Abstract
The type 2 dopamine receptor D2 (D2-R), member of the G protein-coupled receptor (GPCR) superfamily, exists in two isoforms, short (D2S-R) and long (D2L-R). They differ by an additional 29 amino acids (AA) in the third cytoplasmic loop (ICL3) of the D2L-R. These isoforms differ in their intracellular localization and trafficking functionality, as D2L-R possesses a larger intracellular pool, mostly in the endoplasmic reticulum (ER). This review focuses on the evolutionarily conserved motifs in the ICL3 of the D2-R and proteins interacting with the ICL3 of both isoforms, specifically with the 29 AA insert. These motifs might be involved in D2-R exit from the ER and have an impact on cell-surface and intracellular localization and, therefore, also play a role in the function of dopamine receptor signaling, ligand binding and possible homo/heterodimerization. Our recent bioinformatic data on potential new interaction partners for the ICL3 of D2-Rs are also presented. Both are highly relevant, and have clinical impacts on the pathophysiology of several diseases such as Parkinson’s disease, schizophrenia, Tourette’s syndrome, Huntington’s disease, manic depression, and others, as they are connected to a variety of essential motifs and differences in communication with interaction partners.
1. Dopamine Receptors
G-protein-coupled receptors (GPCRs), also termed seven-transmembrane receptors (7TMRs) are by far the largest family of membrane-bound receptors, which are involved in the regulation of the neurotransmitter dopamine effects as one of their targets []. Based on functional, structural, and pharmacological properties, five types of dopamine receptors have been described, that belong to the D1- or D2-like subfamily of receptors (D1-R and D2-R respectively), with differing abilities of stimulation or inhibition of adenylyl cyclase (AC), respectively.
The D1-R subfamily is comprised of D1 and D5 receptors (D1-R and D5-R), and the D2 subfamily includes D2, D3 and D4 receptors (D2-R, D3-R, and D4-R). Members of the D1-R subfamily have a short third cytoplasmic loop (ICL3) and a very long C-terminal cytoplasmic end. In contrast, D2-Rs have a very long ICL3 and a short C-terminal end and include the receptor variants generated by alternative splicing (D2 and D3) or polymorphic variation (D4) (reviewed by Beaulieu et al.) []. The D2-R subgroup has a long ICL3 whose structure is common to the receptor interaction with the heterotrimeric protein Gαi []. In D1-R, with its characteristically short ICL3, coupling with Gαs proteins occurs [,]. The C-terminal end is approximately seven times longer in D1-Rs than in D2-Rs. Both the ICL3 and the C-terminal end are thought to serve as possible communication points for interaction with intracellular proteins. The N-terminal tail has a similar number of amino acids in all receptor subtypes and contains sites for N-glycosylation. D1-R and D5-R have two glycosylation sites, located at the N-terminal end and in extracellular loop 2 (ECL2). D2-R has three potential N-linked glycosylation sites, all in the N-terminus: N5, N17, and N23, D3-R has four potential glycosylation sites: N12 and N19 in the N-terminus, N97 in the first extracellular loop (ECL1), and N173 in the second extracellular loop (ECL2) [] and D4-R only one in the N-terminus []. Cysteines located in the first and second extracellular loops (ECL1 and ECL2) are linked by a disulfide bond that stabilizes the receptor structure []. The endogenous ligand for the dopamine receptors is the neurotransmitter dopamine. After dopamine binds to the D1-R, the signaling pathway is canonically activated via the heterotrimeric protein Gαs and Golf G-proteins, leading to adenylate cyclase (AC) activation and cyclic adenosine monophosphate (cAMP) formation in the cell. Diversity in functional outcomes may also be achieved via selective binding to Gαi and Gαo proteins. Previous work has shown that D2-R can be stabilized by an agonist, which affect the selectivity and amount of coupling with Gαi and Gαo [,]. Although previous work had indicated that Gαi2 was selective for D2L-R [,], experimental data has indicated that selectivity regulation of Gαi is driven by the agonist-activated conformation of D2-R. R(+)-3-PPP hydrochloride stimulation of D2-R resulted in reduced coupling with Gαi1 or Gαi2 and preferential coupling with Gαi3 []. The movement magnitude of the sixth transmembrane helix of the activated receptor was predicted to be the primary modulator of the selectivity of the G-protein subtypes [].
Using cryo-electron microscopy, the structure of an agonist-bound activated D2–Gαi complex reconstituted into a phospholipid membrane has been demonstrated recently [], both as the first experimental model of a GPCR complex embedded in a phospholipid bilayer, as well as the first model of activated D2-R. The models revealed interactions that are unique to the membrane-embedded complex, such as conformational changes in ECL2, TM5, TM6 and TM7, propagating to the opening of the intracellular Gαi-binding site and helix 8 burial in the inner leaflet, ordered lysine and arginine side chains in the membrane interfacial regions, and lipid anchoring of the G-protein in the membrane [].
