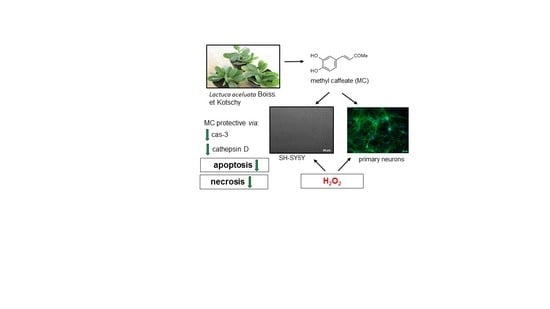
Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Caffeic Acid Derivatives
2.3. SH-SY5Y Cell Culture
2.4. Primary Neuronal Cell Cultures
2.5. Cell Treatment
2.6. Cell Viability Assay
2.7. LDH Release Assay
2.8. Propidium Iodide Staining and Flow Cytometry
2.9. CalceinAM/Hoechst 33,342 Live Imaging and MAP-2/Hoechst 33,342 Immunofluorescence
2.10. Caspase-3 Activity Assay
2.11. Cathepsin D Activity Assay
2.12. Statistical Analysis
3. Results
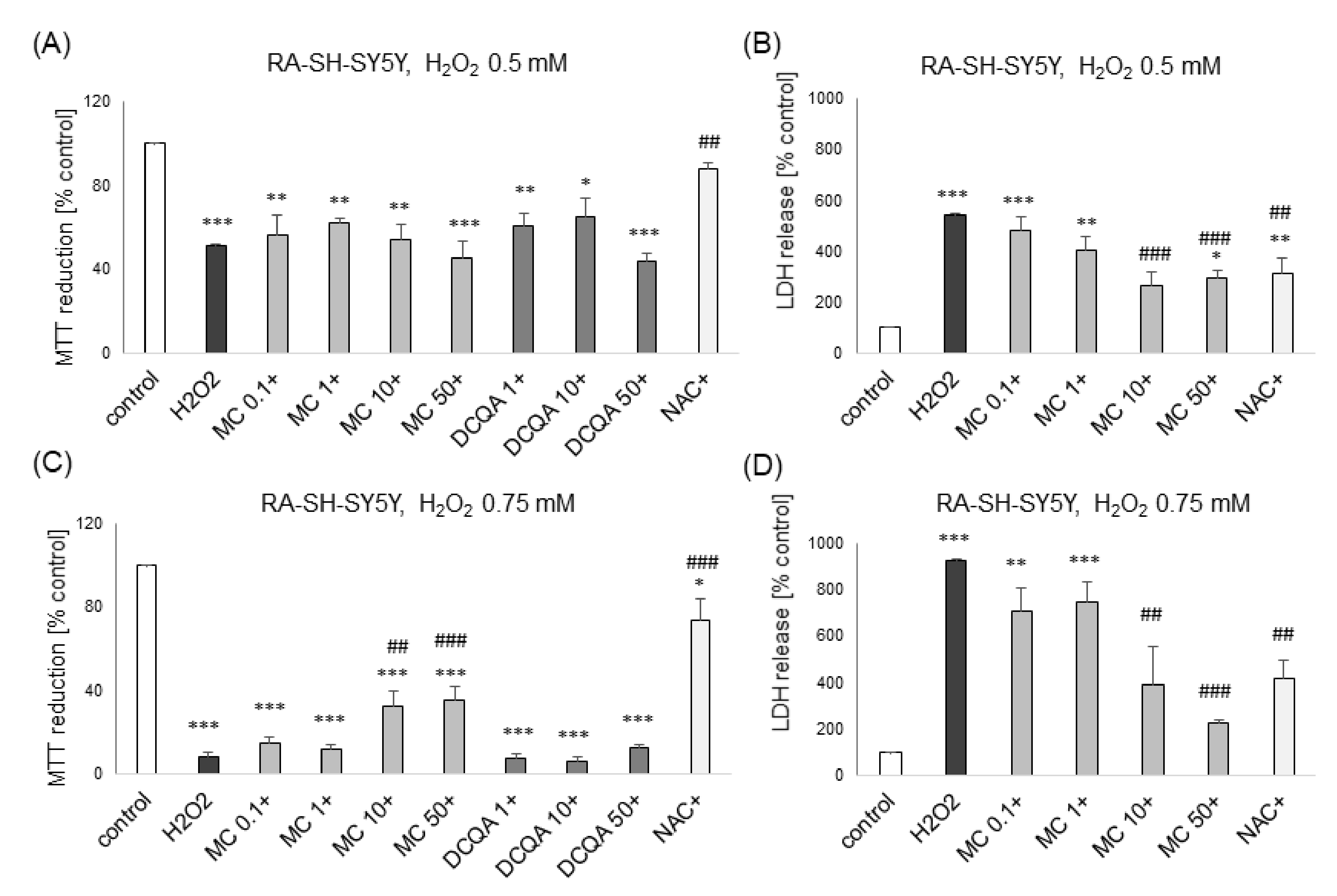
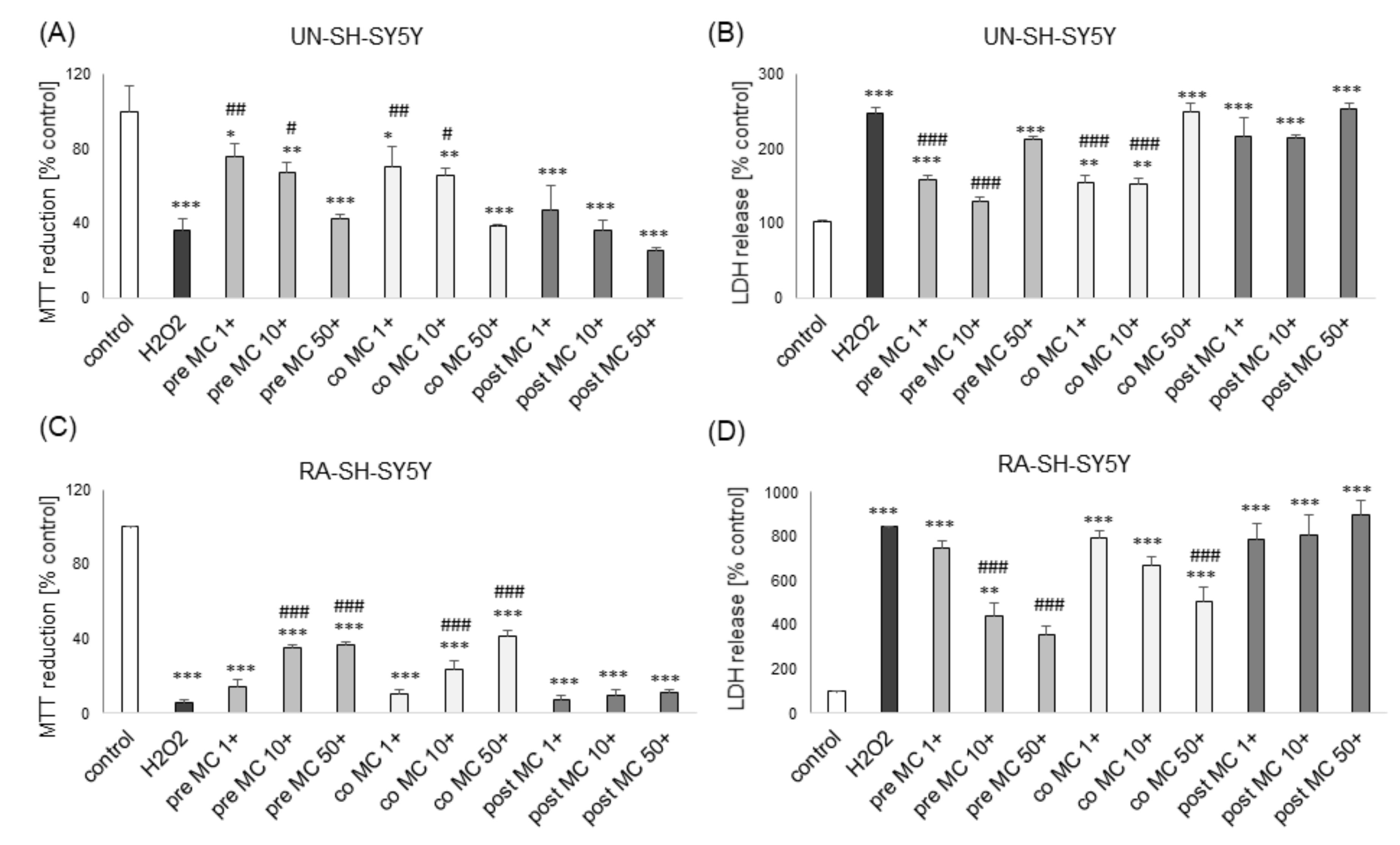
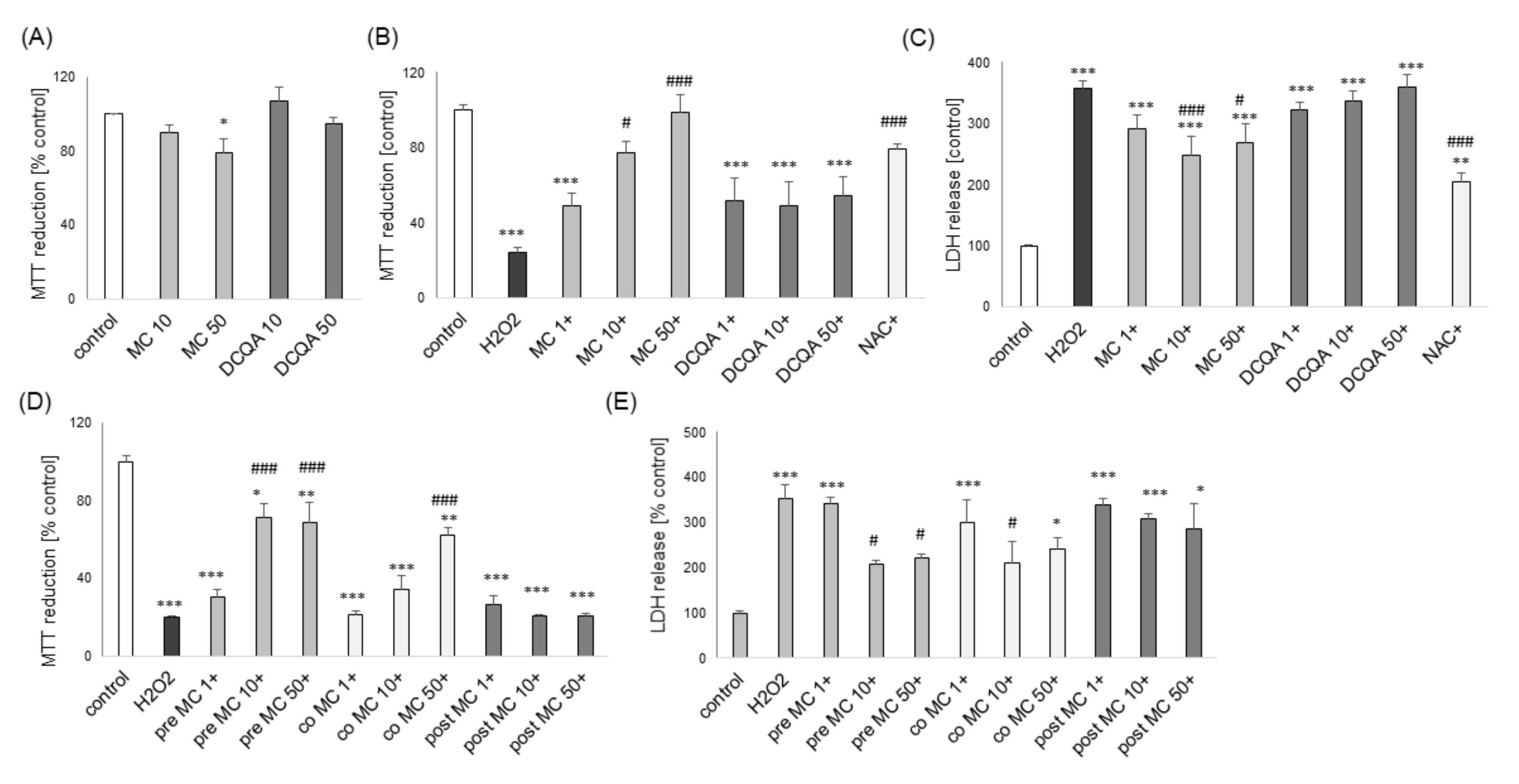
3.1. The Effects of MC and 3,5-DCQA on H2O2-Induced Cell Damage in UN- and RA-SH-SY5Y Cells
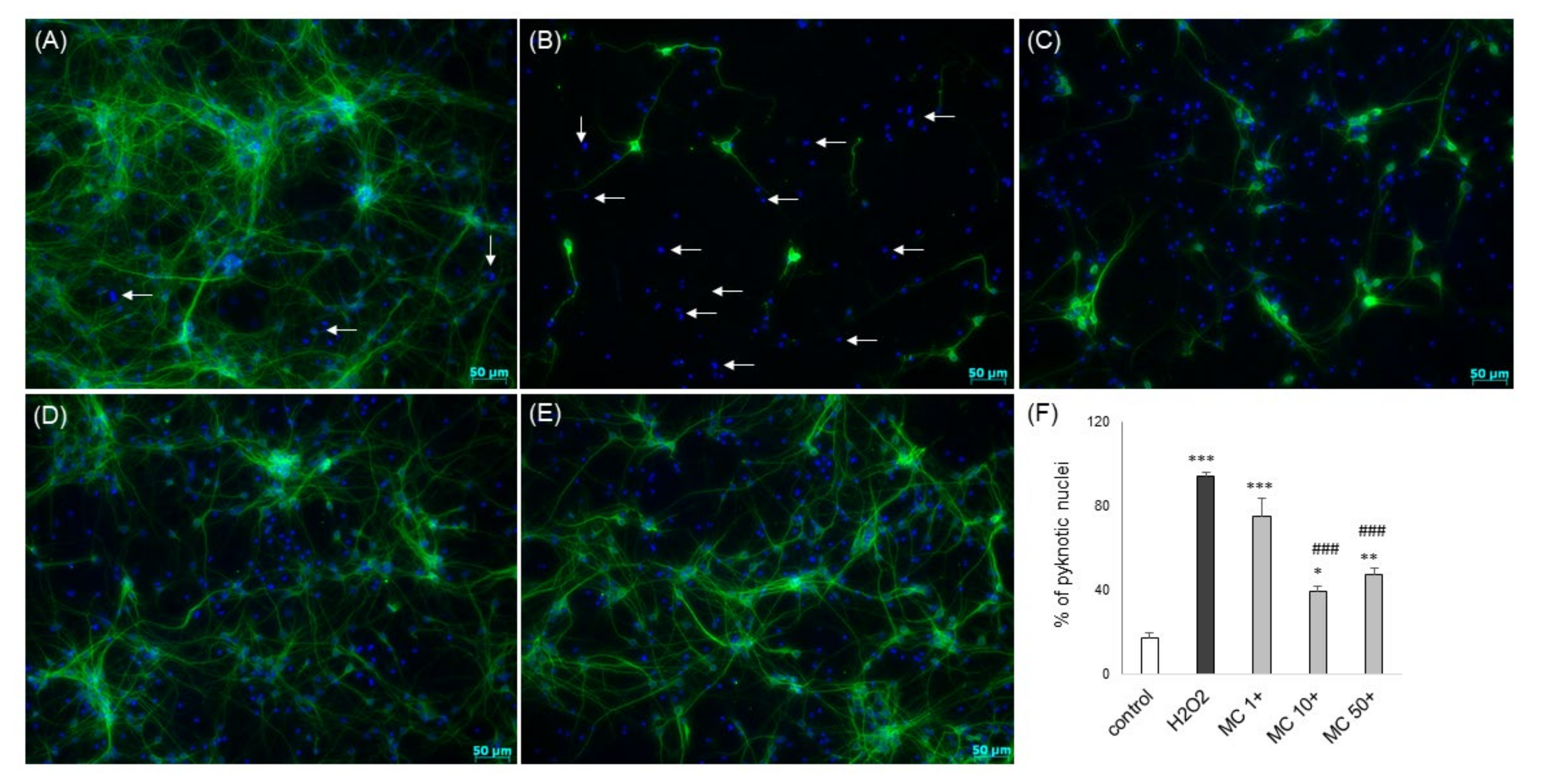
3.2. The MC-Mediated Protection against H2O2-Induced Cell Damage in SH-SY5Y Cells is Connected with Inhibition of Apoptotic and Necrotic Processes
3.3. The Mechanisms of MC-Mediated Protection against H2O2-Induced Cell Damage in SH-SY5Y Cells Involve Inhibition of Cathepsin D but not Activation of PI3-K/Akt Signaling
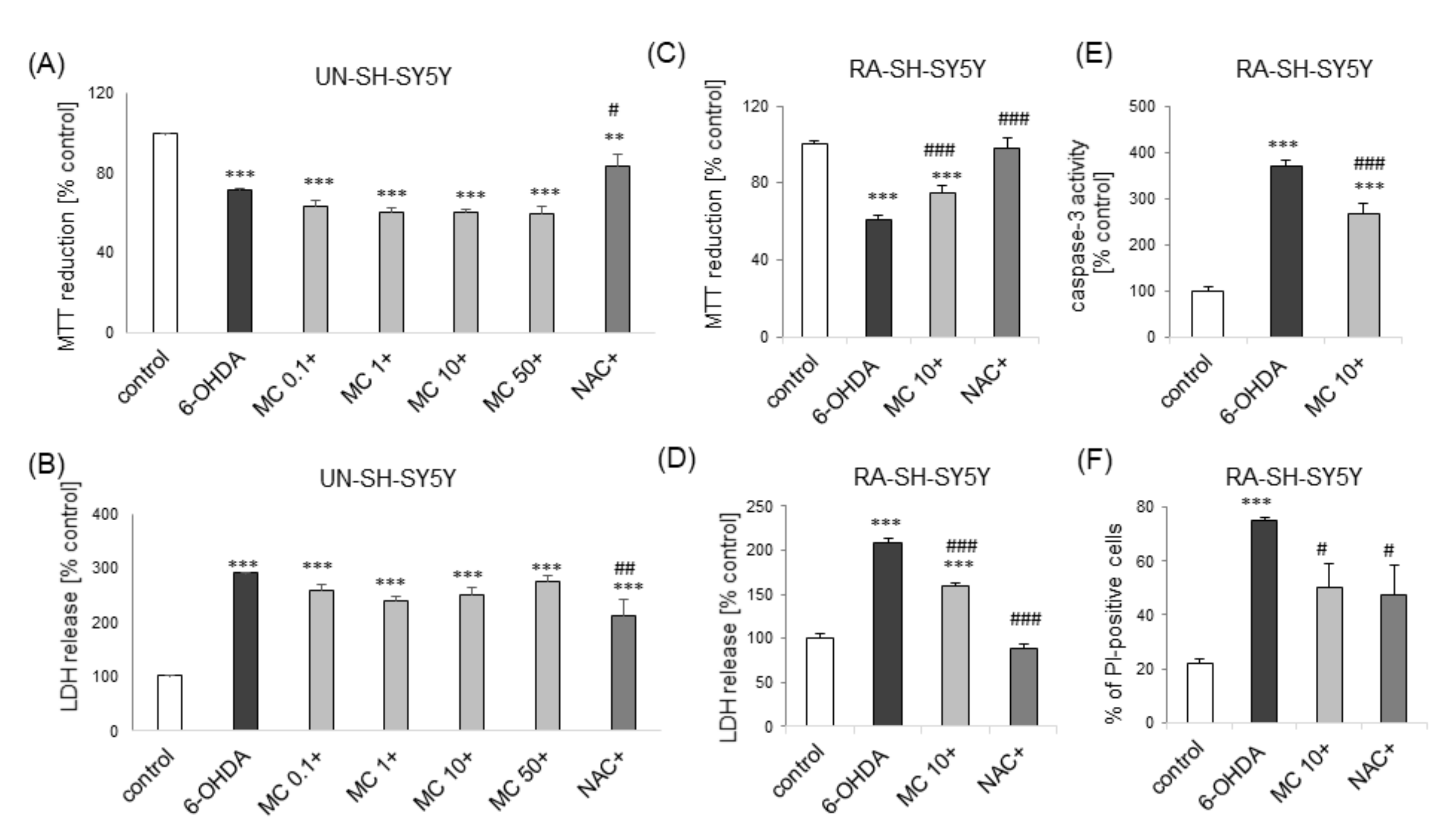
3.4. MC is Protective against 6-OHDA-Induced Cell Damage in SH-SY5Y Cells: The Impact of Cell Differentiation State
3.5. The Effects of MC against Dox- and St-Induced Cell Damage in SH-SY5Y Cells
3.6. The Effects of MC and 3,5-DCQA against Various Types of Neuronal Cell Injury in Primary Neuronal Cell Cultures
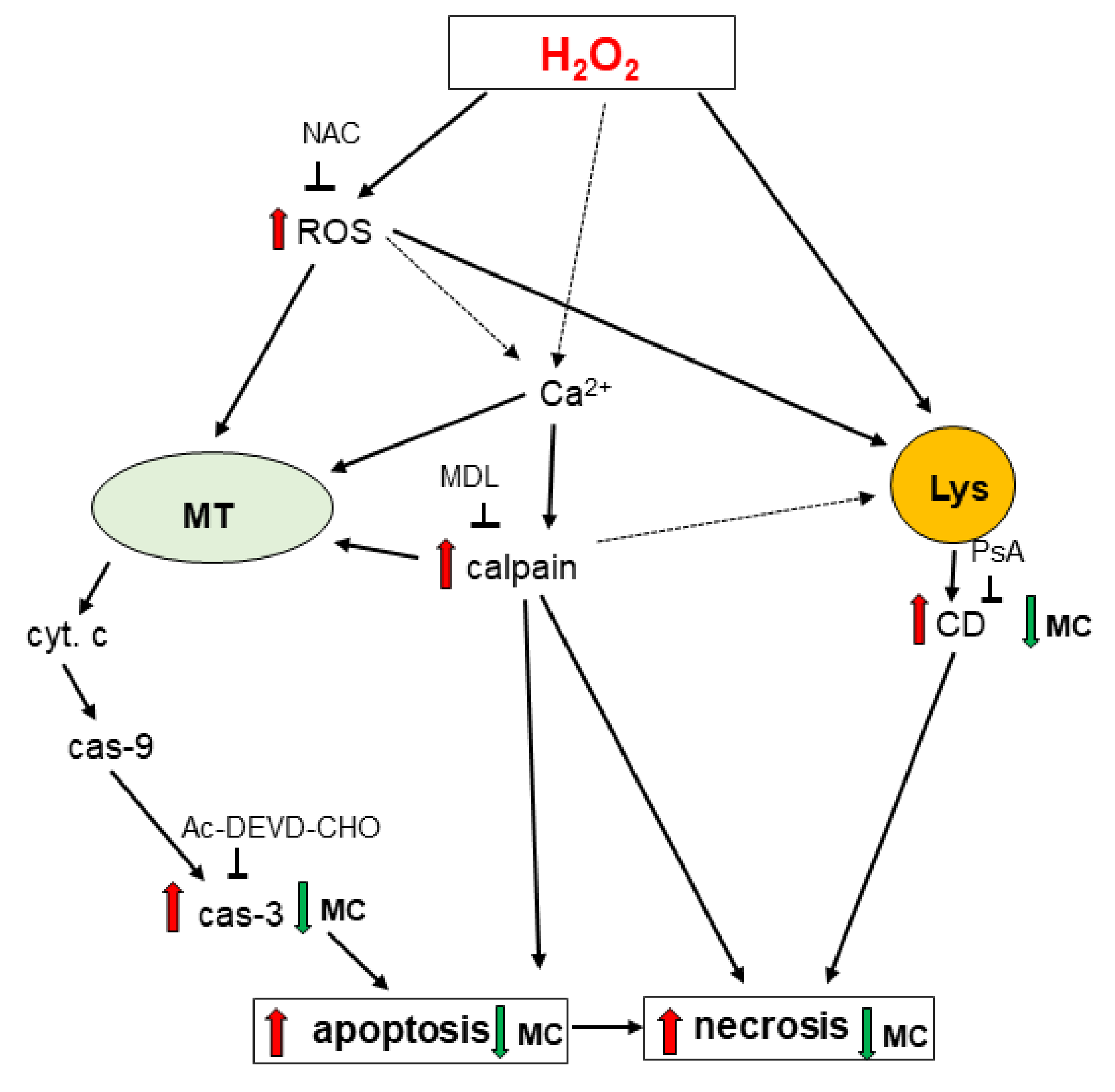
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2019, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C. Neuroprotective phenolics in medicinal plants. Arch. Pharmacal Res. 2010, 33, 1611–1632. