Updating Phospholipase A2 Biology
Abstract
1. Introduction
2. The sPLA2 Family
2.1. General Features
2.2. sPLA2-IB in Digestion and Immunity
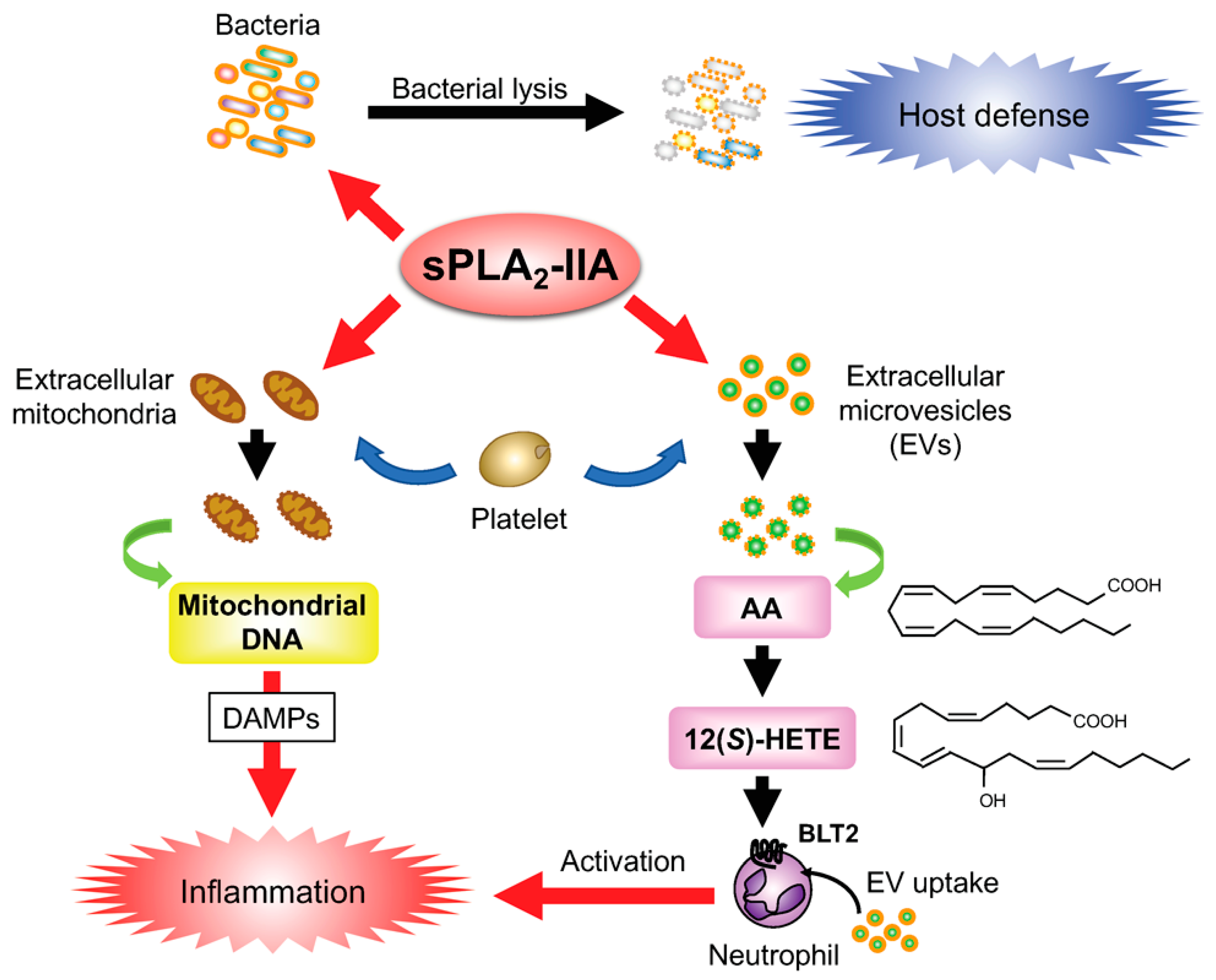
2.3. sPLA2-IIA in Host Defense, Sterile Inflammation, and Colon Cancer
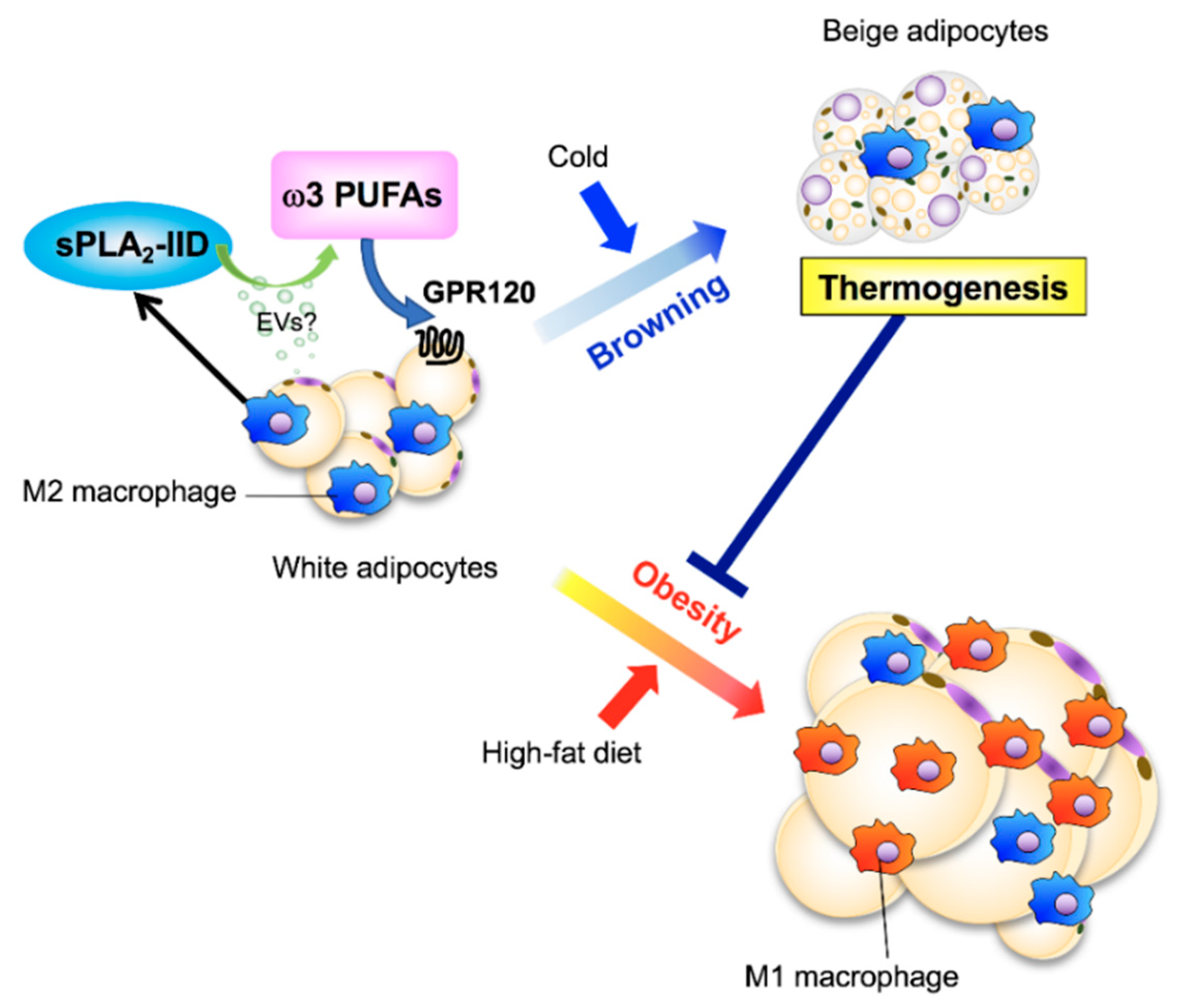
2.4. sPLA2-IID in Immunosuppression, Host Defense, and Adaptive Thermogenesis
2.5. sPLA2-IIE and sPLA2-IIF in the Skin
2.6. sPLA2-III in Male Reproduction, Anaphylaxis, and Colonic Diseases
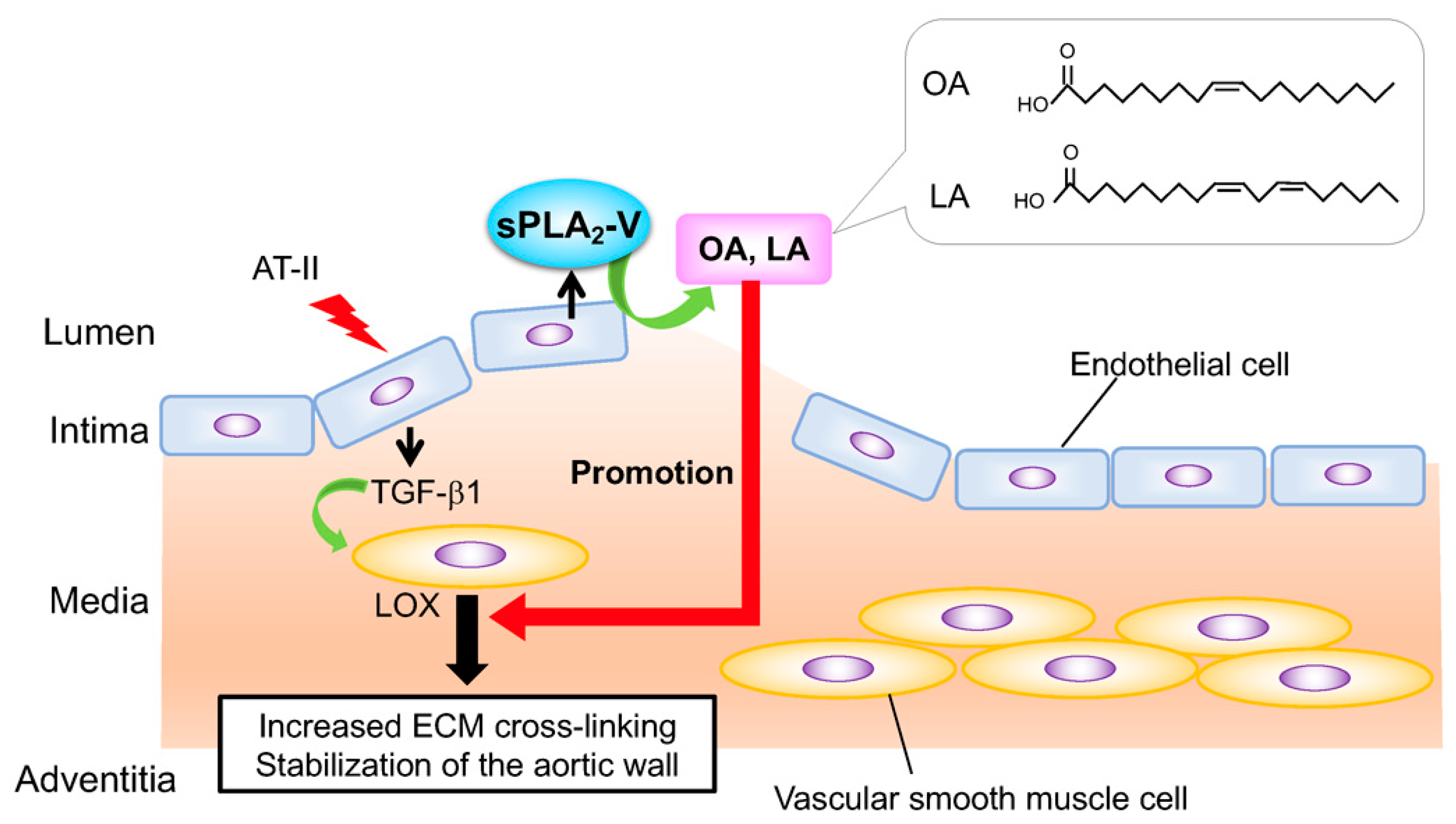
2.7. sPLA2-V in Obesity, Type-2 Immunity, and Aortic Protection
2.8. sPLA2-X in Sperm Activation, Colitis, and Asthma
3. The sPLA2 Family
3.1. General Features
3.2. New Insights into cPLA2α
3.3. cPLA2β Fusion Protein in Carcinoma Cell Proliferation and Survival
3.4. cPLA2γ in Lipid Droplet Formation
3.5. cPLA2δ in Psoriasis
3.6. cPLA2ε as an N-Acyltransferase for N-Acylethanolamine Biosynthesis
3.7. cPLA2ζ in Myocardial Mitochondria
4. The iPLA2/PNPLA Family
4.1. General Features
4.2. iPLA2β in Neurodegeneration and Hepatic Steatosis
4.3. iPLA2γ in Bioenergetics and Signaling
4.4. PNPNA6 and PNPLA7 as Lysophospholipases
4.5. PNPLA2 and PNPLA3 in Triglyceride Metabolism
4.6. PNPLA1 in Acylceramide Synthesis for Skin Barrier Function
5. Other PLA2 Family
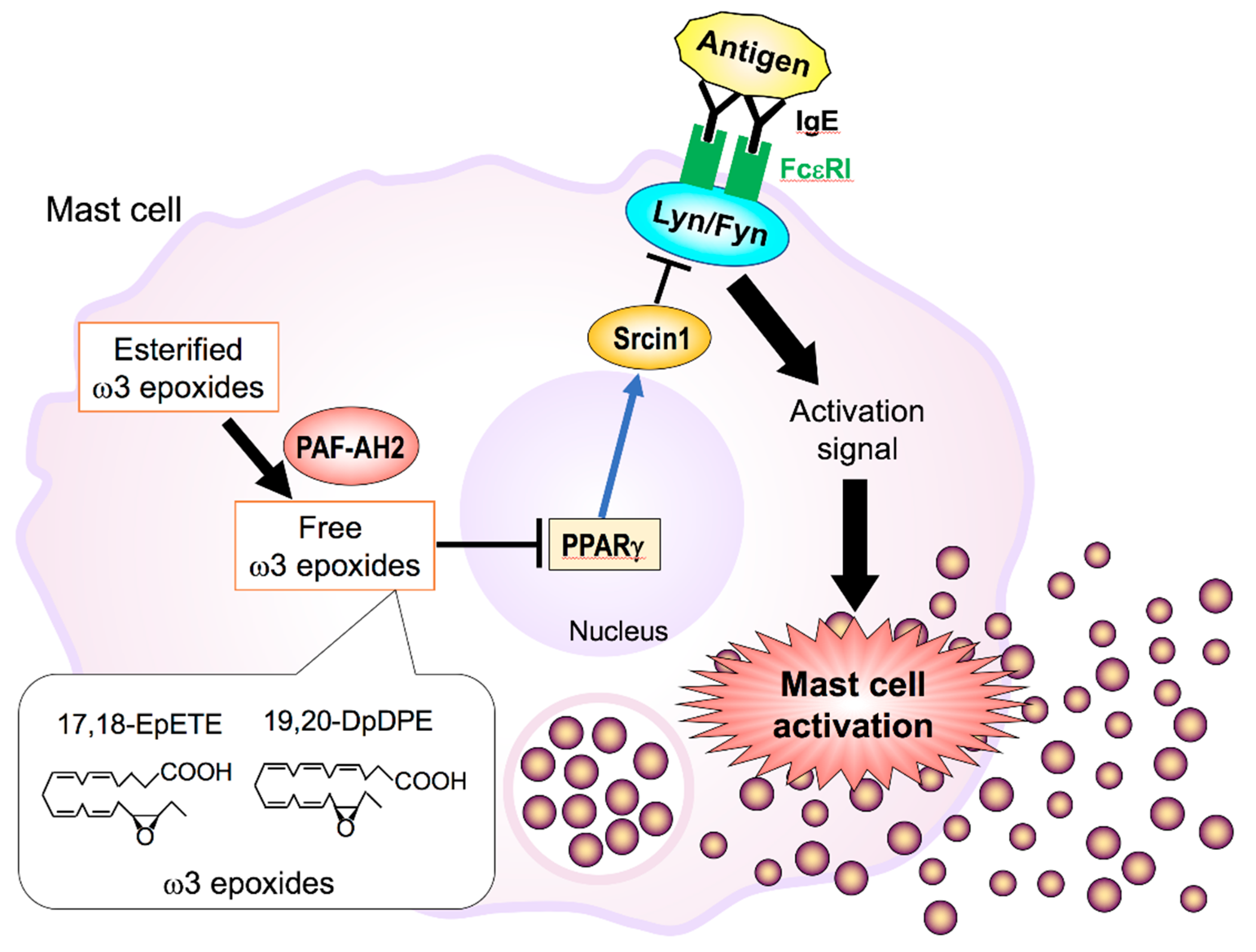
5.1. The PAF-AH Family
5.2. Lysosomal PLA2s
5.3. The PLAAT Family
5.4. The ABHD Family
5.5. GPI-Specific PLA2s
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murakami, M. Novel functions of phospholipase A2s: Overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent progress in phospholipase A2 research: From cells to animals to humans. Prog. Lipid Res. 2011, 50, 152–192. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M. Lipoquality control by phospholipase A2 enzymes. Proc. Jpn. Acad. Ser. B 2017, 93, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Taketomi, Y.; Girard, C.; Yamamoto, K.; Lambeau, G. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie 2010, 92, 561–582. [Google Scholar] [CrossRef]
- Murakami, M.; Taketomi, Y.; Sato, H.; Yamamoto, K. Secreted phospholipase A2 revisited. J. Biochem. 2011, 150, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [CrossRef]
- Murakami, M.; Yamamoto, K.; Miki, Y.; Murase, R.; Sato, H.; Taketomi, Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016, 132, 91–134. [Google Scholar] [CrossRef]
- Lambeau, G.; Gelb, M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008, 77, 495–520. [Google Scholar] [CrossRef]
- Murakami, M.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Yamamoto, K. Group IID, IIE, IIF and III secreted phospholipase A2s. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 803–818. [Google Scholar] [CrossRef]
- Seilhamer, J.J.; Randall, T.L.; Yamanaka, M.; Johnson, L.K. Pancreatic phospholipase A2: Isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA 1986, 5, 519–527. [Google Scholar] [CrossRef]
- Hollie, N.I.; Konaniah, E.S.; Goodin, C.; Hui, D.Y. Group 1B phospholipase A2 inactivation suppresses atherosclerosis and metabolic diseases in LDL receptor-deficient mice. Atherosclerosis. 2014, 234, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Huggins, K.W.; Boileau, A.C.; Hui, D.Y. Protection against diet-induced obesity and obesity- related insulin resistance in group 1B PLA2-deficient mice. Am. J. Physiol. Metab. 2002, 283, E994–E1001. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.Y.; Cope, M.J.; Labonté, E.D.; Chang, H.-T.; Shao, J.; Goka, E.; Abousalham, A.; Charmot, D.; Buysse, J. The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br. J. Pharmacol. 2009, 157, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Labonté, E.D.; Kirby, R.J.; Schildmeyer, N.M.; Cannon, A.M.; Huggins, K.W.; Hui, D.Y. Phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes 2006, 55, 935–941. [Google Scholar] [CrossRef]
- Wilson, S.G.; Adam, G.; Langdown, M.; Reneland, R.; Braun, A.; Andrew, T.; Surdulescu, G.L.; Norberg, M.; Dudbridge, F.; Reed, P.W.; et al. Linkage and potential association of obesity-related phenotypes with two genes on chromosome 12q24 in a female dizygous twin cohort. Eur. J. Hum. Genet. 2006, 14, 340–348. [Google Scholar] [CrossRef]
- Hui, D.Y. Group 1B phospholipase A2 in metabolic and inflammatory disease modulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 784–788. [Google Scholar] [CrossRef]
- Entwistle, L.J.; Pelly, V.S.; Coomes, S.M.; Kannan, Y.; Perez-Lloret, J.; Czieso, S.; Dos Santos, M.S.; Macrae, J.I.; Collinson, L.M.; Sesay, A.; et al. Epithelial-cell-derived phospholipase A2 group 1B is an endogenous anthelmintic. Cell Host Microbe 2017, 22, 484–493.e5. [Google Scholar] [CrossRef]
- Pothlichet, J.; Rose, T.; Bugault, F.; Jeammet, L.; Meola, A.; Haouz, A.; Saul, F.; Geny, D.; Alcami, J.; Ruiz-Mateos, E.; et al. PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients. J. Clin. Investig. 2020, 130, 2872–2887. [Google Scholar] [CrossRef]
- Laine, V.J.; Grass, D.S.; Nevalainen, T.J. Protection by group II phospholipase A2 against Staphylococcus aureus. J. Immunol. 1999, 162, 7402–7408. [Google Scholar]
- Weinrauch, Y.; Abad, C.; Liang, N.S.; Lowry, S.F.; Weiss, J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J. Clin. Investig. 1998, 102, 633–638. [Google Scholar] [CrossRef]
- Pernet, E.; Guillemot, L.; Burgel, P.-R.; Martin, C.; Lambeau, G.; Sermet-Gaudelus, I.; Sands, D.; LeDuc, D.; Morand, P.C.; Jeammet, L.; et al. Pseudomonas aeruginosa eradicates Staphylococcus aureus by manipulating the host immunity. Nat. Commun. 2014, 5, 5105. [Google Scholar] [CrossRef]
- Hamaguchi, K.; Kuwata, H.; Yoshihara, K.; Masuda, S.; Shimbara, S.; Oh-Ishi, S.; Murakami, M.; Kudo, I. Induction of distinct sets of secretory phospholipase A2 in rodents during inflammation. Biochim. Biophys. Acta Bioenerg. 2003, 1635, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Duchez, A.-C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Duchez, A.-C.; Boudreau, L.H.; Naika, G.S.; Bollinger, J.; Belleannée, C.; Cloutier, N.; Laffont, B.; Mendoza-Villarroel, R.E.; Lévesque, T.; Rollet-Labelle, E.; et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. USA 2015, 112, E3564–E3573. [Google Scholar] [CrossRef]
- Dore, E.; Boilard, E. Roles of secreted phospholipase A2 group IIA in inflammation and host defense. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, M.; Sinou, V.; Payré, C.; Jeammet, L.; Parzy, D.; Grellier, P.; Deregnaucourt, C.; Lambeau, G. Antimalarial activity of human group IIA secreted phospholipase A2 in relation to enzymatic hydrolysis of oxidized lipoproteins. Infect. Immun. 2019, 87, e00556-19. [Google Scholar] [CrossRef] [PubMed]