Although all D-Rs recognize the same ligand, they have a differential tissue distribution and are involved in different functions in vivo [,]. By binding to various types of D-Rs, dopamine controls locomotor system functions, cognition, emotion, hunger, satiety, and endocrine secretion [,]. Impaired D2-R signaling is associated with the pathophysiology of many psychiatric and neurological diseases or states, including Parkinson’s disease, schizophrenia, Tourette’s syndrome, Huntington’s disease, bipolar disorder, depression, dementia, as well as others, such as restless leg syndrome and sexual dysfunction. D-Rs are an essential target for currently available modern drugs, including the dopamine precursor levodopa [,] for Parkinson’s disease, where dopaminergic neurons are damaged and a dopamine deficiency leads to a combination of movement and psychiatric pathologies. Thus, D-Rs are targets for motor deficits, cognitive, and motivational deficits in neuropsychiatric disorders []. In schizophrenia and psychosis inhibitors of D2-R are used to reduce increased dopaminergic signaling [].
2. Dopamine Receptor Type 2 (D2-R)
The D2-R is a key component of the dopamine system that is present in two alternatively spliced transcripts of the Drd2 gene and classified as short (D2S-R) and long (D2L-R) receptor isoforms. The long isoform differs from the short one only by the presence of an additional 29 amino acids (AA) encoded by exon 5 in the ICL3 of the D2L-R [,,]. The inclusion is interspersed between the AA lysine (K241) and glutamic acid (E271). D2S-R in mice and rats are made up of 415 AA and D2L-R is made up of 444 AAs. Human D2S-R and D2L-R are shorter than murine and rat equivalents by one AA, consisting of 414 and 443 AAs, respectively. The isoleucine is missing between lysine (K331) and aspartic acid (D332). This region might have an essential role in the functional differences between both D2-R isoforms such as interactions related to G-proteins [,,], post-translation modification and cell localization [,]. D2-R isoforms also indicate different in vivo functions, whereby D2L-R primarily acts at postsynaptic and D2S-R in presynaptic dopaminergic transmissions [,]. Data acquired on genetically engineered D2-R mouse model indicates additional evidence for different roles of two isoforms in cognitive and motor functions [], responsiveness to cocaine exposure [], and therapeutic effects of antipsychotic drugs []. Furthermore, they are expressed in the same cell types with more abundant expression of the D2L-R isoform over D2S-R, but with differences in their intracellular localization. While D2S-R is primarily localized on the plasma membrane (PM), a substantial fraction of D2L-R is located intracellularly, especially in the perinuclear compartments around the Golgi apparatus (GA) [] and endoplasmic reticulum (ER) [].
The D2-R is the most commonly studied dopamine receptor subtype since the majority of antipsychotic drugs act as D2-R antagonists in the mesolimbic dopaminergic system []. As a primary target for atypical and typical antipsychotic drugs and treatment of the Parkinson’s disease, many of those agents can cause potentially life-threatening and severe side effects due to the promiscuous activities against related D2-Rs []. Precisely because of this reason, it is necessary to be familiar with the details of the dopamine receptor’s complex structure and functions.
3. Localization Differences Between D2S-R and D2L-R
D2-R isoforms localization is neither species nor tissue specific. They were found in different tissues, but in highly variable ratios [,,]. D2S-R is predominantly localized in the PM [,,], whereas an intracellular D2L-R reservoir has been reported in the primates brain [] and several cell lines [,,].
The primary intracellular localization of the D2L-R in transiently transfected HEK-293, COS-7, and HeLa is the ER [], whereas in transfected NG108-15 cells intracellularly localized D2L-R is predominantly co-localized with the GA matrix protein marker GM130 []. At the level of confocal microscopy, higher proportions of D2L-R than D2S-R were retained intracellularly in heterologous cell lines [,]. This finding could be due to the retention of overexpressed or incorrectly folded tagged receptors in the ER. However, immunoelectron microscopy also revealed the predominant intracellular localization of the D2L-R [] in monkey dopaminergic neurons, and that these sites are cisterns of GA and ER. Ligand-promoted recruitment of the D2L-R [], D4-R [] and other GPCRs, such as thrombin receptors (PAR1 and PAR2), D1-Rs, and opioid receptors on the PM presented additional evidence for the existence of functional, pre-existing intracellular stores (reviewed by Achour, 2008) [].
N-terminal glycosylation of different GPCR’s has a vital role in cell surface receptor expression. Mutations of potential N-terminal glycosylation sites found for D2-R (N5, N17, and N23) lead to decreased surface expression for D2-R, showing their important role in receptor distribution []. Fishburn et al. performed studies regarding post-translational processing of the D2L-R and D2S-R isoforms []. Three post-translational states were observed in both receptor isoforms: a newly synthesized protein (35 kDa), a partially glycosylated product (45 kDa) and a fully glycosylated receptor (70 kDa) [].
A difference in the processing of the mature receptor was observed. The initial N-glycosylation of the newly synthesized receptor protein occurred shortly after synthesis in both D2-R isoforms, suggesting that a rapid and efficient maturation towards partially glycosylated product occurs in D2-R. However, a marked difference was observed in subsequent N-linked glycosylation, where D2L-R showed slower production of fully glycosylated proteins in comparison to the D2S-R. Additionally, they showed that 20% of the D2L-R remains in the partially processed form and never undergoes the second stage of N-linked glycosylation.