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Moosavi, F.; Rajaian, H.; Silva, T.; E Silva, D.M.; Soares, P.; Saso, L.; Edraki, N.; Miri, R.; Borges, F.; et al. Discovery of neurotrophic agents based on hydroxycinnamic acid scaffold. Chem. Biol. Drug Des. 2016, 88, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Moosavi, F.; Silva, T.; Rajaian, H.; Hosseini, S.Y.; Bina, S.; Saso, L.; Miri, R.; Borges, F.; Firuzi, O. Modulation of ERK1/2 and Akt Pathways Involved in the Neurotrophic Action of Caffeic Acid Alkyl Esters. Molecules 2018, 23, 3340. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Rajaian, H.; Silva, T.; E Silva, D.M.; Saso, L.; Edraki, N.; Miri, R.; Borges, F.; Firuzi, O. Derivatives of caffeic acid, a natural antioxidant, as the basis for the discovery of novel nonpeptidic neurotrophic agents. Bioorganic Med. Chem. 2017, 25, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer’s disease. Bioorganic Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant Properties of Hydroxycinnamic Acids: A Review of Structure- Activity Relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Fabiszewska, A.U.; Zieniuk, B. Phenolic Acids Derivatives-Biotechnological Methods of Synthesis and Bioactivity. Curr. Pharm. Biotechnol. 2019, 19, 1098–1113. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2009, 9, 147–170. [Google Scholar] [CrossRef]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Patents 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric Acid Ameliorates Lipopolysaccharide-Induced Oxidative Stress via Promoting the Keap1/Nrf2 Transcriptional Signaling Pathway in BV-2 Microglial Cells and Mouse Brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharm. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Mikami, Y.; Kakizawa, S.; Yamazawa, T. Essential Roles of Natural Products and Gaseous Mediators on Neuronal Cell Death or Survival. Int. J. Mol. Sci. 2016, 17, 1652. [Google Scholar] [CrossRef]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef]
