cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease
Abstract
:1. Introduction
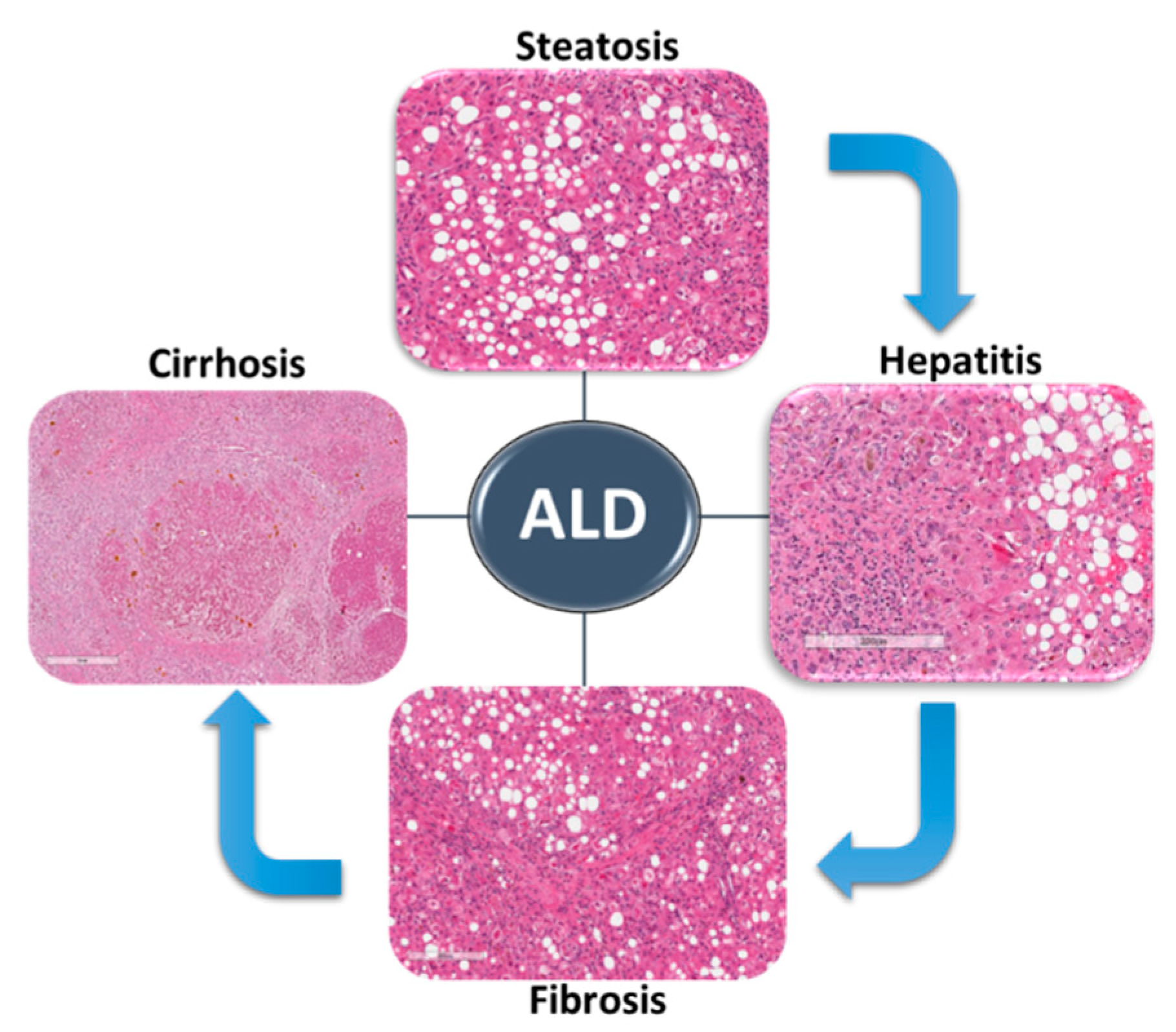
2. Alcohol-Associated Liver Disease
2.1. Alcohol Metabolism
2.2. ALD Spectrum
2.3. Mechanisms of ALD
2.3.1. Liver Steatosis
2.3.2. Alcohol-Associated Hepatitis and Immune Cells
2.3.3. Fibrosis and Hepatic Stellate Cells
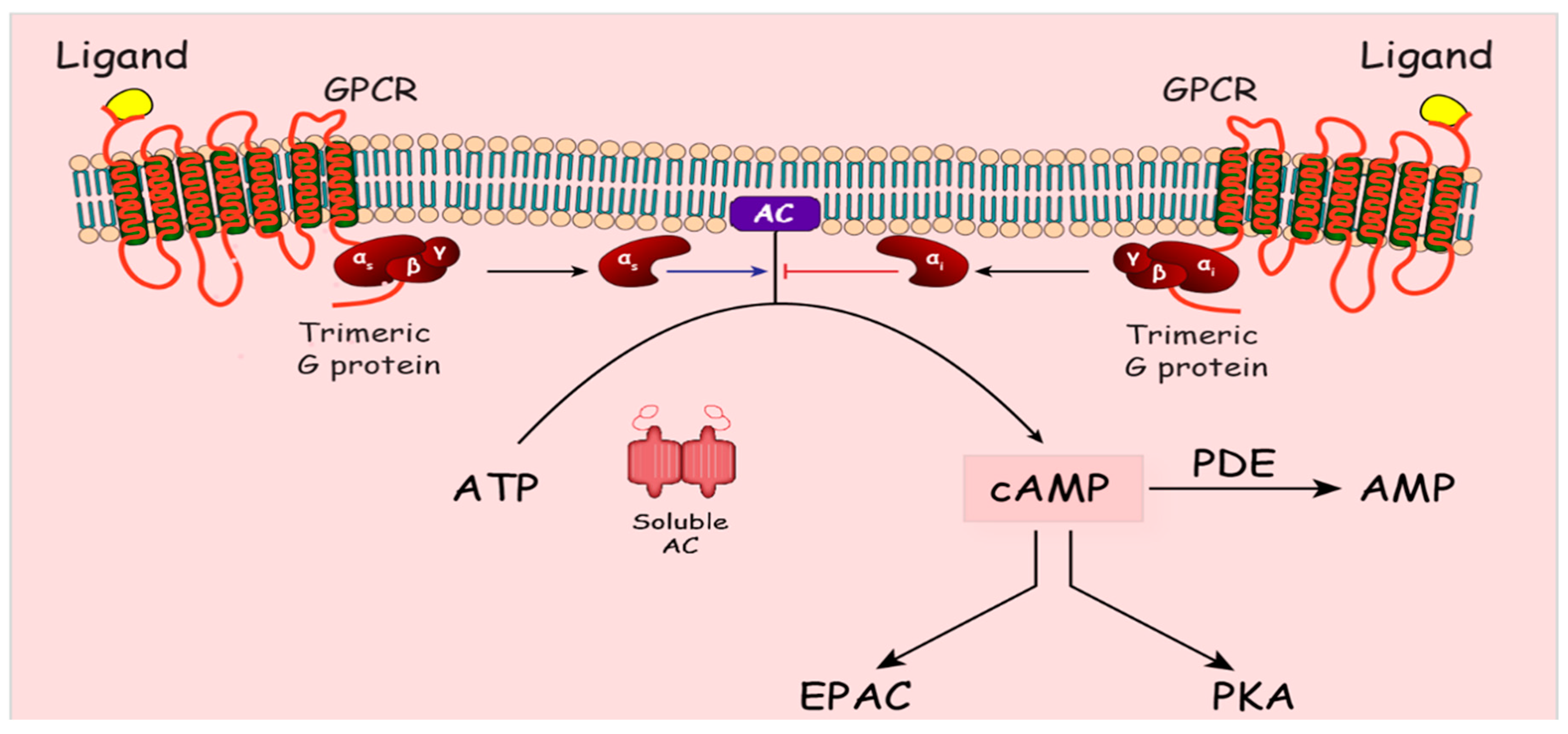
3. Cyclic AMP Signaling
4. cAMP Signaling in ALD
4.1. Hepatocytes: Regeneration/Steatosis
4.2. Immune Cells/Alcohol Associated Hepatitis (AH)
4.3. Stellate Cells/Fibrosis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayiner, M.; Golabi, P.; Younossi, Z.M. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig. Dis. Sci. 2019, 64, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Brown, R.S., Jr.; Terrault, N.A.; El-Serag, H. Burden of liver disease in the United States: Summary of a workshop. Hepatology 2002, 36, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Pincock, S. Binge drinking on rise in UK and elsewhere. Government report shows increases in alcohol consumption, cirrhosis, and premature deaths. Lancet 2003, 362, 1126–1127. [Google Scholar] [CrossRef]
- Kong, L.Z.; Chandimali, N.; Han, Y.H.; Lee, D.H.; Kim, J.S.; Kim, S.U.; Kim, T.D.; Jeong, D.K.; Sun, H.N.; Lee, D.S.; et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, 2712. [Google Scholar] [CrossRef] [Green Version]
- Ntandja Wandji, L.C.; Gnemmi, V.; Mathurin, P.; Louvet, A. Combined alcoholic and non-alcoholic steatohepatitis. JHEP Rep. 2020, 2, 100101. [Google Scholar] [CrossRef]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef] [Green Version]
- Galicia-Moreno, M.; Gutierrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mexico 2014, 79, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Pohorecky, L.A.; Brick, J. Pharmacology of ethanol. Pharmacol. Ther. 1988, 36, 335–427. [Google Scholar] [CrossRef]
- Sofer, W.; Martin, P.F. Analysis of alcohol dehydrogenase gene expression in drosophila. Annu. Rev. Genet. 1987, 21, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 2001, 276, 68–75. [Google Scholar] [CrossRef] [Green Version]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef] [Green Version]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef] [Green Version]
- Celli, R.; Zhang, X. Pathology of Alcoholic Liver Disease. J. Clin. Transl. Hepatol. 2014, 2, 103–109. [Google Scholar] [CrossRef]
- Naveau, S.; Giraud, V.; Borotto, E.; Aubert, A.; Capron, F.; Chaput, J.C. Excess weight risk factor for alcoholic liver disease. Hepatology 1997, 25, 108–111. [Google Scholar] [CrossRef]
- Mehta, M.; Satsangi, S.; Duseja, A.; Taneja, S.; Dhiman, R.K.; Chawla, Y. Can Alcoholic Liver Disease and Nonalcoholic Fatty Liver Disease Co-Exist? J. Clin. Exp. Hepatol. 2017, 7, 121–126. [Google Scholar] [CrossRef]
- Lu, X.L.; Luo, J.Y.; Tao, M.; Gen, Y.; Zhao, P.; Zhao, H.L.; Zhang, X.D.; Dong, N. Risk factors for alcoholic liver disease in China. World J. Gastroenterol. 2004, 10, 2423–2426. [Google Scholar] [CrossRef]
- Mihm, S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef] [Green Version]
- Gudowska, M.; Wojtowicz, E.; Cylwik, B.; Gruszewska, E.; Chrostek, L. The Distribution of Liver Steatosis, Fibrosis, Steatohepatitis and Inflammation Activity in Alcoholics According to FibroMax Test. Adv. Clin. Exp. Med. Off. Organ Wroc. Med Univ. 2015, 24, 823–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomes, P.G.; Osna, N.A.; Davis, J.S.; Donohue, T.M., Jr. Cellular steatosis in ethanol oxidizing-HepG2 cells is partially controlled by the transcription factor, early growth response-1. Int. J. Biochem. Cell Biol. 2013, 45, 454–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in Liver Diseases: A Mini-review. J. Clin. Transl. Hepatol. 2018, 6, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Wang, F.; Li, X.; Rogers, C.Q.; Liang, X.; Finck, B.N.; Mitra, M.S.; Zhang, R.; Mitchell, D.A.; You, M. Regulation of hepatic lipin-1 by ethanol: Role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology 2012, 55, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferre, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 83–92. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, C.; Thomes, P.G.; Kharbanda, K.K.; Casey, C.A.; McNiven, M.A.; Donohue, T.M., Jr. Lipophagy and Alcohol-Induced Fatty Liver. Front. Pharmacol. 2019, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Nan, Y.-M.; Wang, R.-Q.; Fu, N. Peroxisome proliferator-activated receptor α, a potential therapeutic target for alcoholic liver disease. World J. Gastroenterol. 2014, 20, 8055–8060. [Google Scholar] [CrossRef]