4. Functional Differences between D2S-R and D2L-R
Identifying probable functional differences between D2S-R and D2L-R has been the subject of numerous studies. Depending on the site of action and the effect on D2-R-mediated responses, isoforms have different and likely antagonistic functions in vivo []. D2S-R is mainly a presynaptic receptor, but at the postsynaptic level, it negatively modulates D1-R-dependent responses. In contrast, D2L-R is found predominantly at postsynaptic sites where it acts synergistically with D1-Rs [].
The location of the inclusion in ICL3 also led to the assumption that it may affect the specificity of the interaction with G-proteins and the sequential activation of specific effector proteins.
Several studies have shown that structural differences between isoforms can determine the specificity of interactions with G-proteins [,]. A recent study showed the preferential coupling of both D2-R isoforms with G-proteins (Gi1 and Gi2), due to the differences in ICL3, which affect receptor behavior [,]. Results obtained with the messenger gene construct controlled by the cAMP response promoter suggest constitutive, i.e., agonist-independent D2L-R activity []. Studies with D2-R knockout mice provided additional evidence for their diverse roles in motor and cognitive functions [], sensitivity to cocaine [], and therapeutic/side effects of antipsychotic agents [].
5. The ER Retention Motifs in GPCRs and Both D2-R Isoforms
The synthesis and transfer of newly formed proteins via secretory pathways from the ER to the PM is a complex process involving different mechanisms and many additional proteins and motifs that are important in protein interaction and enable their proper formation, quality control, selective retention, and transport []. Mechanisms that regulate the secretory transport of GPCRs or their transfer to the cell surface are poorly elucidated [,]. It is known that the interconnection of the same or different GPCRs, homo- and heterodimerization, is essential in the transport of GPCRs in families A and C, but not for representatives of family B on the PM []. Transfer of the protein to the PM requires control of transport from the ER via the GA to the PM. This process is regulated by COPI and COPII vesicles []. COPII-coated transfer vesicles serve anterograde transport from the ER to GA, whilst transport between GA cisternae and retrograde transport from GA to ER takes place with COPI-coated vesicles. Improperly synthesized proteins or those having exposed sequences encoding motives for retention in the ER are transported retrogradely into the ER by COPI-coated vesicles []. Moreover, correctly folded proteins might be retained in the ER because they hold ER retention motifs, which prevent their export from the ER. Three types of ER retention motifs have been identified in the intracellular domains of various proteins: KDEL, KKXX, and RXR type motifs []. The presence of specific conserved sequences, so-called ER retention signals, could be responsible for preventing D2-R proteins from leaving the ER. Since the level of GPCR expression dictates the magnitude of cellular responses elicited by a signal at the PM, which is the balance of elaborately regulated endocytic and exocytic trafficking, it is crucial to know the motifs as well as the proteins involved in this interplay.
6. KDEL and KKXX Motifs
The KDEL motif is a short C-terminal retrieval signal (Lys-Asp-Glu-Leu) identified in ER luminal chaperone proteins, such as immunoglobulin heavy chain-binding protein (BiP) and other soluble ER resident proteins [,,]. The KDEL receptor recognizes this motif in the post-ER compartments, which mediates retrograde transport to the ER by COPI coatomer structures []. For the proper sorting of cargo into COPI vesicles, a Ras-like small GTPase ADP-ribosylation factor 1 (ARF1) activation is required []. ARFGAP1 activates ARF1 by hydrolysis of GTP to GDP []. Ligand binding on the luminal side of the KDEL receptor induces interaction with ARFGTP1 on the cytoplasmic side of the receptor, resulting in the recruitment of ARFGTP1 from the cytosol to the PM leading to ARF1 activation [].
In zebrafish D2-Rs and D3-Rs, the expression of gene Hsp47 was identified, which is an ER-resident collagen-specific chaperone with a C-terminal KDEL retention motif and plays a fundamental role in the folding, stability, and intracellular transport of procollagen triple helices [].
The KDEL receptor cycles between the ER and the GA and its affinity for KDEL containing proteins changes between these two compartments. Retrieval of proteins mediated by the KDEL receptor can occur from different sites, ranging from early Golgi complex locations to trans Golgi networks []. In the GA, the KDEL receptor could associate with Gαo, one of the abundant Gα subunit and regulate receptor trafficking through G-proteins [].
In addition to KDEL, the di-lysine motif (KKXX), have been identified as retrieval signal, important for recycling proteins from the GA back to the ER []. These signals are necessary for determining the localization of modified secretory and PM proteins in the ER [].
Type I integral membrane proteins, ERGIC53, and p24 family proteins contain di-lysine KKXX motifs []. This carboxyl-terminal retrieval signal usually consists of two lysine residues on positions -3 and -4 relative to the C-terminus, followed by any amino acid []. The KKXX signal is evolutionarily conserved as it also appears in yeast []. Like KDEL, the KKXX motif also serves as a retrieval signal for the transport of proteins from GA by COPI vesicles, although it binds directly with coatomer structures and does not require a receptor [].