- Taram, F.; Ignowski, E.; Duval, N.; Linseman, D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Colonnello, A.; Aguilera-Portillo, G.; Rubio-López, L.C.; Robles-Bañuelos, B.; Rangel-López, E.; Cortez-Núñez, S.; Evaristo-Priego, Y.; Silva-Palacios, A.; Galván-Arzate, S.; García-Contreras, R.; et al. Comparing the Neuroprotective Effects of Caffeic Acid in Rat Cortical Slices and Caenorhabditis elegans: Involvement of Nrf2 and SKN-1 Signaling Pathways. Neurotox. Res. 2020, 37, 326–337. [Google Scholar] [CrossRef]
- Firuzi, O.; Moosavi, F.; Hosseini, R.; Saso, L. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef]
- Sul, D.; Kim, H.-S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.-Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, R.; Takakura, K.; Takatou, S.; Le, T.M.; Nishiuchi, T.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester induce preconditioning ER stress and autophagy in SH-SY5Y cells. J. Cell. Physiol. 2017, 233, 1671–1684. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Ahmed, E.; Elsherbiny, N.M.; Mehanna, E.T.; El-Kherbetawy, M.K.; Elsayed, M.H.; Alshareef, D.M.; Moustafa, Y.M. Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson’s disease therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Szewczyk, A.; Kisiel, W. Caffeic acid derivatives from a hairy root culture of Lactuca virosa. Acta Physiol. Plant. 2011, 34, 291–298. [Google Scholar] [CrossRef][Green Version]
- Stojakowska, A.; Malarz, J. Bioactive phenolics from in vitro cultures of Lactuca aculeata Boiss. et Kotschy. Phytochem. Lett. 2017, 19, 7–11. [Google Scholar] [CrossRef]
- Balachandran, C.; Emi, N.; Arun, Y.; Yamamoto, Y.; Ahilan, B.; Sangeetha, B.; Duraipandiyan, V.; Inaguma, Y.; Okamoto, A.; Ignacimuthu, S.; et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem. Interact. 2015, 242, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Toillon, R.-A.; Tomavo, O.; Jouy, N.; Hondermarck, H.; Cotelle, P. Antiproliferative and apoptotic effects of the oxidative dimerization product of methyl caffeate on human breast cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 574–578. [Google Scholar] [CrossRef]