- Nishise, Y.; Saito, T.; Makino, N.; Okumoto, K.; Ito, J.-I.; Watanabe, H.; Saito, K.; Togashi, H.; Ikeda, C.; Kubota, I.; et al. Relationship between Alcohol Consumption and Serum Adiponectin Levels: The Takahata Study—A Cross-Sectional Study of a Healthy Japanese Population. J. Clin. Endocrinol. Metab. 2010, 95, 3828–3835. [Google Scholar] [CrossRef] [Green Version]
- Shepard, B.D.; Fernandez, D.J.; Tuma, P.L. Alcohol consumption impairs hepatic protein trafficking: Mechanisms and consequences. Genes Nutr. 2010, 5, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasineni, K.; Casey, C.A. Molecular mechanism of alcoholic fatty liver. Indian J. Pharmacol. 2012, 44, 299–303. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, J.H.; Febbraio, M.; Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. 2011, 236, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Parlesak, A.; Schafer, C.; Schutz, T.; Bode, J.C.; Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol. Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Thurman, R.G. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 1998, 275, G605–G611. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Uesugi, T.; Froh, M.; Arteel, G.E.; Bradford, B.U.; Thurman, R.G. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 2001, 34, 101–108. [Google Scholar] [CrossRef]
- Iimuro, Y.; Gallucci, R.M.; Luster, M.I.; Kono, H.; Thurman, R.G. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology 1997, 26, 1530–1537. [Google Scholar] [CrossRef]
- Yin, M.; Wheeler, M.D.; Kono, H.; Bradford, B.U.; Gallucci, R.M.; Luster, M.I.; Thurman, R.G. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 1999, 117, 942–952. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.J.; Gao, B.; Zakhari, S.; Nagy, L.E. Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 2012, 32, 343–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, S.L.; Blanco, A.M.; Azorin, I.; Guasch, R.; Pascual, M.; Gomez-Lechon, M.J.; Renau-Piqueras, J.; Guerri, C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol. Clin. Exp. Res. 2003, 27, 1979–1986. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Ju, C.; Mandrekar, P. Macrophages and Alcohol-Related Liver Inflammation. Alcohol. Res. Curr. Rev. 2015, 37, 251–262. [Google Scholar]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Kim, A.; Saikia, P.; Nagy, L.E. miRNAs Involved in M1/M2 Hyperpolarization Are Clustered and Coordinately Expressed in Alcoholic Hepatitis. Front. Immunol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. 50M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; French, B.; Morgan, T.; French, S.W. The liver is populated by a broad spectrum of markers for macrophages. In alcoholic hepatitis the macrophages are M1 and M2. Exp. Mol. Pathol. 2014, 96, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, X.; Hoque, R.; Garcia-Martinez, I.; Yousaf, M.N.; Tonack, S.; Offermanns, S.; Dubuquoy, L.; Louvet, A.; Mathurin, P.; et al. beta-Hydroxybutyrate protects from alcohol-induced liver injury via a Hcar2-cAMP dependent pathway. J. Hepatol. 2018, 69, 687–696. [Google Scholar] [CrossRef]
- Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001, 21, 311–335. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Nobili, V.; Alisi, A. Toll-like receptor-mediated signaling cascade as a regulator of the inflammation network during alcoholic liver disease. World J. Gastroenterol. 2014, 20, 16443–16451. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013, 305, C789–C799. [Google Scholar] [CrossRef] [Green Version]
- Rockey, D.C.; Fouassier, L.; Chung, J.J.; Carayon, A.; Vallee, P.; Rey, C.; Housset, C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: Potential for autocrine and paracrine effects on stellate cells. Hepatology 1998, 27, 472–480. [Google Scholar] [CrossRef]