7. RXR and RSRR Motifs
The RXR motif, and in some proteins, RSRR, has been found on different proteins where it disables the exit of proteins from the ER. Initially, they were found in ion channels and also in several GPCRs [,]. The first discovered of two arginine retention signals (the RXR and RSRR) are known to be located at the C-terminal end of the Kir 6.2 potassium channel (RXR signal) and a GPCR representative, the gamma-aminobutyric acid type B1 receptor (GABAB1; RSRR signal) []. The GABAB1 receptor contains the C-terminal RXR type ER retention motif RSRR, which prevents protein release from the ER. GABAB1 is functionally impaired in terms of ligand binding when expressed alone, whereas GABAB2 is nonfunctional in its signaling properties. Only when co-expressed with GABAB2, GABAB1 receptor releases from the ER and translocates to the cell surface [,]. Upon co-expression, the RSRR retention signal of GABAB1 is proposed to be masked by interaction of the C-terminus of both subunits due to highly stable α coil-coil interactions. Therefore, GABAB1 and GABAB are functionally combined of distinct subunits as obligatory constitutive heterodimers [,,,].
There are more examples of the RXR-type retention motif in GPCR receptors. A published study showed that in the C-terminal end of the type 2c α-adrenergic receptor (α2C-AR), there is a set of five arginine residues (RRRRR), which represent a possible retention signal of the ER type RXR []. The RXR-type retention motif has also been described in the ICL3 of the kainate receptor, which is a ligand-dependent ion channel []. Disease-causing vasopressin type 2 receptors (V2R) mutations are retained in different compartments of the early secretory pathway []. V2R mutants connected to nephrogenic diabetes insipidus in the contrast with the wild-type V2R are less expressed on the cell surface. Additionally, the D2-R RXR motifs have been revealed. In our study [], we showed that the evolutionarily conserved arginine cluster in the insert of ICL3 of D2L-R (R267-R269) acts as an ER retention signal and is potentially crucial for anterograde trafficking of the D2-R and receptor PM availability. However, we must take into account that ER exit is a highly regulated process and that one motif is not responsible solely for it. Other proposed mechanisms involving interaction with the ER-resident gatekeeper prenylated Rab acceptor 1 domain family member 3 (PRAF3) or other D2L-R binding and interaction proteins, such as fatty acid-binding protein 3 (FABP3), which possibly bind to 29 AA in ICL3 in D2L-R may be included (Figure 1) [].
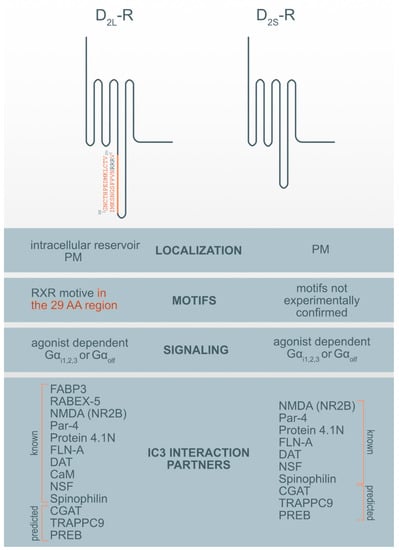
Figure 1.
Motif and interaction partners’ differences between D2L-R and D2S-R. CaM—Ca2+-binding protein calmodulin; CGAT—Chromaffin granule amine transporter; DAT—dopamine transporter; FABP3-Fatty acid binding protein 3; FLN-A—filamin A; NMDA (NR2B)—NR2B subunit of the NMDA glutamate (N-methyl-D-aspartate); NSF—N-ethylmaleimide-sensitive factor; Par-4—Prostate apoptosis response-4; PM-plasma membrane; PREB—prolactin regulatory element-binding protein; Rabex-5-Rabaptin-5 interacting protein; TRAPPC9—Trafficking protein particle complex subunit 9.
Additionally, the mutational analysis revealed that heteromers between the dopamine D2-R, adenosine A2A and cannabinoid CB1 receptors are stabilized by electrostatic interactions between arginine-rich motifs in the ICL3 of D2-R and A2A receptors and phosphorylated casein kinase 1/2 sites in ICL3 and C-tail of the CB1 receptor, and the C-terminus of the A2A receptor []. The RXR-rich motif in ICL3 of the D2-R is also involved in stabilizing electrostatic interactions with a di-glutamate motif in the C-terminus of the serotonin 5-HT2A and D1-R [,].
Likhite, N. et al. [] used bioinformatics analysis to identify 583 RGG and RXR-type motifs in GPCRs. Approximately 34% of those were conserved in human GPCRs within the ICL3 and could serve as arginine methylation motifs. They showed that R217 and R219 within the ICL3 N-terminal end common to both D2-R isoforms serve as an arginine N-methyltransferase 5 (PRMT5) methylation motif important for modulating receptor signaling []. This corroborates with R217 and R219 location of within the Gα interaction domain (reviewed in []).
Furthermore, Table 1 summarizes selected motifs from the eukaryotic linear motif (ELM) [] resources, which are potentially involved in ER-GA trafficking of the D2-R. The experimentally validated short linear motifs (SLiM) were manually curated after globular domain filtering, structural filtering, and context filtering.

Table 1.
Motifs obtained from eukaryotic linear motif (ELM) bioinformatics analysis of type 2 dopamine receptor D2 (D2-R) sequences.