- Grass, D.S.; Felkner, R.H.; Chiang, M.Y.; Wallace, R.E.; Nevalainen, T.J.; Bennett, C.F.; Swanson, M.E. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Investig. 1996, 97, 2233–2241. [Google Scholar] [CrossRef]
- Chovatiya, G.L.; Sunkara, R.R.; Roy, S.; Godbole, S.R.; Waghmare, S.K. Context-dependent effect of sPLA2-IIA induced proliferation on murine hair follicle stem cells and human epithelial cancer. EBioMedicine 2019, 48, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Kuefner, M.S.; Deng, X.; Stephenson, E.J.; Pham, K.; Park, E.A. Secretory phospholipase A2 group IIA enhances the metabolic rate and increases glucose utilization in response to thyroid hormone. FASEB J. 2018, 33, 738–749. [Google Scholar] [CrossRef]
- Macphee, M.; Chepenik, K.P.; Liddell, R.A.; Nelson, K.K.; Siracusa, L.D.; Buchberg, A.M. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell 1995, 81, 957–966. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miki, Y.; Sato, M.; Taketomi, Y.; Nishito, Y.; Taya, C.; Muramatsu, K.; Ikeda, K.; Nakanishi, H.; Taguchi, R.; et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 2015, 212, 1901–1919. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Franken, P.F.; Sacchetti, A.; Schmitt, M.; Joosten, R.; Böttcher, R.; Van Royen, M.E.; Jeammet, L.; Payre, C.; Scott, P.M.; et al. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell 2016, 19, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Murase, R.; Sato, H.; Yamamoto, K.; Ushida, A.; Nishito, Y.; Ikeda, K.; Kobayashi, T.; Yamamoto, T.; Taketomi, Y.; Murakami, M. Group X secreted phospholipase A2 releases ω3 polyunsaturated fatty acids, suppresses colitis, and promotes sperm fertility. J. Biol. Chem. 2016, 291, 6895–6911. [Google Scholar] [CrossRef] [PubMed]
- Rouault, M.; Le Calvez, C.; Boilard, E.; Surrel, F.; Singer, A.; Ghomashchi, F.; Bezzine, S.; Scarzello, S.; Bollinger, J.; Gelb, M.H.; et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry 2007, 46, 1647–1662. [Google Scholar] [CrossRef]
- Kishi, H.; Yamaguchi, K.; Watanabe, K.; Nakamura, K.; Fujioka, D.; Kugiyama, K. Deficiency of phospholipase A2 receptor exacerbates autoimmune myocarditis in mice. Inflammation 2020, 43, 1097–1109. [Google Scholar] [CrossRef]
- Miki, Y.; Yamamoto, K.; Taketomi, Y.; Sato, H.; Shimo, K.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; Nakanishi, H.; Ikeda, K.; et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013, 210, 1217–1234. [Google Scholar] [CrossRef]
- Miki, Y.; Kidoguchi, Y.; Sato, M.; Taketomi, Y.; Taya, C.; Muramatsu, K.; Gelb, M.H.; Yamamoto, K.; Murakami, M. Dual roles of group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 2016, 291, 15588–15601. [Google Scholar] [CrossRef]
- Vijay, R.; Hua, X.; Meyerholz, D.K.; Miki, Y.; Yamamoto, K.; Gelb, M.; Murakami, M.; Perlman, S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome–CoV infection. J. Exp. Med. 2015, 212, 1851–1868. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Miki, Y.; Murase, R.; Yamamoto, K.; Murakami, M. Secreted phospholipase PLA2G2D contributes to metabolic health by mobilizing ω3 polyunsaturated fatty acids in WAT. Cell Rep. 2020, 31, 107579. [Google Scholar] [CrossRef]
- Takabatake, N.; Sata, M.; Inoue, S.; Shibata, Y.; Abe, S.; Wada, T.; Machiya, J.-I.; Ji, G.; Matsuura, T.; Takeishi, Y.; et al. A novel polymorphism in secretory phospholipase A2-IID is associated with body weight loss in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 172, 1097–1104. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Ushida, A.; Isogai, Y.; Kojima, T.; Hirabayashi, T.; Miki, Y.; Yamamoto, K.; Nishito, Y.; Kobayashi, T.; et al. The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity. Cell Metab. 2014, 20, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Miki, Y.; Sato, H.; Nishito, Y.; Gelb, M.H.; Taketomi, Y.; Murakami, M. Expression and function of group IIE phospholipase A2 in mouse skin. J. Biol. Chem. 2016, 291, 15602–15613. [Google Scholar] [CrossRef]
- Valentin, E. Novel secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 2000, 275, 7492–7496. [Google Scholar] [CrossRef]
- Murakami, M.; Masuda, S.; Shimbara, S.; Bezzine, S.; Lazdunski, M.; Lambeau, G.; Gelb, M.H.; Matsukura, S.; Kokubu, F.; Adachi, M.; et al. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII). J. Biol. Chem. 2003, 278, 10657–10667. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Isogai, Y.; Miki, Y.; Yamamoto, K.; Masuda, S.; Hosono, T.; Arata, S.; Ishikawa, Y.; Ishii, T.; et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Investig. 2010, 120, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, Y.; Ueno, N.; Kojima, T.; Sato, H.; Murase, R.; Yamamoto, K.; Tanaka, S.; Sakanaka, M.; Nakamura, M.; Nishito, Y.; et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2–DP1 receptor paracrine axis. Nat. Immunol. 2013, 14, 554–563. [Google Scholar] [CrossRef]
- Murakami, M.; Masuda, S.; Shimbara, S.; Ishikawa, Y.; Ishii, T.; Kudo, I. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A2. J. Biol. Chem. 2005, 280, 24987–24998. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Kitayama, J.; Hiyoshi, M.; Taketomi, Y.; Murakami, M.; Nishikawa, T.; Tanaka, T.; Tanaka, J.; Kiyomatsu, T.; Kawai, K.; et al. Phospholipase A2 group III and group X have opposing associations with prognosis in colorectal cancer. Anticancer Res. 2015, 35, 2983–2990. [Google Scholar] [PubMed]
- Hoeft, B.; Linseisen, J.; Beckmann, L.; Müller-Decker, K.; Canzian, F.; Hüsing, A.; Kaaks, R.; Vogel, U.; Jakobsen, M.U.; Overvad, K.; et al. Polymorphisms in fatty acid metabolism-related genes are associated with colorectal cancer risk. Carcinog. 2009, 31, 466–472. [Google Scholar] [CrossRef]
- Murase, R.; Taketomi, Y.; Miki, Y.; Nishito, Y.; Saito, M.; Fukami, K.; Yamamoto, K.; Murakami, M. Group III phospholipase A2 promotes colitis and colorectal cancer. Sci. Rep. 2017, 7, 12261. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, D.; Iyer, S.; Ghaleb, A.M.; Shim, H.; Yang, V.W.; Chun, J.; Yun, C.C. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterol. 2009, 136, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Stančić, A.; Jandl, K.; Hasenöhrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.A.; Schicho, R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Koduri, R.S.; Enomoto, A.; Shimbara, S.; Seki, M.; Yoshihara, K.; Singer, A.; Valentin, E.; Ghomashchi, F.; Lambeau, G.; et al. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 2000, 276, 10083–10096. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Shimbara, S.; Kambe, T.; Kuwata, H.; Winstead, M.V.; Tischfield, J.A.; Kudo, I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2s are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 1998, 273, 14411–14423. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Taketomi, Y.; Arata, S.; Masuda, S.; Ishikawa, Y.; Ishii, T.; Takanezawa, Y.; Aoki, J.; Arai, H.; Yamamoto, K.; et al. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 2006, 281, 36420–36433. [Google Scholar] [CrossRef]
