GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Primary Culture of Cortical Neurons
2.2. HEK293 Cultures and Transfections
2.3. Patch-Clamp Recordings
2.4. Calcium Imaging
2.5. Data Analysis
2.6. Reagents
3. Results
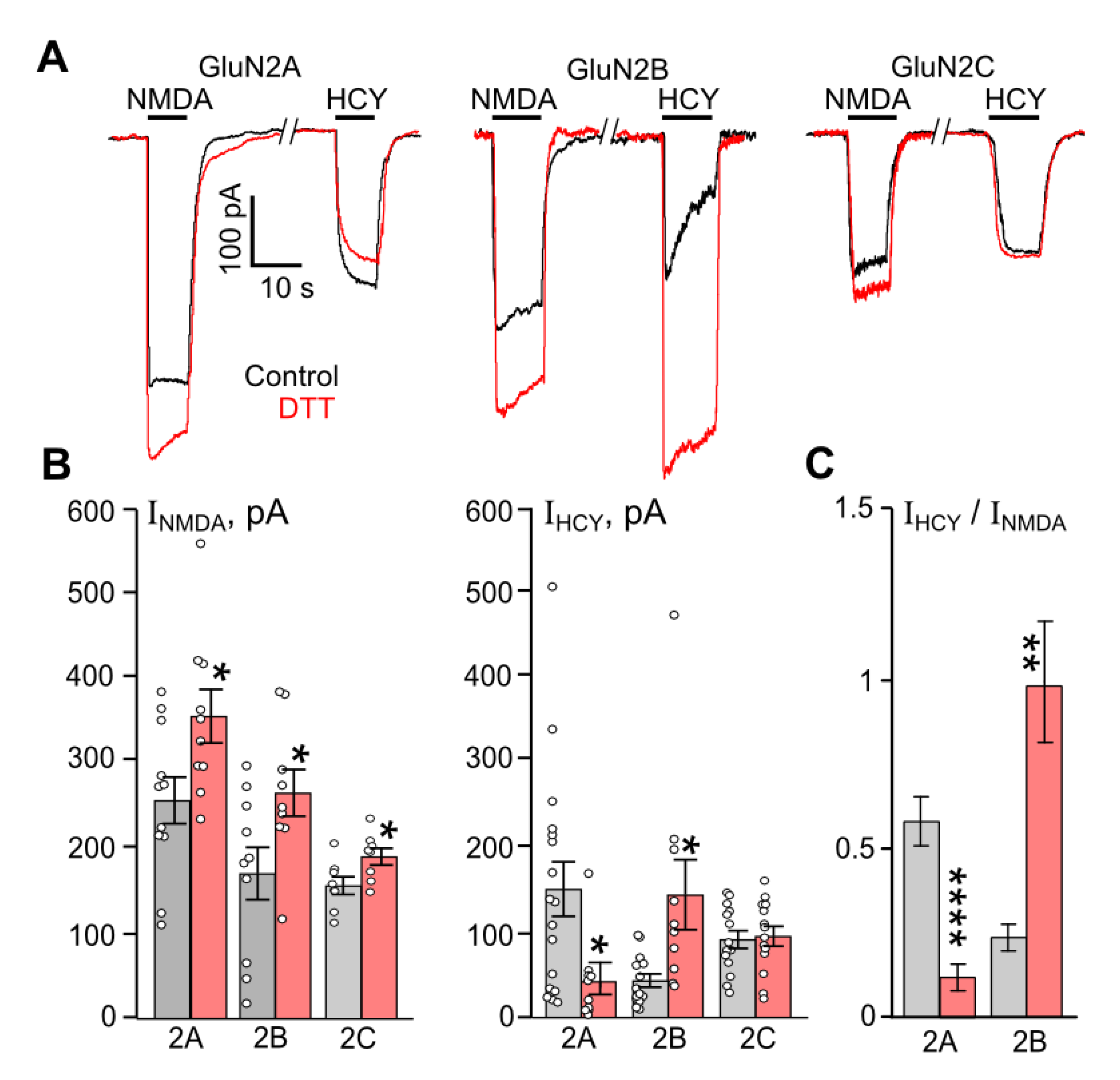
3.1. Redox Modulation of HCY-Elicited Currents Is GluN2 Subtype-Specific
3.2. Redox Modulation of HCY-Induced Desensitization of Native NMDARs
3.3. The Contribution of GluN2A and GluN2B-Containing NMDARs to HCY Redox Effects in Neurons
4. Discussion
4.1. Molecular Basis of Redox Agent Effect on NMDARs
4.2. HCY as NMDAR Agonist and Reducing Agent
4.3. HCY Redox Modulation of NMDARs Is GluN2 Subunit-Specific
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [Ca2+] | free intracellular calcium concentration |
| DTT | dithiothreitol |
| EC50 | half-maximal effective concentration |
| GluN1, GluN2A, GluN2B, and GluN2C | subunits of N-methyl-d-aspartate receptors |
| GSH | glutathione |
| HCY | homocysteine |
| [HCY] | homocysteine concentration |
| H2S | hydrogen sulfide |
| Ifen | ifenprodil |
| NMDAR | N-methyl-d-aspartate receptor |
| SEM | standard error of mean |
| Vh | membrane holding potential |
References
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; Mcpartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; Mcpartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowa, H.; Yasui, K.; Takeshima, T.; Urakami, K.; Sakai, F.; Nakashima, K. The homozygous C677T mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for migraine. Am. J. Med. Genet. 2000, 96, 762–764. [Google Scholar] [CrossRef]
- Surtees, R.; Leung, D.Y.M.; Bowron, A.; Leonard, J. Cerebrospinal fluid and plasma total homocysteine and related metabolites in children with cystathionine b-synthase deficiency: The effect of treatment. Pediatr Res. 1997, 42, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosten, E.; Lesaffre, E.; Riezler, R.; Ghekiere, V.; Dereymaeker, L.; Pelemans, W.; Dejaeger, E. Is metabolic evidence for vitamin B-12 and folate deficiency more frequent in elderly patients with Alzheimer’s disease? J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M76–M79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdev, P. Homocysteine, cerebrovascular disease and brain atrophy. J. Neurol. Sci. 2004, 226, 25–29. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Yu, M.-S.; Yang, X.-F.; So, K.-F.; Yuen, W.-H.; Chang, R.C.-C. Neuroprotective effects of polysaccharides from wolfberry, the fruits of Lycium barbarum, against homocysteine-induced toxicity in rat cortical neurons. J. Alzheimers Dis. 2010, 19, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Abushik, P.A.; Niittykoski, M.; Giniatullina, R.; Shakirzyanova, A.; Bart, G.; Fayuk, D.; Sibarov, D.A.; Antonov, S.M.; Giniatullin, R. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J. Neurochem. 2014, 129, 264–274. [Google Scholar] [CrossRef]
- Oldreive, C.E.; Doherty, G.H. Neurotoxic effects of homocysteine on cerebellar Purkinje neurons in vitro. Neurosci. Lett. 2007, 413, 52–57. [Google Scholar] [CrossRef]
