IL-37 Gene and Cholesterol Metabolism: Association of Polymorphisms with the Presence of Hypercholesterolemia and Cardiovascular Risk Factors. The GEA Mexican Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genetic Analysis
2.3. Functional Prediction Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
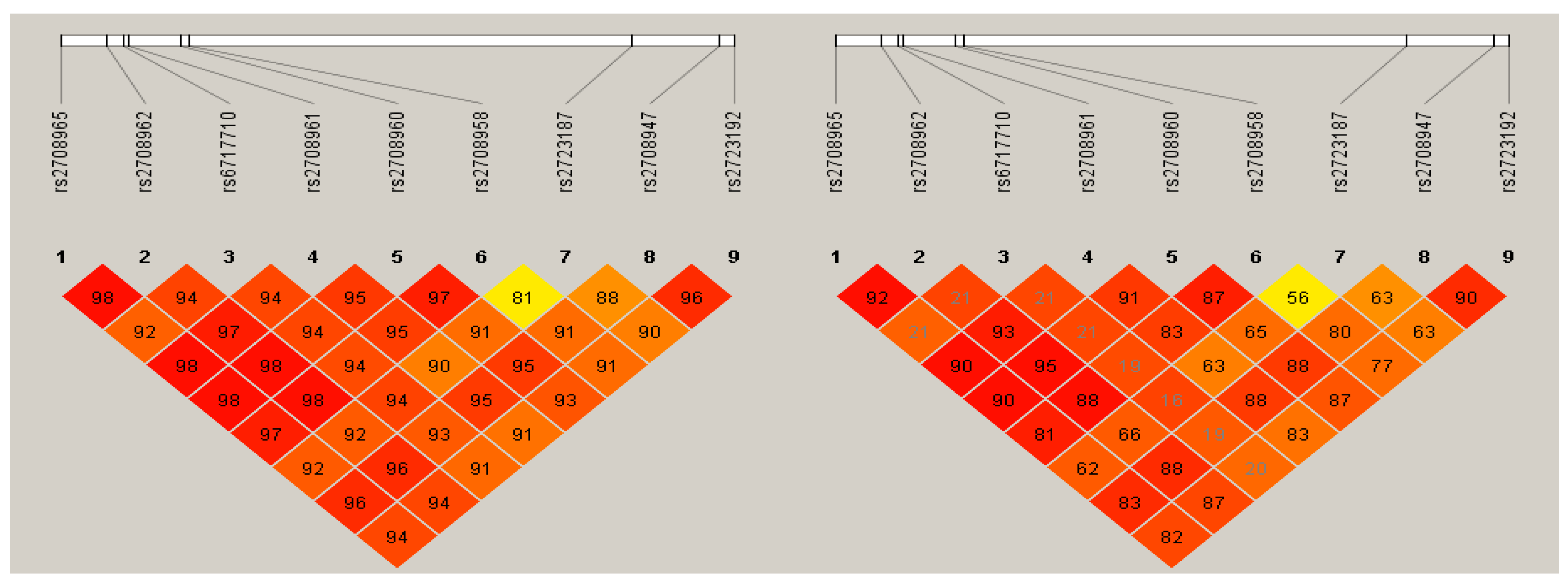
3.2. Association of the IL-37 Polymorphisms and Haplotypes with HC
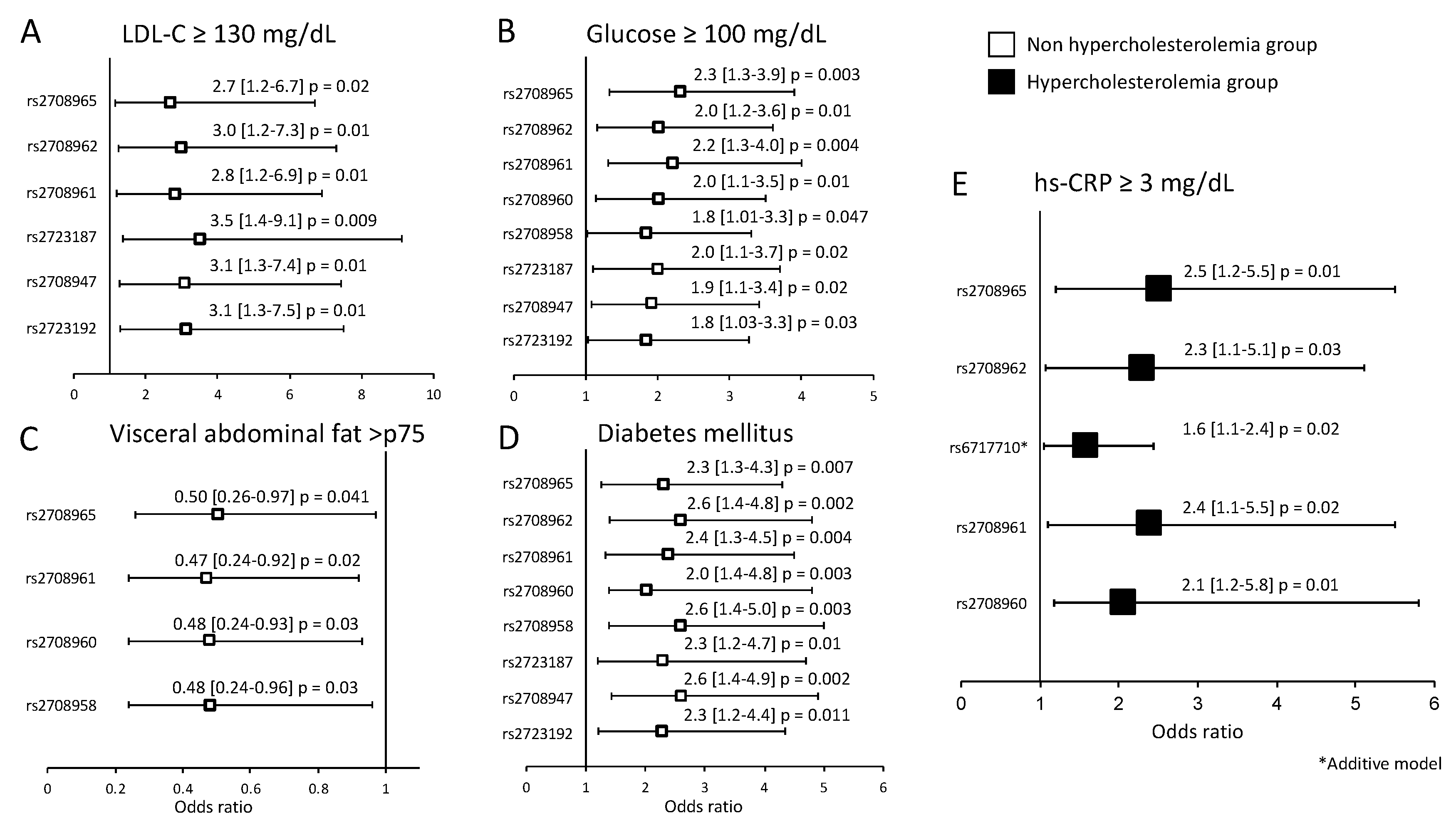
3.3. Association of the IL-37 Polymorphisms with Cardiovascular Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, R.; Ferrari, F.; Scolari, F. Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights. Curr. Cardiol. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low-density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Kruth, H.S. Fluid-phase pinocytosis of LDL by macrophages: A novel target to reduce macrophage cholesterol accumulation in atherosclerotic lesions. Curr. Pharm. Des. 2013, 19, 5865–5872. [Google Scholar] [CrossRef]
- Shalhoub, J.; Falck-Hansen, M.A.; Davies, A.H.; Monaco, C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J. Inflamm. 2011, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Michael, D.R.; Ashlin, T.G.; Davies, C.S.; Gallagher, H.; Stoneman, T.W.; Buckley, M.L.; Ramji, D.P. Differential regulation of macropinocytosis in macrophages by cytokines: Implications for foam cell formation and atherosclerosis. Cytokine 2013, 64, 357–361. [Google Scholar] [CrossRef] [Green Version]
- van de Veerdonk, F.L.; Netea, M.G. New Insights in the Immunobiology of IL-1 Family Members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.M.; Fujita, M. IL-37: A new player in immune tolerance. Cytokine 2015, 72, 113–114. [Google Scholar] [CrossRef]