8. ER Export Motifs in GPCRs and D2-R
GPCRs originate in the ER, where they are synthesized, folded, and assembled. Properly folded receptors are recruited and packaged into ER-derived COPII-coated vesicles. Transport vesicles carrying cargo receptors then migrate from the ER to the ER-Golgi intermediate complex (ERGIC), the GA, and the trans Golgi network (TGN). During their transport, receptors undergo post-translational modifications (e.g., glycosylation). Mature receptors then move from the TGN to their destination at the PM []. For this process, conserved sequences and motifs essential for the exit of GPCRs from the ER are critical. Export from the ER is the first step in the intracellular trafficking of GPCRs These motifs are found on the C- or N-terminal tail of the receptors and are therefore common to both D2-R isoforms. A triple phenylalanine motif [F(X)3F(X)3F] [] has been identified in the membrane-proximal C-terminus of the D1-R that is required for receptor cell-surface expression []. However, no motifs have yet been identified on the D2-R. A newly identified ER-membrane-associated protein, DRiP78, binds to this motif, as described later.
9. D2-R Interaction Proteins (DRIPs)
More than 20 dopamine receptor-interacting membrane-associated or cytoplasmic D2-R interaction proteins (DRIPs) are known and several of them bind the ICL3 of the D2-R []. Using the informational spectrum method (ISM), a virtual spectroscopy method for investigating protein-protein interactions, the analysis of known interaction partners of IC3 of D2-R [] was performed as previously described [,] and obtained the results presented in Table 2. ISM analysis of the IC3 D2-R interaction with protein partners corroborates with published data (reviewed in []) (Table 2, Figure 1). However, in addition to previously identified protein partners it has also been suggested that there are some new potential interaction partners.
Among previously described interaction partners, the highest affinity for the interaction with the D2-R was ascribed to N-methyl-D-aspartate (NMDA) receptor NR2B subunits. It was shown that a distinct region within the first 32 AA of the D2-R ICL3 interacts with the NR2B and disrupts the association of Ca2+/calmodulin-dependent protein kinase II (CaMKII) with NR2B, reduces NR2B phosphorylation at a CaMKII-sensitive site (Ser1303), and inhibits NMDA receptor-mediated currents in medium-sized striatal neurons. The D2-R-NR2B interaction is therefore critical for modulating NMDA receptor-mediated currents and behavioral responsiveness to cocaine []. The second highest propensity for interaction with the D2-R was observed for prostate apoptosis response-4 (Par-4). Par-4 is a protein expressed in the nervous system, where it is known to be a regulatory component in dopaminergic signaling. It is a mediator of neuronal degeneration, and is associated with the pathogenesis of Alzheimer’s disease []. Par-4 directly interacts with the D2-R via the calmodulin-binding motif in the ICL3. Furthermore, Par-4 constitutes a molecular link between impaired dopaminergic signaling and depression []. The N-terminal segment of the D2-Rs and D3-R was also shown to interact with neuronally enriched 4.1N protein; an interaction that contributes to the localization and stability of D2-Rs at the neuronal PM []. Similarly, filamin-A (FLN-A) also interacts with the N-terminal segment of the ICL3 of the D2-R and D3-R, and connects D-Rs with some other GPCRs, such as rhodopsin and, metabotropic glutamate receptors to the cytoskeleton, and therefore participate in their final subcellular localization []. The dopamine transporter (DAT) is a membrane-spanning protein that facilitate the reuptake of extracellular dopamine to the cytosol and is therefore, an essential target for cocaine, amphetamine, and some other drugs of abuse. One study showed a direct interaction between the DAT and the ICL3 (I340-Q373) of both D2-R isoforms. However, D2L-R is more capable of physically interacting with the DAT [].
The Ca2+-binding protein calmodulin (CaM) binds to the N-terminal portion of the ICL3 of the D2L-R, within an Arg-rich epitope (VLRRRRKRVN) that is also involved in the binding to Gi/o proteins and the adenosine A2A receptor, with the formation of A2A-D2-R heteromers [,]. N-ethylmaleimide-sensitive factor (NSF) is an ATPase and an essential part of the protein network responsible for different membrane fusion events, including transport through the GA and exocytosis []. Using immunoprecipitation and in vitro binding assays, it has been shown that NSF binds to the ICL3 of D-R (F341-Q373) and has a putative role in the interaction of D2-R and the Glu2 AMPA receptor []. Agonist stimulation of D2-R promotes the formation of direct protein-protein interactions between the ICL3 of the D2-R and the ATPase N-ethylmaleimide-sensitive factor (NSF). Spinophilin is F-actin and protein phosphatase-1-binding protein with a single PDZ domain that was identified as a protein associated with the ICL3 region of the D2-R. It is hypothesized to be necessary for establishing signaling complexes for dopaminergic neurotransmission through D2-Rs by linking receptors to downstream signaling molecules and the actin cytoskeleton [].