- Shao, R.; Yan, W.; Rockey, D.C. Regulation of endothelin-1 synthesis by endothelin-converting enzyme-1 during wound healing. J. Biol. Chem. 1999, 274, 3228–3234. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Tsukamoto, H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: Implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology 1990, 11, 599–605. [Google Scholar] [CrossRef]
- Friedman, S.L.; Arthur, M.J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J. Clin. Investig. 1989, 84, 1780–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Jerez, D.; Rojas-Valencia, L.; Solis-Herruzo, J.A.; Greenwel, P.; Rojkind, M. Ethanol induces the expression of alpha 1(I) procollagen mRNA in a co-culture system containing a liver stellate cell-line and freshly isolated hepatocytes. Biochim. Biophys. Acta 1997, 1362, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Svegliati-Baroni, G.; Inagaki, Y.; Rincon-Sanchez, A.R.; Else, C.; Saccomanno, S.; Benedetti, A.; Ramirez, F.; Rojkind, M. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology 2005, 42, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem. J. 2002, 368, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.; Mann, D.A. Transcriptional regulation of hepatic stellate cells. Adv. Drug Deliv. Rev. 2009, 61, 497–512. [Google Scholar] [CrossRef]
- Reyes-Gordillo, K.; Shah, R.; Arellanes-Robledo, J.; Hernandez-Nazara, Z.; Rincon-Sanchez, A.R.; Inagaki, Y.; Rojkind, M.; Lakshman, M.R. Mechanisms of action of acetaldehyde in the up-regulation of the human alpha2(I) collagen gene in hepatic stellate cells: Key roles of Ski, SMAD3, SMAD4, and SMAD7. Am. J. Pathol. 2014, 184, 1458–1467. [Google Scholar] [CrossRef] [Green Version]
- Kharbanda, K.K.; Todero, S.L.; Shubert, K.A.; Sorrell, M.F.; Tuma, D.J. Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol 2001, 25, 123–128. [Google Scholar] [CrossRef]
- Sutherland, E.W.; Rall, T.W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958, 232, 1077–1091. [Google Scholar]
- McKnight, G.S. Cyclic AMP second messenger systems. Curr. Opin. Cell Biol. 1991, 3, 213–217. [Google Scholar] [CrossRef]
- Braun, T.; Dods, R.F. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc. Natl. Acad. Sci. USA 1975, 72, 1097–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.; Meili, D.; Salathe, M. Soluble adenylyl cyclase in health and disease. Biochim. Biophys. Acta 2014, 1842, 2584–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steegborn, C. Structure, mechanism, and regulation of soluble adenylyl cyclases—Similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta 2014, 1842, 2535–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Taylor, S.S.; Knighton, D.R.; Zheng, J.; Ten Eyck, L.F.; Sowadski, J.M. Structural framework for the protein kinase family. Annu. Rev. Cell Biol. 1992, 8, 429–462. [Google Scholar] [CrossRef]
- Tasken, K.; Skalhegg, B.S.; Tasken, K.A.; Solberg, R.; Knutsen, H.K.; Levy, F.O.; Sandberg, M.; Orstavik, S.; Larsen, T.; Johansen, A.K.; et al. Structure, function, and regulation of human cAMP-dependent protein kinases. Adv. Second Messenger Phosphoprot. Res. 1997, 31, 191–204. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. Transcription factors responsive to cAMP. Annu. Rev. Cell Dev. Biol. 1995, 11, 355–377. [Google Scholar] [CrossRef]
- Robichaux, W.G., 3rd; Cheng, X. Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Breitkopf-Heinlein, K.; Li, Y.; Zhang, J.; Avila, D.V.; Dooley, S.; McClain, C.J. Rolipram attenuates bile duct ligation-induced liver injury in rats: A potential pathogenic role of PDE4. J. Pharmacol. Exp. Ther. 2013, 347, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Talha, S.; Geny, B. Cyclic nucleotide phosphodiesterases: New targets in the metabolic syndrome? Pharmacol. Ther. 2020, 208, 107475. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cong, L.N.; Stenson Holst, L.; Wang, L.M.; Rahn Landstrom, T.; Pierce, J.H.; Quon, M.J.; Degerman, E.; Manganiello, V.C. Cyclic nucleotide phosphodiesterase 3B is a downstream target of protein kinase B and may be involved in regulation of effects of protein kinase B on thymidine incorporation in FDCP2 cells. J. Immunol. 2000, 164, 4678–4688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, T.B.; Agarwal, S.R.; Harvey, R.D.; Ostrom, R.S. cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes. Mol. Pharmacol. 