Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels
Abstract
:1. Introduction—Skin Wounds as a Medical Challenge
- (1)
- Stable under room temperature storage;
- (2)
- Suitable for topical or injectable delivery;
- (3)
- Antimicrobial, analgesic, or hemostatic properties;
- (4)
- Accelerates wound repair by promoting conductive or inductive regenerative mechanisms.
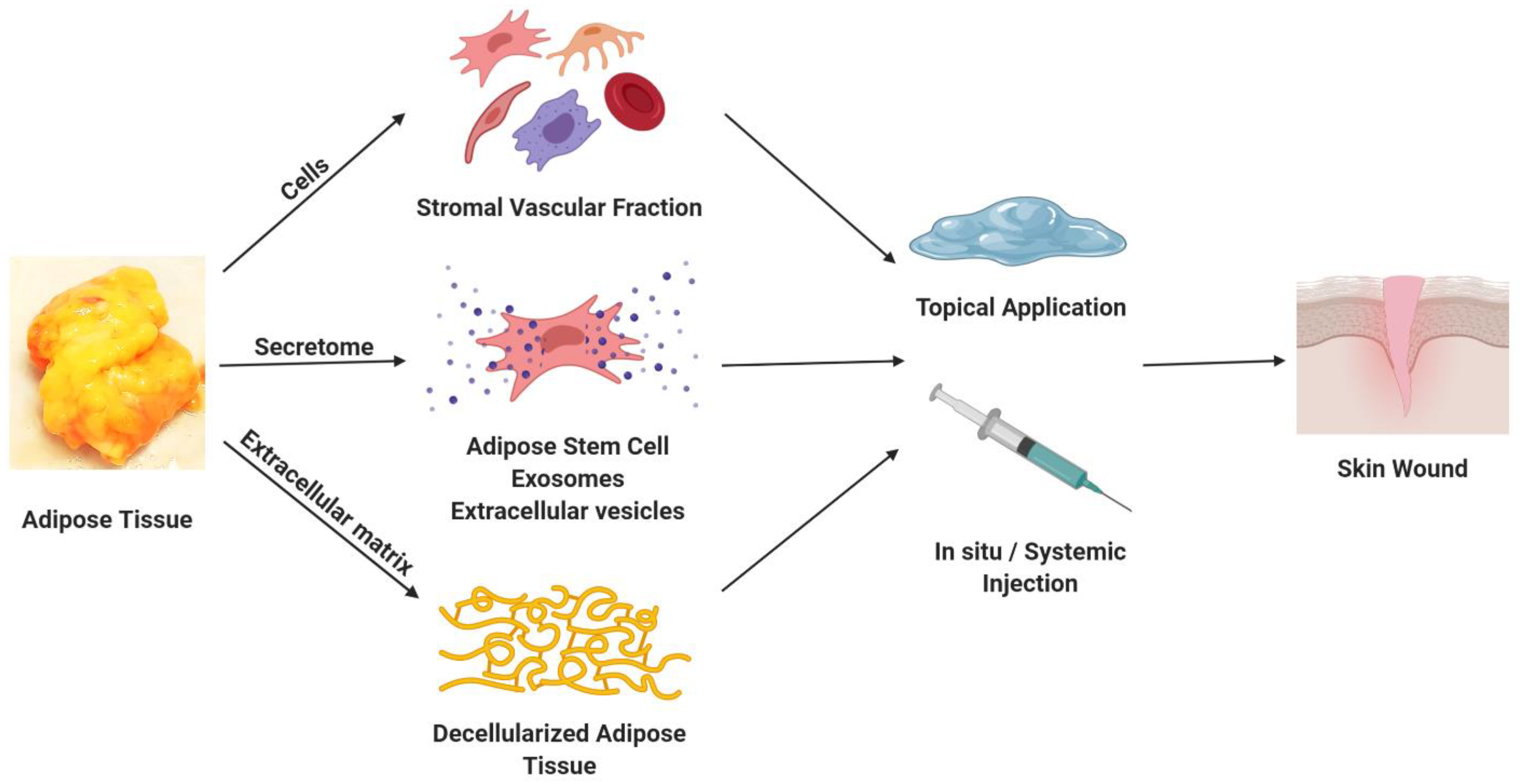
2. Adipose-Derived Cells
3. Adipose-Derived Secretomes, Exosomes, and Microvascular Tissues
4. Extracellular Matrix (ECM) from Decellularized Adipose Tissue
5. Blood Products
6. Modified Cellulose Products
7. Cellulose in Wound Healing
8. Cellulose Tissue Engineering Applications
9. TEMPO-Oxidized Nanocellulose
10. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atiyeh, B.S.; Hayek, S.N. Management of war-related burn injuries: Lessons learned from recent ongoing conflicts providing exceptional care in unusual places. J. Craniofacial Surg. 2010, 21, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- Tribble, D.R.; Krauss, M.R.; Murray, C.K.; Warkentien, T.E.; Lloyd, B.A.; Ganesan, A.; Greenberg, L.; Xu, J.; Li, P.; Carson, M.L.; et al. Epidemiology of Trauma-Related Infections among a Combat Casualty Cohort after Initial Hospitalization: The Trauma Infectious Disease Outcomes Study. Surg. Infect. Larchmt 2018, 19, 494–503. [Google Scholar] [CrossRef]
- Tribble, D.R.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Mende, K.; Blyth, D.M.; Petfield, J.L.; McDonald, J. After the Battlefield: Infectious Complications among Wounded Warriors in the Trauma Infectious Disease Outcomes Study. Mil. Med. 2019, 184 (Suppl. 2), 18–25. [Google Scholar] [CrossRef]
- McDonald, J.R.; Liang, S.Y.; Li, P.; Maalouf, S.; Murray, C.K.; Weintrob, A.C.; Schnaubelt, E.R.; Kuhn, J.; Ganesan, A.; Bradley, W.; et al. Infectious Complications after Deployment Trauma: Following Wounded US Military Personnel into Veterans Affairs Care. Clin. Infect. Dis. 2018, 67, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weintrob, A.C.; Murray, C.K.; Xu, J.; Krauss, M.; Bradley, W.; Warkentien, T.E.; Lloyd, B.A.; Tribble, D.R. Early Infections Complicating the Care of Combat Casualties from Iraq and Afghanistan. Surg. Infect. (Larchmt) 2018, 19, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, D.S.; Wade, C.E.; Baer, D.G. Burn hazards of the deployed environment in wartime: Epidemiology of noncombat burns from ongoing United States military operations. J. Am. Coll. Surg. 2009, 209, 453–460. [Google Scholar] [CrossRef] [PubMed]
- White-Chu, E.F.; Flock, P.; Struck, B.; Aronson, L. Pressure ulcers in long-term care. Clin. Geriatr. Med. 2011, 27, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Trueman, P.; Whitehead, S.J. The economics of pressure relieving surfaces: An illustrative case study of the impact of high-specification surfaces on hospital finances. Int. Wound J. 2010, 7, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Flack, S.; Apelqvist, J.; Keith, M.; Trueman, P.; Williams, D. An economic evaluation of VAC therapy compared with wound dressings in the treatment of diabetic foot ulcers. J. Wound Care 2008, 17, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Boudra, R.; Ramsey, M.R. Understanding Transcriptional Networks Regulating Initiation of Cutaneous Wound Healing. Yale J. Biol. Med. 2020, 93, 161–173. [Google Scholar]
- Huayllani, M.T.; Sarabia-Estrada, R.; Restrepo, D.J.; Boczar, D.; Sisti, A.; Nguyen, J.H.; Rinker, B.D.; Moran, S.L.; Quinones-Hinojosa, A.; Forte, A.J. Adipose-derived stem cells in wound healing of full-thickness skin defects: A review of the literature. J. Plast. Surg. Hand Surg. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Annacontini, L.; Carbone, A.; Beccia, E.; Cecchino, L.R.; Parisi, D.; Di Gioia, S.; Lembo, F.; Angiolillo, A.; Mastrangelo, F.; et al. The Role of Adipose-Derived Stem Cells, Dermal Regenerative Templates, and Platelet-Rich Plasma in Tissue Engineering-Based Treatments of Chronic Skin Wounds. Stem Cells Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef]