- Wootton, P.T.; Arora, N.L.; Drenos, F.; Thompson, S.R.; Cooper, J.A.; Stephens, J.W.; Hurel, S.J.; Hurt-Camejo, E.; Wiklund, O.; Humphries, S.E.; et al. Tagging SNP haplotype analysis of the secretory PLA2-V gene, PLA2G5, shows strong association with LDL and oxLDL levels, suggesting functional distinction from sPLA2-IIA: Results from the UDACS study. Hum. Mol. Genet. 2007, 16, 1437–1444. [Google Scholar] [CrossRef]
- Ohta, S.; Imamura, M.; Xing, W.; Boyce, J.A.; Balestrieri, B. Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation. J. Immunol. 2013, 190, 5927–5938. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Samuchiwal, S.K.; Quehenberger, O.; Boyce, J.A.; Balestrieri, B. Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms. Mucosal Immunol. 2017, 11, 615–626. [Google Scholar] [CrossRef]
- Samuchiwal, S.K.; Balestrieri, B. Harmful and protective roles of group V phospholipase A2: Current perspectives and future directions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 819–826. [Google Scholar] [CrossRef]
- Balestrieri, B.; Maekawa, A.; Xing, W.; Gelb, M.H.; Katz, H.R.; Arm, J.P. Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. Immunol. 2009, 182, 4891–4898. [Google Scholar] [CrossRef]
- Boilard, E.; Lai, Y.; Larabee, K.; Balestrieri, B.; Ghomashchi, F.; Fujioka, D.; Gobezie, R.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; et al. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol. Med. 2010, 2, 172–187. [Google Scholar] [CrossRef]
- Degousee, N.; Kelvin, D.J.; Geisslinger, G.; Hwang, D.M.; Stefanski, E.; Wang, X.-H.; Danesh, A.; Angioni, C.; Schmidt, H.; Lindsay, T.F.; et al. Group V phospholipase A2 in bone marrow-derived myeloid cells and bronchial epithelial cells promotes bacterial clearance after Escherichia coli pneumonia. J. Biol. Chem. 2011, 286, 35650–35662. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Rodríguez, J.P.; Gil-De-Gómez, L.; Guijas, C.; Balboa, M.A.; Balsinde, J. Group V secreted phospholipase A2 is upregulated by IL-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids. J. Immunol. 2015, 194, 3327–3339. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.M.; et al. Aortic dissection. Nat. Rev. Dis. Prim. 2016, 2, 16054. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taketomi, Y.; Miki, Y.; Kugiyama, K.; Murakami, M. Group V secreted phospholipase A2 plays a protective role against aortic dissection. J. Biol. Chem. 2020, 295, 10092–10111. [Google Scholar] [CrossRef] [PubMed]
- Jemel, I.; Ii, H.; Oslund, R.C.; Payré, C.; Dabert-Gay, A.-S.; Douguet, D.; Chargui, K.; Scarzello, S.; Gelb, M.H.; Lambeau, G. Group X secreted phospholipase A2 proenzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK293 cells. J. Biol. Chem. 2011, 286, 36509–36521. [Google Scholar] [CrossRef]
- Escoffier, J.; Jemel, I.; Tanemoto, A.; Taketomi, Y.; Payré, C.; Coatrieux, C.; Sato, H.; Yamamoto, K.; Masuda, S.; Pernet-Gallay, K.; et al. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Investig. 2010, 120, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Nahed, R.A.; Martinez, G.; Escoffier, J.; Yassine, S.; Karaouzene, T.; Hograindleur, J.-P.; Turk, J.; Kokotos, G.; Ray, P.F.; Bottari, S.P.; et al. Progesterone-induced acrosome exocytosis requires sequential involvement of calcium-independent phospholipase A2β (iPLA2β) and group X secreted phospholipase A2 (sPLA2). J. Biol. Chem. 2015, 291, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Isogai, Y.; Masuda, S.; Taketomi, Y.; Miki, Y.; Kamei, D.; Hara, S.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; et al. Physiological roles of group X-secreted phospholipase A2 in reproduction, gastrointestinal phospholipid digestion, and neuronal function. J. Biol. Chem. 2011, 286, 11632–11648. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Lahoute, C.; Coatrieux, C.; Loyer, X.; Joffre, J.; Laurans, L.; Ramkhelawon, B.; Blanc-Brude, O.; Karabina, S.; et al. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor–null mice. Arter. Thromb. Vasc. Biol. 2013, 33, 466–473. [Google Scholar] [CrossRef]
- Li, X.; Shridas, P.; Forrest, K.; Bailey, W.; Webb, N.R. Group X secretory phospholipase A2 negatively regulates adipogenesis in murine models. FASEB J. 2010, 24, 4313–4324. [Google Scholar] [CrossRef]
- Zack, M.; Boyanovsky, B.B.; Shridas, P.; Bailey, W.; Forrest, K.; Howatt, D.A.; Gelb, M.H.; De Beer, F.C.; Daugherty, A.; Webb, N.R. Group X secretory phospholipase A2 augments angiotensin II-induced inflammatory responses and abdominal aortic aneurysm formation in apoE-deficient mice. Atheroscler. 2011, 214, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R.; Chi, E.Y.; Bollinger, J.G.; Tien, Y.-T.; Ye, X.; Castelli, L.; Rubtsov, Y.; Singer, A.G.; Chiang, G.K.; Nevalainen, T.; et al. Importance of group X–secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 2007, 204, 865–877. [Google Scholar] [CrossRef]
- Nolin, J.D.; Lai, Y.; Ogden, H.L.; Manicone, A.M.; Murphy, R.C.; An, D.; Frevert, C.W.; Ghomashchi, F.; Naika, G.S.; Gelb, M.H.; et al. Secreted PLA2 group X orchestrates innate and adaptive immune responses to inhaled allergen. JCI Insight 2017, 2, e94929. [Google Scholar] [CrossRef] [PubMed]
- Ogden, H.L.; Lai, Y.; Nolin, J.D.; An, D.; Frevert, C.W.; Gelb, M.H.; Altemeier, W.A.; Hallstrand, T.S. Secreted phospholipase A2 group X acts as an adjuvant for type 2 inflammation, leading to an allergen-specific immune response in the lung. J. Immunol. 2020, 204, 3097–3107. [Google Scholar] [CrossRef]
- Nolin, J.D.; Murphy, R.C.; Gelb, M.H.; Altemeier, W.A.; Henderson, W.R.; Hallstrand, T.S. Function of secreted phospholipase A2 group-X in asthma and allergic disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ohto, T.; Uozumi, N.; Hirabayashi, T.; Shimizu, T. Identification of novel cytosolic phospholipase A2s, murine cPLA2δ, ϵ, and ζ, which form a gene cluster with cPLA2β. J. Biol. Chem. 2005, 280, 24576–24583. [Google Scholar] [CrossRef]
- Ghosh, M.; Loper, R.; Gelb, M.H.; Leslie, C.C. Identification of the expressed form of human cytosolic phospholipase A2β (cPLA2β): cPLA2β3 is a novel variant localized to mitochondria and early endosomes. J. Biol. Chem. 2006, 281, 16615–16624. [Google Scholar] [CrossRef]
