Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Plasmid Constructs
2.2.1. Mammalian Expression Vectors
2.2.2. Reporter Vectors
2.2.3. RNA Interference (RNAi) Expression Vectors
2.2.4. Validation of Constructs
2.3. Transfection
2.4. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
2.5. Immunofluorescence
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western and Dot Blotting
2.8. Phospho-Receptor Tyrosine Kinase (pRTK) Array
2.9. Zymography
2.10. Cell Proliferation Assay
2.11. Statistical Analysis
3. Results
3.1. Expression of Wild-Type and Truncated Syndecan-1 in Hepatoma Cells
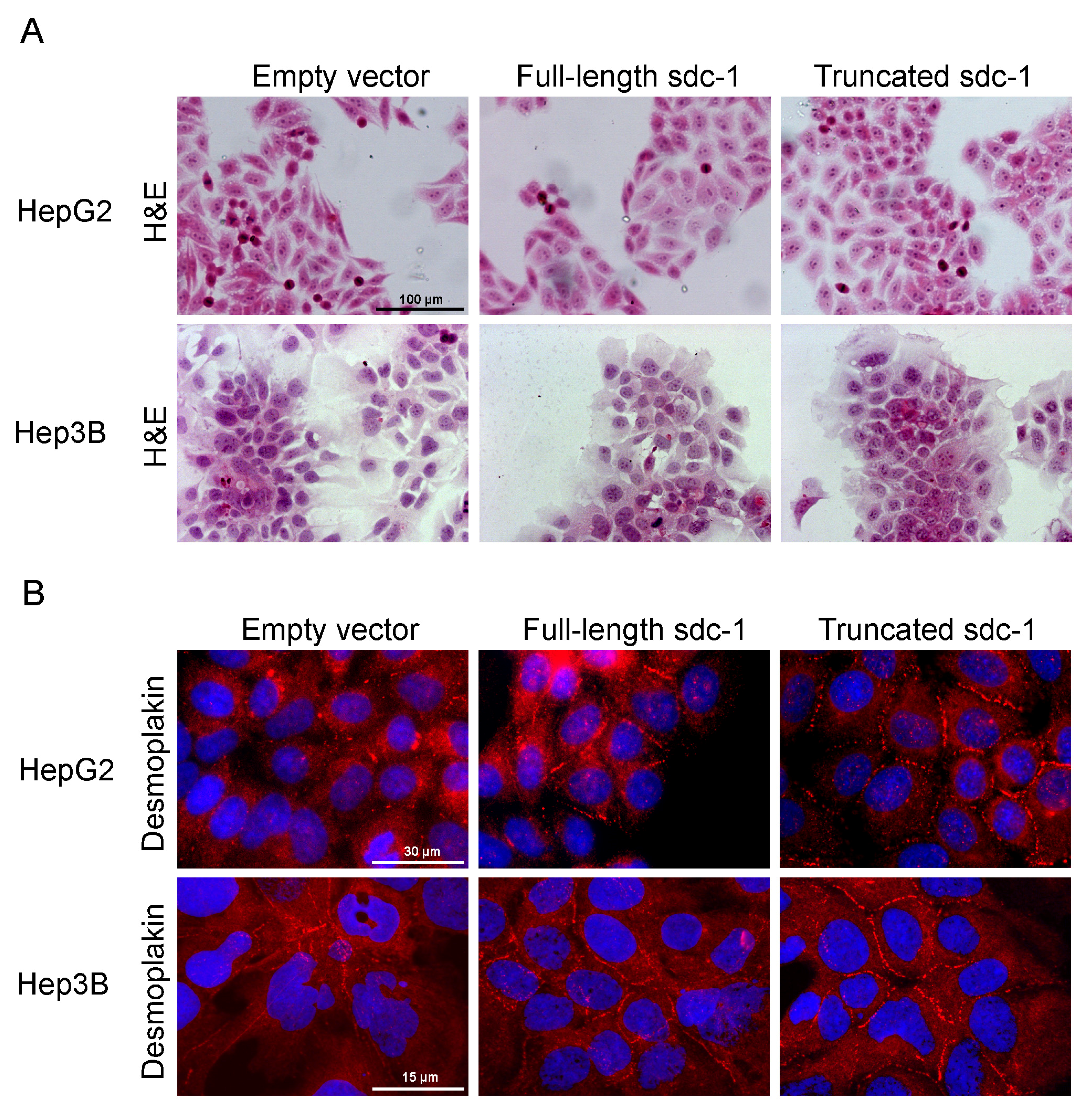
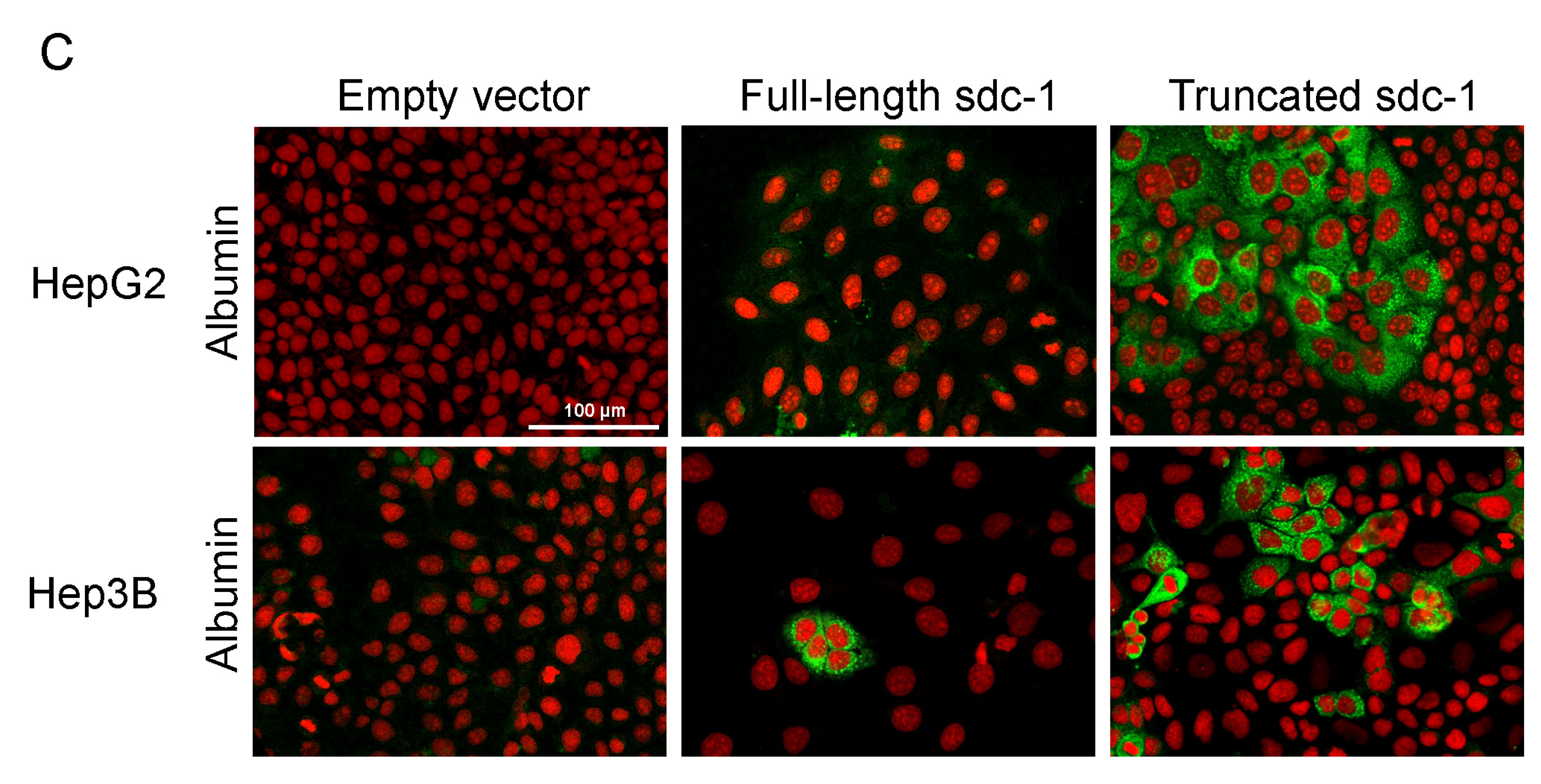
3.2. Overexpression of Full-Length or Truncated Syndecan-1 Induces a Hepatocyte-Like Cell Morphology
3.3. Syndecan-1 Status of the Transfectant Cells
3.4. Shift of Heparan Sulfate Epitope Abundance in Syndecan-1-Transfected Hep3B Cells
3.5. Syndecan-1 Shedding is Inhibited by Truncated Syndecan-1 in Hepatoma Cells
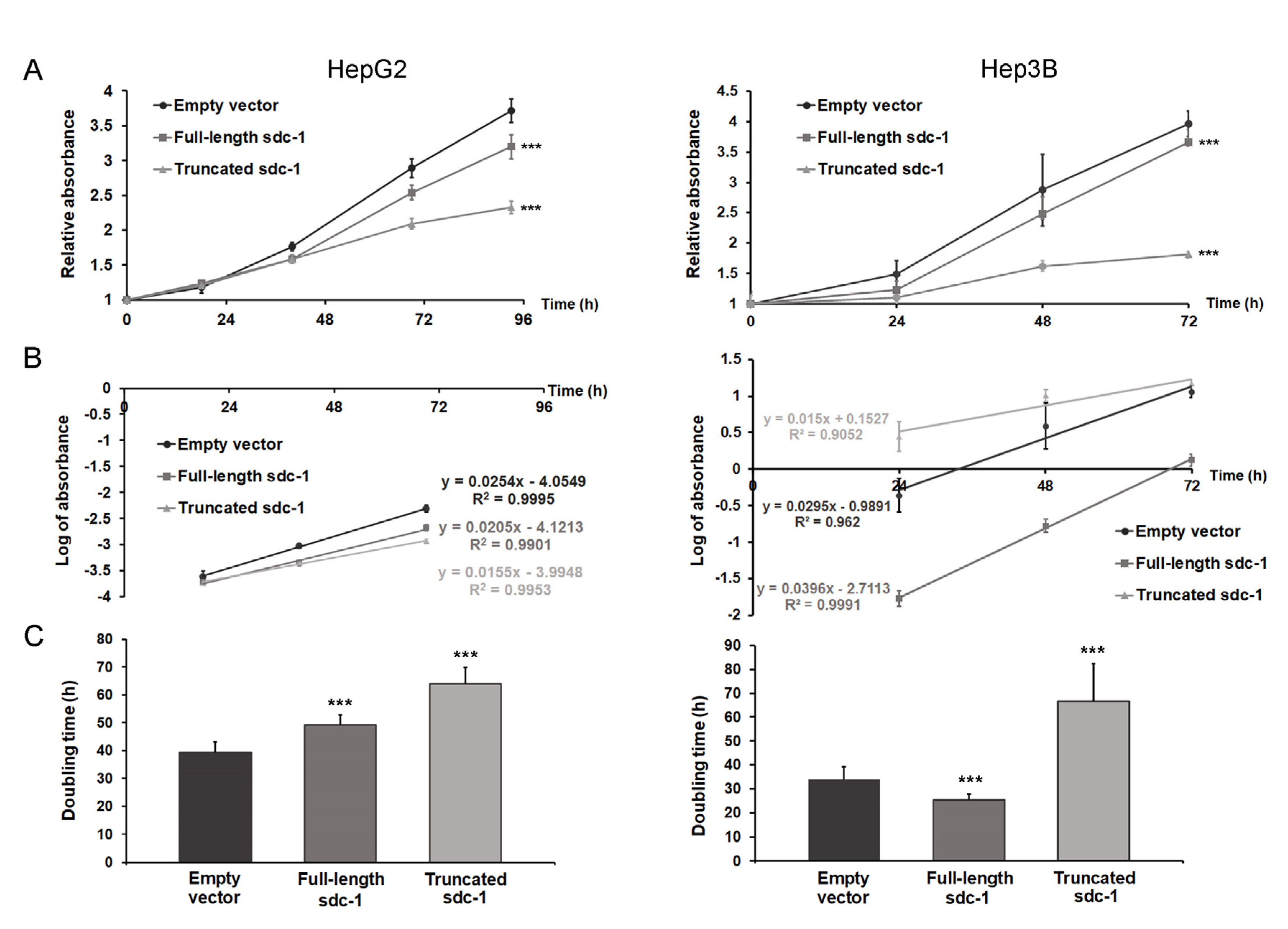
3.6. Truncated Syndecan-1 Suppresses Proliferation of Hepatoma Cells
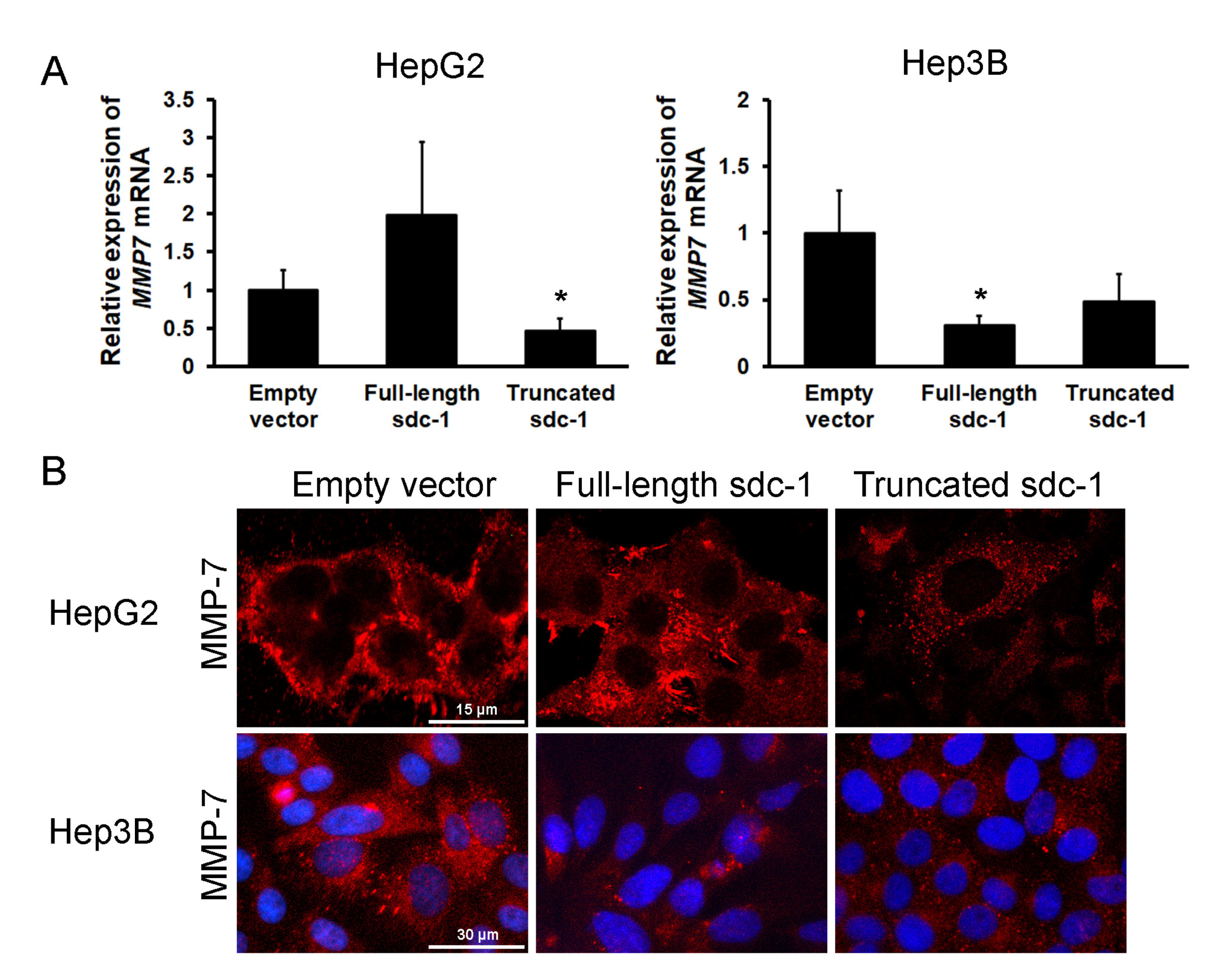
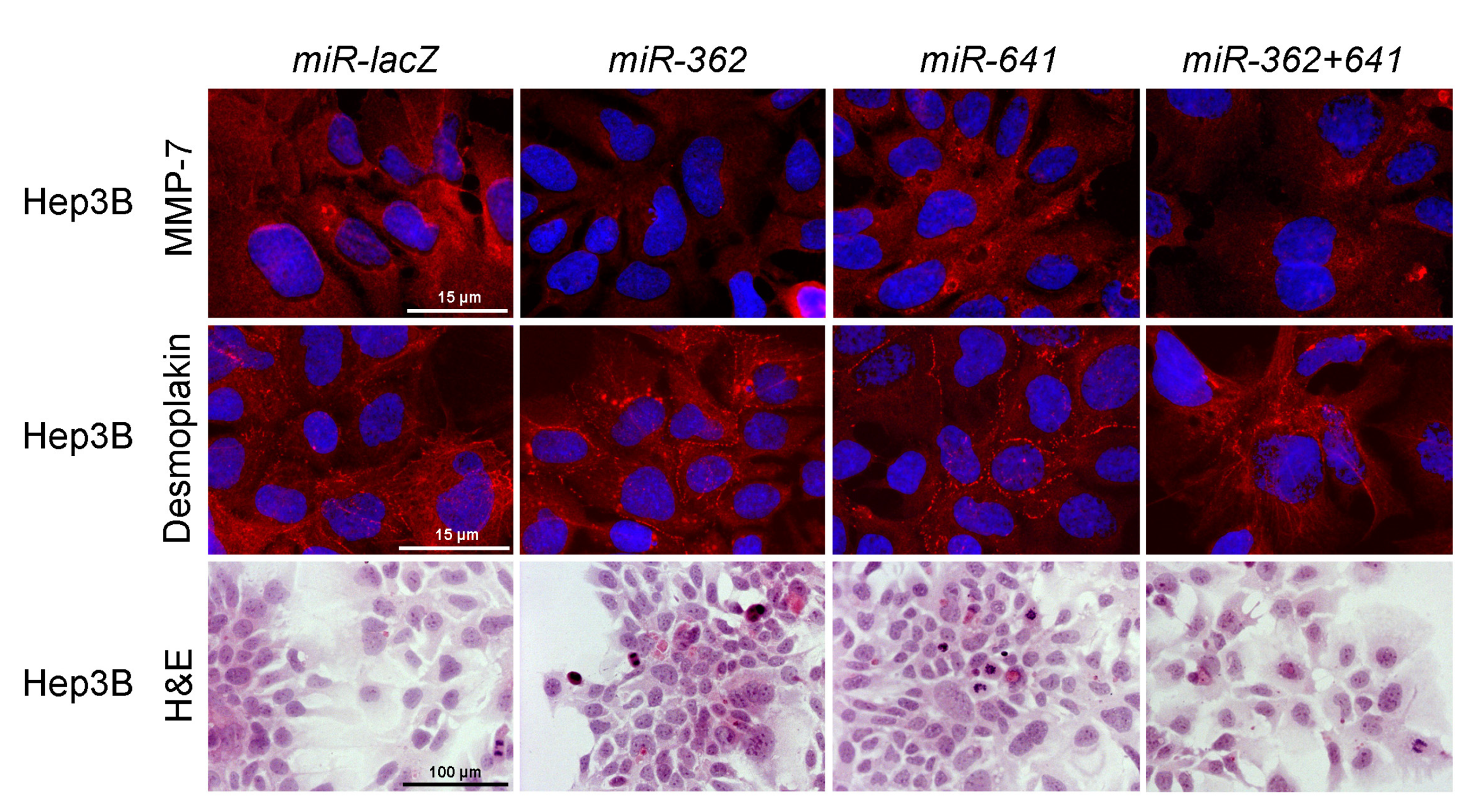
3.7. Overexpression of Truncated Syndecan-1 Downregulates MMP-7 Expression in HCC Cells
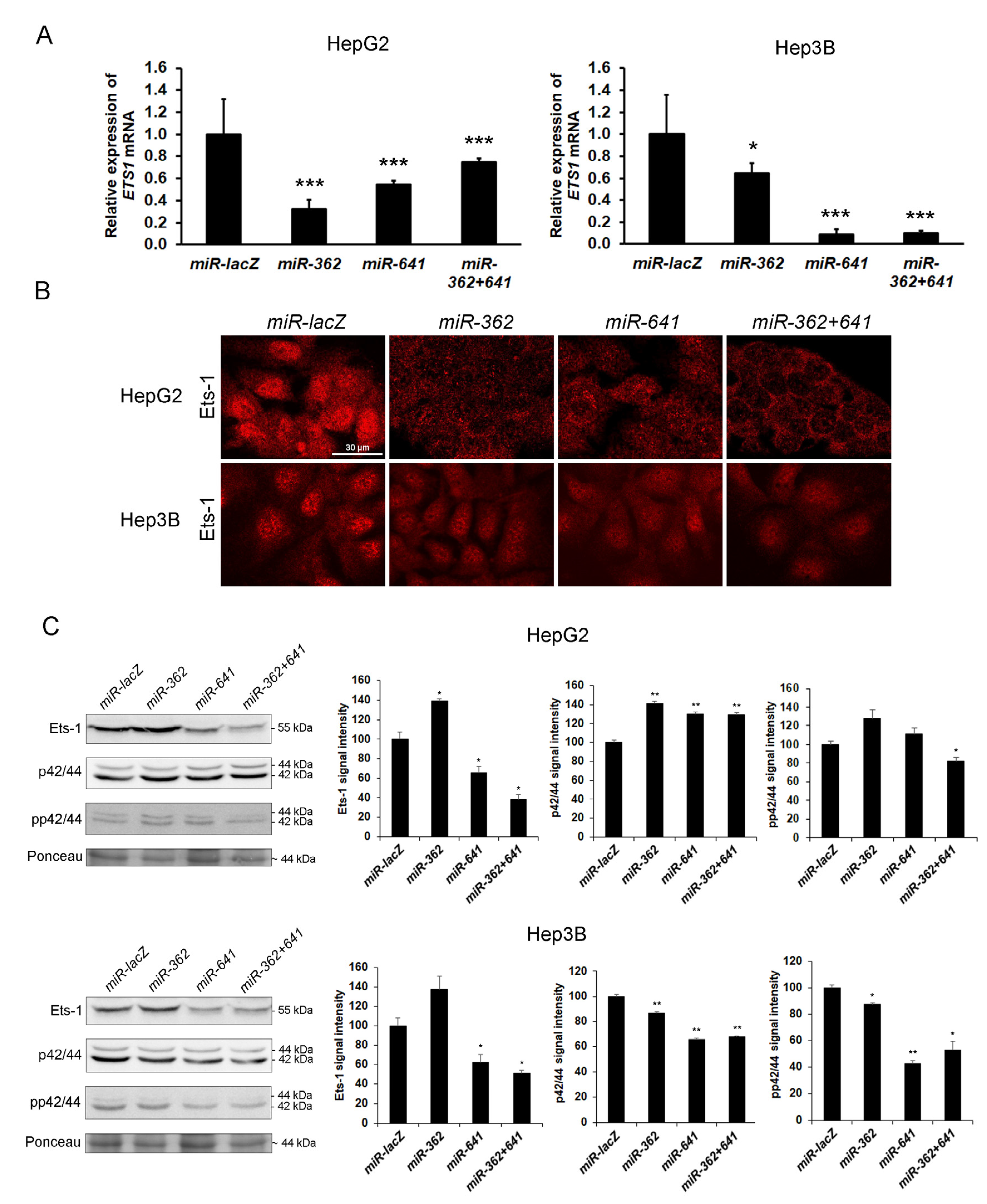
3.8. Signaling Pathways Converging to Ets-1 Are Downregulated in Hepatoma Cells Overexpressing Full-Length or Truncated Syndecan-1
3.9. Silencing of Ets-1 Alone is Insufficient to Induce Epithelial Differentiation in Hepatoma Cell Lines
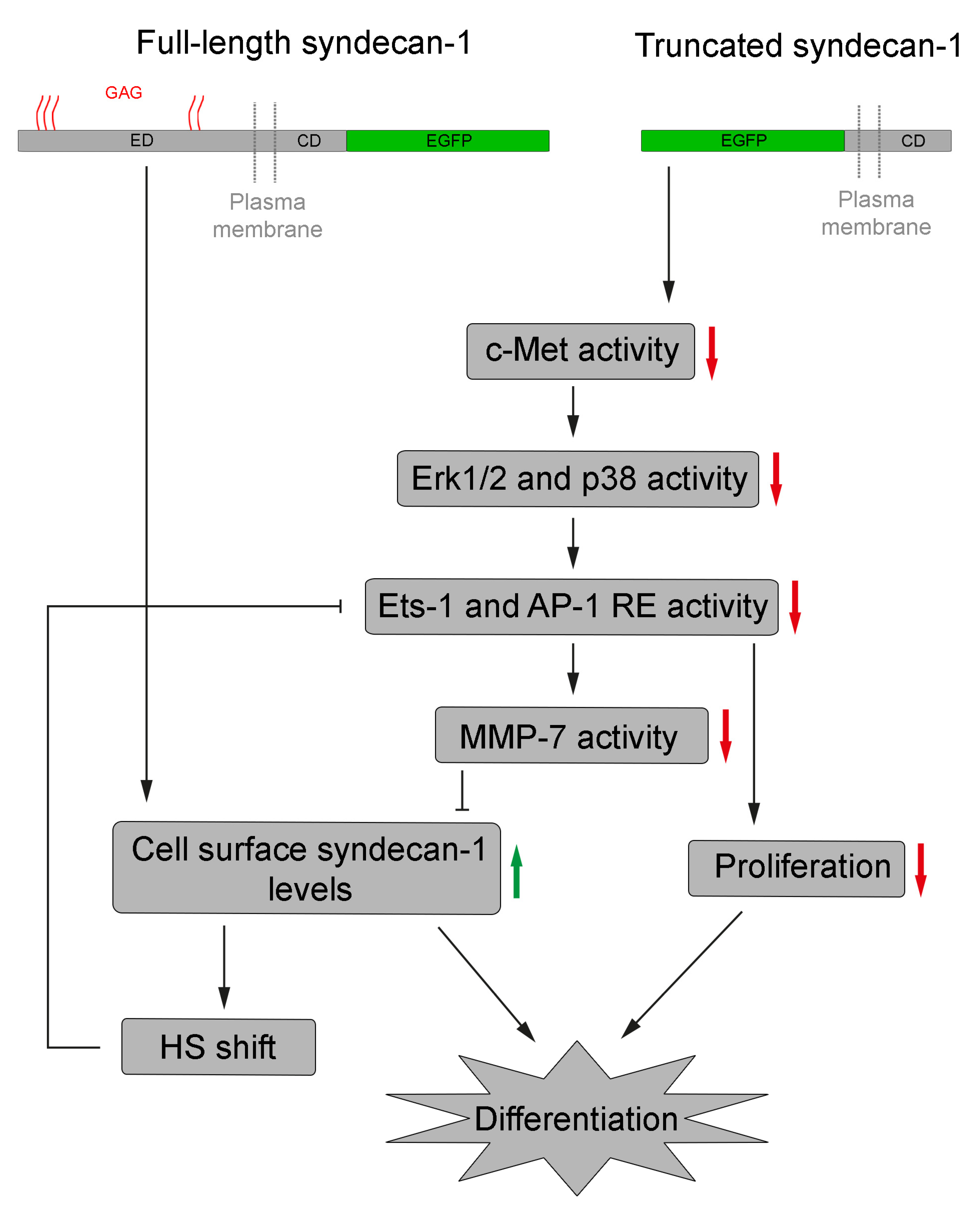
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D.; Curado, M.P.; Franceschi, S.; Hytiroglou, P.; Kudo, M.; Park, Y.N.; Sakamoto, M.; Torbenson, M.; Wee, A. Tumours of the liver and intrahepatic bile ducts: Hepatocellular carcinoma. In World Health Organization Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; Volume 3, pp. 205–216. [Google Scholar]
- Laurent-Puig, P.; Zucman-Rossi, J. Genetics of hepatocellular tumors. Oncogene 2006, 25, 3778–3786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravalli, R.N.; Steer, C.J.; Cressman, E.N. Molecular mechanisms of hepatocellular carcinoma. Hepatology 2008, 48, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Rapraeger, A.; Jalkanen, M.; Endo, E.; Koda, J.; Bernfield, M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J. Biol. Chem. 1985, 260, 11046–11052. [Google Scholar] [PubMed]
- Saunders, S.; Jalkanen, M.; O’Farrell, S.; Bernfield, M. Molecular cloning of syndecan, an integral membrane proteoglycan. J. Cell Biol. 1989, 108, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, M.; Jalkanen, M.; Firestone, J.H.; Trelstad, R.L.; Bernfield, M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J. Histochem. Cytochem. 1987, 35, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskams, T.; Moshage, H.; De Vos, R.; Guido, D.; Yap, P.; Desmet, V. Heparan sulfate proteoglycan expression in normal human liver. Hepatology 1995, 21, 950–958. [Google Scholar] [CrossRef]
- Roskams, T.; De Vos, R.; David, G.; Van Damme, B.; Desmet, V. Heparan sulphate proteoglycan expression in human primary liver tumours. J. Pathol. 1998, 185, 290–297. [Google Scholar] [CrossRef]