- Boraschi, D.; Lucchesi, D.; Hainzl, S.; Leitner, M.; Maier, E.; Mangelberger, D.; Oostingh, G.J.; Pfaller, T.; Pixner, C.; Posselt, G.; et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur. Cytokine Netw. 2011, 22, 127–147. [Google Scholar] [CrossRef]
- Ballak, D.B.; Van Diepen, J.A.; Moschen, A.; Jansen, H.J.; Hijmans, A.; Groenhof, G.J.; Leenders, F.; Bufler, P.; Boekschoten, M.V.; Muller, M.; et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK activation enhances the anti-atherogenic effects of high-density lipoproteins in apoE−/− mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, D.; Naji, D.H.; Xia, Y.; Li, S.; Bai, Y.; Jiang, G.; Zhao, Y.; Wang, X.; Huang, Y.; Chen, S.; et al. Genomic Variant in IL-37 Confers A Significant Risk of Coronary Artery Disease. Sci. Rep. 2017, 7, 42175. [Google Scholar] [CrossRef] [PubMed]
- Medina-Urrutia, A.; Posadas-Romero, C.; Posadas-Sánchez, R.; Jorge-Galarza, E.; Villarrreal-Molina, T.; Gónzalez-Salazar, M.C.; Cardoso-Saldaña, G.; Vargas-Alarcón, G.; Torres-Tamayo, M.; Juárez-Rojas, G. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 2015, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadas-Sánchez, R.; López-Uribe, A.R.; Posadas-Romero, C.; Pérez-Hernánez, N.; Rodríguez-Pérez, J.M.; Ocampo-Arcos, W.A.; Fragoso, J.M.; Cardoso-Saldaña, G.; Vargas-Alarcón, G. Association of the I148M/PNPLA3 (rs738409) polymorphism with premature coronary artery disease, fatty liver, and insulin resistance in type 2 diabetic patients and healthy controls. The GEA study. Immunobiology 2017, 222, 960–966. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Angeles-Martínez, J.; López-bautista, F.; Villarreal-Molina, T.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Posadas-Romero, C.; Vargas-Alarcón, G. Interleukin 35 Polymorphisms Are Associated with Decreased Risk of Premature Coronary Artery Disease, Metabolic Parameters, and IL-35 Levels: The Genetics of Atherosclerotic Disease (GEA) Study. Mediat. Inflamm. 