- Hu, P.; Yang, Q.; Wang, Q.; Shi, C.; Wang, D.; Armato, U.; Pra, I.D.; Chiarini, A. Mesenchymal stromal cells-exosomes: A promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burn. Trauma 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Coalson, E.; Bishop, E.; Liu, W.; Feng, Y.; Spezia, M.; Liu, B.; Shen, Y.; Wu, D.; Du, S.; Li, A.J.; et al. Stem cell therapy for chronic skin wounds in the era of personalized medicine: From bench to bedside. Genes Dis. 2019, 6, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Iorio, M.L.; Shuck, J.; Attinger, C.E. Wound healing in the upper and lower extremities: A systematic review on the use of acellular dermal matrices. Plast. Reconstr. Surg. 2012, 130 (Suppl. 2), 232S–241S. [Google Scholar] [CrossRef]
- Waycaster, C.R.; Gilligan, A.M.; Motley, T.A. Cost-Effectiveness of Becaplermin Gel on Diabetic Foot Ulcer HealingChanges in Wound Surface Area. J. Am. Podiatr. Med. Assoc. 2016, 106, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldo, B.A. Side effects of cytokines approved for therapy. Drug Saf. 2014, 37, 921–943. [Google Scholar] [CrossRef]
- VanGilder, C.; Lachenbruch, C.; Algrim-Boyle, C.; Meyer, S. The International Pressure Ulcer Prevalence Survey: 2006-2015: A 10-Year Pressure Injury Prevalence and Demographic Trend Analysis by Care Setting. J. Wound Ostomy Cont. Nurs. 2017, 44, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Vangilder, C.; Macfarlane, G.D.; Meyer, S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manag. 2008, 54, 40–54. [Google Scholar]
- Rayman, G.; Vas, P.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Apelqvist, J.; Attinger, C.; Game, F.; et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3283. [Google Scholar] [CrossRef] [Green Version]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Skardal, A.; Nelson, R.A., Jr.; Sunnon, K.; Reid, T.; Clouse, C.; Kock, N.D.; Jackson, J.; Soker, S.; Atala, A. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl. Med. 2020, 9, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.A.; Samsell, B.; Wallis, G.; Triplett, S.; Chen, S.; Jones, A.L.; Qin, X. Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: A review. Cell Tissue Bank. 2015, 16, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef]
- Eweida, A.M.; Marei, M.K. Naturally Occurring Extracellular Matrix Scaffolds for Dermal Regeneration: Do They Really Need Cells? Biomed. Res. Int. 2015, 2015, 839694. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed] [Green Version]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.R.; Frazier, T.; Rowan, B.G.; Gimble, J.M. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev. Clin. Immunol. 2013, 9, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mesimaki, K.; Lindroos, B.; Tornwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef]
- Sandor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerstrom, B.; Patrikoski, M.; Seppanen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.F.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Lindroos, B.; Boucher, S.; Chase, L.; Kuokkanen, H.; Huhtala, H.; Haataja, R.; Vemuri, M.; Suuronen, R.; Miettinen, S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009, 11, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Glovinski, P.V.; Herly, M.; Mathiasen, A.B.; Svalgaard, J.D.; Borup, R.; Talman, M.M.; Elberg, J.J.; Kolle, S.T.; Drzewiecki, K.T.; Fischer-Nielsen, A. Overcoming the bottleneck of platelet lysate supply in large-scale clinical expansion of adipose-derived stem cells: A comparison of fresh versus three types of platelet lysates from outdated buffy coat-derived platelet concentrates. Cytotherapy 2017, 19, 222–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicok, K.C.; Hedrick, M.H. Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol. Biol. 2011, 702, 87–105. [Google Scholar] [PubMed]
