Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of High-Purity Gyp LXXV and Ginsenoside Rg3 Compounds
2.3. Cell Culture and Viability Assay
2.4. ELISA for IL-1β-Detection
2.5. RNA Isolation and Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.6. Animal Preparation
2.7. Animal NASH Model
2.8. Blood Chemistry Assay
2.9. Immunohistochemistry (IHC)
2.10. Oil Red O Staining
2.11. Western Blot Analysis
2.12. Hepatic Triglyceride (TG) Assay
2.13. Statistics
3. Results and Discussion
3.1. Characterization of High-Purity Gyp LXXV and Ginsenoside Rg3 Compounds
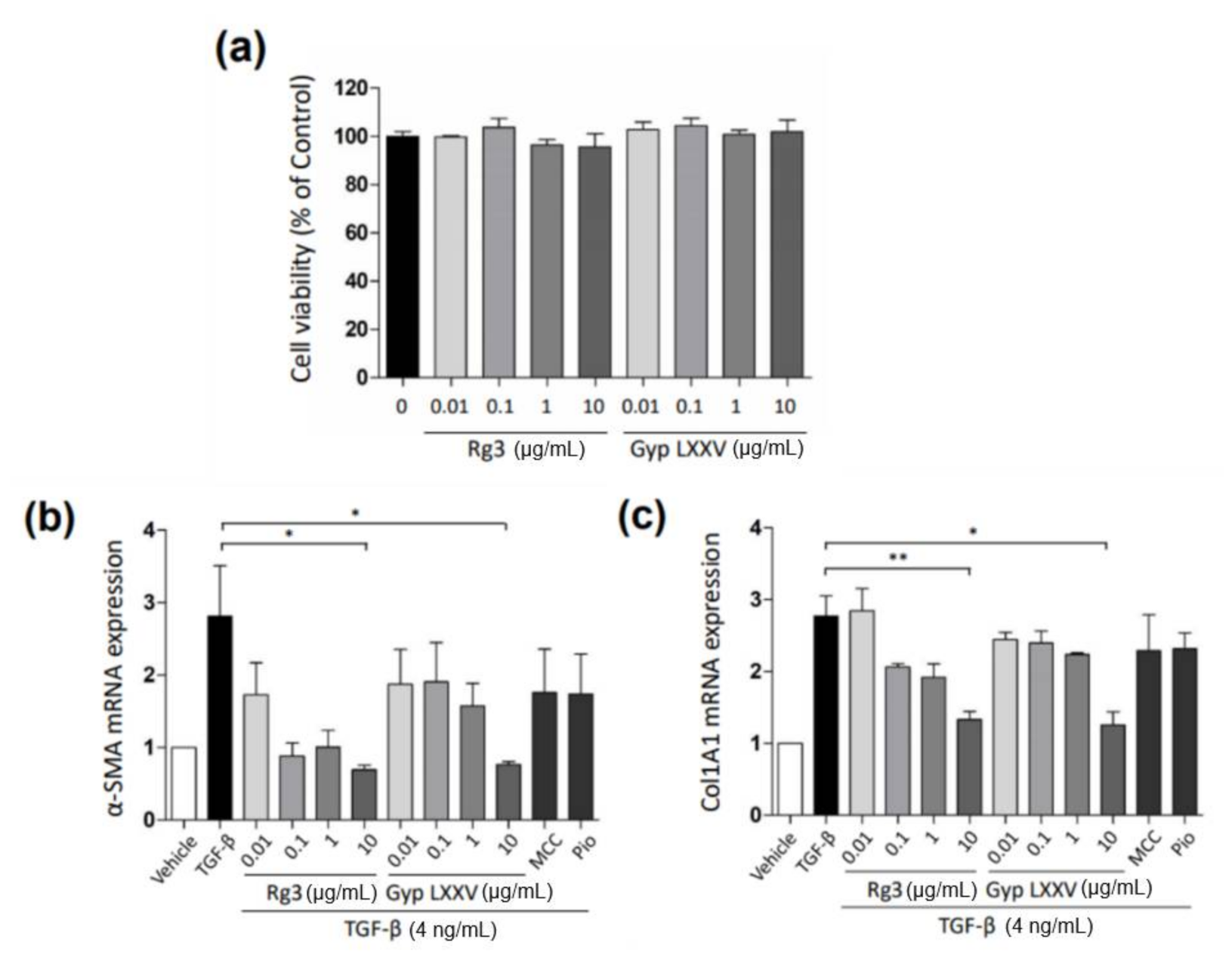
3.2. Gyp LXXV Inhibits the TGF-β-Induced Activation of Hepatic Stellate Cells
3.3. Gyp LXXV Inhibits the TGF-β-Induced Activation of Hepatocytes (HepG2 Cells)
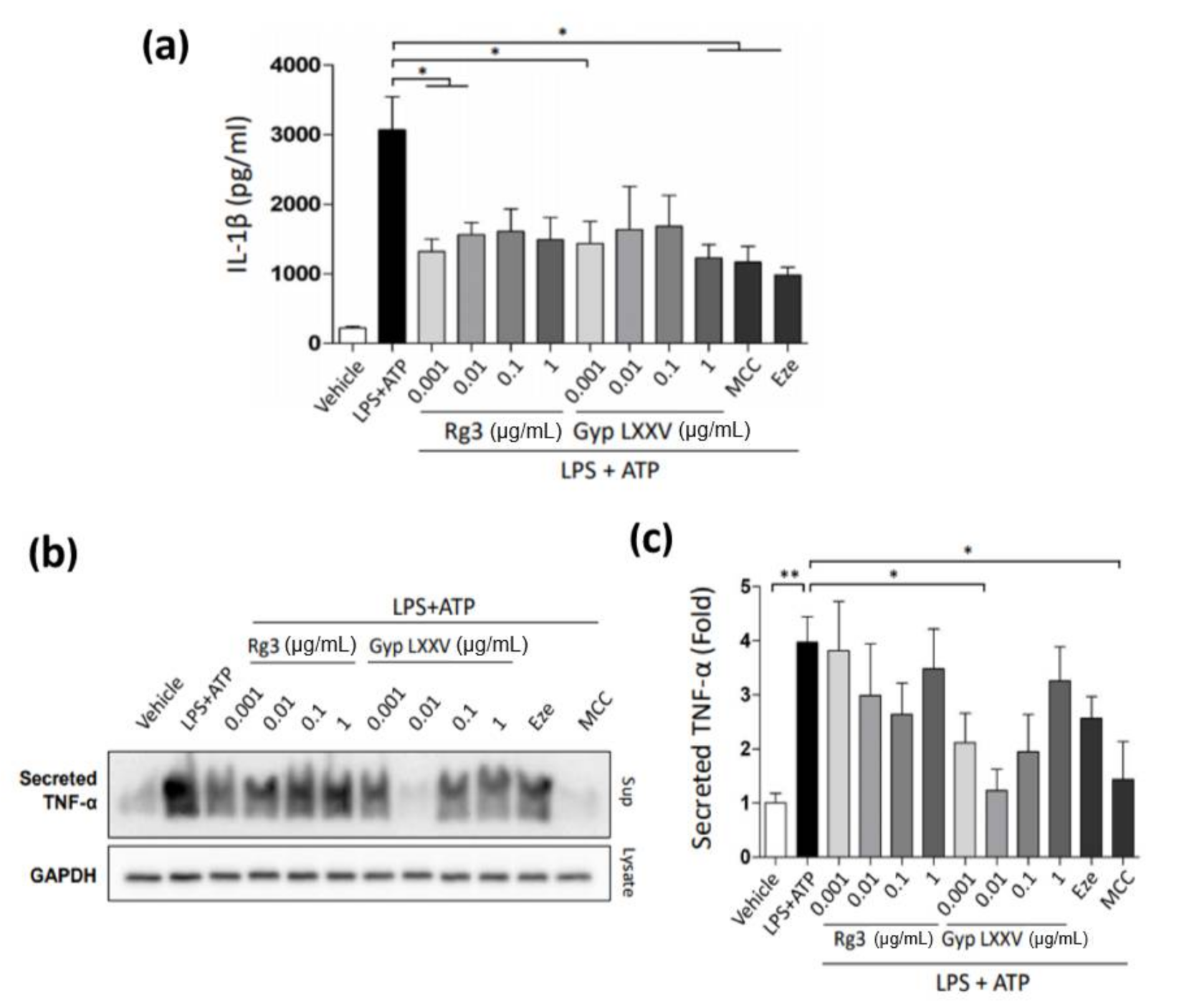
3.4. Inflammasome Activity in Hepatic Macrophages (THP-1 Cells)
3.5. Effects of Gyp LXXV on Inflammation and Endoplasmic Reticulum (ER) Stress in Hepatocytes (HepG2 Cells)
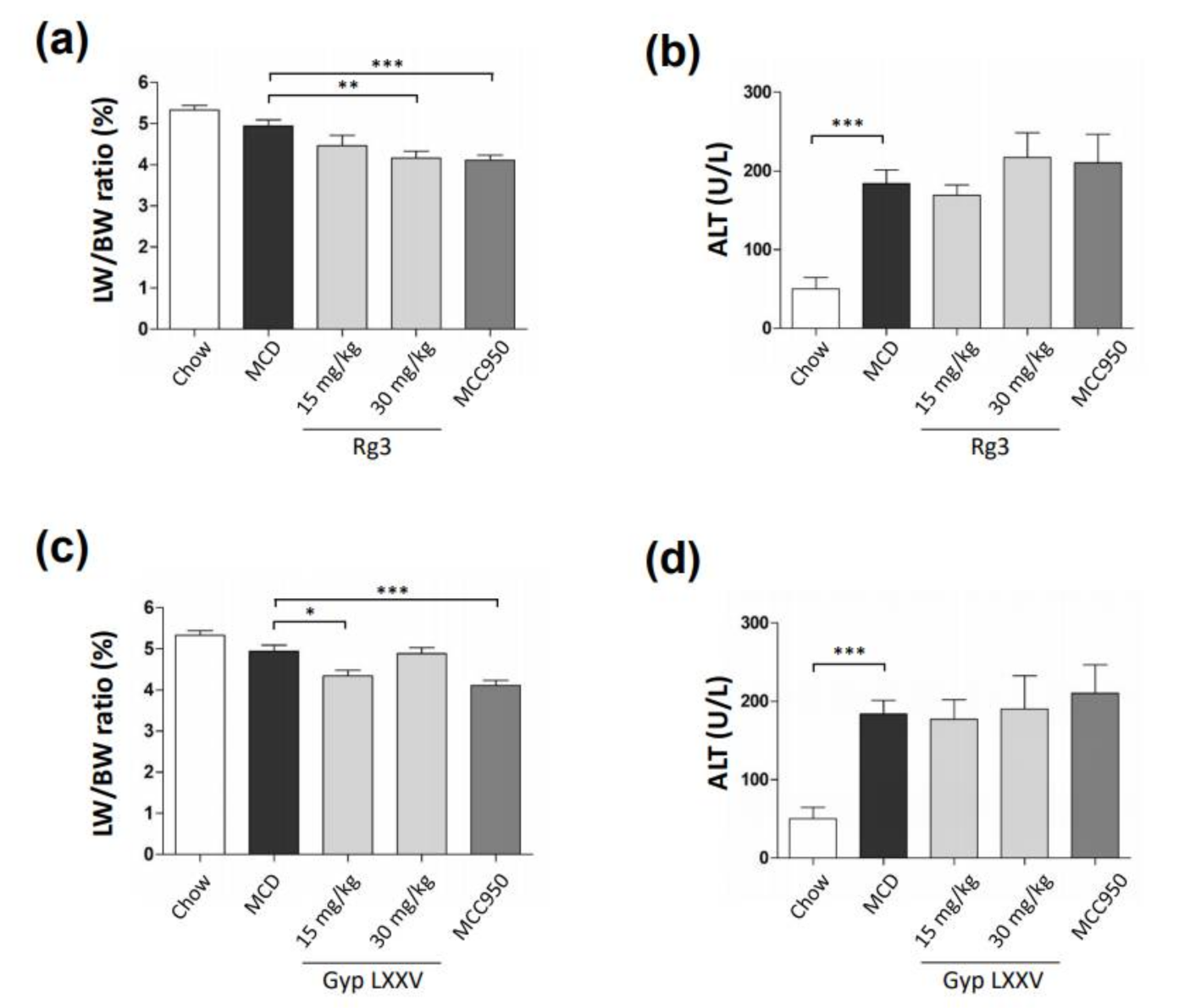
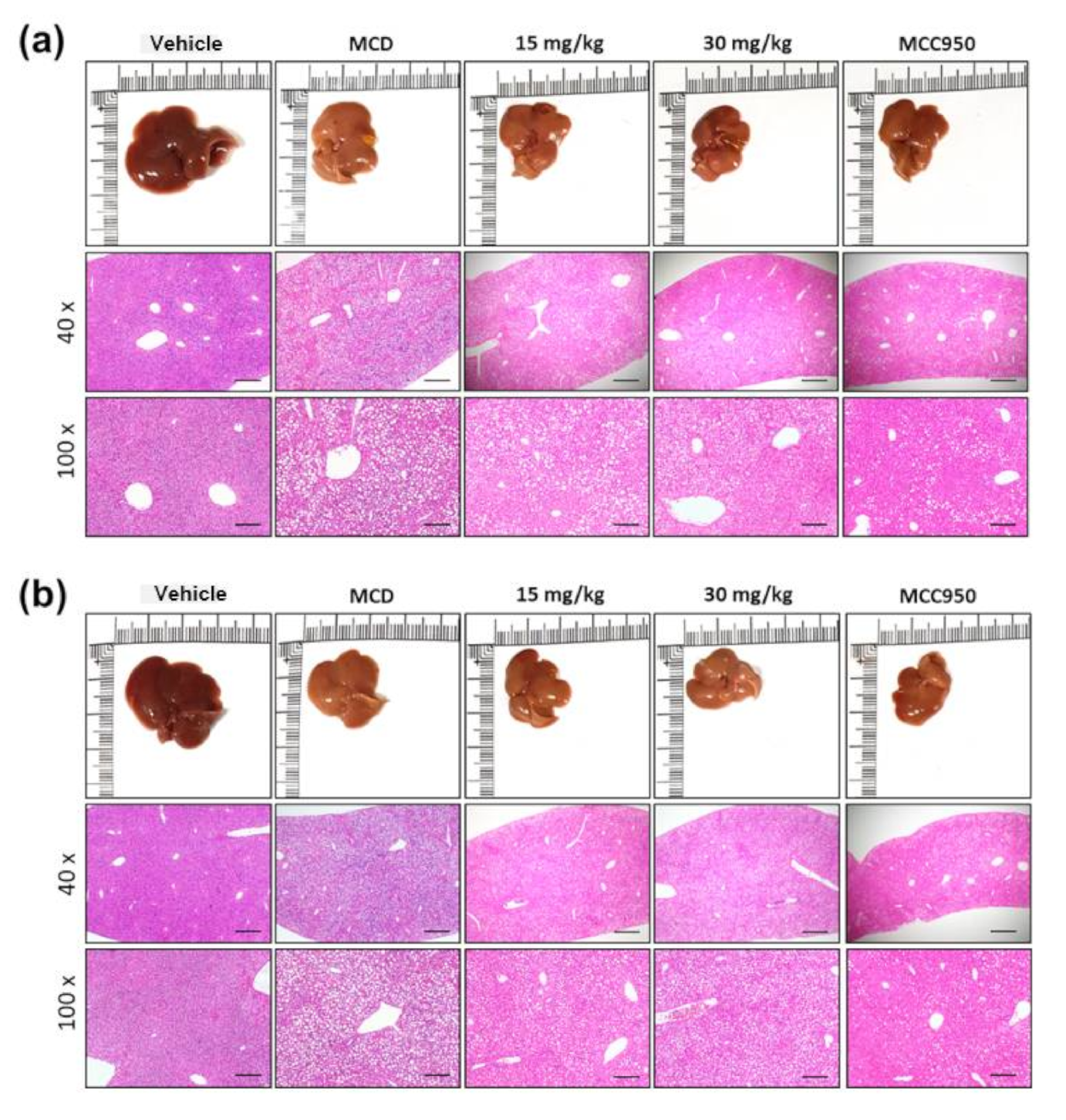
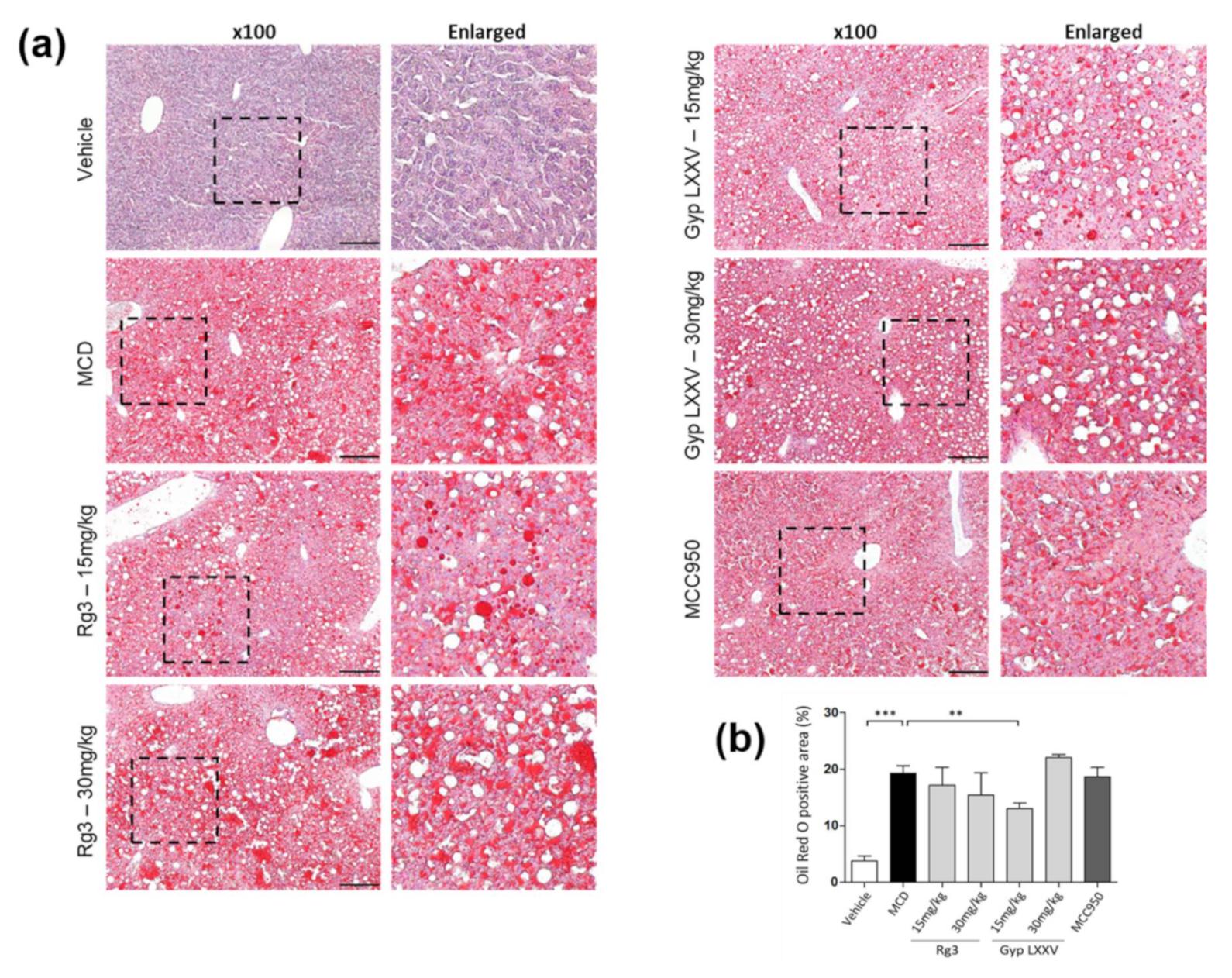