2018, 93, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessauer, C.W. Adenylyl cyclase—A-kinase anchoring protein complexes: The next dimension in cAMP signaling. Mol. Pharmacol. 2009, 76, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariga, M.; Neitzert, B.; Nakae, S.; Mottin, G.; Bertrand, C.; Pruniaux, M.P.; Jin, S.L.; Conti, M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J. Immunol. 2004, 173, 7531–7538. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.L.; Conti, M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc. Natl. Acad. Sci. USA 2002, 99, 7628–7633. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.L.; Lan, L.; Zoudilova, M.; Conti, M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J. Immunol. 2005, 175, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Mehats, C.; Jin, S.L.; Wahlstrom, J.; Law, E.; Umetsu, D.T.; Conti, M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003, 17, 1831–1841. [Google Scholar] [CrossRef]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell Signal 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Farokhnia, M.; Browning, B.D.; Leggio, L. Prospects for pharmacotherapies to treat alcohol use disorder: An update on recent human studies. Curr. Opin. Psychiatry 2019, 32, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Link, J.; Dennis, L.E.; McCready, H.; Huckans, M.; Hoffman, W.F.; Loftis, J.M. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol. Biochem. Behav. 2019, 179, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Briones, M.S.; Heinzerling, K.G.; Kalmin, M.M.; Shoptaw, S.J. Ibudilast attenuates peripheral inflammatory effects of methamphetamine in patients with methamphetamine use disorder. Drug Alcohol Depend. 2020, 206, 107776. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.C.; Friedman, L.S.; McClain, C.J. Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin. Gastroenterol. Hepatol. 2017, 15, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Khera, R.; Allen, A.M.; Murad, M.H.; Loomba, R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology 2015, 62, 1417–1432. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Chandar, A.K.; Bongiorno, C.M.; Singal, A.K.; Atkinson, S.R.; Thursz, M.R.; Loomba, R.; Shah, V.H. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015, 149, 958–970.e12. [Google Scholar] [CrossRef]
- Zein, C.O.; Lopez, R.; Fu, X.; Kirwan, J.P.; Yerian, L.M.; McCullough, A.J.; Hazen, S.L.; Feldstein, A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology 2012, 56, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Zein, C.O.; Yerian, L.M.; Gogate, P.; Lopez, R.; Kirwan, J.P.; Feldstein, A.E.; McCullough, A.J. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology 2011, 54, 1610–1619. [Google Scholar] [CrossRef]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef]
- Diehl, A.M.; Yang, S.Q.; Cote, P.; Wand, G.S. Chronic ethanol consumption disturbs G-protein expression and inhibits cyclic AMP-dependent signaling in regenerating rat liver. Hepatology 1992, 16, 1212–1219. [Google Scholar]
- Lu, C.; Xia, J.; Zhou, Y.; Lu, X.; Zhang, L.; Gou, M.; Li, L.; Zhang, X.; Ji, H.; Zhu, K.; et al. Loss of Gsalpha impairs liver regeneration through a defect in the crosstalk between cAMP and growth factor signaling. J. Hepatol. 2016, 64, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E. Role of adenosine A1 receptors in inhibition of receptor-stimulated cyclic AMP production by ethanol in hepatocytes. Biochem. Pharmacol. 1994, 48, 2091–2096. [Google Scholar] [CrossRef]
- Nagy, L.E.; DeSilva, S.E. Ethanol increases receptor-dependent cyclic AMP production in cultured hepatocytes by decreasing G(i)-mediated inhibition. Biochem. J. 1992, 286 Pt 3, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, E.; Johansson, I.; Ingelman-Sundberg, M. Substrate-, hormone-, and cAMP-regulated cytochrome P450 degradation. Proc. Natl. Acad. Sci. USA 1990, 87, 3225–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menez, J.F.; Machu, T.K.; Song, B.J.; Browning, M.D.; Deitrich, R.A. Phosphorylation of cytochrome P4502E1 (CYP2E1) by calmodulin dependent protein kinase, protein kinase C and cAMP dependent protein kinase. Alcohol Alcohol. 1993, 28, 445–451. [Google Scholar] [PubMed]