- Brown, J.C.; Shang, H.; Li, Y.; Yang, N.; Patel, N.; Katz, A.J. Isolation of Adipose-Derived Stromal Vascular Fraction Cells Using a Novel Point-of-Care Device: Cell Characterization and Review of the Literature. Tissue Eng. Part C Methods 2017, 23, 125–135. [Google Scholar] [CrossRef]
- Bukowska, J.; Alarcon Uquillas, A.; Wu, X.; Frazier, T.; Walendzik, K.; Vanek, M.; Gaupp, D.; Bunnell, B.A.; Kosnik, P.; Mehrara, B.; et al. Safety of Human Adipose Stromal Vascular Fraction Cells Isolated with a Closed System Device in an Immunocompetent Murine Pressure Ulcer Model. Stem Cells Dev. 2020, 29, 452–461. [Google Scholar] [CrossRef]
- Bukowska, J.; Alarcon Uquillas, A.; Wu, X.; Frazier, T.; Walendzik, K.; Vanek, M.; Gaupp, D.; Bunnell, B.A.; Kosnik, P.; Mehrara, B.; et al. Safety and Efficacy of Human Adipose-Derived Stromal/Stem Cell Therapy in an Immunocompetent Murine Pressure Ulcer Model. Stem Cells Dev. 2020, 29, 440–451. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Salgado, A.J.; Gimble, J.M. Secretome of mesenchymal stem/stromal cells in regenerative medicine. Biochimie 2013, 95, 2195. [Google Scholar] [CrossRef]
- Salgado, A.J.; Gimble, J.M.; Costa, B.M. The cell secretome in personalized and regenerative medicine. Biochimie 2018, 155, 1. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Casado-Diaz, A.; Quesada-Gomez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Martin, E.C.; Qureshi, A.T.; Dasa, V.; Freitas, M.A.; Gimble, J.M.; Davis, T.A. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie 2016, 124, 98–111. [Google Scholar] [CrossRef]
- Gimble, J.M.; Frazier, T.; Wu, X.; Uquillas, A.A.; Llamas, C.; Brown, T.; Nguyen, D.; Tucker, H.A.; Arm, D.M.; Peterson, D.R.; et al. A Novel, Sterilized Microvascular Tissue Product Improves Healing in a Murine Pressure Ulcer Model. Plast. Reconstr. Surg. Glob. Open 2018, 6, e2010. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Jia, J.; Hao, X.Y.; Yu, X.Y.; Liu, X.Y.; Shu, M.G. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/beta-catenin pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A., Jr.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Brown, C.F.; Yan, J.; Han, T.T.; Marecak, D.M.; Amsden, B.G.; Flynn, L.E. Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomed. Mater. 2015, 10, 45010. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.K.; Han, T.T.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.E.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials 2008, 29, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.M.; Omidi, E.; Flynn, L.E.; Samani, A. Comparative biomechanical study of using decellularized human adipose tissues for post-mastectomy and post-lumpectomy breast reconstruction. J. Mech. Behav. Biomed. Mater. 2016, 57, 235–245. [Google Scholar] [CrossRef]
- Han, T.T.; Toutounji, S.; Amsden, B.G.; Flynn, L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 2015, 72, 125–137. [Google Scholar] [CrossRef]
- Morissette Martin, P.; Shridhar, A.; Yu, C.; Brown, C.; Flynn, L.E. Decellularized Adipose Tissue Scaffolds for Soft Tissue Regeneration and Adipose-Derived Stem/Stromal Cell Delivery. In Adipose-Derived Stem Cells; Humana Press: New York, NY, USA, 2018; Volume 1773, pp. 53–71. [Google Scholar]
- Omidi, E.; Fuetterer, L.; Reza Mousavi, S.; Armstrong, R.C.; Flynn, L.E.; Samani, A. Characterization and assessment of hyperelastic and elastic properties of decellularized human adipose tissues. J. Biomech. 2014, 47, 3657–3663. [Google Scholar] [CrossRef]
