α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Experimental Animals
2.3. Experimental Procedure
2.4. Tissue Collection
2.5. Biochemical Studies
2.6. Assessment of Malondialdehyde (MDA), a Product of Lipid Peroxidation
2.7. Assessment of Glutathione (GSH) Levels
2.8. Assessment of Antioxidant Enzymes Activity
2.9. Assessment of Nitrite Levels (NO)
2.10. Assessment of Matrix Metalloproteinase-9 (MMP-9) Activity
2.11. Assessment of Proinflammatory Cytokines
2.12. Immunocytochemistry of Tyrosine Hydroxylase (TH)
2.13. Immunofluorescence Staining of GFAP and Iba-1
2.14. Assessment of Tyrosine Hydroxylase-immunoreactive (TH-ir) DA Neurons and TH-ir DA Fibers
2.15. Assessment of Activated Astrocytes and Microglia
2.16. Western Blot Analysis of COX-2, iNOS, Bcl2, Bax, Cleaved Caspase-3 and 9, and Cytochrome-C
2.17. Estimation of MC-I and Adenosine Triphosphate (ATP)
2.18. Estimation of Mitochondrial Lipid Peroxidation
2.19. Estimation of Protein Concentration
2.20. Total Antioxidant Activity (2, 2-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) of BSB
2.21. Statistical Analysis
3. Results
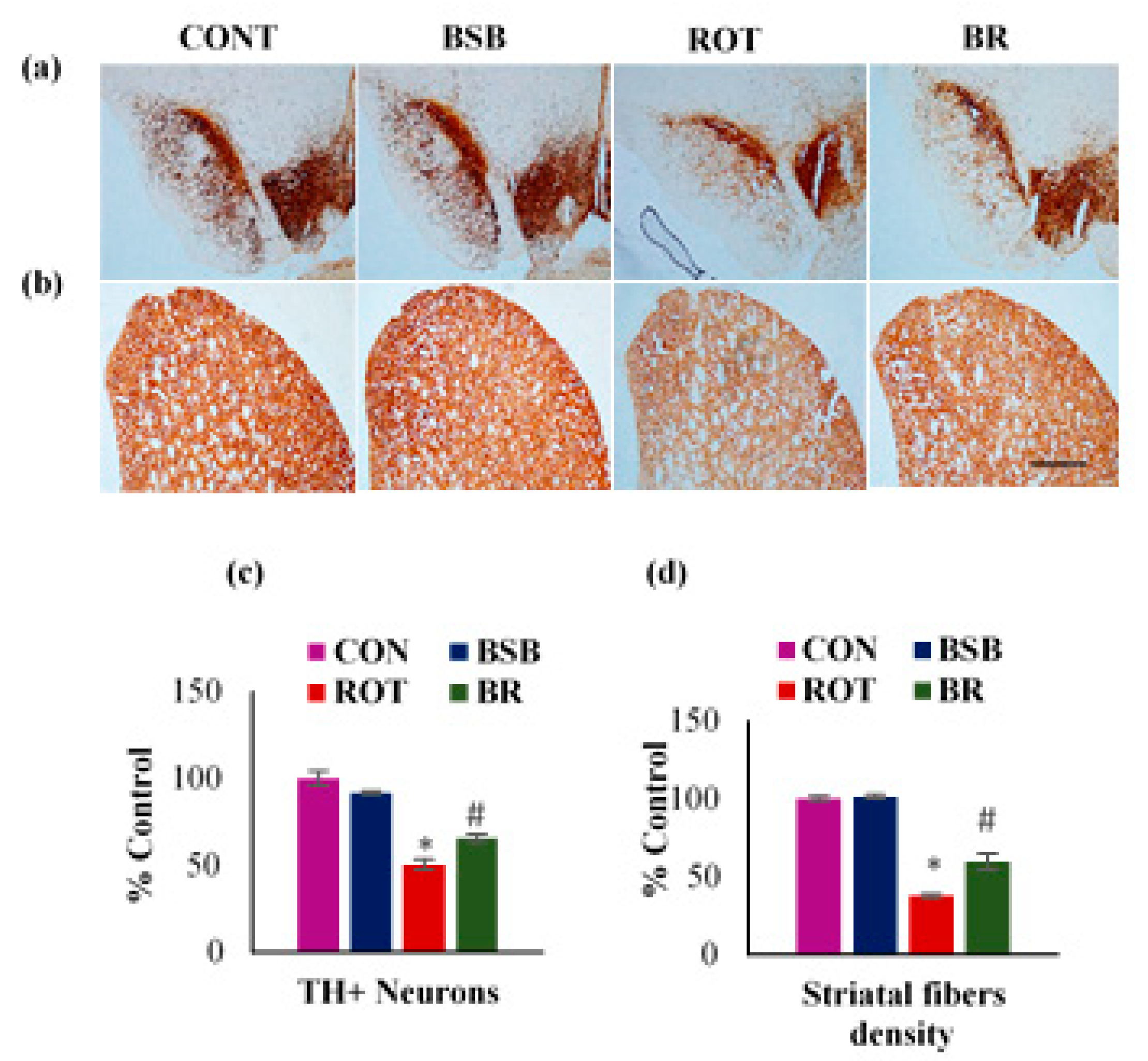
3.1. BSB Mitigates the Loss of TH-ir Neurons in the SNc and TH-ir Fibres in the Striatum
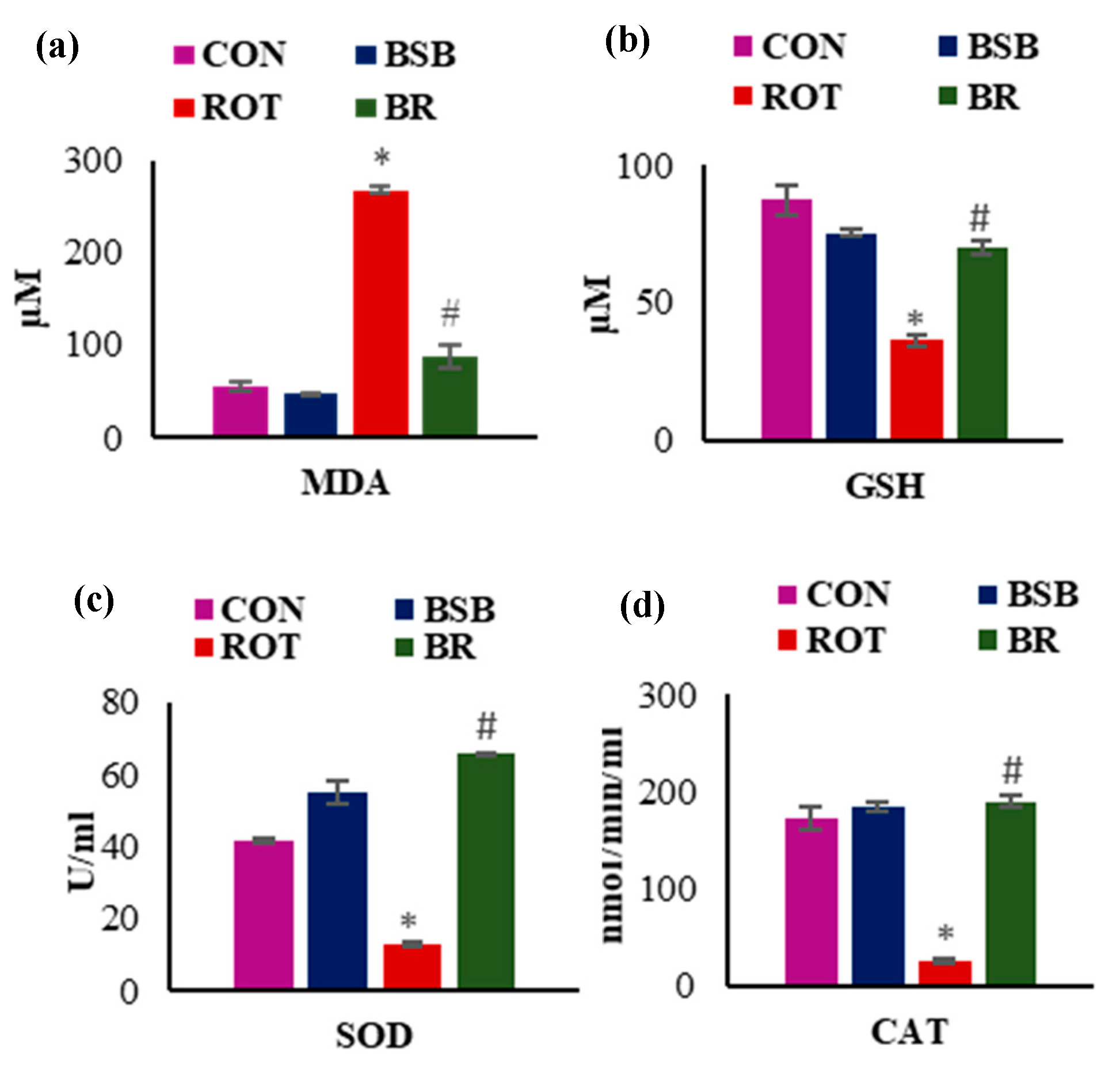
3.2. BSB Attenuates Lipid Peroxidation and Improved Levels and Activities of Antioxidants in the Midbrain
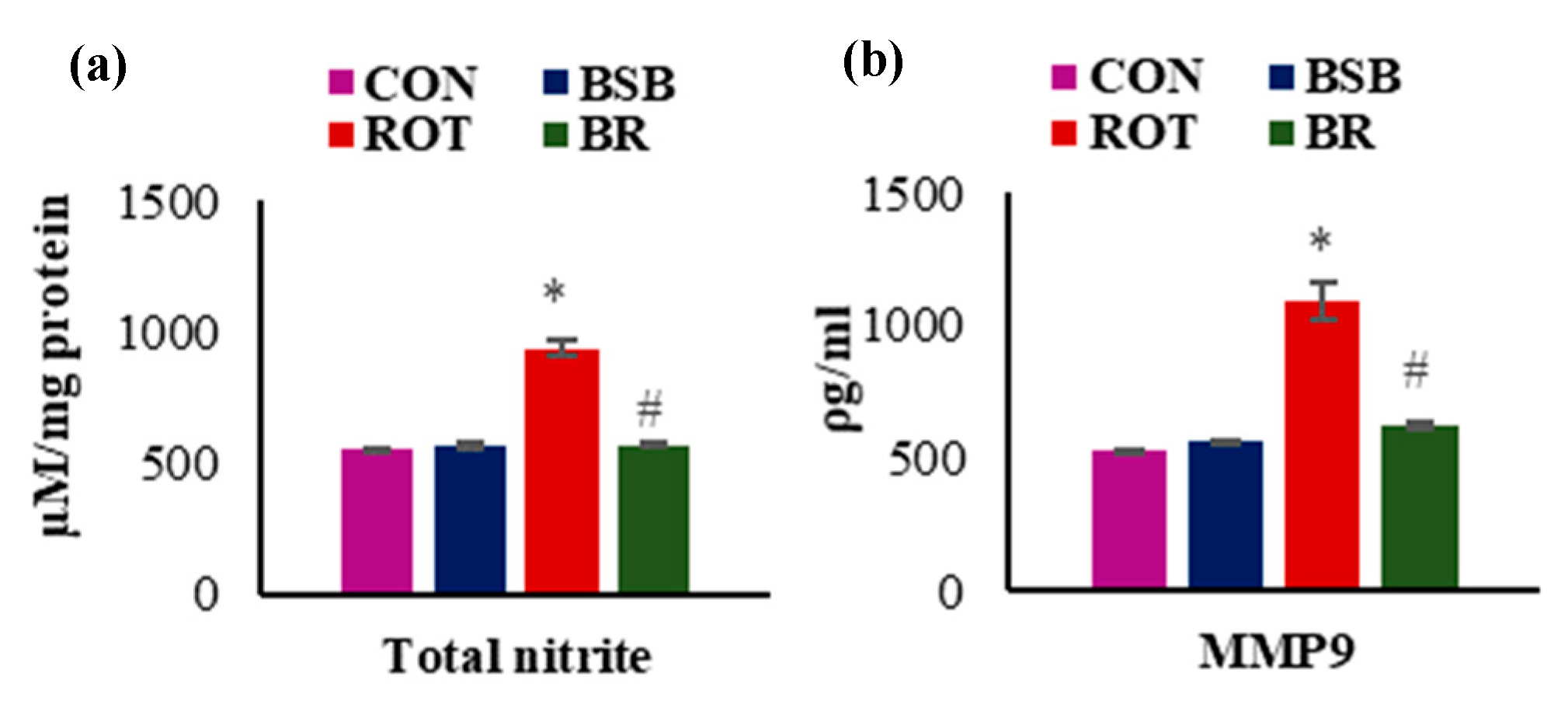
3.3. BSB Inhibits the Levels of Total Nitric Oxide (NO) and MMP-9
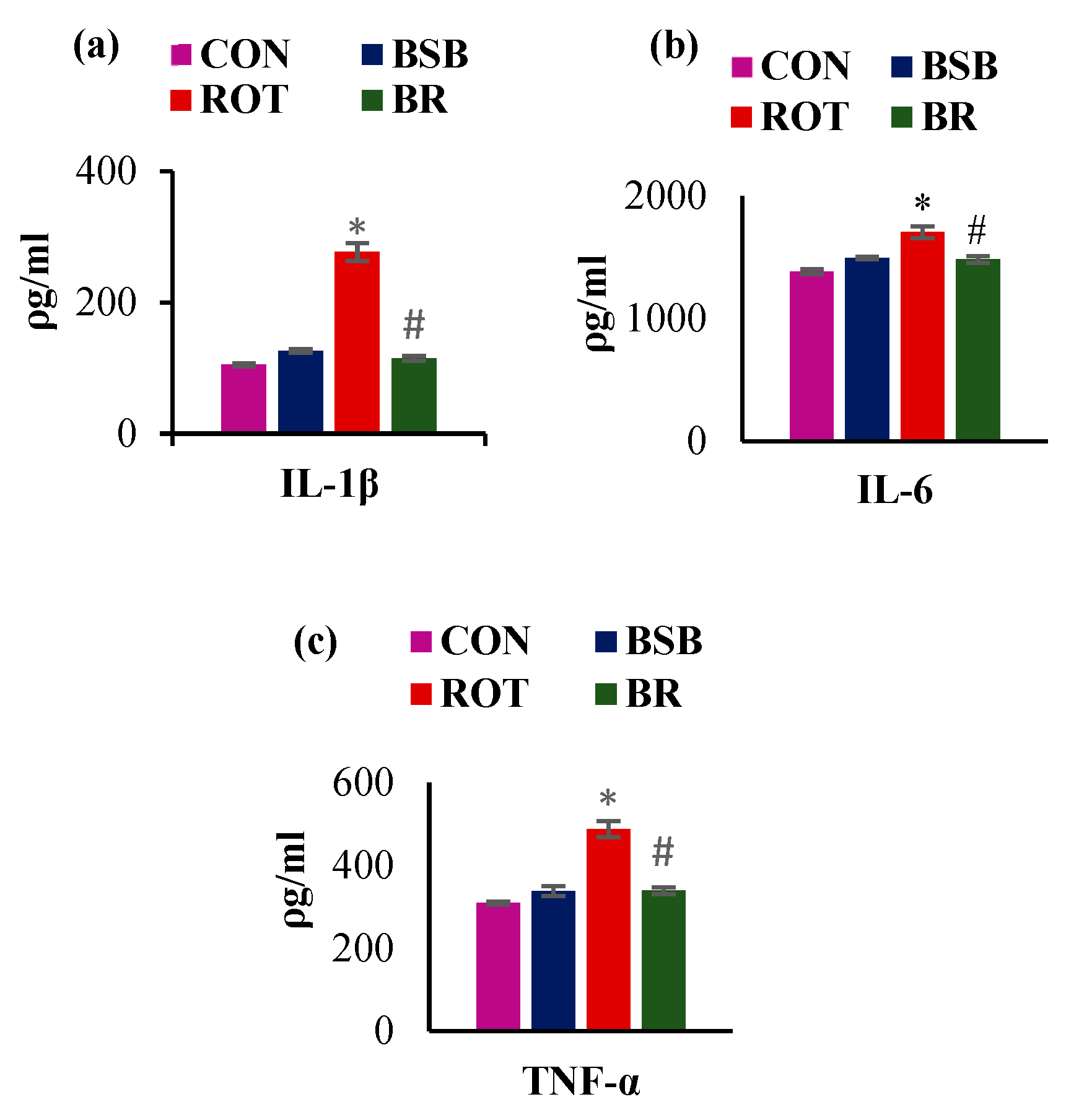
3.4. BSB Inhibits the Activation of Glial Cells and Attenuates the Release of Proinflammatory Cytokines in the Striatum
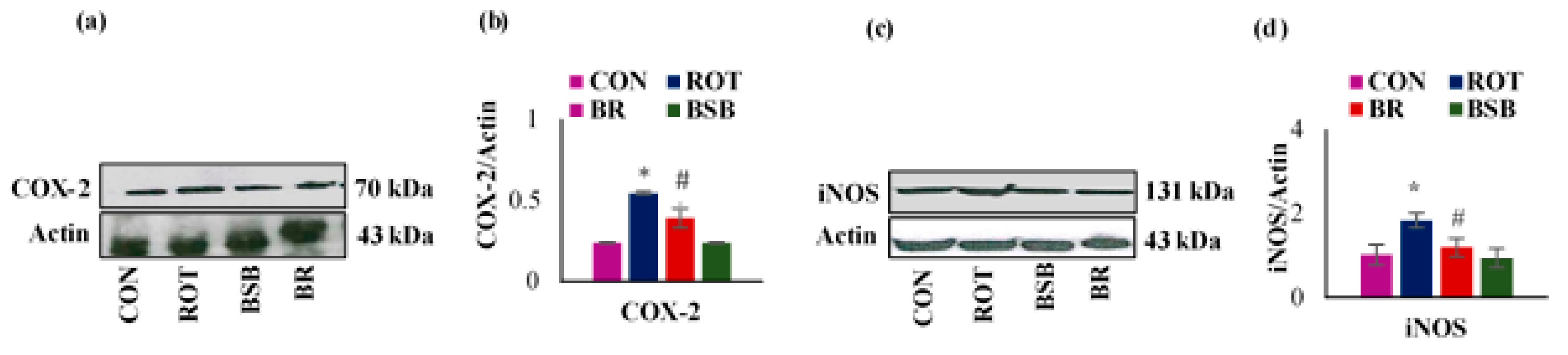
3.5. BSB Attenuated the ROT-induced Expression of Inflammatory Mediators COX-2 and iNOS in the Striatum
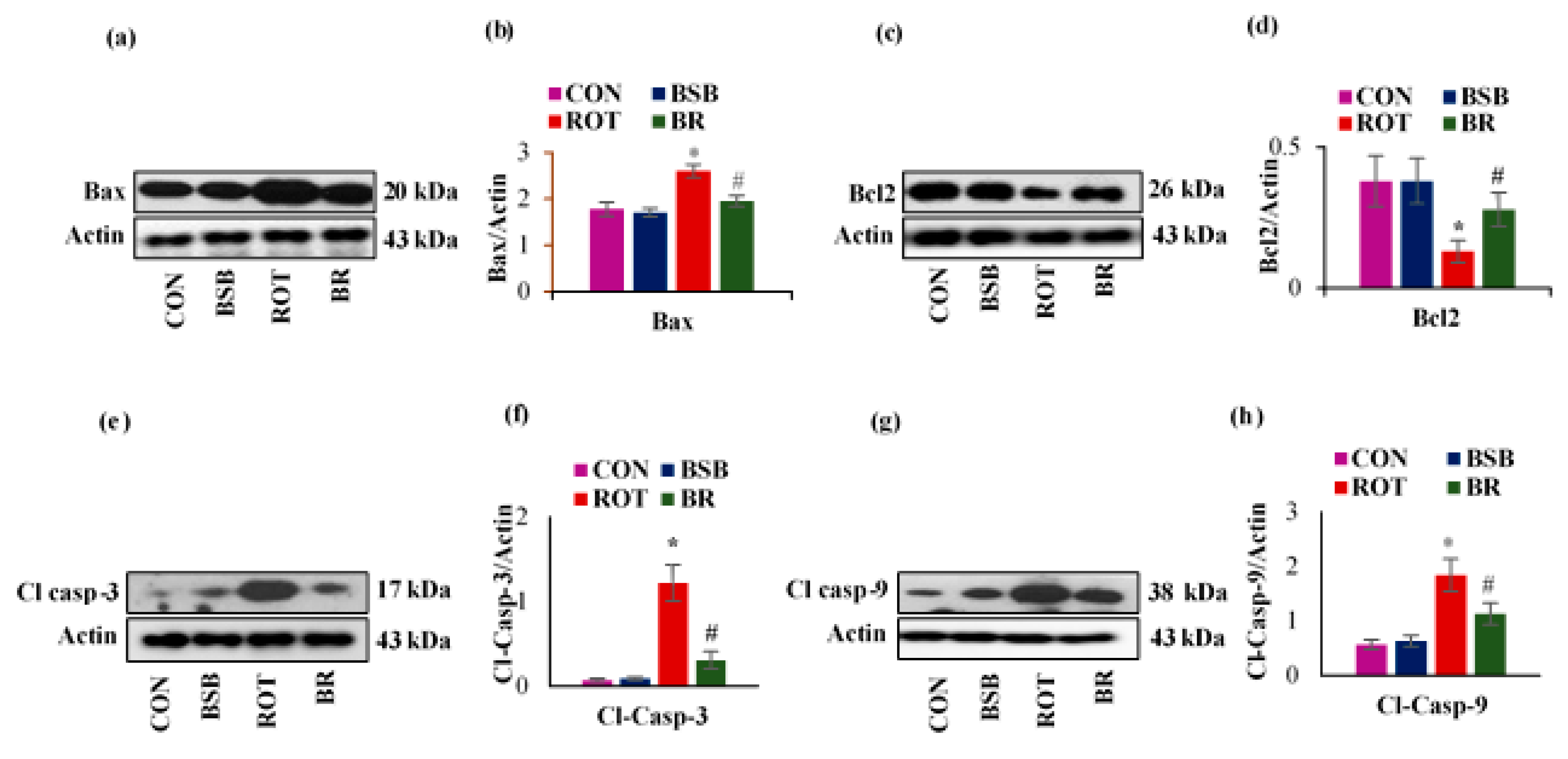
3.6. BSB Attenuates ROT-Induced Expression of Apoptotic Markers Bax, Bcl-2, Cleaved Caspase-3 and 9
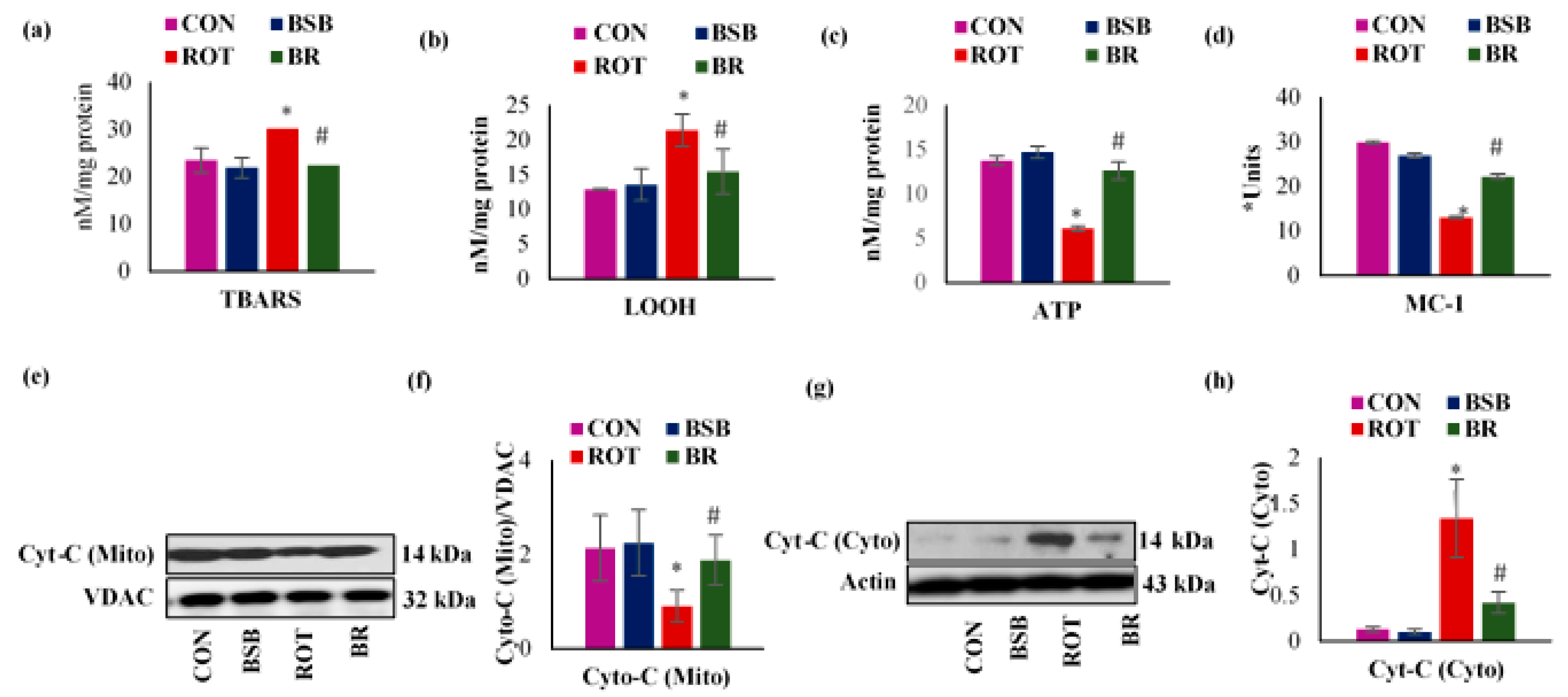
3.7. BSB Attenuates ROT-Induced Mitochondrial Dysfunction
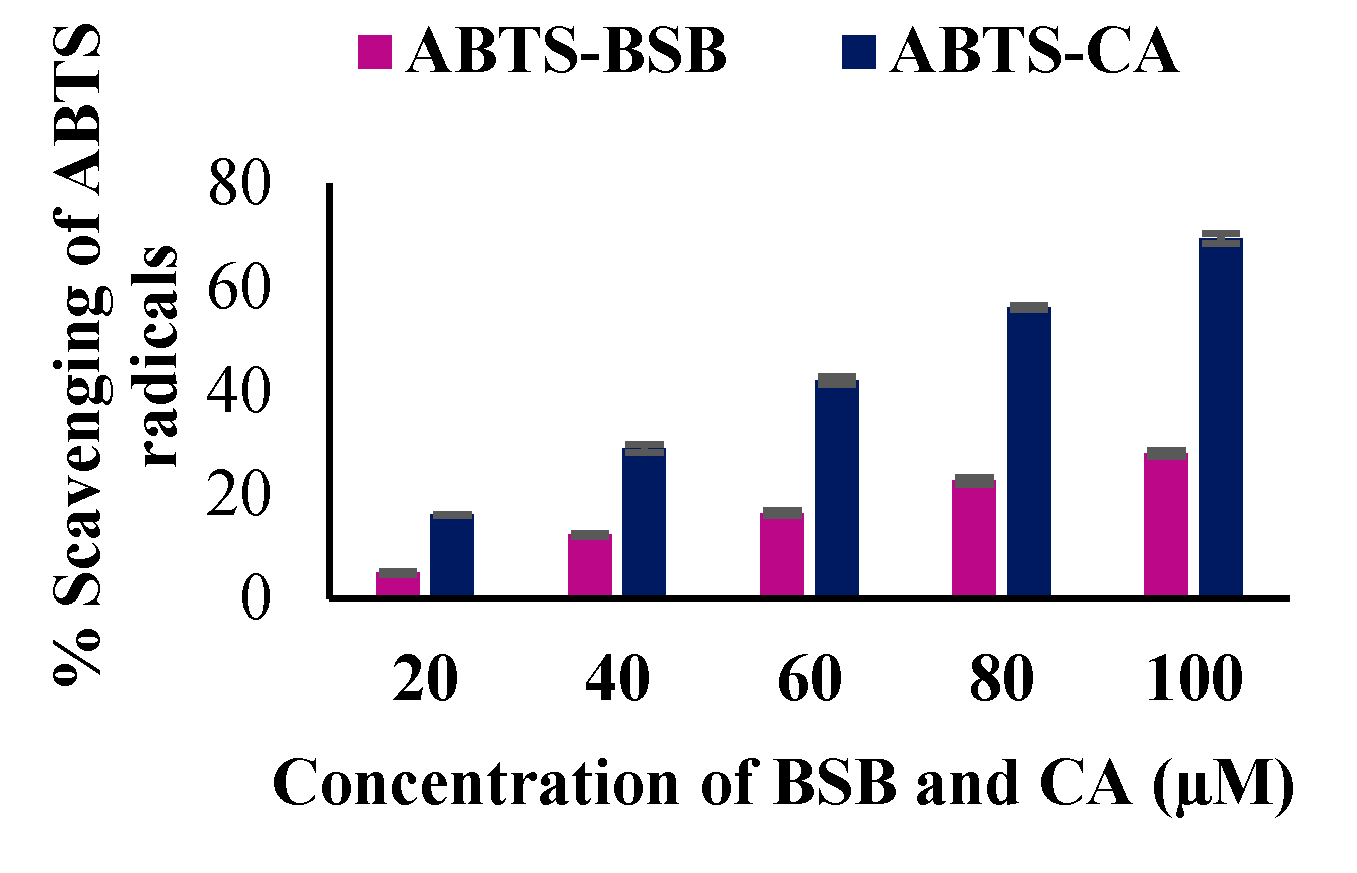
3.8. The In Vitro Antioxidant Potential of BSB