2017, 2017, 6012795. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. American Heart Association, National Heart, Lung, and Blood Institute, Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Castillo, C.P.; Velázquez-Monroy, O.; Berber, A.; Lara-Esqueda, A.; Tapia-Conyer, R.; James, W.P.T. Encuesta Nacional de Salud (ENSA) 2000 Working Group Anthropometric cutoff points for predicting chronic diseases in the Mexican National Health Survey 2000. Obes. Res. 2003, 11, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Kvist, H.; Chowdhury, B.; Grangård, U.; Tylén, U.; Sjöström, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: Predictive equations. Am. J. Clin. Nutr. 1988, 48, 1351–1361. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Chiou, J.J.; Tsengetal, W.-H. FASTSNP: An always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006, 34, W635–W641. [Google Scholar] [CrossRef]
- Human-transcriptome Database for Alternative Splicing. Available online: http://www.h-invitational.jp/h-dbas (accessed on 5 May 2018).
- SNP Function Prediction. National Institute of Environmental Health Science. Available online: http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html (accessed on 9 May 2018).
- Human Splicing Finder. INSERM. Available online: http://www.umd.be/HSF (accessed on 13 May 2018).
- SNPs3D. Available online: http://www.snps3d.org (accessed on 25 May 2018).
- Dogan, R.I.; Getoor, L.; Wilbur, W.J.; Mount, S.M. Splice Port: An Interactive Splice Site Analysis Tool. Nucleic Acids Res. 2007, 35, W285–W291. Available online: http://spliceport.cbcb.umd.edu/SplicingAnalyser (accessed on 18 May 2018).