- Shridhar, A.; Amsden, B.G.; Gillies, E.R.; Flynn, L.E. Investigating the Effects of Tissue-Specific Extracellular Matrix on the Adipogenic and Osteogenic Differentiation of Human Adipose-Derived Stromal Cells Within Composite Hydrogel Scaffolds. Front. Bioeng. Biotechnol. 2019, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Thomas-Porch, C.; Li, J.; Zanata, F.; Martin, E.C.; Pashos, N.; Genemaras, K.; Poche, J.N.; Totaro, N.P.; Bratton, M.R.; Gaupp, D.; et al. Comparative proteomic analyses of human adipose extracellular matrices decellularized using alternative procedures. J. Biomed. Mater. Res. Part A 2018, 106, 2481–2493. [Google Scholar] [CrossRef]
- Turner, A.E.; Yu, C.; Bianco, J.; Watkins, J.F.; Flynn, L.E. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials 2012, 33, 4490–4499. [Google Scholar] [CrossRef]
- Yu, C.; Bianco, J.; Brown, C.; Fuetterer, L.; Watkins, J.F.; Samani, A.; Flynn, L.E. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013, 34, 3290–3302. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, B.S.; Kim, J.Y.; Kim, J.D.; Choi, Y.C.; Yang, H.J.; Park, K.; Lee, H.Y.; Cho, Y.W. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J. Biomed. Mater. Res. Part A 2011, 97, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yang, H.J.; Kim, B.S.; Kim, J.D.; Kim, J.Y.; Yoo, B.; Park, K.; Lee, H.Y.; Cho, Y.W. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J. Control. Release 2009, 139, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Choi, J.S.; Kim, B.S.; Kim, J.D.; Yoon, H.I.; Cho, Y.W. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng. Part C Methods 2012, 18, 866–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Choi, Y.C.; Cho, Y.W. Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res. 2012, 348, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.; Nahas, Z.; Kimmerling, K.A.; Rosson, G.D.; Elisseeff, J.H. An injectable adipose matrix for soft-tissue reconstruction. Plast. Reconstr. Surg. 2012, 129, 1247–1257. [Google Scholar] [CrossRef] [Green Version]
- Banyard, D.A.; Borad, V.; Amezcua, E.; Wirth, G.A.; Evans, G.R.; Widgerow, A.D. Preparation, Characterization, and Clinical Implications of Human Decellularized Adipose Tissue Extracellular Matrix (hDAM): A Comprehensive Review. Aesthet Surg. J. 2016, 36, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Yu, M.; Zhang, Y.; Yin, Y.; Tian, W. Recent developments and clinical potential on decellularized adipose tissue. J. Biomed. Mater. Res. A 2018, 106, 2563–2574. [Google Scholar] [CrossRef]
- Sharath, S.S.; Ramu, J.; Nair, S.V.; Iyer, S.; Mony, U.; Rangasamy, J. Human Adipose Tissue Derivatives as a Potent Native Biomaterial for Tissue Regenerative Therapies. Tissue Eng. Regen. Med. 2020, 17, 123–140. [Google Scholar] [CrossRef]
- Kokai, L.E.; Schilling, B.K.; Chnari, E.; Huang, Y.C.; Imming, E.A.; Karunamurthy, A.; Khouri, R.K.; D’Amico, R.A.; Coleman, S.R.; Marra, K.G.; et al. Injectable Allograft Adipose Matrix Supports Adipogenic Tissue Remodeling in the Nude Mouse and Human. Plast. Reconstr. Surg. 2019, 143, 299e–309e. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Ma, M.; Rogers, E.; Gaupp, D.; Harrison, M.A.A.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 2019, 7, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Thomas-Porch, C.; Hayes, D.A.; Bunnell, B.A.; Gimble, J.M. Decellularized Adipose Tissue: Biochemical Composition, in vivo Analysis and Potential Clinical Applications. Cell Biol. Transl. Med. 2020, 6, 57–70. [Google Scholar]
- Mohiuddin, O.A.; O’Donnell, B.T.; Poche, J.N.; Iftikhar, R.; Wise, R.M.; Motherwell, J.M.; Campbell, B.; Savkovic, S.D.; Bunnell, B.A.; Hayes, D.J.; et al. Human Adipose-Derived Hydrogel Characterization Based on In Vitro ASC Biocompatibility and Differentiation. Stem Cells Int. 2019, 2019, 9276398. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, O.A.; Motherwell, J.M.; Rogers, E.; Bratton, M.R.; Zhang, Q.; Wang, G.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Characterization And Proteomic Analysis Of Decellularized Adipose Tissue Hydrogels Derived From Lean And Overweight/Obese Human Donors. Adv. Biosyst. 2020. accepted. [Google Scholar] [CrossRef]