3.6. Effect of Gyp LXXV on NASH in Methionine- and Choline-Deficient (MCD) Diet-Induced Mice
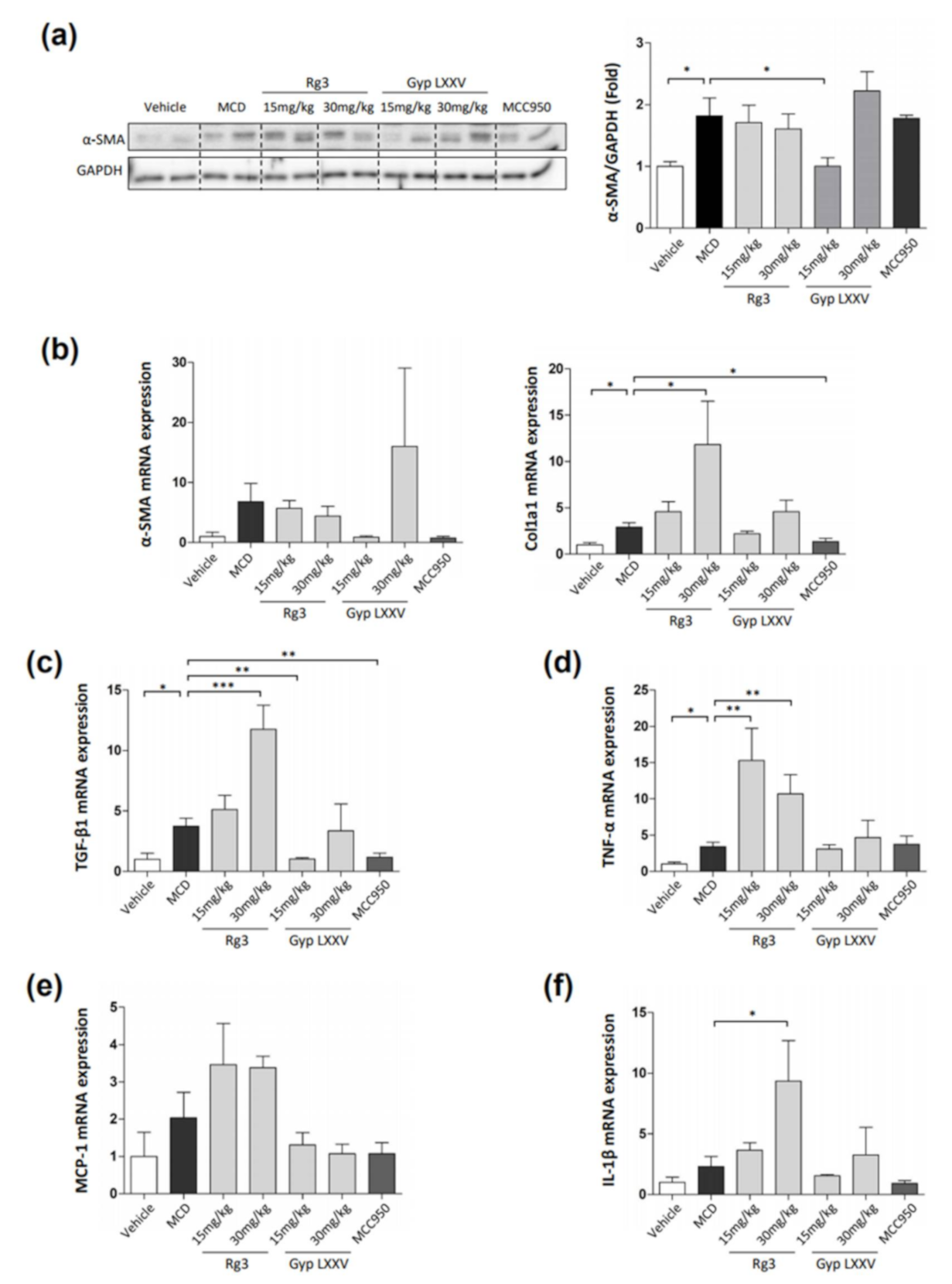
3.7. Effect of Gyp LXXV in Hepatic Fibrosis in MCD Diet-Induced Mice
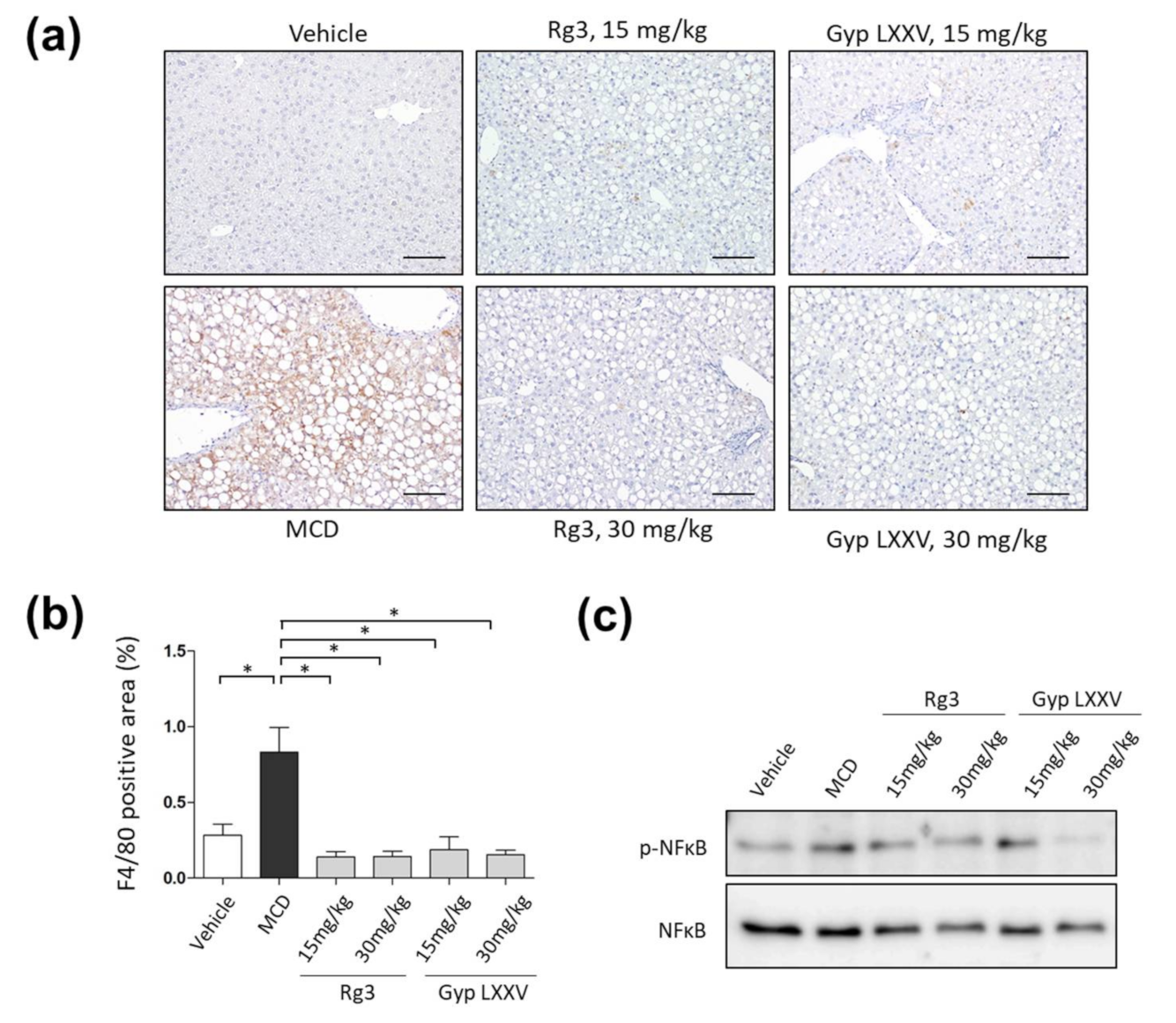
3.8. Effect of Gyp LXXV on the Activation of Liver Macrophages in MCD Diet Models
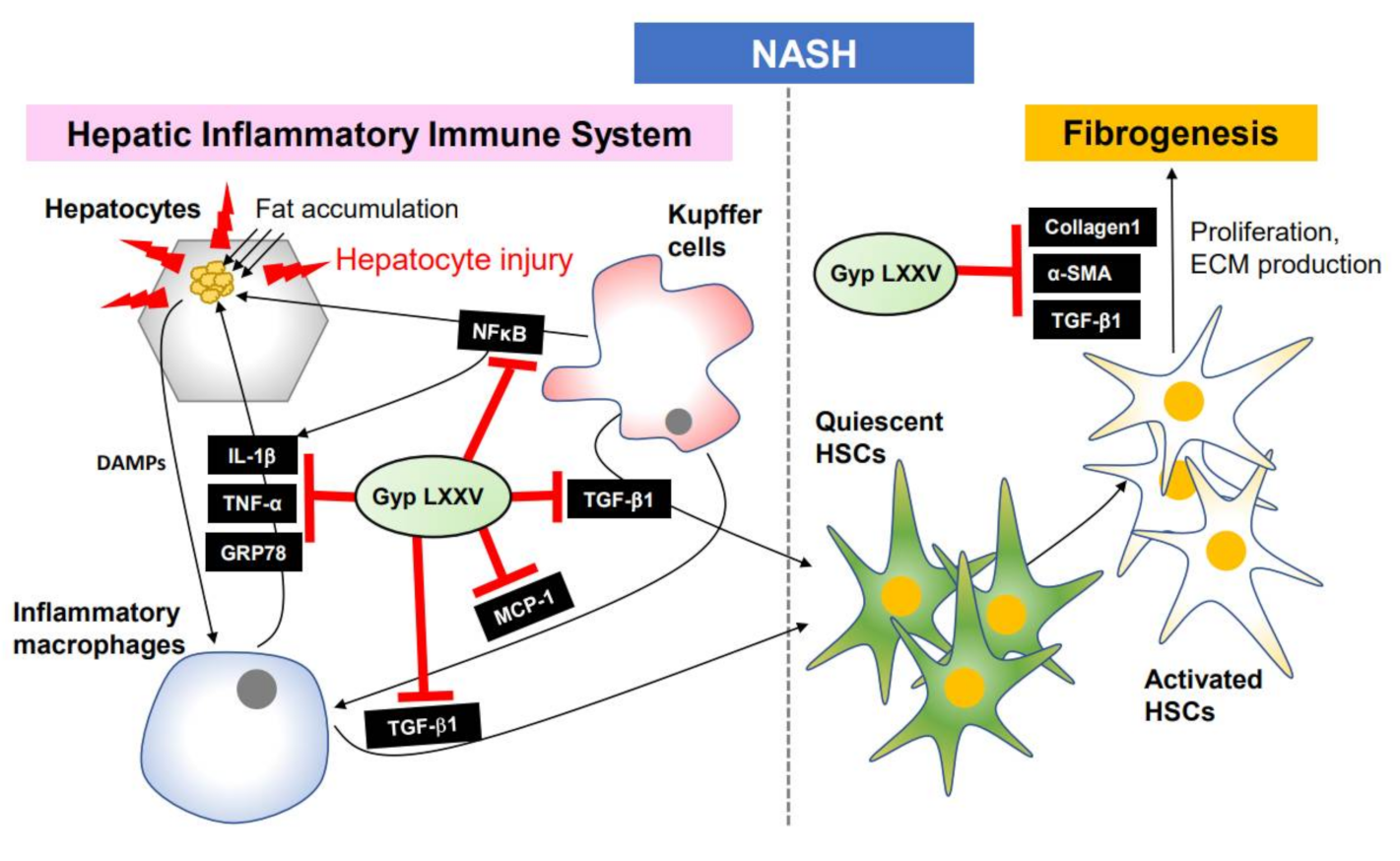
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef] [Green Version]
- Ignat, S.R.; Dinescu, S.; Hermenean, A.; Costache, M. Cellular Interplay as a Consequence of Inflammatory Signals Leading to Liver Fibrosis Development. Cells 2020, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Veal, J.; Mcbride, C.; Lazic, M.; Povero, D.; Ambrus, G.; Santini, A.; Stansfield, R.; Trzoss, L.; Johnson, C.D.; Stafford, J.; et al. Discovery of Inhibitors of NLRP3 inflammasome assembly for the treatment of NASH and liver fibrosis. J. Hepatol. 2018, 68, S347–S348. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can J. Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, M.; Szabo, G. Immune and inflammatory pathways in NASH. Hepatol. Int. 2013, 7 (Suppl. 