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut–brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J.; Chen, L.; Zhang, P.-P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Herrero, M.T.; García-Martín, E.; Agúndez, J.A. An Update on the Role of Nitric Oxide in the Neurodegenerative Processes of Parkinson’s Disease. Curr. Med. Chem. 2016, 23, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef]
- Carrarini, C.; Russo, M.; Dono, F.; Di Pietro, M.; Rispoli, M.G.; Di Stefano, V.; Ferri, L.; Barbone, F.; Vitale, M.; Thomas, A.; et al. A Stage-Based Approach to Therapy in Parkinson’s Disease. Biomolecules 2019, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Meeran, M.F.N.; Azimullah, S.; Adem, A.; Sadek, B.; Ojha, S. Plant Extracts and Phytochemicals Targeting α-Synuclein Aggregation in Parkinson’s Disease Models. Front. Pharmacol. 2019, 9, 1555. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.E.; Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Dev. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef] [Green Version]
- Asai, M.; Iwata, N.; Yoshikawa, A.; Aizaki, Y.; Ishiura, S.; Saido, T.C.; Maruyama, K. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease Aβ secretion. Biochem. Biophys. Res. Commun. 2007, 352, 498–502. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.-S.; Lee, K.-G.; Choi, C.Y.; Jang, S.S.; Kim, Y.-H.; Lee, S.-E. Effects of Naturally Occurring Compounds on Fibril Formation and Oxidative Stress of β-Amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of Curcumin (Curcuma longa) on Learning and Spatial Memory as Well as Cell Proliferation and Neuroblast Differentiation in Adult and Aged Mice by Upregulating Brain-Derived Neurotrophic Factor and CREB Signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol.-Res. Pr. 2014, 210, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin Exerts Differential Neuroprotective Effects Against H2O2 and Aβ Aggregates in Hippocampal Neurons: The Role of Mitochondria. Mol. Neurobiol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Abin-Carriquiry, J.A.; Arredondo, F.; Blasina, F.; Echeverry, C.; Martínez, M.; Rivera, F.; Vaamonde, L. Quercetin in brain diseases: Potential and limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar]