- Li, S.; Poche, J.N.; Liu, Y.; Scherr, T.; McCann, J.; Forghani, A.; Smoak, M.; Muir, M.; Berntsen, L.; Chen, C.; et al. Hybrid Synthetic-Biological Hydrogel System for Adipose Tissue Regeneration. Macromol. Biosci. 2018, 18, e1800122. [Google Scholar] [CrossRef]
- Kokai, L.E.; Sivak, W.N.; Schilling, B.K.; Karunamurthy, A.; Egro, F.M.; Schusterman, M.A.; Minteer, D.M.; Simon, P.; D’Amico, R.A.; Rubin, J.P. Clinical Evaluation of an Off-the-Shelf Allogeneic Adipose Matrix for Soft Tissue Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2574. [Google Scholar] [CrossRef]
- Giatsidis, G.; Succar, J.; Haddad, A.; Lago, G.; Schaffer, C.; Wang, X.; Schilling, B.; Chnari, E.; Matsumine, H.; Orgill, D.P. Preclinical Optimization of a Shelf-Ready, Injectable, Human-Derived, Decellularized Allograft Adipose Matrix. Tissue Eng. Part A 2019, 25, 271–287. [Google Scholar] [CrossRef]
- Banerjee, J.; Seetharaman, S.; Wrice, N.L.; Christy, R.J.; Natesan, S. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS ONE 2019, 14, e0217965. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In Situ Delivery of Fibrin-Based Hydrogels Prevents Contraction and Reduces Inflammation. J. Burn. Care Res. 2018, 39, 40–53. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Stone, R., 2nd; Wrice, N.; Laborde, A.; Becerra, S.C.; Natesan, S.; Christy, R.J. Delivery of Allogeneic Adipose Stem Cells in Polyethylene Glycol-Fibrin Hydrogels as an Adjunct to Meshed Autografts After Sharp Debridement of Deep Partial Thickness Burns. Stem Cells Transl. Med. 2018, 7, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Natesan, S.; Li, J.; Valdes, J.; Harding, A.; Solis, M.; Davis, S.C.; Christy, R.J. A PEGylated fibrin hydrogel-based antimicrobial wound dressing controls infection without impeding wound healing. Int. Wound J. 2017, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Stone, R.; Coronado, R.E.; Wrice, N.L.; Kowalczewski, A.C.; Zamora, D.O.; Christy, R.J. PEGylated Platelet-Free Blood Plasma-Based Hydrogels for Full-Thickness Wound Regeneration. Adv. Wound Care 2019, 8, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Samberg, M.; Stone, R., 2nd; Natesan, S.; Kowalczewski, A.; Becerra, S.; Wrice, N.; Cap, A.; Christy, R. Platelet rich plasma hydrogels promote in vitro and in vivo angiogenic potential of adipose-derived stem cells. Acta Biomater. 2019, 87, 76–87. [Google Scholar] [CrossRef]
- Bender, R.M.M.; Brown, T.; Bukowska, J.; Smith, S.; Abbott, R.D.; Kaplan, D.L.; Williams, C.; Wade, J.W.; Alarcon, A.; Wu, X.; et al. Human Adipose Derived Cells in Two- and Three-Dimensional Cultures: Functional Validation of an In Vitro Fat Construct. Stem Cells Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013, 41, D377–D386. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S. Wound Management and Dressings; Pharmaceutical Press: Ann Arbor, MI, USA, 1990; p. 211. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.; Nam, S.; Concha, M.; Condon, B. Synthesis and assessment of peptide nanocellulosic biosensors. In Nanocellulose, Cellulose Nanofibers and Cellulose Nanocomposites: Synthesis and Applications; Mondal, I.H., Ed.; Nova Science: New York, NY, USA, 2016; pp. 475–494. [Google Scholar]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose—Structure and characterization. Cell. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Edwards, J.V.; Castro, N.J.; Condon, B.; Costable, C.; Goheen, S.C. Chromatographic and traditional albumin isotherms on cellulose: A model for wound protein adsorption on modified cotton. J. Biomater. Appl. 2012, 26, 939–961. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Laine, J.; Rojas, O.J. Modification of cellulose films by adsorption of CMC and chitosan for controlled attachment of biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef] [Green Version]