- Ghosh, M.; Loper, R.; Ghomashchi, F.; Tucker, D.E.; Bonventre, J.V.; Gelb, M.H.; Leslie, C.C. Function, activity, and membrane targeting of cytosolic phospholipase A2ζ in mouse lung fibroblasts. J. Biol. Chem. 2007, 282, 11676–11686. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.D.; Lin, L.-L.; Kriz, R.W.; Ramesha, C.S.; Sultzman, L.A.; Lin, A.Y.; Milona, N.; Knopf, J.L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 1991, 65, 1043–1051. [Google Scholar] [CrossRef]
- Lin, L.-L.; Wartmann, M.; Lin, A.Y.; Knopf, J.L.; Seth, A.; Davis, R.J. cPLA2 is phosphorylated and activated by MAP kinase. Cell 1993, 72, 269–278. [Google Scholar] [CrossRef]
- Casas, J.; Gijón, M.A.; Vigo, A.G.; Crespo, M.S.; Balsinde, J.; Balboa, M.A. Phosphatidylinositol 4,5-bisphosphate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Mol. Biol. Cell 2006, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Slatter, D.A.; Aldrovandi, M.; O’Connor, A.; Allen, S.M.; Brasher, C.J.; Murphy, R.C.; Mecklemann, S.; Ravi, S.; Darley-Usmar, V.; O’Donnell, V.B. Mapping the human platelet lipidome reveals cytosolic phospholipase A2 as a regulator of mitochondrial bioenergetics during activation. Cell Metab. 2016, 23, 930–944. [Google Scholar] [CrossRef]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef]
- Macknight, H.P.; Stephenson, D.J.; Hoeferlin, L.A.; Benusa, S.D.; DeLigio, J.T.; Maus, K.D.; Ali, A.N.; Wayne, J.S.; Park, M.A.; Hinchcliffe, E.H.; et al. The interaction of ceramide 1-phosphate with group IVA cytosolic phospholipase A2 coordinates acute wound healing and repair. Sci. Signal. 2019, 12, eaav5918. [Google Scholar] [CrossRef]
- Chao, C.-C.; Gutiérrez-Vázquez, C.; Rothhammer, V.; Mayo, L.; Wheeler, M.A.; Tjon, E.C.; Zandee, S.E.; Blain, M.; De Lima, K.A.; Takenaka, M.C.; et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell 2019, 179, 1483–1498.e22. [Google Scholar] [CrossRef]
- Koundouros, N.; Karali, E.; Tripp, A.; Valle, A.; Inglese, P.; Perry, N.J.; Magee, D.J.; Virmouni, S.A.; Elder, G.A.; Tyson, A.L.; et al. Metabolic fingerprinting links oncogenic PIK3CA with enhanced arachidonic acid-derived eicosanoids. Cell 2020, 181, 1596–1611.e27. [Google Scholar] [CrossRef]
- Ghomashchi, F.; Naika, G.S.; Bollinger, J.G.; Aloulou, A.; Lehr, M.; Leslie, C.C.; Gelb, M.H. Interfacial Kinetic and Binding Properties of mammalian group IVB phospholipase A2 (cPLA2β) and comparison with the other cPLA2 isoforms. J. Biol. Chem. 2010, 285, 36100–36111. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Li, J.; Chang, I.; Wang, C.-Y. A novel read-through transcript JMJD7-PLA2G4B regulates head and neck squamous cell carcinoma cell proliferation and survival. Oncotarget 2016, 8, 1972–1982. [Google Scholar] [CrossRef]
- Saare, M.; Tserel, L.; Haljasmägi, L.; Taalberg, E.; Peet, N.; Eimre, M.; Vetik, R.; Kingo, K.; Saks, K.; Tamm, R.; et al. Monocytes present age-related changes in phospholipid concentration and decreased energy metabolism. Aging Cell 2020, 19, e13127. [Google Scholar] [CrossRef] [PubMed]
- Underwood, K.W.; Song, C.; Kriz, R.W.; Chang, X.J.; Knopf, J.L.; Lin, L.-L. A novel calcium-independent phospholipase A2, cPLA2γ, that is prenylated and contains homology to cPLA2. J. Biol. Chem. 1998, 273, 21926–21932. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Hirabayashi, T.; Houjou, T.; Uozumi, N.; Taguchi, R.; Shimizu, T. Human group IVC phospholipase A2 (cPLA2γ). Roles in the membrane remodeling and activation induced by oxidative stress. J. Biol. Chem. 2003, 278, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Lebrero, P.; Astudillo, A.M.; Rubio, J.M.; Fernández-Caballero, L.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cellular plasmalogen content does not influence arachidonic acid levels or distribution in macrophages: A role for cytosolic phospholipase A2γ in phospholipid remodeling. Cells 2019, 8, 799. [Google Scholar] [CrossRef]
- Vitale, A.; Perlin, J.; Leonelli, L.; Herr, J.; Wright, P.; Digilio, L.; Coonrod, S. Mouse cPLA2γ, a novel oocyte and early embryo-abundant phospholipase A2γ-like protein, is targeted to the nuclear envelope during germinal vesicle breakdown. Dev. Biol. 2005, 282, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, S.; Zhang, X.; Lam, S.M.; Hu, X.; Zhou, Y.; Chen, J.; Wang, Y.; Wu, C.; Shui, G.; et al. Requirement of cytosolic phospholipase A2γ in lipid droplet formation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017, 1862, 692–705. [Google Scholar] [CrossRef]
- Xu, S.; Pei, R.; Guo, M.; Han, Q.; Lai, J.; Wang, Y.; Wu, C.; Zhou, Y.; Lu, M.; Chen, X. Cytosolic phospholipase A2γ is involved in hepatitis C virus replication and assembly. J. Virol. 2012, 86, 13025–13037. [Google Scholar] [CrossRef]
- Chiba, H.; Michibata, H.; Wakimoto, K.; Seishima, M.; Kawasaki, S.; Okubo, K.; Mitsui, H.; Torii, H.; Imai, Y. Cloning of a gene for a novel epithelium-specific cytosolic phospholipase A2, cPLA2δ, induced in psoriatic skin. J. Biol. Chem. 2004, 279, 12890–12897. [Google Scholar] [CrossRef]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.-L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Parsons, W.H.; Kamat, S.S.; Cravatt, B.F. A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat. Chem. Biol. 2016, 12, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Mustafiz, S.S.B.; Uyama, T.; Morito, K.; Takahashi, N.; Kawai, K.; Hussain, Z.; Tsuboi, K.; Araki, N.; Yamamoto, K.; Tanaka, T.; et al. Intracellular Ca2+-dependent formation of N-acyl-phosphatidylethanolamines by human cytosolic phospholipase A2ε. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158515. [Google Scholar] [CrossRef] [PubMed]
- Binte Mustafiz, S.S.; Uyama, T.; Hussain, Z.; Kawai, K.; Tsuboi, K.; Araki, N.; Ueda, N. The role of intracellular anionic phospholipids in the production of N-acyl-phosphatidylethanolamines by cytosolic phospholipase A2ε. J. Biochem. 2019, 165, 343–352. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Kawai, K.; Mustafiz, S.S.B.; Tsuboi, K.; Earaki, N.; Ueda, N. Phosphatidylserine-stimulated production of N-acyl-phosphatidylethanolamines by Ca2+-dependent N-acyltransferase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 493–502. [Google Scholar] [CrossRef]
- Capestrano, M.; Mariggio, S.; Perinetti, G.; Egorova, A.V.; Iacobacci, S.; Santoro, M.; Di Pentima, A.; Iurisci, C.; Egorov, M.V.; Di Tullio, G.; et al. Cytosolic phospholipase A2ε drives recycling through the clathrin-independent endocytic route. J. Cell Sci. 2014, 127, 977–993. [Google Scholar] [CrossRef]
- Everard, A.; Plovier, H.; Rastelli, M.; Van Hul, M.; D’Oplinter, A.D.W.; Geurts, L.; Druart, C.; Robine, S.; Delzenne, N.M.; Muccioli, G.G.; et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019, 10, 457. [Google Scholar] [CrossRef]