Three additional hypothetical ICL3 D2-R interaction partners were suggested by ISM: prolactin regulatory element-binding protein (PREB), chromaffin granule amine transporter (CGAT) and trafficking protein particle complex subunit 9 (TRAPPC9). Among prospective partners, CGAT displayed the highest affinity for interacting with the ICL3 D2-R, followed by TRAPPC9 and PREB. For all three prospective interaction partners we were unable to find experimental evidence for the direct interaction with the ICL3 of the D2-R but only some indirect indication for their involvement in dopamine synthesis, transport, or D2-R binding. PREB is an ubiquitously expressed protein and, a member of the WD-repeat protein family, that acts as a transcriptional regulator and suppresses the expression of the adiponectin gene [], regulates prolactin (PRL) gene expression [] and functions as a transcriptional regulator of PRL promoter activity, and therefore might be involved in thyrotropin-releasing hormone (TRH)-induced PRL gene transcription []. PRL gene expression and secretion are regulated by various hormones and growth factors, including dopamine, epidermal growth factor, and thyrotropin-releasing hormone (TRH) []. PREB is highly expressed in the anterior pituitary. Prolactinomas are the most common pituitary tumors and are treated with the selective dopamine D2-R agonist cabergoline []. Mutation of the PREB-binding site within the promoter abrogated the ability of cabergoline to inhibit PRL promoter activity. The chromaffin granule amine transporter (CGAT), also named the vesicular monoamine transporter 1 (VMAT1), is involved in the transport of biogenic monoamines, such as serotonin, from the cytoplasm into the secretory vesicles of neuroendocrine and endocrine cells. It has a positive impact on dopamine synthesis, secretion, and transport to storage vesicles, which releases neurotransmitters into synapses as chemical messages to postsynaptic neurons []. The pharmaceutical industry also targets VMATs for treating hypertension, drug addiction, psychiatric disorders, Parkinson’s disease, and other neurological disorders. The trafficking protein particle complex subunit 9 (TRAPPC9), also known as NIBP, belongs to the TRAPPII multiprotein complex. TRAPPC9 is involved in vesicular trafficking from the ER to the GA and promotes the activation of NFκB signaling. It is highly expressed in the postmitotic neurons of the cerebral cortex [].
To the best of our knowledge, only two proteins have been identified that specifically interact only with the D2L-R i.e., 29 AA within its ICL3. These proteins are fatty acid-binding protein 3 (FABP3) [] and Rabaptin-5 interacting protein (Rabex-5) []. Fatty acid-binding protein 3 (FABP3), also named the heart-type FABP (H-FAB), is one of the novel 29 AA insert binding protein on the position (G242-V270), which also alters D2L-R function []. D2L-R, when activated with a ligand, is known to activate the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) pathways, which are enhanced by FABP3 in FABP3-overexpressed cells, showing that FABP3 enhances D2L-R signaling []. A co-expression study of D2L-R and D2S-R with this protein in NG108-15 cells shows overexpression and colocalization of endogenous FABP only with the D2L-R in the GA and ER but not in the PM []. Dysfunction of FABP3 protein binding to D2L-R was shown in FABP3 KO mice [], which affects emotional behavior, and is characteristic of neurodegenerative diseases such as schizophrenia and Alzheimer’s disorder. These KO mice, which showed altered sensory, motor, and emotional behaviors, also exhibited decreased methamphetamine-induced sensitization and enhanced haloperidol-induced catalepsy due to D2-R dysfunction. Impaired FABP brain function was observed as an essential factor in the perturbation of D2-R signaling []. Rabaptin-5 interacting protein (Rabex-5) was identified in mouse brain lysates as another protein binding the 29 AA of D2L-R and has been shown to promote the early-endosome formation and Rab5 activation []. Both proteins are essential for prolonged D2L-R mediated ERK signaling.
DRIPs have the propensity to bind to conserved motifs in receptors. For D1-R it was shown that the ER-membrane-associated protein DRiP78 binds to a FXXXFXXXF motif in the C-terminus of D1-R and other GPCRs. Overexpression or down-modulation of this putative two-TM domain protein leads to ER retention of D1-Rs, reduced ligand binding, and impaired kinetics of receptor glycosylation []. This mechanism acts as a chaperone and may control PM receptor targeting without traveling to the cell surface.
Some of the DRIPs are also possible “private” chaperones with other functions, escorting proteins for D2L-R or proteins of the quality-control machinery involved in its retention within intracellular compartments [] and facilitating receptor cell surface expression by enabling their trafficking to the PM. Pools of intracellular D1-R exist in renal tubular cells, and receptor recruitment to the PM is independent of agonist activation elicited by the activation of cell surface receptors and via atrial natriuretic peptide-dependent heterologous activation [,].

Table 2.
The bioinformatics approach-informational spectrum method (ISM) analysis of interaction partners of the third cytoplasmic loop (ICL3) of the D2-R. A lower signal to noise S/N ratio suggests a lower interaction affinity between tested protein partners.
Table 2.