- Cartegni, L.; Wang, J.; Zhu, Z.; Zhang, M.Q.; Krainer, A.R. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acid Res. 2003, 31, 3568–3571. Available online: http://rulai.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi (accessed on 25 May 2018). [CrossRef] [PubMed] [Green Version]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265, Haploview version 4.2. Available online: https://www.broadinstitute.org/haploview/haploview (accessed on 5 January 2020).
- McCurdy, S.; Liu, C.A.; Yap, J.; Boisvert, W.A. Potential role of IL-37 in atherosclerosis. Cytokine 2019, 122, 154169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, M.; Figueroa, J.A.; Cobos, E.; Uretsky, B.F.; Chiriva-Internati, M.; Hermonat, P.L. AAV2/8-hSMAD3 gene delivery attenuates aortic atherogenesis, enhances Th2 response without fibrosis, in LDLR-KO mice on high cholesterol diet. J. Transl. Med. 2014, 12, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Kang, L.; Wang, Y.; Xiang, J.; Wu, Q.; Xu, C.; Zhou, Y.; Chem, S.; Fang, H.; Liu, J.; et al. Role of IL-37 in Cardiovascular Disease Inflammation. Can. J. Cardiol. 2019, 35, 923–930. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Li, W.; Chen, S.; Feng, T.; Jiao, W.; Wu, C.; Dong, J.; Li, Y.; Li, S.; et al. Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol. Immunol. 2019, 112, 322–329. [Google Scholar] [CrossRef]
- McCurdy, S.; Baumer, Y.; Toulmin, E.; Lee, B.H.; Boisvert, W.A. Macrophage-Specific Expression of IL-37 in Hyperlipidemic Mice Attenuates Atherosclerosis. J. Immunol. 2017, 199, 3604–3613. [Google Scholar] [CrossRef]
- Després, J.P.; Moorjani, S.; Lupien, P.J.; Tremblay, A.; Nadeau, A.; Bouchard, C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990, 10, 497–511. [Google Scholar] [CrossRef] [Green Version]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef]
| No HC n = 778 | HC n = 514 | p | |
|---|---|---|---|
| Demographic | |||
| Gender (% male) | 51.1 | 48.8 | 0.41 |
| Age (years) | 53 ± 9.8 | 54.2 ± 8.4 | 0.02 |
| Clinical and Biochemical Characteristics | |||
| Body mass index (kg/m2) | 28.1 ± 4.1 | 28.5 ± 3.8 | 0.10 |
| Systolic blood pressure (mmHg) | 116 ± 17 | 118.7 ± 18.2 | 0.02 |
| Diastolic blood pressure (mmHg) | 71 ± 9.2 | 73.2 ± 9.8 | 0.001 |
| Triglycerides (mg/dL) | 132 (98–184) | 175 (134–239) | <0.001 |
| Total cholesterol (mg/dL) | 169.5 ± 21.7 | 227.5 ± 24.6 | <0.001 |
| HDL-cholesterol (mg/dL) | 44.4 ± 12.6 | 48.6 ± 13.7 | <0.001 |
| LDL-cholesterol (mg/dL) | 100.3 ± 20.9 | 145.9 ± 25.9 | <0.001 |
| Glucose (mg/dL) | 99.2 ± 34.1 | 99.8 ± 33.7 | 0.75 |
| Insulin (μU/mL) | 17.2 (12.3–23.8) | 17.3 (12.5–23) | 0.92 |
| HOMA-IR | 3.9 (2.65–5.71) | 4.0 (2.7–5.8) | 0.59 |
| hs-CRP (mg/L) | 1.39 (0.78–2.9) | 1.65 (0.85–3.37) | 0.16 |
| Visceral adipose tissue (cm2) | 146 (105–188) | 155 (116–204) | 0.003 |