- Mock, E.D.; Mustafa, M.; Gunduz-Cinar, O.; Cinar, R.; Petrie, G.N.; Kantae, V.; Di, X.; Ogasawara, D.; Varga, Z.V.; Paloczi, J.; et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat. Chem. Biol. 2020, 16, 667–675. [Google Scholar] [CrossRef]
- Perez-Gonzalez, M.; Mendioroz, M.; Badesso, S.; Sucunza, D.; Roldan, M.; Espelosín, M.; Ursua, S.; Lujan, R.; Cuadrado-Tejedor, M.; García-Osta, A. PLA2G4E, a candidate gene for resilience in Alzheimer´s disease and a new target for dementia treatment. Prog. Neurobiol. 2020, 191, 101818. [Google Scholar] [CrossRef]
- Ghosh, M.; Tucker, D.E.; Burchett, S.A.; Leslie, C.C. Properties of the group IV phospholipase A2 family. Prog. Lipid Res. 2006, 45, 487–510. [Google Scholar] [CrossRef]
- Moon, S.H.; Liu, X.; Cedars, A.M.; Yang, K.; Kiebish, M.A.; Joseph, S.M.; Kelley, J.; Jenkins, C.M.; Gross, R.W. Heart failure–induced activation of phospholipase iPLA2γ generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J. Biol. Chem. 2017, 293, 115–129. [Google Scholar] [CrossRef]
- Kienesberger, P.C.; Oberer, M.; Lass, A.; Zechner, R. Mammalian patatin domain containing proteins: A family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 2008, 50, S63–S68. [Google Scholar] [CrossRef]
- Ramanadham, S.; Ali, T.; Ashley, J.W.; Bone, R.N.; Hancock, W.D.; Lei, X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J. Lipid Res. 2015, 56, 1643–1668. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.K.A.; Claesson, H.-E.; Kennedy, B.P. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J. Biol. Chem. 1998, 273, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kriz, R.W.; Wolfman, N.; Shaffer, M.; Seehra, J.; Jones, S.S. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J. Biol. Chem. 1997, 272, 8567–8575. [Google Scholar] [CrossRef] [PubMed]
- Malley, K.R.; Koroleva, O.; Miller, I.; Sanishvili, R.; Jenkins, C.M.; Gross, R.W.; Korolev, S. The structure of iPLA2β reveals dimeric active sites and suggests mechanisms of regulation and localization. Nat. Commun. 2018, 9, 765. [Google Scholar] [CrossRef]
- Turk, J.; White, T.D.; Nelson, A.J.; Lei, X.; Ramanadham, S. iPLA2β and its role in male fertility, neurological disorders, metabolic disorders, and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 846–860. [Google Scholar] [CrossRef]
- Morgan, N.V.; Westaway, S.K.; Morton, J.E.V.; Gregory, A.; Gissen, P.; Sonek, S.; Cangul, H.; Coryell, J.; Canham, N.; Nardocci, N.; et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat. Genet. 2006, 38, 752–754. [Google Scholar] [CrossRef]
- Zhou, Q.; Yen, A.; Rymarczyk, G.; Asai, H.; Trengrove, C.; Aziz, N.; Kirber, M.T.; Mostoslavsky, G.; Ikezu, T.; Wolozin, B.; et al. Impairment of PARK14-dependent Ca2+ signalling is a novel determinant of Parkinson’s disease. Nat. Commun. 2016, 7, 10332. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, K.J.; Castillo-Quan, J.I.; Bartolome, F.; Angelova, P.R.; Li, L.; Pope, S.; Cochemé, H.M.; Khan, S.; Asghari, S.; Bhatia, K.P.; et al. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 2015, 138, 1801–1816. [Google Scholar] [CrossRef]
- Lin, G.; Lee, P.-T.; Chen, K.; Mao, D.; Tan, K.L.; Zuo, Z.; Lin, W.-W.; Wang, L.; Bellen, H.J. Phospholipase PLA2G6, a parkinsonism-associated gene, affects Vps26 and Vps35, retromer function, and ceramide levels, similar to α-synuclein gain. Cell Metab. 2018, 28, 605–618.e6. [Google Scholar] [CrossRef]
- Mori, A.; Hatano, T.; Inoshita, T.; Shiba-Fukushima, K.; Koinuma, T.; Meng, H.; Kubo, S.-I.; Spratt, S.; Cui, C.; Yamashita, C.; et al. Parkinson’s disease-associated iPLA2-VIA/PLA2G6 regulates neuronal functions and α-synuclein stability through membrane remodeling. Proc. Natl. Acad. Sci. USA 2019, 116, 20689–20699. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, J.; Jiao, L.; Utaipan, T.; Tuma-Kellner, S.; Schmitz, G.; Liebisch, G.; Stremmel, W.; Chamulitrat, W. iPLA2β deficiency attenuates obesity and hepatic steatosis in ob/ob mice through hepatic fatty-acyl phospholipid remodeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 449–461. [Google Scholar] [CrossRef]
- Jiao, L.; Gan-Schreier, H.; Zhu, X.; Wei, W.; Tuma-Kellner, S.; Liebisch, G.; Stremmel, W.; Chamulitrat, W. Ageing sensitized by iPLA2β deficiency induces liver fibrosis and intestinal atrophy involving suppression of homeostatic genes and alteration of intestinal lipids and bile acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1520–1533. [Google Scholar] [CrossRef]
- Otto, A.-C.; Gan-Schreier, H.; Zhu, X.; Tuma-Kellner, S.; Staffer, S.; Ganzha, A.; Liebisch, G.; Chamulitrat, W. Group VIA phospholipase A2 deficiency in mice chronically fed with high-fat-diet attenuates hepatic steatosis by correcting a defect of phospholipid remodeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gan-Schreier, H.; Otto, A.-C.; Cheng, Y.; Staffer, S.; Tuma-Kellner, S.; Ganzha, A.; Liebisch, G.; Chamulitrat, W. iPLA2β deficiency in mice fed with MCD diet does not correct the defect of phospholipid remodeling but attenuates hepatocellular injury via an inhibition of lipid uptake genes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 677–687. [Google Scholar] [CrossRef]
- Liu, X.; Moon, S.H.; Jenkins, C.M.; Sims, H.F.; Gross, R.W. Cyclooxygenase-2 mediated oxidation of 2-arachidonoyl-lysophospholipids identifies unknown lipid signaling pathways. Cell Chem. Biol. 2016, 23, 1217–1227. [Google Scholar] [CrossRef]
- Moon, S.H.; Jenkins, C.M.; Liu, X.; Guan, S.; Mancuso, D.J.; Gross, R.W. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 2012, 287, 14880–14895. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.J.; Sims, H.F.; Han, X.; Jenkins, C.M.; Guan, S.P.; Yang, K.; Moon, S.H.; Pietka, T.; Abumrad, N.A.; Schlesinger, P.H.; et al. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J. Biol. Chem. 2007, 282, 34611–34622. [Google Scholar] [CrossRef] [PubMed]
- Bione, S.; D’Adamo, P.; Maestrini, E.; Gedeon, A.K.; Bolhuis, P.A.; Toniolo, D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 1996, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.J.; Moon, S.H.; Liu, X.; Thiffault, I.; Coffman, K.; LePichon, J.B.; Taboada, E.; Smith, L.D.; Farrow, E.G.; Miller, N.; et al. Loss of function variants in human PNPLA8 encoding calcium-independent phospholipase A2γ recapitulate the mitochondriopathy of the homologous null mouse. Hum. Mutat. 2015, 36, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoda, E.; Rai, K.; Ogawa, M.; Takakura, Y.; Kuwata, H.; Suzuki, H.; Nakatani, Y.; Murakami, M.; Hara, S. Group VIB Calcium-independent phospholipase A2 (iPLA2γ) regulates platelet activation, hemostasis and thrombosis in mice. PLoS ONE 2014, 9, e109409. [Google Scholar] [CrossRef]