The bioinformatics approach-informational spectrum method (ISM) analysis of interaction partners of the third cytoplasmic loop (ICL3) of the D2-R. A lower signal to noise S/N ratio suggests a lower interaction affinity between tested protein partners.
| Interaction Partner | S/N Ratio | Function | Reference |
|---|---|---|---|
| Glutamate, NMDA (NR2B) | 62.39 | ionotropic glutamate receptor | Liu, X.Y. et al. (2006) [] |
| Par-4 | 48.63 | regulatory component in dopamine signaling | Guo, Q. et al. (1998) [] Park, S.K. et al. (2005) [] |
| Protein 4.1N | 38.61 | membrane-cytoskeleton adaptor | Binda, A.V. et al. (2002) [] |
| FLN-A | 26.65 | actin binding protein | Lin, R. et al. (2001) [] |
| DAT | 20.29 | facilitating reuptake of extracellular dopamine back in the cytosol | Lee, F.J. et al. (2007) [] |
| Gα i/z/o | 17.85 | binding GPCRs | |
| CaM | 13.36 | intermediate calcium-binding messenger | Navarro, G. et al. (2009) [] |
| NSF | 13.03 | ATPase | Hanson, P.I. et. al. (1995) [] Zou S. et al. (2005) [] |
| Spinophilin | 12.14 | F-actin and protein phosphatase-1-binding protein | Smith, F.D. et al. (1999) [] |
| Predicted Interaction Partner | 12.14 | ||
| CGAT | 19.90 | involved in the transport of biogenic monoamines | |
| TRAPPC9 | 19.73 | involved in vesicular trafficking from ER to GA | |
| PREB | 18.78 | transcriptional regulator |
Legend: Glutamate, NMDA (NR2B)—NR2B subunit of the NMDA glutamate receptor (N-methyl-D-aspartate); FLN-A—filamin-A; Par-4—prostate apoptosis response-4; DAT—dopamine transporter; CGAT—chromaffin granule amine transporter; TRAPPC9—trafficking protein particle complex subunit 9; PREB—prolactin regulatory element-binding protein; NSF—N-ethylmaleimide-sensitive factor; CaM—Ca2+-binding protein calmodulin.
10. Interaction with ER-Resident Gatekeeper Proteins
ER gatekeeper proteins tightly control receptor cell-surface export. GTRAP3-18, an integral ER membrane protein, was introduced as a protein both in vivo and in vitro, and is dynamically induced by retinoic acid and inhibits the activity of EAAC1 in a dose dependent manner. GTRAP3-18 forms an oligomeric complex with D2-R before exiting the ER, increasing the population of high-mannose oligosaccharide state proteins, and restricting its subcellular localization to the ER []. There is evidence that the specific gatekeeper protein PRAF2 binds to subunit GABA1 of the GABAB receptor and prevents its progression in the biosynthetic pathway []. Dupre, J.D. et al. showed that one of the ER-resident proteins, which is known to regulate trafficking via a FXXXFXXXF motif of D-Rs and interact mostly with the Gγ subunit and not Gα or Gβ subunits in HEK-293 cells, is dopamine-receptor interaction protein 78 (DRiP78) []. Another ER-gatekeeper candidate of the D2-D3 heterodimer is an activator of G protein signaling 3 (AGS3), which binds to GiαGDP and inhibits GDP dissociation in the prefrontal cortex during late withdrawal from repeated cocaine administration. However, this actual mechanism in D2-R signaling is still unknown []. In familiar, as well as in sporadic Parkinson’s disease, a mutation in the leucine-reach repeat kinase 2 gene (LRRK2) represents the most frequent genetic cause of disease. LRRK2 is a member of the Roco superfamily of proteins, a novel multi-domain family of Ras-like G-proteins, involved in vesicle-mediated transport to the cell membrane. Because LRRK2 could affect D2-R turnover by decreasing this rate of trafficking from the GA to the CM, the LRRK2 could have an essential function in one of the possible retention mechanisms [].
11. Clinical Relevance
This review examines the role of different types of conserved retention motifs and DRIPs on D2-R and roles in routing regulation. The impact of both is of high importance for the physiological functions of D2L-R and its export trafficking and precise localization in the cell. Defective transport of D2L-R, as well as many other GPCRs from the ER to the cell surface, is a highly regulated, dynamic process and is associated with the pathogenesis of a variety of human diseases, therefore advances in our understanding of GPCR export. Thus, the secretory pathway and its role in proper cell function are of high importance.
So far, we are not aware of any known mutation in the 29 AA region that would be associated with a disease. No variant of D2L-R has been linked or associated with schizophrenia, substance abuse, or alcoholism, including the most extensively investigated Ser311Cys polymorphisms of the D2-R gene. In vitro studies showed that the Cys311-type D2-R impairs dopamine-induced sequestration, which appears to be consistent with the dopamine hypothesis []. A naturally occurring synonymous mutation of the human D2-R gene (C957T, P319P) is postulated to correlate with the schizophrenia phenotype, and was shown to markedly change mRNA stability via changes in mRNA secondary structure and reduced dopamine-induced up-regulation of D2-R expression []. We also conducted a GPCR database (GPCRdb) search to find additional mutants of the D2-R and presented them in Table 3.

Table 3.
A summary of mutations within the ICL3 of the D2-R (source: G protein-coupled receptor (GPCR) database (GPCRdb); http://gpcrdb.org/mutations/render).
We have not found additional mutations located within the insert (AA 241–270). Further elucidation of the regulatory mechanism underlying GPCR export trafficking may provide an essential foundation for developing new therapeutic strategies in treating diseases.