| Lifestyle | |||
| Physical Activity Index | 7.8 ± 1.27 | 7.8 ± 1.2 | 0.99 |
| Alcohol, gr/day | 0.29 (0.01–1.47) | 0.45 (0.09–1.47) | 0.01 |
| Smoke, % | |||
| Current | 172 (22.1) | 111 (21.6) | 0.93 |
| Past | 290 (37.8) | 189 (36.7) | |
| Never | 316 (40.6) | 214 (41.6) | |
| Saturated fat intake (kcal) | 226.8 ± 83.2 | 221.5 ± 85.3 | 0.27 |
| Medical History, % | |||
| Hypoalphalipoproteinemia | 56 | 42 | <0.001 |
| Hypertriglyceridemia | 40 | 64 | <0.001 |
| LDL-cholesterol ≥ 130 mg/dL | 5.1 | 76 | <0.001 |
| Obesity | 30.4 | 32.4 | 0.13 |
| Type 2 Diabetes Mellitus | 15 | 11.2 | 0.03 |
| Hypertension | 23.5 | 25.4 | 0.22 |
| hsCRP ≥ 3 mg/dL | 24.6 | 29.1 | 0.042 |
| Lipid-lowering therapy | 14 | 20 | 0.001 |
| Polymorphism | Genotype Frequency | MAF | Model | OR (95% CI) | p | ||
|---|---|---|---|---|---|---|---|
| rs2708961 | TT | TC | CC | ||||
| No (n = 778) | 0.91 | 0.08 | 0.001 | 0.04 | Codominant 1 | 0.51 (0.28–0.93) | 0.02 |
| Yes (n = 514) | 0.94 | 0.05 | 0 | 0.02 | |||
| rs2723187 | CC | CT | TT | ||||
| No (n = 778) | 0.93 | 0.06 | 0.001 | 0.03 | Codominant 1 | 0.35 (0.17–0.73) | 0.005 |
| Yes (n = 514) | 0.96 | 0.03 | 0 | 0.01 | |||
| rs2708947 | TT | TC | CC | ||||
| No (n = 778) | 0.91 | 0.07 | 0.001 | 0.04 | Codominant 1 | 0.49 (0.26–0.92) | 0.02 |
| Yes (n = 514) | 0.94 | 0.04 | 0.001 | 0.02 | |||
| Haplotype | Sequence | HC YES | NO | OR (95%CI) | p |
|---|---|---|---|---|---|
| H1 | C-C-T-T-C-A-C-T-G | 0.874 | 0.859 | 1.13 (0.89–1.43) | 0.142 |
| H2 | C-C-C-T-C-A-C-T-G | 0.097 | 0.102 | 0.93 (0.71–1.22) | 0.310 |
| H3 | A-T-C-C-T-G-T-C-A | 0.013 | 0.027 | 0.46 (0.25–0.88) | 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Bautista, F.; Posadas-Sánchez, R.; Vázquez-Vázquez, C.; Fragoso, J.M.; Rodríguez-Pérez, J.M.; Vargas-Alarcón, G. IL-37 Gene and Cholesterol Metabolism: Association of Polymorphisms with the Presence of Hypercholesterolemia and Cardiovascular Risk Factors. The GEA Mexican Study. Biomolecules 2020, 10, 1409. https://doi.org/10.3390/biom10101409
López-Bautista F, Posadas-Sánchez R, Vázquez-Vázquez C, Fragoso JM, Rodríguez-Pérez JM, Vargas-Alarcón G. IL-37 Gene and Cholesterol Metabolism: Association of Polymorphisms with the Presence of Hypercholesterolemia and Cardiovascular Risk Factors. The GEA Mexican Study. Biomolecules. 2020; 10(10):1409. https://doi.org/10.3390/biom10101409
Chicago/Turabian StyleLópez-Bautista, Fabiola, Rosalinda Posadas-Sánchez, Christian Vázquez-Vázquez, José Manuel Fragoso, José Manuel Rodríguez-Pérez, and Gilberto Vargas-Alarcón. 2020. "IL-37 Gene and Cholesterol Metabolism: Association of Polymorphisms with the Presence of Hypercholesterolemia and Cardiovascular Risk Factors. The GEA Mexican Study" Biomolecules 10, no. 10: 1409. https://doi.org/10.3390/biom10101409