- Kuszczyk, M.; Gordon-Krajcer, W.; Lazarewicz, J.W. Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem. Int. 2009, 55, 174–180. [Google Scholar] [CrossRef]
- Kim, W.K.; Pae, Y.S. Involvement of N-methyl-D-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci. Lett. 1996, 216, 117–120. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D’Emilia, D.M.; Rayuda, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieminska, E.; Stafiej, A.; Lazarewicz, J.W. Role of group I metabotropic glutamate receptors and NMDA receptors in homocysteine-evoked acute neurodegeneration of cultured cerebellar granule neurones. Neurochem. Int. 2003, 43, 481–492. [Google Scholar] [CrossRef]
- Yeganeh, F.; Nikbakht, F.; Bahmanpour, S.; Rastegar, K.; Namavar, R. Neuroprotective effects of NMDA and group I metabotropic glutamate receptor antagonists against neurodegeneration induced by homocysteine in rat hippocampus: In vivo study. J. Mol. Neurosci. 2013, 50, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, L.S.; Ivanova, M.A.; Stepanenko, Y.D.; Karelina, T.V.; Giniatullin, R.; Sibarov, D.A.; Abushik, P.A.; Antonov, S.M. Collapse of neuronal energy balance as a basis of L-homocysteine neurotoxicity. Biochem. Suppl. Ser. A Membr. Cell Biol. 2018, 12, 360–368. [Google Scholar] [CrossRef]
- Ivanova, M.A.; Kokorina, A.D.; Timofeeva, P.D.; Karelina, T.V.; Abushik, P.A.; Stepanenko, J.D.; Sibarov, D.A.; Antonov, S.M. Calcium export from neurons and multi-kinase signaling cascades contribute to ouabain neuroprotection in hyperhomocysteinemia. Biomolecules 2020, 10, 1104. [Google Scholar] [CrossRef]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O., Jr.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef] [Green Version]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [Green Version]
- Sibarov, D.A.; Abushik, P.A.; Giniatullin, R.; Antonov, S.M. GluN2A subunit-containing NMDA receptors are the preferential neuronal targets of homocysteine. Front. Cell. Neurosci. 2016, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Poddar, R.; Chen, A.; Winter, L.; Rajagopal, S.; Paul, S. Role of AMPA receptors in homocysteine-NMDA receptor-induced crosstalk between ERK and p38 MAPK. J. Neurochem. 2017, 142, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, S.; Fitzgerald, A.A.; Deep, S.N.; Paul, S.; Poddar, R. Role of GluN2A NMDA receptor in homocysteine-induced prostaglandin E2 release from neurons. J. Neurochem. 2019, 150, 44–55. [Google Scholar] [CrossRef]
- Deep, S.N.; Mitra, S.; Rajagopal, S.; Paul, S.; Poddar, R. GluN2A-NMDA receptor-mediated sustained Ca2+ influx leads to homocysteine-induced neuronal cell death. J. Biol. Chem. 2019, 294, 11154–11165. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Rajagopal, S.; Winter, L.; Miller, J.W.; Jacobsen, D.W.; Brigman, J.; Allan, A.M.; Paul, S.; Poddar, R. Hyperhomocysteinemia leads to exacerbation of ischemic brain damage: Role of GluN2A NMDA receptors. Neurobiol. Dis. 2019, 127, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Sonkar, V.K.; Katare, P.B.; Kumar, R.; Kruger, W.D.; Arning, E.; Bottiglieri, T.; Lentz, S.R.; Dayal, S. Memantine protects from exacerbation of ischemic stroke and blood brain barrier disruption in mild but not severe hyperhomocysteinemia. J. Am. Heart Assoc. 2020, 9, e013368. [Google Scholar] [CrossRef] [PubMed]