- Tátrai, P.; Egedi, K.; Somorácz, A.; van Kuppevelt, T.H.; Ten Dam, G.; Lyon, M.; Deakin, J.A.; Kiss, A.; Schaff, Z.; Kovalszky, I. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2010, 58, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Kambham, N.; Kong, C.; Longacre, T.A.; Natkunam, Y. Utility of syndecan-1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: A tissue microarray study of 1754 cases. Appl. Immunohistochem. Mol. Morphol. AIMM 2005, 13, 304–310. [Google Scholar] [CrossRef]
- Ramalingam, P.; Adeagbo, B.; Bollag, R.; Lee, J.; Reid-Nicholson, M. Metastatic hepatocellular carcinoma with CD138 positivity: An unusual mimic of multiple myeloma? Diagn. Cytopathol. 2008, 36, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ono, M.; Fujimoto, Y.; Gallo, R.L.; Bernfield, M.; Kohgo, Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int. J. Cancer 1997, 74, 482–491. [Google Scholar] [CrossRef]
- Li, H.G.; Xie, D.R.; Shen, X.M.; Li, H.H.; Zeng, H.; Zeng, Y.J. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, M.; Rapraeger, A.; Saunders, S.; Bernfield, M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J. Cell Biol. 1987, 105, 3087–3096. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.W.; Goldberger, O.A.; Gallo, R.L.; Bernfield, M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 1994, 5, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Metwaly, H.A.; Al-Gayyar, M.M.; Eletreby, S.; Ebrahim, M.A.; El-Shishtawy, M.M. Relevance of serum levels of interleukin-6 and syndecan-1 in patients with hepatocellular carcinoma. Sci. Pharm. 2012, 80, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Nault, J.C.; Guyot, E.; Laguillier, C.; Chevret, S.; Ganne-Carrie, N.; N’Kontchou, G.; Beaugrand, M.; Seror, O.; Trinchet, J.C.; Coelho, J.; et al. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Zong, F.; Fthenou, E.; Wolmer, N.; Hollósi, P.; Kovalszky, I.; Szilák, L.; Mogler, C.; Nilsonne, G.; Tzanakakis, G.; Dobra, K. Syndecan-1 and FGF-2, but not FGF receptor-1, share a common transport route and co-localize with heparanase in the nuclei of mesenchymal tumor cells. PLoS ONE 2009, 4, e7346. [Google Scholar] [CrossRef] [Green Version]
- Zong, F.; Fthenou, E.; Castro, J.; Péterfia, B.; Kovalszky, I.; Szilák, L.; Tzanakakis, G.; Dobra, K. Effect of syndecan-1 overexpression on mesenchymal tumour cell proliferation with focus on different functional domains. Cell Prolif. 2010, 43, 29–40. [Google Scholar] [CrossRef]
- Zong, F.; Fthenou, E.; Mundt, F.; Szatmári, T.; Kovalszky, I.; Szilák, L.; Brodin, D.; Tzanakakis, G.; Hjerpe, A.; Dobra, K. Specific syndecan-1 domains regulate mesenchymal tumor cell adhesion, motility and migration. PLoS ONE 2011, 6, e14816. [Google Scholar] [CrossRef]
- Péterfia, B.; Füle, T.; Baghy, K.; Szabadkai, K.; Fullár, A.; Dobos, K.; Zong, F.; Dobra, K.; Hollósi, P.; Jeney, A.; et al. Syndecan-1 enhances proliferation, migration and metastasis of HT-1080 cells in cooperation with syndecan-2. PLoS ONE 2012, 7, e39474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatmári, T.; Mundt, F.; Heidari-Hamedani, G.; Zong, F.; Ferolla, E.; Alexeyenko, A.; Hjerpe, A.; Dobra, K. Novel genes and pathways modulated by syndecan-1: Implications for the proliferation and cell-cycle regulation of malignant mesothelioma cells. PLoS ONE 2012, 7, e48091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatmári, T.; Dobra, K. The role of syndecan-1 in cellular signaling and its effects on heparan sulfate biosynthesis in mesenchymal tumors. Front. Oncol. 2013, 3, 310. [Google Scholar] [CrossRef] [Green Version]