- Khan, R.S.; Senthi, M.; Rao, P.C.; Basha, A.; Alvala, M.; Tummuri, D.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic constituents of Abutilon indicum leaves against U87MG human glioblastoma cells. Nat. Prod. Res. 2014, 29, 1069–1073. [Google Scholar] [CrossRef]
- Znati, M.; Ben Jannet, H.; Cazaux, S.; Souchard, J.-P.; Harzallah-Skhiri, F.; Bouajila, J. Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef]
- Fiuza, S.; Gomes, C.; Teixeira, L.; Da Cruz, M.G.; Cordeiro, M.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic acid derivatives with potential anticancer properties––a structure–activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Park, B.K.; Shin, S.Y.; Kwon, Y.S.; Kim, H.P. Methyl caffeate and some plant constituents inhibit age-related inflammation: Effects on senescence-associated secretory phenotype (SASP) formation. Arch. Pharmacal Res. 2017, 40, 524–535. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Al-Dhabi, N.A.; Balakrishna, K.; Kalia, N.P.; Rajput, V.S.; Khan, I.A.; Ignacimuthu, S. Antimicrobial and Antimycobacterial Activities of Methyl Caffeate Isolated from Solanum torvum Swartz. Fruit. Indian J. Microbiol. 2012, 52, 676–681. [Google Scholar] [CrossRef]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In-vitro and in-vivo antimalarial activity of caffeic acid and some of its derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Ignacimuthu, S.; Paulraj, M.G.; Sasikumar, P. Antihyperglycemic activity and antidiabetic effect of methyl caffeate isolated from Solanum torvum Swartz. fruit in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 670, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.K.; Lee, Y.; Yun-Choi, H.S. Anti-platelet effect of the constituents isolated from the barks and fruits ofMagnolia obovata. Arch. Pharmacal Res. 2002, 25, 325–328. [Google Scholar] [CrossRef]