- Čolić, M.; Mihajlović, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating thecytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Palamand, S.; Brenden, R.; Reed, A. Intelligent wound dressings and their physical characteristics. Wounds Compend. Clin. Res. Pract. 1992, 3, 149–156. [Google Scholar]
- Edwards, J.V. Future structure and properties of mechanism-based wound dressing. In Modified Fibers with Medical and Specialty Applications; Edwards, J.V., Buschle-Diller, G., Goheen, S.C., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 11–33. [Google Scholar]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, M.; Helbert, W.; Imai, T.; Sugiyama, J.; Henrissat, B. Parallel-up structure evidences the molecular directionality during biosynthesis of bacterial cellulose. Proc. Natl. Acad. Sci. USA 1997, 94, 9091–9095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulos Ib from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. A Revised Structure and Hydrogen-Bonding System in Cellulose II from a Neutron Fiber Diffraction Analysis. J. Am. Chem. Soc. 1999, 121, 9940–9946. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray Structure of Mercerized Cellulose II at 1 Å Resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Perez, S.; Samain, D. Structure and Enginnering of Cellulloses. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2010; Volume 64, pp. 25–116. [Google Scholar]
- El-Hoseny, S.M.; Basmaji, P.; Olyveira, G.M.D.; Costa, L.M.M.; Alwahedi, A.M.; Oliveira, J.D.D.C.; Francozo, G.B. Natural ECM-Bacterial Cellulose Wound Healing—Dubai Study. J. Biomater. Nanobiotechnol. 2015, 6, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Ávila, H.M.; Schwarz, S.; Feldmann, E.-M.; Mantas, A.; Bomhard, A.V.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotechnol. 2014, 98, 7423–7435. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Álemvila, H.M.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose−Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Backdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.A.H.; Kramer, F.; Fried, W.; Wippermann, J.; Kinne, R.W. Bacterial nanocellulose hydrogels designed as bioartificial medical implants. In Bacterial Nanocellulose: A Sophisticated Multifunctional Material. Perspectives in Nanotechnology; Gama, M.G.P., Klemm, D., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 175–196. [Google Scholar]
- Gatenholm, P.B.J.; Rojas, R.; Sano, M.B.; Davalos, R.V.; Johnson, K.; Rourke, L.O. Bacterial nanocellulose biomaterials with controlled architecture for tissue engineering scaffolds and customizable implants. In Bacterial Nanocellulose: A Sophisticated Multifunctional Material. Perspectives in Nanotechnology; Gama, M.G.P., Klemm, D., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 197–216. [Google Scholar]
- Nimeskern, L.; Martinez Avila, H.; Sundberg, J.; Gatenholm, P.; Muller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstr, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Pathak, V.; Soni, B. Minocycline-loaded cellulose nano whiskers/poly(sodium acrylate) composite hydrogel films as wound dressing. Int. J. Biol. Macromol. 2015, 79, 76–85. [Google Scholar] [CrossRef]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drugdelivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Edwards, J.V.; Yager, D.R.; Cohen, I.K.; Diegelmann, R.F.; Montante, S.; Bertoniere, N.; Bopp, A. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen. 2001, 9, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Bopp, A.F.; Batiste, S.; Ullah, A.J.; Cohen, I.K.; Diegelmann, R.F.; Montante, S.J. Inhibition of elastase by a synthetic cotton-bound serine protease inhibitor: In vitro kinetics and inhibitor release. Wound Repair Regen. 1999, 7, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Wound Care Handbook: Protease Modulating Dressings. Available online: https://www.woundcarehandbook.com/configuration/categories/wound-care/protease-modulating-dressings/ (accessed on 20 August 2020).