- Aden, D.P.; Fogel, A.; Plotkin, S.; Damjanov, I.; Knowles, B.B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 1979, 282, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.B.; Howe, C.C.; Aden, D.P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980, 209, 497–499. [Google Scholar] [CrossRef]
- Fisher, R.J.; Mavrothalassitis, G.; Kondoh, A.; Papas, T.S. High-affinity DNA-protein interactions of the cellular ETS1 protein: The determination of the ETS binding motif. Oncogene 1991, 6, 2249–2254. [Google Scholar]
- Nye, J.A.; Petersen, J.M.; Gunther, C.V.; Jonsen, M.D.; Graves, B.J. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992, 6, 975–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, D.B.; Ghysdael, J.; Owen, M.J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992, 20, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Van Kuppevelt, T.H.; Dennissen, M.A.; van Venrooij, W.J.; Hoet, R.M.; Veerkamp, J.H. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J. Biol. Chem. 1998, 273, 12960–12966. [Google Scholar] [CrossRef] [Green Version]
- Ten Dam, G.B.; Kurup, S.; van de Westerlo, E.M.; Versteeg, E.M.; Lindahl, U.; Spillmann, D.; van Kuppevelt, T.H. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J. Biol. Chem. 2006, 281, 4654–4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurup, S.; Wijnhoven, T.J.; Jenniskens, G.J.; Kimata, K.; Habuchi, H.; Li, J.P.; Lindahl, U.; van Kuppevelt, T.H.; Spillmann, D. Characterization of anti-heparan sulfate phage display antibodies AO4B08 and HS4E4. J. Biol. Chem. 2007, 282, 21032–21042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Park, P.W.; Wilson, C.L.; Parks, W.C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002, 111, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Ding, K.; Lopez-Burks, M.; Sánchez-Duran, J.A.; Korc, M.; Lander, A.D. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J. Cell Biol. 2005, 171, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Leppä, S.; Mali, M.; Miettinen, H.M.; Jalkanen, M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc. Natl. Acad. Sci. USA 1992, 89, 932–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalkanen, M.; Elenius, K.; Inki, P.; Kirjavainen, J.; Leppä, S. Syndecan, a regulator of cell behaviour, is lost in malignant transformation. Biochem. Soc. Trans. 1991, 19, 1069–1072. [Google Scholar] [CrossRef]
- Inki, P.; Joensuu, H.; Grénman, R.; Klemi, P.; Jalkanen, M. Association between syndecan-1 expression and clinical outcome in squamous cell carcinoma of the head and neck. Br. J. Cancer 1994, 70, 319–323. [Google Scholar] [CrossRef]