- Gouillon, Z.Q.; Miyamoto, K.; Donohue, T.M.; Wan, Y.J.; French, B.A.; Nagao, Y.; Fu, P.; Reitz, R.C.; Hagbjork, A.; Yap, C.; et al. Role of CYP2E1 in the pathogenesis of alcoholic liver disease: Modifications by cAMP and ubiquitin-proteasome pathway. Front. Biosci. 1999, 4, A16–A25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Borea, P.A.; Varani, K.; Wilder, T.; Yee, H.; Chiriboga, L.; Blackburn, M.R.; Azzena, G.; Resta, G.; Cronstein, B.N. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J. Clin. Investig. 2009, 119, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [Green Version]
- Aroor, A.R.; Jackson, D.E.; Shukla, S.D. Dysregulated phosphorylation and nuclear translocation of cyclic AMP response element binding protein (CREB) in rat liver after chronic ethanol binge. Eur. J. Pharmacol. 2012, 679, 101–108. [Google Scholar] [CrossRef]
- Louet, J.F.; Hayhurst, G.; Gonzalez, F.J.; Girard, J.; Decaux, J.F. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB). J. Biol. Chem. 2002, 277, 37991–38000. [Google Scholar] [CrossRef] [Green Version]
- Avila, D.V.; Barker, D.F.; Zhang, J.; McClain, C.J.; Barve, S.; Gobejishvili, L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J. Pathol. 2016, 240, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, W.E.; Wahlang, B.; Wang, Y.; Zhang, J.; Vadhanam, M.V.; Joshi-Barve, S.; Bauer, P.; Cannon, R.; Ahmadi, A.R.; Sun, Z.; et al. Phosphodiesterase 4 Inhibition as a Therapeutic Target for Alcoholic Liver Disease: From Bedside to Bench. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Gobejishvili, L.; Barve, S.; Joshi-Barve, S.; Uriarte, S.; Song, Z.; McClain, C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: Relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G681–G688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, M.C.; Caton, P.W.; Holness, M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010, 204, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Attia, R.R.; Connaughton, S.; Niesen, M.I.; Ness, G.C.; Elam, M.B.; Hori, R.T.; Cook, G.A.; Park, E.A. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol. Cell. Endocrinol. 2010, 325, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazennec, G.; Canaple, L.; Saugy, D.; Wahli, W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase an activators. Mol. Endocrinol. 2000, 14, 1962–1975. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Gao, R.; Li, H.; Dunn, T.; Wu, P.; Smith, R.G.; Sarkar, P.S.; Fang, X. Ethanol suppresses PGC-1alpha expression by interfering with the cAMP-CREB pathway in neuronal cells. PLoS ONE 2014, 9, e104247. [Google Scholar] [CrossRef]
- Chaung, W.W.; Jacob, A.; Ji, Y.; Wang, P. Suppression of PGC-1alpha by Ethanol: Implications of Its Role in Alcohol Induced Liver Injury. Int. J. Clin. Exp. Med. 2008, 1, 161–170. [Google Scholar]
- Lieber, C.S.; Leo, M.A.; Wang, X.; Decarli, L.M. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem. Biophys. Res. Commun. 2008, 370, 44–48. [Google Scholar] [CrossRef]
- Kang, X.; Zhong, W.; Liu, J.; Song, Z.; McClain, C.J.; Kang, Y.J.; Zhou, Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology 2009, 50, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, M.; Crabb, D.W. Recent advances in alcoholic liver disease II. Minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schott, M.B.; Rasineni, K.; Weller, S.G.; Schulze, R.J.; Sletten, A.C.; Casey, C.A.; McNiven, M.A. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J. Biol. Chem. 2017, 292, 11815–11828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Xu, X.; Sun, C.; Yu, Z. Protective effects of cilostazol on ethanol-induced damage in primary cultured hepatocytes. Cell Stress Chaperones 2018, 23, 203–211. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shu, M.S.; Kim, J.Y.; Kim, Y.H.; Sim, K.H.; Sung, W.J.; Eun, J.R. Cilostazol protects hepatocytes against alcohol-induced apoptosis via activation of AMPK pathway. PLoS ONE 2019, 14, e0211415. [Google Scholar] [CrossRef]
- Mandal, S.; Nelson, V.K.; Mukhopadhyay, S.; Bandhopadhyay, S.; Maganti, L.; Ghoshal, N.; Sen, G.; Biswas, T. 14-Deoxyandrographolide targets adenylate cyclase and prevents ethanol-induced liver injury through constitutive NOS dependent reduced redox signaling in rats. Food Chem. Toxicol. 2013, 59, 236–248. [Google Scholar] [CrossRef]
- Braun, W.; Ishizuka, M.; Winchurch, R.; Webb, D. On the role of cyclic AMP in immune responses. Ann. N. Y. Acad. Sci. 1971, 185, 417–422. [Google Scholar] [CrossRef]