- Sibarov, D.A.; Giniatullin, R.; Antonov, S.M. High sensitivity of cerebellar neurons to homocysteine is determined by expression of GluN2C and GluN2D subunits of NMDA receptors. Biochem. Biophys. Res. Commun. 2018, 506, 648–652. [Google Scholar] [CrossRef]
- Glushchenko, A.V.; Jacobsen, D.W. Molecular targeting of proteins by L-homocysteine: Mechanistic implications for vascular disease. Antioxid. Redox. Signal. 2007, 9, 1883–1898. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Rossi, R.; Giustarini, D.; Milzani, A.; Dalle-Donne, I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J. Cell. Mol. Med. 2009, 13, 3131–3140. [Google Scholar] [CrossRef] [Green Version]
- Köhr, G.; Eckardt, S.; Lüddens, H.; Monyer, H.; Seeburg, P.H. NMDA receptor channels: Subunit-specific potentiation by reducing agents. Neuron 1994, 12, 1031–1040. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.M.; Traynelis, S.F.; Chen, H.S.V.; Escobar, W.; Heinemann, S.F.; Lipton, A. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron 1994, 13, 929–936. [Google Scholar] [CrossRef]
- Talukder, I.; Kazi, R.; Wollmuth, L.P. GluN1-specific redox effects on the kinetic mechanism of NMDA receptor activation. Biophys. J. 2011, 101, 2389–2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakovlev, A.V.; Kurmasheva, E.; Ishchenko, Y.; Giniatullin, R.; Sitdikova, G. Age-dependent, subunit specific action of hydrogen sulfide on GluN1/2A and GluN1/2B NMDA receptors. Front. Cell. Neurosci. 2017, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.B.; Chen, H.S.V.; Lipton, S.A. Three pairs of cysteine residues mediate both redox and Zn2+ modulation of the NMDA receptor. J. Neurosci. 2001, 21, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Mironova, E.V.; Evstratova, A.A.; Antonov, S.M. A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J. Neurosci. Methods 2007, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, E.B.; Stevens, C.F. Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 10817–10822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, A.; Buller, A.L.; Johnson, J.W. NR2 subunit dependence of NMDA receptor channel block by external Mg2+. J. Physiol. 2005, 562, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Inoue, Y.; Sakimura, K.; Mishina, M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 1992, 3, 1138–1140. [Google Scholar] [CrossRef]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Hansen, K.B.; Ogden, K.K.; Yuan, H.; Traynelis, S.F. Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 2014, 81, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K. Separating dual effects of zinc at recombinant N-methyl-D-aspartate receptors. Neurosci. Lett. 1996, 215, 9–12. [Google Scholar] [CrossRef]