- Anttonen, A.; Kajanti, M.; Heikkilä, P.; Jalkanen, M.; Joensuu, H. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br. J. Cancer 1999, 79, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, H.; Zhang, M.; Matsumoto, S.; Yamashita, Y.; Tanaka, T.; Takamori, K.; Igawa, K.; Yoshida, M.; Fukuyama, H.; Takahashi, T.; et al. Reduced syndecan-1 expression is correlated with the histological grade of malignancy at the deep invasive front in oral squamous cell carcinoma. J. Oral Pathol. Med. 2006, 35, 301–306. [Google Scholar] [CrossRef]
- Binder Gallimidi, A.; Nussbaum, G.; Hermano, E.; Weizman, B.; Meirovitz, A.; Vlodavsky, I.; Götte, M.; Elkin, M. Syndecan-1 deficiency promotes tumor growth in a murine model of colitis-induced colon carcinoma. PLoS ONE 2017, 12, e0174343. [Google Scholar] [CrossRef]
- Day, R.M.; Hao, X.; Ilyas, M.; Daszak, P.; Talbot, I.C.; Forbes, A. Changes in the expression of syndecan-1 in the colorectal adenoma-carcinoma sequence. Virchows Arch. Int. J. Pathol. 1999, 434, 121–125. [Google Scholar] [CrossRef]
- Fujiya, M.; Watari, J.; Ashida, T.; Honda, M.; Tanabe, H.; Fujiki, T.; Saitoh, Y.; Kohgo, Y. Reduced expression of syndecan-1 affects metastatic potential and clinical outcome in patients with colorectal cancer. Jpn. J. Cancer Res. 2001, 92, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.; Nordling, S.; Lundin, J.; Isola, J.; Wiksten, J.P.; Haglund, C. Epithelial syndecan-1 expression is associated with stage and grade in colorectal cancer. Oncology 2005, 68, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Shinyo, Y.; Kodama, J.; Hasengaowa; Kusumoto, T.; Hiramatsu, Y. Loss of cell-surface heparan sulfate expression in both cervical intraepithelial neoplasm and invasive cervical cancer. Gynecol. Oncol. 2005, 96, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Juuti, A.; Nordling, S.; Lundin, J.; Louhimo, J.; Haglund, C. Syndecan-1 expression—A novel prognostic marker in pancreatic cancer. Oncology 2005, 68, 97–106. [Google Scholar] [CrossRef]
- Gökden, N.; Greene, G.F.; Bayer-Garner, I.B.; Spencer, H.J.; Sanderson, R.D.; Gökden, M. Expression of CD138 (Syndecan-1) in renal cell carcinoma is reduced with increasing nuclear grade. Appl. Immunohistochem. Mol. Morphol. 2006, 14, 173–177. [Google Scholar] [CrossRef]
- Szarvas, T.; Reis, H.; Kramer, G.; Shariat, S.F.; Vom Dorp, F.; Tschirdewahn, S.; Schmid, K.W.; Kovalszky, I.; Rübben, H. Enhanced stromal syndecan-1 expression is an independent risk factor for poor survival in bladder cancer. Hum. Pathol. 2014, 45, 674–682. [Google Scholar] [CrossRef]
- Ito, Y.; Yoshida, H.; Nakano, K.; Takamura, Y.; Miya, A.; Kobayashi, K.; Yokozawa, T.; Matsuzuka, F.; Matsuura, N.; Kuma, K.; et al. Syndecan-1 expression in thyroid carcinoma: Stromal expression followed by epithelial expression is significantly correlated with dedifferentiation. Histopathology 2003, 43, 157–164. [Google Scholar] [CrossRef]
- Zellweger, T.; Ninck, C.; Mirlacher, M.; Annefeld, M.; Glass, A.G.; Gasser, T.C.; Mihatsch, M.J.; Gelmann, E.P.; Bubendorf, L. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate 2003, 55, 20–29. [Google Scholar] [CrossRef]
- Szarvas, T.; Reis, H.; Vom Dorp, F.; Tschirdewahn, S.; Niedworok, C.; Nyirady, P.; Schmid, K.W.; Rübben, H.; Kovalszky, I. Soluble syndecan-1 (SDC1) serum level as an independent pre-operative predictor of cancer-specific survival in prostate cancer. Prostate 2016, 76, 977–985. [Google Scholar] [CrossRef]