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Condon, B.D.; Qureshi, H.; Yager, D. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: Implications of material selection for dressing and protease sensor design. J. Biomater. Appl. 2017, 32, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Grad, S.; Stoddart, M.J.; Farè, S.; Altomare, L.; Azzimonti, B.; Alini, M.; Rimondini, L. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermoreversible methylcellulose-based hydrogel. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Contessi, N.; Altomare, L.; Fillipponi, A.; Fare, S. Thermo-responsive properties of methylcellulose hydrogels for cell sheet engineering. Mater. Lett. 2017, 207, 157–160. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Shin, S.; Hyun, J. Effect of negatively charged cellulose nanofibers on the dispersion of hydroxyapatite nanoparticles for scaffolds in bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 130, 222–228. [Google Scholar] [CrossRef]
- Huang, Y.; Jing, W.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041. [Google Scholar] [CrossRef]
- Hoshiba, T.; Lu, H.; Kawazoe, N.; Chen, G. Decellularized matrices for tissue engineering. Expert Opin. Biol. Ther. 2010, 10, 1717–1728. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, L.C.; Khan, S.; Chinga-Carrasco, G.; Wright, C.J.; Hill, K.E.; Thomas, D.W. An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohydr. Polym. 2016, 137, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Powell, K.A.; Kishnani, H.; Johnsen, P.O.; Pukstad, B.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohydr. Polym. 2017, 157, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Makitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef]
- Hooshmand, S.; Aitomäki, Y.; Berglund, L.; Mathew, A.P.; Oksman, K. Enhanced alignment and mechanical properties through the use of hydroxyethyl cellulose in solvent-free native cellulose spun filaments. Compos. Sci. Technol. 2017, 150, 79–86. [Google Scholar] [CrossRef]
- Minelli, M.; Baschetti, M.G.; Doghieri, F.; Ankerfors, M.; Lindström, T.; Siró, I.; Plackett, D. Investigation of mass transport properties of microfibrillated cellulose (MFC) films. Science 2010, 358, 67–75. [Google Scholar] [CrossRef]
- Sun, F.; Nordli, H.R.; Pukstad, B.; Gamstedt, E.K.; Chinga-Carrasco, G. Mechanical characteristics of nanocellulose-PEG bionanocomposite wound dressings in wet conditions. J. Mech. Behav. Biomed. 2017, 69, 377–384. [Google Scholar] [CrossRef]
- Miettinen, A.; Chinga-Carrasco, G.; Kataja, M. Three-dimensional microstructural properties of nanofibrillated cellulose films. Int. J. Mol. Sci. 2014, 15, 6423–6440. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly (vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Huang, R.; Huang, Z.; Hu, B.; Zheng, W.; Yang, G.; Jiang, X. Evaluation of the Effect of the Structure of Bacterial Cellulose on Full Thickness Skin Wound Repair on a Microfluidic Chip. Biomacromolecules 2015, 16, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Mertaniemi, H.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Gandía, C.; Mäkitie, A.; Partanen, J.; Ikkala, O.; Yliperttula, M. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016, 82, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, M.; Fournier, C.; Driemeier, C.; Picart, C.; Foster, E.J.; Bras, J. Tunable structural and mechanical properties of cellulose nanofiber substrates in aqueous condition for stem cell culture. Biomacromolecules 2017, 18, 2034–2044. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Urtti, A.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]

| Type | Description | Commercial Products |
|---|---|---|
