRef]
- Goretti, E.; Vausort, M.; Wagner, D.R.; Devaux, Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int. J. Cardiol. 2013, 168, 4548–4550. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Spazzafumo, L.; Santini, G.; Rippo, M.R.; Galeazzi, R.; Giovagnetti, S.; D’Alessandra, Y.; Marcheselli, F.; Capogrossi, M.C.; et al. Admission levels of circulating miRNA-499-5p and risk of death in elderly patients after acute non-ST elevation myocardial infarction. Int. J. Cardiol. 2014, 172, 276–278. [Google Scholar]
- Matsumoto, S.; Sakata, Y.; Nakatani, D.; Suna, S.; Mizuno, H.; Shimizu, M.; Usami, M.; Sasaki, T.; Sato, H.; Kawahara, Y.; et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 427, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infaction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [PubMed]
- Dong, Y.M.; Liu, X.X.; Wei, G.Q.; Da, Y.N.; Cha, L.; Ma, C.S. Prediction of long-term outcome after acute myocardial infaction using circulating miRNA-145. Scand. J. Clin. Lab. Investig. 2015, 75, 85–91. [Google Scholar]
- Cortez-Dias, N.; Costa, M.C.; Carrilho-Ferreira, P.; Silva, D.; Jorge, C.; Calisto, C.; Pessoa, T.; Martins, S.R.; De Sousa, J.C.; Da Silva, P.C.; et al. Circulating miR-122-5p/miR-133b Ratio Is a Specific Early Prognostic Biomarker in Acute Myocardial Infarction. Circ. J. 2016, 80, 2183–2191. [Google Scholar] [CrossRef]
- Eitel, L.; Adams, V.; Dieterich, P.; Fuernau, G.; de Waha, S.; Desch, S.; Schuler, G.; Thiele, H. Relation of circular microRNS-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infaction. Am. Heart J. 2012, 164, 706–714. [Google Scholar]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long Noncoding RNAs in Patients with Acute Myocardial Infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef]
- Zhai, H.; Li, X.-M.; Liu, F.; Chen, B.-D.; Zheng, H.; Wang, X.-M.; Liao, W.; Chen, Q.-J.; Ma, Y.-T.; Yang, Y.-N. Expression pattern of genome-scale long noncoding RNA following acute myocardial infarction in Chinese Uyghur patients. Oncotarget 2017, 8, 31449–31464. [Google Scholar] [CrossRef]
- Qu, X.; Song, X.; Yuan, W.; Shu, Y.; Wang, Y.; Zhao, X.; Gao, M.; Lu, R.; Luo, S.; Zhao, W.; et al. Expression signature of lncRNAs and their potential roles in cardiac fibrosis of post-infarct mice. Biosci. Rep. 2016, 36, 00337. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Kelly, D.; Collignon, O.; Ng, L.L.; Wagner, R.D.; Squire, I.B. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS ONE 2013, 8, e70644. [Google Scholar]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, D.H.; Park, Y.; Han, J.; Lee, H.; Kang, H.; Park, H.S.; Cho, Y.; Chae, S.C.; Jun, J.-E.; et al. Incremental Prognostic Value of C-Reactive Protein and N-Terminal ProB-Type Natriuretic Peptide in Acute Coronary Syndrome. Circ. J. 2006, 70, 1379–1384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Donoghue, M.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Desai, N.R.; Sherwood, M.W.; Murphy, S.A.; Gerszten, R.E.; Sabatine, M.S. Multimarker Risk Stratification in Patients with Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e002586. [Google Scholar] [CrossRef]
- Aldous, S.J. Cardiac biomarkers in acute myocardial infarction. Int. J. Cardiol. 2013, 164, 282–294. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Feistritzer, H.-J.; Reindl, M.; Klug, G.; Mayr, A.; Mair, J.; Jaschke, W.; Metzler, B. Combined biomarker testing for the prediction of left ventricular remodelling in ST-elevation myocardial infarction. Open Heart 2016, 3, e000485. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lichtenauer, M.; Wernly, B.; Paar, V.; Pistulli, R.; Rohm, I.; Jung, C.; Figulla, H.-R.; Yilmaz, A.; Cadamuro, J.; et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2017, 47, 638–648. [Google Scholar] [CrossRef]