- Barbareschi, M.; Maisonneuve, P.; Aldovini, D.; Cangi, M.G.; Pecciarini, L.; Angelo Mauri, F.; Veronese, S.; Caffo, O.; Lucenti, A.; Palma, P.D.; et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer 2003, 98, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Lendorf, M.E.; Manon-Jensen, T.; Kronqvist, P.; Multhaupt, H.A.; Couchman, J.R. Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2011, 59, 615–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, E.J.; Blackhall, F.H.; Shanks, J.H.; David, G.; McGown, A.T.; Swindell, R.; Slade, R.J.; Martin-Hirsch, P.; Gallagher, J.T.; Jayson, G.C. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin. Cancer Res. 2004, 10, 5178–5186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, A.; Lendorf, M.E.; Couchman, J.R.; Multhaupt, H.A. Breast and ovarian cancers: A survey and possible roles for the cell surface heparan sulfate proteoglycans. J. Histochem. Cytochem. 2012, 60, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Regős, E.; Abdelfattah, H.H.; Reszegi, A.; Szilák, L.; Werling, K.; Szabó, G.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. Syndecan-1 inhibits early stages of liver fibrogenesis by interfering with TGFβ1 action and upregulating MMP14. Matrix Biol. 2018, 68, 474–489. [Google Scholar] [CrossRef]
- Regős, E.; Karászi, K.; Reszegi, A.; Kiss, A.; Schaff, Z.; Baghy, K.; Kovalszky, I. Syndecan-1 in Liver Diseases. Pathol. Oncol. Res. POR 2020, 26, 813–819. [Google Scholar] [CrossRef]
- Charni, F.; Friand, V.; Haddad, O.; Hlawaty, H.; Martin, L.; Vassy, R.; Oudar, O.; Gattegno, L.; Charnaux, N.; Sutton, A. Syndecan-1 and syndecan-4 are involved in RANTES/CCL5-induced migration and invasion of human hepatoma cells. Biochim. Biophys. Acta 2009, 1790, 1314–1326. [Google Scholar] [CrossRef]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef] [Green Version]
- Dews, I.C.; Mackenzie, K.R. Transmembrane domains of the syndecan family of growth factor coreceptors display a hierarchy of homotypic and heterotypic interactions. Proc. Natl. Acad. Sci. USA 2007, 104, 20782–20787. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, A.P.; Green, K.J. Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 2013, 116, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Chidgey, M.; Dawson, C. Desmosomes: A role in cancer? Br. J. Cancer 2007, 96, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chang, H.; Li, L.; Cheng, R.C.; Fan, X.N. Alteration of adhesion molecule expression and cellular polarity in hepatocellular carcinoma. Histopathology 2007, 51, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, A.; Shrinet, J.; Parniewska, M.M.; Fuxe, J.; Dobra, K.; Hjerpe, A. Mapping the Interactome of the Nuclear Heparan Sulfate Proteoglycan Syndecan-1 in Mesothelioma Cells. Biomolecules 2020, 10, 1034. [Google Scholar] [CrossRef]
- Elenius, V.; Götte, M.; Reizes, O.; Elenius, K.; Bernfield, M. Inhibition by the soluble syndecan-1 ectodomains delays wound repair in mice overexpressing syndecan-1. J. Biol. Chem. 2004, 279, 41928–41935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manon-Jensen, T.; Multhaupt, H.A.; Couchman, J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef]