- Bispo, V.S.; Dantas, L.S.; Filho, A.B.C.; Pinto, I.F.; Da Silva, R.P.; Otsuka, F.A.; Santos, R.B.; Santos, A.C.; Trindade, D.J.; Matos, H.R. Reduction of the DNA damages, Hepatoprotective Effect and Antioxidant Potential of the Coconut Water, ascorbic and Caffeic Acids in Oxidative Stress Mediated by Ethanol. Anais da Academia Brasileira de Ciências 2017, 89, 1095–1109. [Google Scholar] [CrossRef]
- Lázaro, D.F.; Pavlou, M.A.S.; Outeiro, T.F. Cellular models as tools for the study of the role of alpha-synuclein in Parkinson’s disease. Exp. Neurol. 2017, 298, 162–171. [Google Scholar] [CrossRef]
- Kim, S.-S.; Park, R.-Y.; Jeon, H.-J.; Kwon, Y.-S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef]
- Chwastek, J.; Jantas, D.; Lasoń, W. The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a γH2AX/p-p53/caspase-3-independent mechanism: Inhibition of calpain and cathepsin D. Int. J. Biochem. Cell Biol. 2017, 87, 38–53. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Grygier, B.; Lasoń, W. Neuroprotective Effects of Necrostatin-1 Against Oxidative Stress-Induced Cell Damage: An Involvement of Cathepsin D Inhibition. Neurotox. Res. 2020, 37, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Lech, T.; Gołda, S.; Pilc, A.; Lasoń, W. New evidences for a role of mGluR7 in astrocyte survival: Possible implications for neuroprotection. Neuropharmacology 2018, 141, 223–237. [Google Scholar] [CrossRef]
- Domin, H.; Jantas, D.; Smiałowska, M. Neuroprotective effects of the allosteric agonist of metabotropic glutamate receptor 7 AMN082 on oxygen-glucose deprivation- and kainate-induced neuronal cell death. Neurochem. Int. 2015, 88, 110–123. [Google Scholar] [CrossRef]