- Barreto, R.S.; Quintans, J.D.S.S.; Amarante, R.K.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.M.; Duarte, M.C.; Coutinho, H.D.M.; Menezes, I.R.; et al. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-α-bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; De Oliveira, G.V.; De Araújo, F.Y.R.; Rios, E.R.V.; Carvalho, A.M.R.; Vasconcelos, L.F.; Macêdo, D.S.; Soares, P.M.G.; De Sousa, D.P.; De Sousa, F.C.F. (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur. J. Pharm. Sci. 2011, 44, 455–461. [Google Scholar] [CrossRef]
- Rottini, M.M.; Amaral, A.C.F.; Ferreira, J.L.P.; Silva, J.R.D.A.; Taniwaki, N.; Souza, C.D.S.F.D.; D’Escoffier, L.N.; Almeida-Souza, F.; Hardoim, D.D.J.; Da Costa, S.C.G.; et al. In vitro evaluation of (−)α-bisabolol as a promising agent against Leishmania amazonensis. Exp. Parasitol. 2015, 148, 66–72. [Google Scholar] [CrossRef]
- Piochon, M.; Legault, J.; Gauthier, C.; Pichette, A. Synthesis and cytotoxicity evaluation of natural alpha-bisabolol beta-D-fucopyranoside and analogues. Phytochemistry 2009, 70, 228–236. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E.; Ojha, S.; Fizur, N.M.M. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. 2020, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.; Viljoen, A.M.; Gono-Bwalya, A.; Van Zyl, R.L.; Van Vuuren, S.; Lourens, A.; Baser, K.H.C.; Demirci, B.; Lindsey, K.; Van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Solovăstru, L.G.; Stîncanu, A.; De Ascentii, A.; Capparé, G.; Mattana, P.; Vâţă, D. Randomized, Controlled Study of Innovative Spray Formulation Containing Ozonated Oil and α-Bisabolol in the Topical Treatment of Chronic Venous Leg Ulcers. Adv. Ski. Wound Care 2015, 28, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Suryanarayanan, V.; Sathya, S.; Narenkumar, M.; Singh, S.K.; Ruckmani, K.; Kasi, P.D. Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018, 143, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.D.O.; Ecker, A.; Seeger, R.L.; Krum, B.N.; Lugokenski, T.H.; Fachinetto, R.; Sudati, J.H.; Barbosa, N.V.; Wagner, C. Protective effect of (−)-α-bisabolol on rotenone-induced toxicity in Drosophila melanogaster. Can. J. Physiol. Pharmacol. 