- Brule, S.; Charnaux, N.; Sutton, A.; Ledoux, D.; Chaigneau, T.; Saffar, L.; Gattegno, L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology 2006, 16, 488–501. [Google Scholar] [CrossRef] [Green Version]
- Endo, K.; Takino, T.; Miyamori, H.; Kinsen, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003, 278, 40764–40770. [Google Scholar] [CrossRef] [Green Version]
- Pruessmeyer, J.; Martin, C.; Hess, F.M.; Schwarz, N.; Schmidt, S.; Kogel, T.; Hoettecke, N.; Schmidt, B.; Sechi, A.; Uhlig, S.; et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J. Biol. Chem. 2010, 285, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Gaire, M.; Magbanua, Z.; McDonnell, S.; McNeil, L.; Lovett, D.H.; Matrisian, L.M. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J. Biol. Chem. 1994, 269, 2032–2040. [Google Scholar]
- Fitzgerald, M.L.; Wang, Z.; Park, P.W.; Murphy, G.; Bernfield, M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J. Cell Biol. 2000, 148, 811–824. [Google Scholar] [CrossRef]
- Wolfe, M.S. Intramembrane-cleaving proteases. J. Biol. Chem. 2009, 284, 13969–13973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasqualon, T.; Pruessmeyer, J.; Jankowski, V.; Babendreyer, A.; Groth, E.; Schumacher, J.; Koenen, A.; Weidenfeld, S.; Schwarz, N.; Denecke, B.; et al. A cytoplasmic C-terminal fragment of Syndecan-1 is generated by sequential proteolysis and antagonizes Syndecan-1 dependent lung tumor cell migration. Oncotarget 2015, 6, 31295–31312. [Google Scholar] [CrossRef] [PubMed]
- Pasqualon, T.; Pruessmeyer, J.; Weidenfeld, S.; Babendreyer, A.; Groth, E.; Schumacher, J.; Schwarz, N.; Denecke, B.; Jahr, H.; Zimmermann, P.; et al. A transmembrane C-terminal fragment of syndecan-1 is generated by the metalloproteinase ADAM17 and promotes lung epithelial tumor cell migration and lung metastasis formation. Cell. Mol. Life Sci. 2015, 72, 3783–3801. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Ye, J.; Rawson, R.B.; Goldstein, J.L. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 2000, 100, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Paumelle, R.; Tulasne, D.; Kherrouche, Z.; Plaza, S.; Leroy, C.; Reveneau, S.; Vandenbunder, B.; Fafeur, V. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 2002, 21, 2309–2319. [Google Scholar] [CrossRef] [Green Version]
- Gutman, A.; Wasylyk, B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990, 9, 2241–2246. [Google Scholar] [CrossRef]
- Ozaki, I.; Mizuta, T.; Zhao, G.; Yotsumoto, H.; Hara, T.; Kajihara, S.; Hisatomi, A.; Sakai, T.; Yamamoto, K. Involvement of the Ets-1 gene in overexpression of matrilysin in human hepatocellular carcinoma. Cancer Res. 2000, 60, 6519–6525. [Google Scholar]
- Seth, A.; Papas, T.S. The c-ets-1 proto-oncogene has oncogenic activity and is positively autoregulated. Oncogene 1990, 5, 1761–1767. [Google Scholar]
- Majérus, M.A.; Bibollet-Ruche, F.; Telliez, J.B.; Wasylyk, B.; Bailleul, B. Serum, AP-1 and Ets-1 stimulate the human ets-1 promoter. Nucleic Acids Res. 1992, 20, 2699–2703. [Google Scholar] [CrossRef] [Green Version]
- Bu, S.; Yamanaka, M.; Pei, H.; Bielawska, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.; Trojanowska, M. Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via activation of ERK1/2-Ets1 pathway in human fibroblasts. FASEB J. 2006, 20, 184–186. [Google Scholar] [CrossRef]