- Jantas, D.; Greda, A.; Leskiewicz, M.; Grygier, B.; Pilc, A.; Lasoń, W. Neuroprotective effects of mGluR II and III activators against staurosporine- and doxorubicin-induced cellular injury in SH-SY5Y cells: New evidence for a mechanism involving inhibition of AIF translocation. Neurochem. Int. 2015, 88, 124–137. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; Júnior, M.L.C.D.F.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Leist, M. Consensus report on the future of animal-free systemic toxicity testing. ALTEX 2014, 31, 341–356. [Google Scholar] [CrossRef]
- De Groot, M.W.G.D.M.; Dingemans, M.M.L.; Rus, K.H.; De Groot, A.; Westerink, R.H.S. Characterization of Calcium Responses and Electrical Activity in Differentiating Mouse Neural Progenitor Cells In Vitro. Toxicol. Sci. 2013, 137, 428–435. [Google Scholar] [CrossRef]
- Bi, Y.-M.; Wu, Y.-T.; Chen, L.; Tan, Z.-B.; Fan, H.-J.; Xie, L.-P.; Zhang, W.-T.; Chen, H.-M.; Li, J.; Liu, B.; et al. 3,5-Dicaffeoylquinic acid protects H9C2 cells against oxidative stress-induced apoptosis via activation of the PI3K/Akt signaling pathway. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Sánchez-Gómez, M.V.; Matute, C.; Fattouch, S.; Amri, M. Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem. Res. 2017, 42, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhao, L.; Ma, Z.; Holtzman, D.M.; Yan, C.; Dodel, R.; Hampel, H.; Oertel, W.; Farlow, M.R.; Du, Y. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain 2004, 127, 2629–2635. [Google Scholar] [CrossRef]
- Kim, I.H.; Yan, B.C.; Park, J.H.; Yeun, G.H.; Yim, Y.; Ahn, J.H.; Lee, J.-C.; Hwang, I.K.; Cho, J.H.; Kim, Y.-M.; et al. Neuroprotection of a Novel Synthetic Caffeic Acid-Syringic Acid Hybrid Compound against Experimentally Induced Transient Cerebral Ischemic Damage. Planta Medica 2013, 79, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Lee, D.; Hiep, N.T.; Song, J.H.; Lee, H.-J.; Lee, D.; Kang, K.S. Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1. Molecules 2019, 24, 195. [Google Scholar] [CrossRef]