| Extracellular matrix (ECM) | Human acellular dermis | GRAFTJACKETTM (Wright Medical Technology, Inc., Arlington, TN, USA) AlloDerm® (Biohorizons implant systems Inc., Hoover, AL, USA) DermACELL AWM® (LifeNet Health Inc., Virginia Beach, VA, USA) DermaMatrixTM Acellular matrix (Synthes, Inc., West Chester, PA, USA) Surederm (Hans Biomed Corp., Seoul, Korea) |
| Human acellular amniotic membrane | Neox® (Amniox Medical, Miami, FL, USA) Epifix® (MiMedx Group Inc, Marietta, GA, USA) | |
| Porcine acellular dermis | EZ-DERM® (Mölnlycke Health Care AB, Gothenburg, Sweden), | |
| Porcine acellular small intestinal submucosa | OASIS® Wound Matrix (Healthpoint, Fort Worth, TX, USA) | |
| Porcine acellular urinary bladder matrix | MicroMatrix® (ACell, Columbia, MD, USA) | |
| Porcine dermal collagen cross-linked with fine nylon mesh | Biobrane® (Smith & Nephew, London, UK) | |
| Bovine acellular dermis | Integra® (Integra LifeSciences, Plainsboro, NJ, USA) | |
| Sheep fore-stomach submucosa | Endoform® (Aroa Biosurgery Ltd. Auckland, New Zealand) | |
| Fibrin-based sealant | Tisseel® (Baxter International, Deerfield, IL, USA) Evicel® (Ethicon Inc., Somerville, NJ, USA) | |
| Hyaluronic-acid-based skin substitute | Hyaff® (Fidia Advanced Biopolymers, Abano Terme, Italy) | |
| Cells/ECM | Cultured autologous keratinocytes | Epicel® (Genzyme, Cambridge, MA, USA) |
| Autologous epidermal cells in liquid suspension | ReCell® (Avita Medical, Cambridge, UK) | |
| Bovine collagen I gel seeded with neonatal foreskin fibroblasts and keratinocytes | Apligraf® (Organogenesis, Canton, MA, USA) | |
| Cells/Synthetic matrix | PLGA scaffold seeded with neonatal fibroblasts | Dermagraft® (Organogenesis, Canton, MA, USA) |
| Growth factor | Human recombinant platelet-derived growth factor gel | Regranax® (Ortho-McNeil Pharmaceutical, Raritan, NJ, USA) |
| Properties | AdipoGel | Cellulose | ObaGel | |
|---|---|---|---|---|
| Physicochemical | Source | Adipose | Plant | Blood |
| Enriched proteins or polymers | ECM | Cellulose | Angio/Coag | |
| Chemical modifiability | ++ | ++ | ++ | |
| Potential stability at room temperature | ++ | +++ | ++ | |
| In vitro | Stromal-cell-compatible | ASC | TBD | ASC |
| ASC differentiation | Adipogenic and osteogenic | TBD | Adipogenic and endothelial | |
| In vivo | Angiogenic | + | TBD | +++ |
| Soft tissue regeneration | ++ | TBD | + | |
| Hemostatic | TBD | ++ | TBD | |
| Antimicrobial | TBD | +++ | TBD | |
| Potential for synergy in combination | +++ | +++ | +++ | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazier, T.; Alarcon, A.; Wu, X.; Mohiuddin, O.A.; Motherwell, J.M.; Carlsson, A.H.; Christy, R.J.; Edwards, J.V.; Mackin, R.T.; Prevost, N.; et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules 2020, 10, 1373. https://doi.org/10.3390/biom10101373
Frazier T, Alarcon A, Wu X, Mohiuddin OA, Motherwell JM, Carlsson AH, Christy RJ, Edwards JV, Mackin RT, Prevost N, et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules. 2020; 10(10):1373. https://doi.org/10.3390/biom10101373
Chicago/Turabian StyleFrazier, Trivia, Andrea Alarcon, Xiying Wu, Omair A. Mohiuddin, Jessica M. Motherwell, Anders H. Carlsson, Robert J. Christy, Judson V. Edwards, Robert T. Mackin, Nicolette Prevost, and et al. 2020. "Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels" Biomolecules 10, no. 10: 1373. https://doi.org/10.3390/biom10101373
APA StyleFrazier, T., Alarcon, A., Wu, X., Mohiuddin, O. A., Motherwell, J. M., Carlsson, A. H., Christy, R. J., Edwards, J. V., Mackin, R. T., Prevost, N., Gloster, E., Zhang, Q., Wang, G., Hayes, D. J., & Gimble, J. M. (2020). Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules, 10(10), 1373. https://doi.org/10.3390/biom10101373