- Tanaka, K.; Oda, N.; Iwasaka, C.; Abe, M.; Sato, Y. Induction of Ets-1 in endothelial cells during reendothelialization after denuding injury. J. Cell. Physiol. 1998, 176, 235–244. [Google Scholar] [CrossRef]
- Dudás, J.; Ramadori, G.; Knittel, T.; Neubauer, K.; Raddatz, D.; Egedy, K.; Kovalszky, I. Effect of heparin and liver heparan sulphate on interaction of HepG2-derived transcription factors and their cis-acting elements: Altered potential of hepatocellular carcinoma heparan sulphate. Biochem. J. 2000, 350 Pt 1, 245–251. [Google Scholar] [CrossRef]
- Hirano, K.; Sasaki, N.; Ichimiya, T.; Miura, T.; Van Kuppevelt, T.H.; Nishihara, S. 3-O-sulfated heparan sulfate recognized by the antibody HS4C3 contributes to the differentiation of mouse embryonic stem cells via fas signaling. PLoS ONE 2012, 7, e43440. [Google Scholar] [CrossRef]
- Gulberti, S.; Mao, X.; Bui, C.; Fournel-Gigleux, S. The role of heparan sulfate maturation in cancer: A focus on the 3O-sulfation and the enigmatic 3O-sulfotransferases (HS3STs). Semin. Cancer Biol. 2020, 62, 68–85. [Google Scholar] [CrossRef]
- Heidari-Hamedani, G.; Vivès, R.R.; Seffouh, A.; Afratis, N.A.; Oosterhof, A.; van Kuppevelt, T.H.; Karamanos, N.K.; Metintas, M.; Hjerpe, A.; Dobra, K.; et al. Syndecan-1 alters heparan sulfate composition and signaling pathways in malignant mesothelioma. Cell. Signal. 2015, 27, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, W.; Lu, J.; He, F.; Yang, X. Invasiveness of hepatocellular carcinoma cell lines: Contribution of hepatocyte growth factor, c-met, and transcription factor Ets-1. Biochem. Biophys. Res. Commun. 2001, 286, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, G.; Boccaccio, C.; Giordano, S.; Andŏ, M.; Stella, M.C.; Comoglio, P.M. Ets up-regulates MET transcription. Oncogene 1996, 13, 1911–1917. [Google Scholar] [PubMed]
- Sementchenko, V.I.; Watson, D.K. Ets target genes: Past, present and future. Oncogene 2000, 19, 6533–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pap, Z.; Pávai, Z.; Dénes, L.; Kovalszky, I.; Jung, J. An immunohistochemical study of colon adenomas and carcinomas: E-cadherin, Syndecan-1, Ets-1. Pathol. Oncol. Res. 2009, 15, 579–587. [Google Scholar] [CrossRef]
- Ishii, Y.; Nakasato, Y.; Kobayashi, S.; Yamazaki, Y.; Aoki, T. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2003, 22, 461–470. [Google Scholar]
- Behrens, A.; Sibilia, M.; David, J.P.; Möhle-Steinlein, U.; Tronche, F.; Schütz, G.; Wagner, E.F. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002, 21, 1782–1790. [Google Scholar] [CrossRef] [Green Version]
- Eferl, R.; Ricci, R.; Kenner, L.; Zenz, R.; David, J.P.; Rath, M.; Wagner, E.F. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell 2003, 112, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Yuen, M.F.; Wu, P.C.; Lai, V.C.; Lau, J.Y.; Lai, C.L. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer 2001, 91, 106–112. [Google Scholar] [CrossRef]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karászi, K.; Vigh, R.; Máthé, M.; Fullár, A.; Oláh, L.; Füle, T.; Papp, Z.; Kovalszky, I. Aberrant Expression of Syndecan-1 in Cervical Cancers. Pathol. Oncol. Res. 2020, 20, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Wiksten, J.P.; Lundin, J.; Nordling, S.; Lundin, M.; Kokkola, A.; von Boguslawski, K.; Haglund, C. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int. J. Cancer 2001, 95, 1–6. [Google Scholar] [CrossRef]