- Koh, J.-Y.; Wie, M.B.; Gwag, B.J.; Sensi, S.L.; Canzoniero, L.M.T.; Demaro, J.; Csernansky, C.; Choi, D.W. Staurosporine-Induced Neuronal Apoptosis. Exp. Neurol. 1995, 135, 153–159. [Google Scholar] [CrossRef]
- Castino, R.; Bellio, N.; Nicotra, G.; Follo, C.; Trincheri, N.F.; Isidoro, C. Cathepsin D–Bax death pathway in oxidative stressed neuroblastoma cells. Free. Radic. Biol. Med. 2007, 42, 1305–1316. [Google Scholar] [CrossRef]
- Kumaran, K.S.; Prince, P.S.M. Preventive effect of caffeic acid on lysosomal dysfunction in isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2010, 24, 115–122. [Google Scholar] [CrossRef]
- Airavaara, M.; Parkkinen, I.; Konovalova, J.; Albert, K.; Chmielarz, P.; Domanskyi, A. Back and to the Future: From Neurotoxin-Induced to Human Parkinson’s Disease Models. Curr. Protoc. Neurosci. 2020, 91, e88. [Google Scholar] [CrossRef]
- Wenker, S.D.; Chamorro, M.E.; Vota, D.M.; Callero, M.A.; Vittori, D.C.; Nesse, A.B. Differential antiapoptotic effect of erythropoietin on undifferentiated and retinoic acid-differentiated SH-SY5Y cells. J. Cell. Biochem. 2010, 110, 151–161. [Google Scholar] [CrossRef]
- Turan, D.; Abdik, H.; Şahin, F.; Abdik, E.A. Evaluation of the neuroprotective potential of caffeic acid phenethyl ester in a cellular model of Parkinson’s disease. Eur. J. Pharmacol. 2020, 883, 173342. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, X.; Fontanilla, C.; Noelker, C.; Dodel, R.; Hampel, H.; Du, Y. Caffeic acid phenethyl ester blocks free radical generation and 6-hydroxydopamine-induced neurotoxicity. Life Sci. 2006, 79, 1307–1311. [Google Scholar] [CrossRef]
- Noelker, C.; Bacher, M.; Gocke, P.; Wei, X.; Klockgether, T.; Du, Y.; Dodel, R. The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neurosci. Lett. 2005, 383, 39–43. [Google Scholar] [CrossRef]