- Barlas, N.; Mutchnick, M.G.; Grant, G.J.; Trainin, N. The effect of thymic humoral factor on intracellular lymphocyte cyclic AMP in alcoholic liver disease. Thymus 1983, 5, 433–437. [Google Scholar]
- Li, S.; Tan, H.Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent Insights into the Role of Immune Cells in Alcoholic Liver Disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Joshi-Barve, S.; McClain, C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: Relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G718–G724. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Ghare, S.; Khan, R.; Cambon, A.; Barker, D.F.; Barve, S.; McClain, C.; Hill, D. Misoprostol modulates cytokine expression through a cAMP pathway: Potential therapeutic implication for liver disease. Clin. Immunol. 2015, 161, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Sanchez, I.; Dunkel, Y.; Roh, Y.S.; Mittal, Y.; De Minicis, S.; Muranyi, A.; Singh, S.; Shanmugam, K.; Aroonsakool, N.; Murray, F.; et al. GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis. Nat. Commun. 2014, 5, 4451. [Google Scholar] [CrossRef] [Green Version]
- Houglum, K.; Lee, K.S.; Chojkier, M. Proliferation of hepatic stellate cells is inhibited by phosphorylation of CREB on serine 133. J. Clin. Investig. 1997, 99, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Kawada, N.; Kuroki, T.; Kobayashi, K.; Inoue, M.; Kaneda, K. Inhibition of myofibroblastic transformation of cultured rat hepatic stellate cells by methylxanthines and dibutyryl cAMP. Dig. Dis. Sci. 1996, 41, 1022–1029. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Q.; Xu, K. CREB family: A significant role in liver fibrosis. Biochimie 2019, 163, 94–100. [Google Scholar] [CrossRef]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The Role of Cyclic AMP Signaling in Cardiac Fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- El Awdan, S.A.; Abdel Rahman, R.F.; Ibrahim, H.M.; Hegazy, R.R.; El Marasy, S.A.; Badawi, M.; Arbid, M.S. Regression of fibrosis by cilostazol in a rat model of thioacetamide-induced liver fibrosis: Up regulation of hepatic cAMP, and modulation of inflammatory, oxidative stress and apoptotic biomarkers. PLoS ONE 2019, 14, e0216301. [Google Scholar] [CrossRef]
- Abdel Kawy, H.S. Cilostazol attenuates cholestatic liver injury and its complications in common bile duct ligated rats. Eur. J. Pharmacol. 2015, 752, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Zhang, Y.; Yang, Z. Cilostazol protects rats against alcohol-induced hepatic fibrosis via suppression of TGF-beta1/CTGF activation and the cAMP/Epac1 pathway. Exp. Ther. Med. 2019, 17, 2381–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getz, M.; Rangamani, P.; Ghosh, P. Regulating cellular cyclic adenosine monophosphate: “Sources,” “sinks,” and now, “tunable valves”. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, e1490. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.; Beljaars, L.; Post, E.; Lotersztajn, S.; Reker-Smit, C.; Han, B.; Munoz-Llancao, P.; Schmidt, M.; Poelstra, K. Upregulation of Epac-1 in Hepatic Stellate Cells by Prostaglandin E2 in Liver Fibrosis Is Associated with Reduced Fibrogenesis. J. Pharmacol. Exp. Ther. 2017, 363, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, H.; Nakamuta, M.; Tada, S.; Sugimoto, R.; Enjoji, M.; Nawata, H. A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J. Hepatol. 2000, 32, 762–770. [Google Scholar] [CrossRef]
- van Beuge, M.M.; Prakash, J.; Lacombe, M.; Gosens, R.; Post, E.; Reker-Smit, C.; Beljaars, L.; Poelstra, K. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J. Pharmacol. Exp. Ther. 2011, 337, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Hu, D.; Yu, H.; Xu, W.; Fu, R. Hypoxiainducible factor 1alpha and ROCK1 regulate proliferation and collagen synthesis in hepatic stellate cells under hypoxia. Mol. Med. Rep. 2018, 18, 3997–4003. [Google Scholar] [CrossRef] [Green Version]
- Gortzen, J.; Schierwagen, R.; Bierwolf, J.; Klein, S.; Uschner, F.E.; van der Ven, P.F.; Furst, D.O.; Strassburg, C.P.; Laleman, W.; Pollok, J.M.; et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front. Physiol. 2015, 6, 359. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yang, Y.; Mei, C.; Dong, P.; Mu, S.; Wu, H.; Zhou, Y.; Zheng, Y.; Guo, F.; Yang, J.Q. Inhibition of Rho-Kinase Downregulates Th17 Cells and Ameliorates Hepatic Fibrosis by Schistosoma japonicum Infection. Cells 2019, 8, 1262. [Google Scholar] [CrossRef] [Green Version]