- Hasengaowa; Kodama, J.; Kusumoto, T.; Shinyo, Y.; Seki, N.; Hiramatsu, Y. Prognostic significance of syndecan-1 expression in human endometrial cancer. Ann. Oncol. 2005, 16, 1109–1115. [Google Scholar] [CrossRef]
- Máthé, M.; Suba, Z.; Németh, Z.; Tátrai, P.; Füle, T.; Borgulya, G.; Barabás, J.; Kovalszky, I. Stromal syndecan-1 expression is an adverse prognostic factor in oral carcinomas. Oral Oncol. 2006, 42, 493–500. [Google Scholar] [CrossRef]
- Götte, M.; Kersting, C.; Ruggiero, M.; Tio, J.; Tulusan, A.H.; Kiesel, L.; Wülfing, P. Predictive value of syndecan-1 expression for the response to neoadjuvant chemotherapy of primary breast cancer. Anticancer Res. 2006, 26, 621–627. [Google Scholar]
- Nguyen, T.L.; Grizzle, W.E.; Zhang, K.; Hameed, O.; Siegal, G.P.; Wei, S. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am. J. Clin. Pathol. 2013, 140, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, T.; Kodama, J.; Seki, N.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Clinical significance of syndecan-1 and versican expression in human epithelial ovarian cancer. Oncol. Rep. 2010, 23, 917–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagouassat, M.; Suffee, N.; Hlawaty, H.; Haddad, O.; Charni, F.; Laguillier, C.; Vassy, R.; Martin, L.; Schischmanoff, P.O.; Gattegno, L.; et al. Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int. J. Cancer 2010, 126, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Gharbaran, R. Advances in the molecular functions of syndecan-1 (SDC1/CD138) in the pathogenesis of malignancies. Crit. Rev. Oncol. Hematol. 2015, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, Z.; Fu, H.; Tie, Y.; Zhang, H.; Wu, Y.; Zheng, X. MicroRNA-1 and microRNA-499 downregulate the expression of the ets1 proto-oncogene in HepG2 cells. Oncol. Rep. 2012, 28, 701–706. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollósi, P.; Váncza, L.; Karászi, K.; Dobos, K.; Péterfia, B.; Tátrai, E.; Tátrai, P.; Szarvas, T.; Paku, S.; Szilák, L.; et al. Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1. Biomolecules 2020, 10, 1356. https://doi.org/10.3390/biom10101356
Hollósi P, Váncza L, Karászi K, Dobos K, Péterfia B, Tátrai E, Tátrai P, Szarvas T, Paku S, Szilák L, et al. Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1. Biomolecules. 2020; 10(10):1356. https://doi.org/10.3390/biom10101356
Chicago/Turabian StyleHollósi, Péter, Lóránd Váncza, Katalin Karászi, Katalin Dobos, Bálint Péterfia, Enikő Tátrai, Péter Tátrai, Tibor Szarvas, Sándor Paku, László Szilák, and et al. 2020. "Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1" Biomolecules 10, no. 10: 1356. https://doi.org/10.3390/biom10101356
APA StyleHollósi, P., Váncza, L., Karászi, K., Dobos, K., Péterfia, B., Tátrai, E., Tátrai, P., Szarvas, T., Paku, S., Szilák, L., & Kovalszky, I. (2020). Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1. Biomolecules, 10(10), 1356. https://doi.org/10.3390/biom10101356