- Silva, R.B.; Santos, N.; Martins, N.; Ferreira, D.; Barbosa, F.; Souza, V.O.; Kinoshita, A.; Baffa, O.; Del-Bel, E.; Santos, N.A.G. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef] [PubMed]





| LDH Release [% control] | ||
|---|---|---|
| control | 100.00 ± 0.01 | |
| H2O2 | 386.12 ± 3.67 *** | |
| +MC | 217.54 ± 19.59 ***,###,& | |
| +MDL+MC | 229.89 ± 21.57 ***,###,& | |
| +MDL | 304.18 ± 27.84 ***,# |
| LDH Release [% control] | ||
|---|---|---|
| control | 100.18 ± 0.17 | |
| H2O2 | 386.69 ± 3.05 *** | |
| +MC | 201.81 ± 22.43 **,### | |
| +LY294+MC | 245.54 ± 24.51 ***,### | |
| +LY294 | 427.73 ± 26.58 *** | |
| LY294 | 131.95 ± 10.95 |
| Cell Type Cell Damage | UN-SH-SY5Y | RA-SH-SY5Y | Primary Neurons | |||
|---|---|---|---|---|---|---|
| MC | 3,5-DCQA | MC | 3,5-DCQA | MC | 3,5-DCQA | |
| H2O2 | NP | 0 | NP | 0 | NP | 0 |
| 6-OHDA | 0 | 0 * | NP | n.s. | n.s. | n.s. |
| Staurosporine | D | 0 * | n.s. | n.s. | D | 0 * |
| Doxorubicin | D | 0 * | 0 | 0 * | n.s. | n.s. |
| Glutamate | n.s. | n.s. | n.s. | n.s. | 0 | 0 * |
| OGD | n.s. | n.s. | n.s. | n.s. | 0 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules 2020, 10, 1530. https://doi.org/10.3390/biom10111530
Jantas D, Chwastek J, Malarz J, Stojakowska A, Lasoń W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules. 2020; 10(11):1530. https://doi.org/10.3390/biom10111530
Chicago/Turabian StyleJantas, Danuta, Jakub Chwastek, Janusz Malarz, Anna Stojakowska, and Władysław Lasoń. 2020. "Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition" Biomolecules 10, no. 11: 1530. https://doi.org/10.3390/biom10111530
APA StyleJantas, D., Chwastek, J., Malarz, J., Stojakowska, A., & Lasoń, W. (2020). Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules, 10(11), 1530. https://doi.org/10.3390/biom10111530