- Moon, S.H.; Mancuso, D.J.; Sims, H.F.; Liu, X.; Nguyen, A.L.; Yang, K.; Guan, S.; Dilthey, B.G.; Jenkins, C.M.; Weinheimer, C.J.; et al. Cardiac myocyte-specific knock-out of calcium-independent phospholipase A2γ (iPLA2γ) decreases oxidized fatty acids during ischemia/reperfusion and reduces infarct size. J. Biol. Chem. 2016, 291, 19687–19700. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Moon, S.H.; Jenkins, C.M.; Li, M.; Sims, H.F.; Guan, S.; Gross, R.W. The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J. Biol. Chem. 2017, 292, 10672–10684. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sims, H.F.; Jenkins, C.M.; Guan, S.; Dilthey, B.G.; Gross, R.W. 12-LOX catalyzes the oxidation of 2-arachidonoyl-lysolipids in platelets generating eicosanoid-lysolipids that are attenuated by iPLA2γ knockout. J. Biol. Chem. 2020, 295, 5307–5320. [Google Scholar] [CrossRef] [PubMed]
- Kienesberger, P.C.; Lass, A.; Preiss-Landl, K.; Wolinski, H.; Kohlwein, S.D.; Zimmermann, R.; Zechner, R. Identification of an insulin-regulated lysophospholipase with homology to neuropathy target esterase. J. Biol. Chem. 2007, 283, 5908–5917. [Google Scholar] [CrossRef] [PubMed]
- Quistad, G.B.; Barlow, C.; Winrow, C.J.; Sparks, S.E.; Casida, J.E. Evidence that mouse brain neuropathy target esterase is a lysophospholipase. Proc. Natl. Acad. Sci. USA 2003, 100, 7983–7987. [Google Scholar] [CrossRef]
- Winrow, C.J.; Hemming, M.L.; Allen, D.M.; Quistad, G.B.; Casida, J.E.; Barlow, C. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nat. Genet. 2003, 33, 477–485. [Google Scholar] [CrossRef]
- Gallazzini, M.; Ferraris, J.D.; Kunin, M.; Morris, R.G.; Burg, M.B. Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc. Natl. Acad. Sci. USA 2006, 103, 15260–15265. [Google Scholar] [CrossRef]
- Akassoglou, K.; Malester, B.; Xu, J.; Tessarollo, L.; Rosenbluth, J.; Chao, M.V. Brain-specific deletion of neuropathy target esterase/swisscheese results in neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 5075–5080. [Google Scholar] [CrossRef]
- Moser, M.; Li, Y.; Vaupel, K.; Kretzschmar, D.; Kluge, R.; Glynn, P.; Buettner, R. Placental failure and impaired vasculogenesis result in embryonic lethality for neuropathy target esterase-deficient mice. Mol. Cell. Biol. 2004, 24, 1667–1679. [Google Scholar] [CrossRef]
- Read, D.J.; Li, Y.; Chao, M.V.; Cavanagh, J.B.; Glynn, P. Neuropathy target esterase is required for adult vertebrate axon maintenance. J. Neurosci. 2009, 29, 11594–11600. [Google Scholar] [CrossRef] [PubMed]
- Rainier, S.; Bui, M.; Mark, E.; Thomas, D.; Tokarz, D.; Ming, L.; Delaney, C.E.; Richardson, R.J.; Albers, J.W.; Matsunami, N.; et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am. J. Hum. Genet. 2008, 82, 780–785. [Google Scholar] [CrossRef]
- Kmoch, S.; Majewski, J.; Ramamurthy, V.; Cao, S.; Fahiminiya, S.; Ren, H.; MacDonald, I.M.; Lopez, I.; Sun, V.; Keser, V.; et al. Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness. Nat. Commun. 2015, 6, 5614. [Google Scholar] [CrossRef] [PubMed]
- Heier, C.; Kien, B.; Huang, F.; Eichmann, T.O.; Xie, H.; Zechner, R.; Chang, P.-A. The phospholipase PNPLA7 functions as a lysophosphatidylcholine hydrolase and interacts with lipid droplets through its catalytic domain. J. Biol. Chem. 2017, 292, 19087–19098. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, S.; Li, J.Z.; Seo, Y.-K.; Osborne, T.F.; Cohen, J.C.; Hobbs, H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. USA 2010, 107, 7892–7897. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in adipose tissue Is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Girousse, A.; Tavernier, G.; Valle, C.; Moro, C.; Mejhert, N.; Dinel, A.-L.; Houssier, M.; Roussel, B.; Besse-Patin, A.; Combes, M.; et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013, 11, e1001485. [Google Scholar] [CrossRef]
- Haemmerle, G.; Busemann, H.; Young, A.F.; Alexander, C.M.O.; Hoppe, P.; Mukhopadhyay, S.; Nittler, L.R. Lipolysis and Altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; Van De Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Lombes, M.; Rha, G.B.; Chi, Y.I.; Guerin, T.M.; Smart, E.J.; Liu, J. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Schlager, S.; Goeritzer, M.; Jandl, K.; Frei, R.; Vujic, N.; Kolb, D.; Strohmaier, H.; Dorow, J.; Eichmann, T.O.; Rosenberger, A.; et al. Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis. J. Leukoc. Biol. 2015, 98, 837–850. [Google Scholar] [CrossRef]
- Sugihara, M.; Morito, D.; Ainuki, S.; Hirano, Y.; Ogino, K.; Kitamura, A.; Hirata, H.; Nagata, K. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J. Cell Biol. 2019, 218, 949–960. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Zhang, X.; Xie, X.; Saarinen, A.; Lu, X.; Yang, X.; Liu, J. Defective adipose lipolysis and altered global energy metabolism in mice with adipose overexpression of the lipolytic inhibitor G0/G1 switch gene 2 (G0S2). J. Biol. Chem. 2014, 289, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Haemmerle, G.; Riederer, M.; Schoiswohl, G.; Schweiger, M.; Kienesberger, P.; Strauss, J.G.; Gorkiewicz, G.; Zechner, R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006, 3, 309–319. [Google Scholar] [CrossRef]
- Demerjian, M.; Crumrine, D.A.; Milstone, L.M.; Williams, M.L.; Elias, P.M. Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin–Dorfman Syndrome). J. Investig. Dermatol. 2006, 126, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Young, S.G.; Zechner, R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013, 27, 459–484. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Basantani, M.K.; Sitnick, M.T.; Cai, L.; Brenner, D.S.; Gardner, N.P.; Li, J.Z.; Schoiswohl, G.; Yang, K.; Kumari, M.; Gross, R.W.; et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 2011, 52, 318–329. [Google Scholar] [CrossRef]
- Smagris, E.; Basuray, S.; Li, J.; Huang, Y.; Lai, K.-M.V.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 2014, 61, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Huang, Y.; Karaman, R.; Ivanova, P.T.; Brown, H.A.; Roddy, T.; Castro-Perez, J.; Cohen, J.C.; Hobbs, H.H. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Investig. 2012, 122, 4130–4144. [Google Scholar] [CrossRef]
- Mitsche, M.A.; Hobbs, H.H.; Cohen, J.C. Patatin-like phospholipase domain–containing protein 3 promotes transfers of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. J. Biol. Chem. 2018, 293, 6958–6968. [Google Scholar] [CrossRef] [PubMed]