- van Dijk, F.; Teekamp, N.; Post, E.; Schuppan, D.; Kim, Y.O.; Zuidema, J.; Steendam, R.; Klose, M.H.M.; Meier-Menches, S.M.; Casini, A.; et al. The antifibrotic potential of a sustained release formulation of a PDGFbeta-receptor targeted rho kinase inhibitor. J. Control. Release 2019, 296, 250–257. [Google Scholar] [CrossRef]
- Okimoto, S.; Kuroda, S.; Tashiro, H.; Kobayashi, T.; Taogoshi, T.; Matsuo, H.; Ohdan, H. Vitamin A-coupled liposomal Rho-kinase inhibitor ameliorates liver fibrosis without systemic adverse effects. Hepatol. Res. 2019, 49, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Fausther, M. Extracellular adenosine: A critical signal in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G12–G19. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Irenius, E.; Kull, B.; Schulte, G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001, 61, 443–448. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, P. Adenosine A2B Receptor: From Cell Biology to Human Diseases. Front. Chem. 2016, 4, 37. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Lv, X.; Wang, Q.; Zhao, H.; Yang, F.; Yang, Y.; Li, J. Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie 2015, 115, 59–70. [Google Scholar] [CrossRef]
- Wang, H.; Guan, W.; Yang, W.; Wang, Q.; Zhao, H.; Yang, F.; Lv, X.; Li, J. Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS ONE 2014, 9, e92482. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Saito, S.Y.; Nishiyama, R.; Nakamura, M.; Todoroki, K.; Toyo’oka, T.; Ishikawa, T. Caffeine Suppresses the Activation of Hepatic Stellate Cells cAMP-Independently by Antagonizing Adenosine Receptors. Biol. Pharm. Bull. 2017, 40, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.K.; Mehal, W.Z. Activation of adenosine receptor A2A increases HSC proliferation and inhibits death and senescence by down-regulation of p53 and Rb. Front. Pharmacol. 2014, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.S.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.F.; Schwarzschild, M.A.; et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Perez-Carreon, J.I.; Martinez-Perez, L.; Loredo, M.L.; Yanez-Maldonado, L.; Velasco-Loyden, G.; Vidrio-Gomez, S.; Ramirez-Salcedo, J.; Hernandez-Luis, F.; Velazquez-Martinez, I.; Suarez-Cuenca, J.A.; et al. An adenosine derivative compound, IFC305, reverses fibrosis and alters gene expression in a pre-established CCl(4)-induced rat cirrhosis. Int. J. Biochem. Cell Biol. 2010, 42, 287–296. [Google Scholar] [CrossRef]
- Hernandez-Munoz, R.; Diaz-Munoz, M.; Suarez, J.; Chagoya de Sanchez, V. Adenosine partially prevents cirrhosis induced by carbon tetrachloride in rats. Hepatology 1990, 12, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Munoz, R.; Diaz-Munoz, M.; Lopez, V.; Lopez-Barrera, F.; Yanez, L.; Vidrio, S.; Aranda-Fraustro, A.; Chagoya de Sanchez, V. Balance between oxidative damage and proliferative potential in an experimental rat model of CCl4-induced cirrhosis: Protective role of adenosine administration. Hepatology 1997, 26, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, R.; Fischer, R.; Haussinger, D. Regulation of endothelin-A receptor sensitivity by cyclic adenosine monophosphate in rat hepatic stellate cells. Hepatology 2002, 36, 861–873. [Google Scholar] [CrossRef]
- Yaya, I.; Marcellin, F.; Costa, M.; Morlat, P.; Protopopescu, C.; Pialoux, G.; Santos, M.E.; Wittkop, L.; Esterle, L.; Gervais, A.; et al. Impact of Alcohol and Coffee Intake on the Risk of Advanced Liver Fibrosis: A Longitudinal Analysis in HIV-HCV Coinfected Patients (ANRS HEPAVIH CO-13 Cohort). Nutrients 2018, 10, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomone, F.; Galvano, F.; Li Volti, G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Dranoff, J.A. Coffee Consumption and Prevention of Cirrhosis: In Support of the Caffeine Hypothesis. Gene Expr. 2018, 18, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Asp. Med. 2017, 55, 20–25. [Google Scholar] [CrossRef]