2), 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Ou, Z.; Cai, C.; Li, P.; Gong, J.; Ruan, X.Z.; He, K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018, 332, 111120. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, P.; Nguyen, L.; French, B.; Tillman, B.; French, S.W. Different Roles of Epigenetic Regulators and Inflammasome in Hepatocellular Carcinoma Tumorigenesis in Patients with ASH vs. NASH. FASEB J. 2019, 33, 662.67. [Google Scholar]
- Sircana, A.; Paschetta, E.; Saba, F.; Molinaro, F.; Musso, G. Recent Insight into the Role of Fibrosis in Nonalcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1745. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.-J.; Lee, Y.-H.; Cha, B.-S. Causal Relationship of Non-alcoholic Fatty Liver Disease with Obesity and Insulin Resistance. J. Korean Diabetes. 2014, 15, 76–81. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [PubMed]
- Léveillé, M.; Estall, J.L. Mitochondrial Dysfunction in the Transition from NASH to HCC. Metabolites 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving concepts in the pathogenesis of NASH: Beyond steatosis and inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef] [PubMed]
- Wattacheril, J.; Issa, D.; Sanyal, A. Nonalcoholic Steatohepatitis (NASH) and Hepatic Fibrosis: Emerging Therapies. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 649–662. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, H.; Masaki, M.; Inoue, I.; Nakamura, M.; Mezaki, Y.; Saeki, C.; Oikawa, T.; Saruta, M.; Takahashi, H.; Ikegami, M.; et al. Histological and biochemical evaluation of transforming growth factor-β activation and its clinical significance in patients with chronic liver disease. Heliyon 2019, 5, e01231. [Google Scholar] [CrossRef] [Green Version]
- Gawrieh, S.; Chalasani, N. Emerging Treatments for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Glass, O.; Filozof, C.; Noureddin, M.; Berner-Hansen, M.; Schabel, E.; Omokaro, S.O.; Schattenberg, J.M.; Barradas, K.; Miller, V.; Francque, S.; et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: Recommendations from the Liver Forum. J. Hepatol. 2020, 73, 680–693. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kong, G.; Tran, Q.; Kim, C.; Park, J.; Park, J. Relationship Between Ginsenoside Rg3 and Metabolic Syndrome. Front Pharmacol. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Bae, E.A.; Han, M.J.; Kim, D.H. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol. Pharm. Bull. 2005, 28, 1992–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.C.; Jeon, J.Y.; Yang, W.S.; Kim, C.H.; Eom, D.W. Combined amelioration of ginsenoside (Rg1, Rb1, and Rg3)-enriched Korean red ginseng and probiotic lactobacillus on non-alcoholic fatty liver disease. Curr. Pharm. Biotechnol. 2019, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, S.; Yang, C.; Du, R.; Zhao, J.; Li, J.; Xu, Y.; Qin, Y.; Gao, Y.; Huang, W. Ginsenoside Rg1 Ameliorates Palmitic Acid-Induced Hepatic Steatosis and Inflammation in HepG2 Cells via the AMPK/NF-κB Pathway. Int. J. Endocrinol. 2019, 2019, 7514802. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.-J.; Hu, H.-G.; Xing, S.-F.; Gao, Y.-J.; Xu, S.-F.; Piao, X.-L. Metabolic profiling of Gynostemma pentaphyllum extract in rat serum, urine and faeces after oral administration. J. Chromatogr. B 2014, 969, 42–52. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.-L.; Zhou, P.-P.; Meng, X.-H.; Shi, Y.-P. Further new gypenosides from jiaogulan (Gynostemma pentaphyllum). J. Agr. Food Chem. 2017, 65, 5926–5934. [Google Scholar] [CrossRef]
- Park, S.; Ko, E.; Lee, J.H.; Song, Y.; Cui, C.-H.; Hou, J.; Jeon, B.M.; Kim, H.S.; Kim, S.C. Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway. Molecules 2019, 24, 1595. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ying, H.; Hu, A.; Hu, Y.; Li, D. Therapeutic Effect of Gypenosides on Nonalcoholic Steatohepatitis via Regulating Hepatic Lipogenesis and Fatty Acid Oxidation, Biol. Pharm. Bull. 2017, 40, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chen, W.; Yan, C.; Yang, R.; Chen, Q.; Xu, H.; Huang, Y. Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its progression. BioMed. Pharmacother. 2019, 118, 109258. [Google Scholar] [CrossRef]
- He, Q.; Li, J.K.; Li, F.; Li, R.G.; Zhan, G.Q.; Li, G.; Du, W.X.; Tan, H.B. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J. Gastroentero 2015, 21, 2058–2066. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, W.H.; Han, B.-S.; Lee, J.H.; Doo, E.K.; Kim, J.