- Feistritzer, H.-J.; Reinstadler, S.J.; Klug, G.; Reindl, M.; Wöhrer, S.; Brenner, C.; Mayr, A.; Mair, J.; Metzler, B. Multimarker approach for the prediction of microvascular obstruction after acute ST-segment elevation myocardial infarction: A prospective, observational study. BMC Cardiovasc. Disord. 2016, 16, 239. [Google Scholar] [CrossRef]

| Characteristics of the Ideal Biomarker |
|---|
| High sensitivity |
| Increased myocardial concentrations after heart attack |
| Rapid release to allow early diagnosis |
| Long half-life to allow late diagnosis |
| High specificity |
| Its absence in tissues other than the myocardial one |
| Its absence in healthy patients |
| Assay-related characteristics |
| Good cost-effectiveness ratio |
| Easy to assay |
| Short processing time |
| High precision |
| Clinical characteristics |
| Useful in guiding therapy |
| Useful in predicting the prognosis |
| Category | Biomarker | Prognostic Value |
|---|---|---|
| Cardiac injury and myocardial necrosis | CK-MB [25,27,28,29,30,31,32,33,34,35,36,37,38] | Predictive of mortality and cardiovascular events |
| Troponin [42,43,44,45] | Independent predictor of ventricular remodeling and cardiovascular events | |
| Myoglobin [49] | Predictive only in association with troponin | |
| Ischemia modified albumin [52,53] | Raises the predictive value of troponin when measured together | |
| hFABP [58,59] | Predictive of mortality and major cardiovascular events after 1 year | |
| GDF-15 [61,62,63] | Independent predictor of mortality | |
| Neurohormonal activity | BNP, NT-proBNP [65,66,69,70,71,72,73] | Highly predictive of heart failure, cardiovascular events, and mortality |
| Adrenomedullin [76] | Predictive of cardiovascular events and severity of heart failure | |
| RAAS-related biomarkers [77,78] | The use of its inhibitors is associated with a mortality and morbidity decrease | |
| Inflammatory biomarkers | C-reactive protein [83,84,85,86,87,88] | Predictive of ventricular remodeling and only when associated with other biomarkers, it becomes predictive of mortality |
| IL-6 [89,96] | Predictive of mortality and cardiovascular events | |
| TNF-α [98] | Might be predictive for survival in association with C-reactive protein | |
| IL-13, IL-23, IL-38, fibrinogen, homocysteine [99,100,101,102,103,104] | Might be predictive of ventricular remodeling | |
| Procalcitonin [106,107] | Predictive of mortality, cardiovascular events, and ventricular remodeling | |
| Fibrosis biomarkers | MMP, MPO [116,133] | Might be predictive of ventricular remodeling |
| Collagen peptides [134,135] | Predictive of cardiovascular events and mortality | |
| Galectin-3 [143,144,146] | Predictive of major cardiovascular events. Might be predictive of ventricular remodeling | |
| ST-2 [149] | Predictive of survival | |
| Novel biomarkers | microRNA [156,157,158,160,162,168,169,170,173,174] | Predictive of mortality, heart failure, cardiovascular events, and ventricular remodeling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostan, M.-M.; Stătescu, C.; Anghel, L.; Șerban, I.-L.; Cojocaru, E.; Sascău, R. Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic. Biomolecules 2020, 10, 1587. https://doi.org/10.3390/biom10111587
Bostan M-M, Stătescu C, Anghel L, Șerban I-L, Cojocaru E, Sascău R. Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic. Biomolecules. 2020; 10(11):1587. https://doi.org/10.3390/biom10111587
Chicago/Turabian StyleBostan, Maria-Madălina, Cristian Stătescu, Larisa Anghel, Ionela-Lăcrămioara Șerban, Elena Cojocaru, and Radu Sascău. 2020. "Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic" Biomolecules 10, no. 11: 1587. https://doi.org/10.3390/biom10111587
APA StyleBostan, M.-M., Stătescu, C., Anghel, L., Șerban, I.-L., Cojocaru, E., & Sascău, R. (2020). Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic. Biomolecules, 10(11), 1587. https://doi.org/10.3390/biom10111587