- Montoya, G.A.; Bakuradze, T.; Eirich, M.; Erk, T.; Baum, M.; Habermeyer, M.; Eisenbrand, G.; Richling, E. Modulation of 3′,5′-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br. J. Nutr. 2014, 112, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Rohrig, T.; Liesenfeld, D.; Richling, E. Identification of a Phosphodiesterase-Inhibiting Fraction from Roasted Coffee (Coffea arabica) through Activity-Guided Fractionation. J. Agric. Food Chem. 2017, 65, 3792–3800. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.G.; Matricon, P.; Eddy, M.T.; Carlsson, J. A2A adenosine receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Cui, W.Q.; Wang, S.T.; Pan, D.; Chang, B.; Sang, L.X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, F.; Wu, X.; Lv, X.; Li, J. EPAC activation inhibits acetaldehyde-induced activation and proliferation of hepatic stellate cell via Rap1. Can. J. Physiol. Pharmacol. 2016, 94, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwel, P.; Dominguez-Rosales, J.A.; Mavi, G.; Rivas-Estilla, A.M.; Rojkind, M. Hydrogen peroxide: A link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology 2000, 31, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Iraburu, M.J.; Dominguez-Rosales, J.A.; Fontana, L.; Auster, A.; Garcia-Trevijano, E.R.; Covarrubias-Pinedo, A.; Rivas-Estilla, A.M.; Greenwel, P.; Rojkind, M. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology 2000, 31, 1086–1093. [Google Scholar] [CrossRef]
- Peleli, M.; Fredholm, B.B.; Sobrevia, L.; Carlstrom, M. Pharmacological targeting of adenosine receptor signaling. Mol. Asp. Med. 2017, 55, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Huang, G.; Ma, L.; Dong, L.; Chen, S.; Shen, X.; Zhang, S.; Xue, R.; Sun, D.; Zhang, S. Smurf2, an E3 ubiquitin ligase, interacts with PDE4B and attenuates liver fibrosis through miR-132 mediated CTGF inhibition. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 297–308. [Google Scholar] [CrossRef]
| Hepatocytes | |
| Liver regeneration | Chronic alcohol ↑ Gαi and ↓ Gαs ➔ ↓ cAMP ➔ ↓ CDK2 and cyclin E ➔ ↓ Cell cycle G1 to S transition ➔ impaired liver regeneration |
| Ethanol metabolism | dbcAMP decreased ethanol-mediated increase in CYP2E1 |
| Cell Proliferation | dbcAMP increased PCNA antibody staining in parenchymal and non-parenchymal liver cells ➔ ↑ Cell proliferation and regeneration |
| Fatty acid synthesis | A1 agonist ➔ (? ↓ AC, cAMP) ➔ ↑ SREBP1 ➔ ↑ Fatty acid synthesis |
| Fatty acid oxidation | - A2B agonist ➔(? ↑ AC, cAMP) ➔ ↓ CPT1A and AMPK ➔ ↓ Fatty acid oxidation - ↑ PDE4B ➔ ↓ cAMP ➔ ↓ PKA/CREB phosphorylation ➔ ↓ CPT1A |
| Lipid droplet lipolysis | Ethanol decreased β-adrenergic-inducible PKA activation and lipid droplet lipolysis |
| Immune cells | |
| Immune cells response | - Chronic ethanol ➔ ↑ PDE4 ➔ ↓ cAMP in monocytes and macrophages/Kupffer cells - ↑ PDE4 ➔ ↓ cAMP ➔ ↑ TNF production in response to endotoxin - Roflumilast (PDE4 inhibitor) ➔ ↓ IL1β and TNF in AH patient blood ex vivo |
| Macrophage M1 to M2 polarization | ↓ Beta Hydroxybutyrate (BHB) ➔ ↓ Hcar2/cAMP-mediated M1 to M2 polarization |
| Stellate cells | |
| HSC activation | - ↓ pCREB level ↑ PDE4 ↑ GIV/Girdin ↑ Gi |
| Acetaldehyde-induced HSC activation | - ↑ A2A receptor activation ➔ ↑ HSCs/fibrosis - Adenosine ➔ ↓ liver fibrosis - Adenosine ➔ ↑ cAMP/PKA ➔ ↓ ET-A receptor ➔ ↓ HSC contractility - Caffeine ➔ ↓ A1, A2a, A2b ➔ ↓ Liver fibrosis and acetaldehyde-induced HSC activation |
| HSC proliferation and motility | - Acetaldehyde ➔ ↓ EPAC1 and ↑ EPAC2 ➔ ↑ HSC proliferation, ↑ αSMA, ↑ Collagen |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnagdy, M.; Barve, S.; McClain, C.; Gobejishvili, L. cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease. Biomolecules 2020, 10, 1433. https://doi.org/10.3390/biom10101433
Elnagdy M, Barve S, McClain C, Gobejishvili L. cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease. Biomolecules. 2020; 10(10):1433. https://doi.org/10.3390/biom10101433
Chicago/Turabian StyleElnagdy, Mohamed, Shirish Barve, Craig McClain, and Leila Gobejishvili. 2020. "cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease" Biomolecules 10, no. 10: 1433. https://doi.org/10.3390/biom10101433
APA StyleElnagdy, M., Barve, S., McClain, C., & Gobejishvili, L. (2020). cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease. Biomolecules, 10(10), 1433. https://doi.org/10.3390/biom10101433