- Pirazzi, C.; Valenti, L.; Motta, B.M.; Pingitore, P.; Hedfalk, K.; Mancina, R.M.; Burza, M.A.; Indiveri, C.; Ferro, Y.; Montalcini, T.; et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet. 2014, 23, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kory, N.; Basuray, S.; Cohen, J.C.; Hobbs, H.H. PNPLA3, CGI-58, and inhibition of hepatic triglyceride hydrolysis in mice. Hepatology 2019, 69, 2427–2441. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Mottillo, E.P.; Mladenovic-Lucas, L.; Zhou, L.; Granneman, J.G. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Nat. Metab. 2019, 1, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Grall, A.; Guaguere, E.; Planchais, S.; Grond, S.; Bourrat, E.; Hausser, I.; Hitte, C.; Le Gallo, M.; Derbois, C.; Kim, G.-J.; et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat. Genet. 2012, 44, 140–147. [Google Scholar] [CrossRef]
- Grond, S.; Eichmann, T.O.; Dubrac, S.; Kolb, D.; Schmuth, M.; Fischer, J.; Crumrine, D.; Elias, P.M.; Haemmerle, G.; Zechner, R.; et al. PNPLA1 deficiency in mice and humans leads to a defect in the synthesis of omega-O-acylceramides. J. Investig. Dermatol. 2017, 137, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Anjo, T.; Kaneko, A.; Senoo, Y.; Shibata, A.; Takama, H.; Yokoyama, K.; Nishito, Y.; Ono, T.; Taya, C.; et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017, 8, 14609. [Google Scholar] [CrossRef]
- Ohno, Y.; Kamiyama, N.; Nakamichi, S.; Kihara, A. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat. Commun. 2017, 8, 14610. [Google Scholar] [CrossRef] [PubMed]
- Kien, B.; Grond, S.; Haemmerle, G.; Lass, A.; Eichmann, T.O.; Radner, F.P.W. ABHD5 stimulates PNPLA1-mediated ω-O-acylceramide biosynthesis essential for a functional skin permeability barrier. J. Lipid Res. 2018, 59, 2360–2367. [Google Scholar] [CrossRef]
- Ohno, Y.; Nara, A.; Nakamichi, S.; Kihara, A. Molecular mechanism of the ichthyosis pathology of Chanarin-Dorfman syndrome: Stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. J. Dermatol. Sci. 2018, 92, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Murakami, M.; Kihara, A. The role of PNPLA1 in ω-O-acylceramide synthesis and skin barrier function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 869–879. [Google Scholar] [CrossRef]
- Hattori, M.; Arai, H. Intracellular PAF-acetylhydrolase type I. Enzymes 2015, 38, 23–36. [Google Scholar] [CrossRef]
- Kono, N.; Arai, H. Intracellular Platelet-activating factor acetylhydrolase, type II. Enzymes 2015, 38, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Tjoelker, L.W.; Wilder, C.; Eberhardt, C.; Stafforinit, D.M.; Dietsch, G.; Schimpf, B.; Hooper, S.; Le Trong, H.; Cousens, L.S.; Zimmerman, G.A.; et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nat. Cell Biol. 1995, 374, 549–553. [Google Scholar] [CrossRef]
- Jiang, Z.; Fehrenbach, M.L.; Ravaioli, G.; Kokalari, B.; Redai, I.G.; Sheardown, S.A.; Wilson, S.; Macphee, C.H.; Haczku, A. The effect of lipoprotein-associated phospholipase A2 deficiency on pulmonary allergic responses in aspergillus fumigatus sensitized mice. Respir. Res. 2012, 13, 100. [Google Scholar] [CrossRef][Green Version]
- Corson, M.A. Phospholipase A2 inhibitors in atherosclerosis: The race is on. Lancet 2009, 373, 608–610. [Google Scholar] [CrossRef]
- Wilensky, R.L.; Shi, Y.; Mohler, E.R.; Hamamdzic, D.; Burgert, M.E.; Li, J.; Postle, A.; Fenning, R.S.; Bollinger, J.G.; Hoffman, B.E.; et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 2008, 14, 1059–1066. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Braunwald, E.; White, H.D.; Steen, D.P.; Lukas, M.A.; Tarka, E.; Steg, P.G.; Hochman, J.S.; Bode, C.; Maggioni, A.P.; et al. Effect of darapladib on major coronary events after an acute coronary syndrome. JAMA 2014, 312, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.K.; Levin, E.; Clifford, P.M.; Han, M.; Tourtellotte, R.; Chamberlain, D.; Pollaro, M.; Coretti, N.J.; Kosciuk, M.C.; Nagele, E.P.; et al. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: Beneficial effects of the LpPLA2 inhibitor darapladib. J. Alzheimer’s Dis. 2013, 35, 179–198. [Google Scholar] [CrossRef]
- Canning, P.; Kenny, B.-A.; Prise, V.; Glenn, J.; Sarker, M.H.; Hudson, N.; Brandt, M.; Lopez, F.J.; Gale, D.; Luthert, P.J.; et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 7213–7218. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Reichert, E.C.; Nakano, T.; Lohse, M.; Gardner, A.A.; Revelo, M.P.; Topham, M.K.; Stafforini, D.M. Deficiency of phospholipase A2 group 7 decreases intestinal polyposis and colon tumorigenesis in ApcMin/+ mice. Cancer Res. 2013, 73, 2806–2816. [Google Scholar] [CrossRef]
- Hattori, M.; Adachi, H.; Tsujimoto, M.; Arai, H.; Inoue, K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor. Nat. Cell Biol. 1994, 370, 216–218. [Google Scholar] [CrossRef]
- Koizumi, H.; Yamaguchi, N.; Hattori, M.; Ishikawa, T.-O.; Aoki, J.; Taketo, M.M.; Inoue, K.; Arai, H. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J. Biol. Chem. 2003, 278, 12489–12494. [Google Scholar] [CrossRef]
- Page, R.M.; Münch, A.; Horn, T.; Kuhn, P.-H.; Colombo, A.; Reiner, O.; Boutros, M.; Steiner, H.; Lichtenthaler, S.F.; Haass, C. Loss of PAFAH1B2 reduces amyloid-β generation by promoting the degradation of amyloid precursor protein C-terminal fragments. J. Neurosci. 2012, 32, 18204–18214. [Google Scholar] [CrossRef] [PubMed]
- Livnat, I.; Finkelshtein, D.; Ghosh, I.; Arai, H.; Reiner, O. PAF-AH catalytic subunits modulate the Wnt pathway in developing GABAergic neurons. Front. Cell. Neurosci. 2010, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Inoue, T.; Yoshida, Y.; Sato, H.; Matsusue, T.; Itabe, H.; Niki, E.; Aoki, J.; Arai, H. Protection against oxidative stress-induced hepatic injury by intracellular type II platelet-activating factor acetylhydrolase by metabolism of oxidized phospholipids in vivo. J. Biol. Chem. 2007, 283, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Shimanaka, Y.; Kono, N.; Taketomi, Y.; Arita, M.; Okayama, Y.; Tanaka, Y.; Nishito, Y.; Mochizuki, T.; Kusuhara, H.; Adibekian, A.; et al. Omega-3 fatty acid epoxides are autocrine mediators that control the magnitude of IgE-mediated mast cell activation. Nat. Med. 2017, 23, 1287–1297. [Google Scholar] [CrossRef]
- Kono, N.; Arai, H. Platelet-activating factor acetylhydrolases: An overview and update. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Hiraoka, M.; Wild, S.; Wilcoxen, S.E.; Paine, R.; Shayman, J.A. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J. Biol. Chem. 2004, 279, 42605–42611. [Google Scholar] [CrossRef]
- Hiraoka, M.; Abe, A.; Lu, Y.; Yang, K.; Han, X.; Gross, R.W.; Shayman, J.A. Lysosomal phospholipase A2 and phospholipidosis. Mol. Cell. Biol. 2006, 26, 6139–6148. [Google Scholar] [CrossRef]