- Paoletti, P.; Ascher, P.; Neyton, J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J. Neurosci. 1997, 17, 5711–5725. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ray, R.S.; Sandhir, R. Hydrogen sulfide attenuates homocysteine-induced neurotoxicity by preventing mitochondrial dysfunctions and oxidative damage: In vitro and in vivo studies. Neurochem. Int. 2018, 120, 87–98. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Givvimani, S.; Sathnur, P.B.; Tyagi, S.C.; Tyagi, N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience 2013, 252, 302–319. [Google Scholar] [CrossRef] [Green Version]
- Yakovleva, O.; Bogatova, K.; Mukhtarova, R.; Yakovlev, A.; Shakhmatova, V.; Gerasimova, E.; Ziyatdinova, G.; Hermann, A.; Sitdikova, G. Hydrogen sulfide alleviates anxiety, motor, and cognitive dysfunctions in rats with maternal hyperhomocysteinemia via mitigation of oxidative stress. Biomolecules 2020, 10, 995. [Google Scholar] [CrossRef]
- Brimecombe, J.C.; Boeckman, F.A.; Aizenman, E. Functional consequences of NR2 subunit composition in single recombinant N-methyl- D-aspartate receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 11019–11024. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, F.; Shibata, T.; Kamiya, K.; Yoshitake, J.; Kikuchi, R.; Matsushita, T.; Ishii, I.; Giménez-Bastida, J.A.; Schneider, C.; Uchida, K. Structural and functional insights into S-thiolation of human serum albumins. Sci. Rep. 2018, 932. [Google Scholar] [CrossRef] [Green Version]
- Ueland, P.M.; Mansoor, M.A.; Guttormsen, A.B.; Müller, F.; Aukrust, P.; Refsum, H.; Svardal, A.M. Reduced, oxidized and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status--a possible element of the extracellular antioxidant defense system. J. Nutr. 1996, 126, 1281S–1284S. [Google Scholar] [CrossRef] [Green Version]
- Bolton, A.D.; Phillips, M.A.; Constantine-Paton, M. Homocysteine reduces NMDAR desensitization and differentially modulates peak amplitude of NMDAR currents, depending on GluN2 subunit composition. J. Neurophysiol. 2013, 110, 1567–1582. [Google Scholar] [CrossRef] [Green Version]
- Poddar, R.; Paul, S. Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death. J. Neurochem. 2009, 110, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poddar, R.; Paul, S. Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J. Neurochem. 2013, 124, 558–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar, K.R.; McGinley, M.J.; Westbrook, G.L. Triheteromeric NMDA receptors at hippocampal synapses. J. Neurosci. 2013, 33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibarov, D.A.; Boikov, S.I.; Karelina, T.V.; Antonov, S.M. GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine. Biomolecules 2020, 10, 1441. https://doi.org/10.3390/biom10101441
Sibarov DA, Boikov SI, Karelina TV, Antonov SM. GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine. Biomolecules. 2020; 10(10):1441. https://doi.org/10.3390/biom10101441
Chicago/Turabian StyleSibarov, Dmitry A., Sergei I. Boikov, Tatiana V. Karelina, and Sergei M. Antonov. 2020. "GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine" Biomolecules 10, no. 10: 1441. https://doi.org/10.3390/biom10101441
APA StyleSibarov, D. A., Boikov, S. I., Karelina, T. V., & Antonov, S. M. (2020). GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine. Biomolecules, 10(10), 1441. https://doi.org/10.3390/biom10101441