2018, 96, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.S.; Sindhu, K.M.; Senthilkumar, K.S.; Mohanakumar, K.P. l-deprenyl protects against rotenone-induced, oxidative stress-mediated dopaminergic neurodegeneration in rats. Neurochem. Int. 2006, 49, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Soloway, S.B. Naturally occurring insecticides. Environ. Health Perspect. 1976, 14, 109–117. [Google Scholar] [CrossRef]
- Martinez, T.N.; Greenamyre, J.T. Toxin Models of Mitochondrial Dysfunction in Parkinson’s Disease. Antioxid. Redox Signal. 2012, 16, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Litteljohn, D.; Mangano, E.; Clarke, M.; Bobyn, J.; Moloney, K.; Hayley, S. Inflammatory Mechanisms of Neurodegeneration in Toxin-Based Models of Parkinson’s Disease. Park. Dis. 2010, 2011, 713517. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, G.F.D.; Da Costa, F.N.; Campos, A.R. Corneal antinociceptive effect of (-)-α-bisabolol. Pharm. Biol. 2017, 55, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Khair, S.B.A.; Haque, M.E. Glycyrrhizic acid Attenuates Neuroinflammation and Oxidative Stress in Rotenone Model of Parkinson’s Disease. Neurotox. Res. 2015, 29, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Coorkey, B.E. Assay of intermediates of the citric acid cycle and related compounds by flourimetric enzymatic methods. In Methods Enzymol; Lowenstein, J.M., Ed.; Academic Press: New York, NY, USA, 1967; pp. 488–492. [Google Scholar]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Miller, N.J.; Castelluccio, C.; Tijburg, L.; Rice-Evans, C. The antioxidant properties of theaflavins and their gallate esters - radical scavengers or metal chelators? FEBS Lett. 1996, 392, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.E.; Mount, M.P.; Safarpour, F.; Abdel-Messih, E.; Callaghan, S.; Mazerolle, C.; Kitada, T.; Slack, R.S.; Wallace, V.; Shen, J.; et al. Inactivation of Pink1 Gene in Vivo Sensitizes Dopamine-producing Neurons to 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and Can Be Rescued by Autosomal Recessive Parkinson Disease Genes, Parkin or DJ-1. J. Biol. Chem. 2012, 287, 23162–23170. [Google Scholar] [CrossRef] [Green Version]