- Paduraru, C.; Bezbradica, J.S.; Kunte, A.; Kelly, R.; Shayman, J.A.; Veerapen, N.; Cox, L.R.; Besra, G.S.; Cresswell, P. Role for lysosomal phospholipase A2 in iNKT cell-mediated CD1d recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Behrends, J.; Hagens, K.; Harmel, N.; Shayman, J.A.; Schaible, U.E. Lysosomal phospholipase A2: A novel player in host immunity to Mycobacterium tuberculosis. Eur. J. Immunol. 2014, 44, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Feinstein, S.I.; Ho, Y.-S. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J. Lipid Res. 2005, 46, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Medina, J.P.; Tao, J.-Q.; Patel, P.; Bannitz-Fernandes, R.; Dodia, C.; Sorokina, E.M.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. Genetic inactivation of the phospholipase A2 activity of peroxiredoxin 6 in mice protects against LPS-induced acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L656–L668. [Google Scholar] [CrossRef]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.-Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2017, 14, 41–46. [Google Scholar] [CrossRef]
- Moawad, A.R.; Fernandez, M.C.; Scarlata, E.; Dodia, C.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci. Rep. 2017, 7, 12994. [Google Scholar] [CrossRef]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef]
- Shayman, J.A.; Tesmer, J.J.G. Lysosomal phospholipase A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Ikematsu, N.; Inoue, M.; Shinohara, N.; Jin, X.-H.; Tsuboi, K.; Tonai, T.; Tokumura, A.; Ueda, N. Generation of N-acylphosphatidylethanolamine by members of the phospholipase A/acyltransferase (PLA/AT) Family. J. Biol. Chem. 2012, 287, 31905–31919. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, K.; Ahmadian, M.; Duncan, R.E.; Sarkadi-Nagy, E.; Varady, K.A.; Hellerstein, M.K.; Lee, H.-Y.; Samuel, V.T.; Shulman, G.I.; Kim, K.-H.; et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 2009, 15, 159–168. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Dong, Y.-Q.; Wang, P.; Zhang, X.; Yan, Y.; Sun, L.; Liu, B.; Zhang, D.; Zhang, H.; Liu, H.; et al. Adipocyte-derived lysophosphatidylcholine activates adipocyte and adipose tissue macrophage Nod-like receptor protein 3 inflammasomes mediating homocysteine-induced insulin resistance. EBioMedicine 2018, 31, 202–216. [Google Scholar] [CrossRef]
- Xiong, S.; Tu, H.; Kollareddy, M.; Pant, V.; Li, Q.; Zhang, Y.; Jackson, J.G.; Suh, Y.-A.; Elizondo-Fraire, A.C.; Yang, P.; et al. Pla2g16 phospholipase mediates gain-of-function activities of mutant p53. Proc. Natl. Acad. Sci. USA 2014, 111, 11145–11150. [Google Scholar] [CrossRef]
- Golczak, M.; Sears, A.E.; Kiser, P.D.; Palczewski, K. LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3. Nat. Chem. Biol. 2014, 11, 26–32. [Google Scholar] [CrossRef]
- Uyama, T.; Kawai, K.; Kono, N.; Watanabe, M.; Tsuboi, K.; Inoue, T.; Araki, N.; Arai, H.; Ueda, N. Interaction of phospholipase A/acyltransferase-3 with Pex19p: A possible involvement in the down-regulation of peroxisomes. J. Biol. Chem. 2015, 290, 17520–17534. [Google Scholar] [CrossRef] [PubMed]
- Staring, J.; Von Castelmur, E.; Blomen, V.A.; Hengel, L.G.V.D.; Brockmann, M.; Baggen, J.; Thibaut, H.J.; Nieuwenhuis, J.; Janssen, H.; Van Kuppeveld, F.J.M.; et al. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nat. Cell Biol. 2017, 541, 412–416. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1546–1561. [Google Scholar] [CrossRef]
- Mardian, E.B.; Bradley, R.M.; Duncan, R.E. The HRASLS (PLA/AT) subfamily of enzymes. J. Biomed. Sci. 2015, 22, 99. [Google Scholar] [CrossRef]
- Thomas, G.; Brown, A.L.; Brown, J.M. In vivo metabolite profiling as a means to identify uncharacterized lipase function: Recent success stories within the alpha beta hydrolase domain (ABHD) enzyme family. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Cisar, J.S.; Milliken, D.; Niessen, S.; Wang, C.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. Metabolomics annotates ABHD3 as a physiologic regulator of medium-chain phospholipids. Nat. Chem. Biol. 2011, 7, 763–765. [Google Scholar] [CrossRef]
- Lee, H.-C.; Simon, G.M.; Cravatt, B.F. ABHD4 regulates multiple classes of N-acyl phospholipids in the mammalian central nervous system. Biochemistry 2015, 54, 2539–2549. [Google Scholar] [CrossRef]
- Marrs, W.R.; Blankman, J.L.; Horne, E.A.; Thomazeau, A.; Lin, Y.H.; Coy, J.; Bodor, A.L.; Muccioli, G.G.; Hu, S.S.-J.; Woodruff, G.; et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010, 13, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Long, J.Z.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 2013, 110, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Fiskerstrand, T.; Brahim, D.H.-B.; Johansson, S.; M’Zahem, A.; Haukanes, B.I.; Drouot, N.; Zimmermann, J.; Cole, A.J.; Vedeler, C.; Bredrup, C.; et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 2010, 87, 410–417. [Google Scholar] [CrossRef]
- Kamat, S.S.; Camara, K.; Parsons, W.H.; Chen, D.-H.; Dix, M.M.; Bird, T.D.; Howell, A.R.; Cravatt, B.F. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 2015, 11, 164–171. [Google Scholar] [CrossRef]
- Maeda, Y.; Tashima, Y.; Houjou, T.; Fujita, M.; Yoko-o, T.; Jigami, Y.; Taguchi, R.; Kinoshita, T. Fatty acid remodeling of GPI-anchored proteins is required for their raft association. Mol. Biol. Cell 2007, 18, 1497–1506. [Google Scholar] [CrossRef]
- Wang, Y.; Murakami, Y.; Yasui, T.; Wakana, S.; Kikutani, H.; Kinoshita, T.; Maeda, Y. Significance of glycosylphosphatidylinositol-anchored protein enrichment in lipid rafts for the control of autoimmunity. J. Biol. Chem. 2013, 288, 25490–25499. [Google Scholar] [CrossRef]
- Lee, G.-H.; Fujita, M.; Takaoka, K.; Murakami, Y.; Fujihara, Y.; Kanzawa, N.; Murakami, K.-I.; Kajikawa, E.; Takada, Y.; Saito, K.; et al. A GPI processing phospholipase A2, PGAP6, modulates Nodal signaling in embryos by shedding CRIPTO. J. Cell Biol. 2016, 215, 705–718. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kokotou, M.G.; Vasilakaki, S.; Kokotos, G. Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 941–956. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. https://doi.org/10.3390/biom10101457
Murakami M, Sato H, Taketomi Y. Updating Phospholipase A2 Biology. Biomolecules. 2020; 10(10):1457. https://doi.org/10.3390/biom10101457
Chicago/Turabian StyleMurakami, Makoto, Hiroyasu Sato, and Yoshitaka Taketomi. 2020. "Updating Phospholipase A2 Biology" Biomolecules 10, no. 10: 1457. https://doi.org/10.3390/biom10101457
APA StyleMurakami, M., Sato, H., & Taketomi, Y. (2020). Updating Phospholipase A2 Biology. Biomolecules, 10(10), 1457. https://doi.org/10.3390/biom10101457