-H. Molecular Drug Discovery of Single Ginsenoside Compounds as a Potent Bruton’s Tyrosine Kinase Inhibitor. Int. J. Mol. Sci. 2020, 21, 3065. [Google Scholar] [CrossRef] [PubMed]
- Mridha, A.R.; Wree, A.; Farrell, G.C. NLRP3 inflammasome blockage reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatology. 2017, 66, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, G.; Lee, M. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibiton. Autophagy 2017, 13, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Xiaomin, S.; He, X.; Gao, Y. Macrophage Phenotype and Function in Liver Disorder. Front. Immunol. 2020, 10, 3112. [Google Scholar]
- Ganz, M.; Cask, T.; Szabo, G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J. Gastoenterol. 2014, 20, 8525–8534. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.W.; Nace, G.W. Histones activate the NLRP3 inflammasome in Kupper cells during sterile inflammatory liver injury. J. Immunol. 2013, 191, 2665–2679. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, M.D.; Canbay, A. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Zamin, I., Jr.; Mattos, A.A.; Mattos, A.Z.; Migon, E.; Soares, E.; Perry, M.L. Model of experimental nonalcoholic steatohepatitis from use of methionine and choline deficient diet. Arq. Gastroenterol. 2009, 46, 69–74. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Oh, J.Y.; Kim, S.H.; Oh, I.J.; Lee, Y.-h.; Lee, K.W.; Lee, W.H.; Kim, J.-H. Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH). Biomolecules 2020, 10, 1426. https://doi.org/10.3390/biom10101426
Lee JH, Oh JY, Kim SH, Oh IJ, Lee Y-h, Lee KW, Lee WH, Kim J-H. Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH). Biomolecules. 2020; 10(10):1426. https://doi.org/10.3390/biom10101426
Chicago/Turabian StyleLee, Jin Ha, Ji Young Oh, Soo Hyun Kim, In Jeong Oh, Yong-ho Lee, Keun Woo Lee, Woong Hee Lee, and Jeong-Hwan Kim. 2020. "Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH)" Biomolecules 10, no. 10: 1426. https://doi.org/10.3390/biom10101426
APA StyleLee, J. H., Oh, J. Y., Kim, S. H., Oh, I. J., Lee, Y.-h., Lee, K. W., Lee, W. H., & Kim, J.-H. (2020). Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH). Biomolecules, 10(10), 1426. https://doi.org/10.3390/biom10101426