- Rajasankar, S.; Ramkumar, M.; Gobi, V.V.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Chidambaram, R.; Chidambaram, S.B.; Guillemin, G.J. Demethoxycurcumin, a natural derivative of curcumin abrogates rotenone-induced dopamine depletion and motor deficits by its antioxidative and anti-inflammatory properties in Parkinsonian rats. Pharmacogn. Mag. 2018, 14, 9–16. [Google Scholar] [CrossRef]
- Narasimhan, K.K.S.; Paul, L.; Sathyamoorthy, Y.K.; Srinivasan, A.; Chakrapani, L.N.; Singh, A.; Ravi, D.B.; Krishnan, T.R.; Velusamy, P.; Kaliappan, K.; et al. Amelioration of apoptotic events in the skeletal muscle of intra-nigrally rotenone-infused Parkinsonian rats by Morinda citrifolia – up-regulation of Bcl-2 and blockage of cytochrome c release. Food Funct. 2016, 7, 922–937. [Google Scholar] [CrossRef]
- Chiu, C.C.; Yeh, T.H.; Lai, S.C.; Wu-Chou, Y.H.; Chen, C.H.; Mochly-Rosen, D.; Huang, Y.C.; Chen, Y.J.; Chen, C.L.; Chang, Y.M.; et al. Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp. Neurol. 2015, 263, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Duvigneau, J.C.; Trovato, A.; Müllebner, A.; Miller, I.; Krewenka, C.; Krenn, K.; Zich, W.; Moldzio, R. Cannabidiol Protects Dopaminergic Neurons in Mesencephalic Cultures against the Complex I Inhibitor Rotenone Via Modulation of Heme Oxygenase Activity and Bilirubin. Antioxidants 2020, 9, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Carneiro, M.; Dias, D.M.; De-Oliveira, A.; Paumgartten, F.J. Evaluation of mutagenic and antimutagenic activities of α-bisabolol in the Salmonella/microsome assay. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 585, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Martini, M.V.; De Oliveira, C.; Cunha, S.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Da Silva, C.C. Antitumor activity of (−)-α-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.J.; Freire, W.B.; Vasconcelos-Silva, A.A.; Fonseca-Magalhães, P.A.; Lima, F.J.; Brito, T.S.; Mourão, L.T.; Ribeiro, R.A.; Lahlou, S.; Magalhães, P.J.C. In-vitro characterization of the pharmacological effects induced by (–)-α-bisabolol in rat smooth muscle preparations. Can. J. Physiol. Pharmacol. 2012, 90, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.F.; Marçalo, A.; Martin, J.; Fernández, I.D.; Maldonado, H.; Vaisberg, A.J.; Hammond, G.R. (+)-epi-α-Bisbolol Is the Wound-Healing Principle ofPeperomiagalioides: Investigation of the in Vivo Wound-Healing Activity of Related Terpenoids. J. Nat. Prod. 2001, 64, 1357–1359. [Google Scholar] [CrossRef]
- Nurulain, S.; Prytkova, T.; Sultan, A.; Ievglevskyi, O.; Lorke, D.; Yang, K.-H.; Petroianu, G.; Howarth, F.; Kabbani, N.; Öz, M. Inhibitory actions of bisabolol on α7-nicotinic acetylcholine receptors. Neuroscience 2015, 306, 91–99. [Google Scholar] [CrossRef]
- Thakur, P.; Nehru, B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience 2013, 231, 420–431. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Nicklas, W.J.; Vyas, I.; Duvoisin, R.C. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci. Lett. 1985, 62, 389–394. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.M.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.; Liu, Y.; Fu, X.; Xu, X.; Bao, Z.; Lin, C.; Li, Z.; Liu, Y.; Wang, X.; You, Y.; et al. Lowered iPLA2γ activity causes increased mitochondrial lipid peroxidation and mitochondrial dysfunction in a rotenone-induced model of Parkinson’s disease. Exp. Neurol. 2018, 300, 74–86. [Google Scholar] [CrossRef]
- Perier, C.; Vila, M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009332. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Laham, F.; Al-Taee, H.; Azimullah, S.; Ojha, S. Protective effects of α-bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: In vivo and in vitro evidences. J. Biochem. Mol. Toxicol. 2018, 32, e22200. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Hunot, S.; Michel, P.P.; Muriel, M.P.; Vyas, S.; Faucheux, B.A.; Mouatt-Prigent, A.; Turmel, H.; Srinivasan, A.; Ruberg, M.; et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2875–2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Michel, P.P.; Troadec, J.-D.; Mouatt-Prigent, A.; Faucheux, B.A.; Ruberg, M.; Agid, Y.; Hirsch, E.C. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J. Neurochem. 2001, 76, 1785–1793. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef]
- Ahmadi, F.A.; Linseman, D.A.; Grammatopoulos, T.N.; Jones, S.M.; Bouchard, R.J.; Freed, C.R.; Heidenreich, K.A.; Zawada, W.M. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J. Neurochem. 2004, 87, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Ethell, D.W.; Fei, Q. Parkinson-Linked Genes and Toxins That Affect Neuronal Cell Death Through the Bcl-2 Family. Antioxid. Redox Signal. 2009, 11, 529–540. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Laham, F.; Azimullah, S.; Tariq, S.; Ojha, S. α-Bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol. Cell. Biochem. 2018, 453, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Jang, Y.J.; Kim, H.J.; Hwang, O. Tetrahydrobiopterin Is Released from and Causes Preferential Death of Catecholaminergic Cells by Oxidative Stress. Mol. Pharmacol. 2000, 58, 633–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzl, S.; Calingasan, N.; Yang, L.; Albers, D.S.; Shugama, S.; Gregorio, J.; Krell, H.W.; Chirichigno, J.; Joh, T.; Beal, M.F. Matrix Metalloproteinase-9 Is Elevated in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonism in Mice. NeuroMol. Med. 2004, 5, 119–132. [Google Scholar] [CrossRef]
- Annese, V.; Herrero, M.T.; Di Pentima, M.; Gómez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2014, 220, 703–727. [Google Scholar] [CrossRef]
- Ferger, A.I.; Campanelli, L.; Reimer, V.; Muth, K.N.; Merdian, I.; Ludolph, A.C.; Witting, A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J. Neuroinflamm. 2010, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated α-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef]
- Pereira, J.F.; Carmo, M.R.S.D.; Fonteles, A.A.; Neves, J.C.D.S.; Da Silva, A.T.A.; Pereira, J.F.; Ferreira, E.D.O.; De Lima, N.M.R.; Neves, K.R.T.; De Andrade, G.M. (-)-α-bisabolol prevents neuronal damage and memory deficits through reduction of proinflammatory markers induced by permanent focal cerebral ischemia in mice. Eur. J. Pharmacol. 2019, 842, 270–280. [Google Scholar] [CrossRef]


| Antibody | Host | Source/Catalogue No. | Dilution |
|---|---|---|---|
| Tyrosine hydroxylase (TH) | Rabbit | Millipore, MA, USA (AB-152) | 1:500 |
| Glial fibrillary acidic protein (GFAP) | Rabbit | Abcam, MA, USA (SAB2107063) | 1:1000 |
| Ionized calcium binding adaptor molecule 1 (Iba1) | Rabbit | Wako Chemicals, VA, USA (019-19741) | 1:1000 |
| Cytochrome-C | Mouse | Abcam, MA, USA (AB13575) | 1:1000 |
| Cyclooxygenase-2 (COX-2) | Rabbit | Abcam, MA, USA (AB52237) | 1:1000 |
| Inducible nitric oxide synthase (iNOS) | Rabbit | Sigma, MO, USA (SAB4502011) | 1:1000 |
| B-cell lymphoma 2 (Bcl-2) | Rabbit | Abcam, MA, USA (AB196495) | 1:500 |
| BCL2 Associated X, Apoptosis Regulator (Bax) | Rabbit | Santacruz, Dallas, USA (SC-526) | 1:1000 |
| Cleaved caspase-3 | Rabbit | Abcam, MA, USA (AB49822) | 1:500 |
| Cleaved caspase-9 | Rabbit | Cell signaling Technology, USA (9507S) | 1:500 |
| Voltage-dependent anion channel (VDAC) | Rabbit | Cell signaling Technology, USA (4661S) | 1:2000 |
| β-actin | Mouse | Millipore, MA, USA (MAB1501R) | 1:2000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, H.; Meeran, M.F.N.; Azimullah, S.; Bader Eddin, L.; Dwivedi, V.D.; Jha, N.K.; Ojha, S. α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease. Biomolecules 2020, 10, 1421. https://doi.org/10.3390/biom10101421
Javed H, Meeran MFN, Azimullah S, Bader Eddin L, Dwivedi VD, Jha NK, Ojha S. α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease. Biomolecules. 2020; 10(10):1421. https://doi.org/10.3390/biom10101421
Chicago/Turabian StyleJaved, Hayate, M. F. Nagoor Meeran, Sheikh Azimullah, Lujain Bader Eddin, Vivek Dhar Dwivedi, Niraj Kumar Jha, and Shreesh Ojha. 2020. "α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease" Biomolecules 10, no. 10: 1421. https://doi.org/10.3390/biom10101421
APA StyleJaved, H., Meeran, M. F. N., Azimullah, S., Bader Eddin, L., Dwivedi, V. D., Jha, N. K., & Ojha, S. (2020). α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease. Biomolecules, 10(10), 1421. https://doi.org/10.3390/biom10101421