The described differences in the evolutionarily preserved region within the 29 AA insertion in the ICL3 of D2L-R influences the regulation of D2L-R cellular trafficking. A thorough search through motifs showed a conserved arginine cluster within the 29-AA insert of ICL3 of the D2L-R, which appears to be the ER retention signal. Identifying possible candidates for DRIPs may also reveal “private” chaperones, which often display different functions or escort proteins for D2L-R or proteins of the quality-control machinery that play a role in GPCR retention within intracellular compartments. We speculate that other specific retention mechanisms for D2L-R exist. Thus, improving our knowledge of the routing regulation of these critical receptors will probably elicit the development of new therapeutic approaches in controlling the targeting of D2L-R at the PM.
Author Contributions
Conceptualization, V.K. and M.V.; methodology, K.B.C., M.M., S.G., M.S., and M.M.; Investigation, K.B.C. and M.M.; resources, K.B.C. and M.M.; writing—review and editing, V.K., K.B.C., M.M., M.V., and C.S.R.; visualization, M.M.; project administration, V.K. and K.B.C.; funding acquisition, M.V. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Slovenian Research Agency Programme (P4-0053), Slovenian-Serbian bilateral project (BI-RS/20-21-045), Slovenian Research Agency for PhD funding for M. Mavri and grant (No. 173001) from the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Acknowledgments
The authors would acknowledge above mentioned funding. V. Kubale, M. Vrecl, M. Mavri, and M. Senćanski participate in the European COST Action CA 18133 (ERNEST).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jackson, D.M.; Westlind-Danielsson, A. Dopamine receptors: Molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 1994, 64, 291–370. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Zheng, M.; Zhang, X.; Guo, S.; Kwon, K.J.; Shin, C.Y.; Kim, H.S.; Cheon, S.H.; Kim, K.M. N-linked Glycosylation on the N-terminus of the dopamine D2 and D3 receptors determines receptor association with specific microdomains in the plasma membrane. Biochim. Biophys. Acta 2015, 1853, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Ferone, D.; Lombardi, G.; Colao, A.; Lamberts, S.W.J.; Hofland, L.J. Novel insights in dopamine receptor physiology. Eur. J. Endocrinol. 2007, 156 (Suppl. 1), S13–S21. [Google Scholar] [CrossRef]
- Dohlman, H.G.; Caron, M.G.; DeBlasi, A.; Frielle, T.; Lefkowitz, R.J. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry 1990, 29, 2335–2342. [Google Scholar] [CrossRef]
- Gazi, L.; Nickolls, S.A.; Strange, P.G. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: Evidence for agonist regulation of G protein selectivity. Br. J. Pharmacol. 2003, 138, 775–786. [Google Scholar] [CrossRef][Green Version]
- Jiang, M.; Spicher, K.; Boulay, G.; Wang, Y.; Birnbaumer, L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc. Natl. Acad. Sci. USA 2001, 98, 3577–3582. [Google Scholar] [CrossRef]
- Senogles, S.E.; Spiegel, A.M.; Padrell, E.; Iyengar, R.; Caron, M.G. Specificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted system. J. Biol. Chem. 1990, 265, 4507–4514. [Google Scholar]
- Montmayeur, J.P.; Guiramand, J.; Borrelli, E. Preferential coupling between dopamine D2 receptors and G-proteins. Mol. Endocrinol. 1993, 7, 161–170. [Google Scholar]
- Zuk, J.; Bartuzi, D.; Matosiuk, D.; Kaczor, A.A. Preferential Coupling of Dopamine D2S and D2L Receptor Isoforms with Gi1 and Gi2 Proteins-In Silico Study. Int. J. Mol. Sci. 2020, 21, 436. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.I.; et al. Common activation mechanism of class A GPCRs. eLife 2019, 8, e50279. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, K.M.; Clark, M.J.; Hijazi, M.; Kumari, P.; Bai, X.C.; Sunahara, R.K.; Barth, P.; Rosenbaum, D.M. Structure of a D2 dopamine receptor-G-protein complex in a lipid membrane. Nature 2020, 584, 125–129. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fukunaga, K. Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells. J. Neurochem. 2003, 85, 1064–1074. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Jorg, M.; Capuano, B. The dopamine D2 receptor dimer and its interaction with homobivalent antagonists: Homology modeling, docking and molecular dynamics. J. Mol. Model 2016, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Madras, B.K. History of the discovery of the antipsychotic dopamine D2 receptor: A basis for the dopamine hypothesis of schizophrenia. J. Hist. Neurosci. 2013, 22, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Giros, B.; Sokoloff, P.; Martres, M.P.; Riou, J.F.; Emorine, L.J.; Schwartz, J.C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 1989, 342, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Monsma, F.J., Jr.; McVittie, L.D.; Gerfen, C.R.; Mahan, L.C.; Sibley, D.R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature 1989, 342, 926–929. [Google Scholar] [CrossRef]
- Dal Toso, R.; Sommer, B.; Ewert, M.; Herb, A.; Pritchett, D.B.; Bach, A.; Shivers, B.D.; Seeburg, P.H. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J. 1989, 8, 4025–4034. [Google Scholar] [CrossRef]
- Pelham, H.R. Sorting and retrieval between the endoplasmic reticulum and Golgi apparatus. Curr. Opin. Cell Biol. 1995, 7, 530–535. [Google Scholar] [CrossRef]
- Montmayeur, J.P.; Bausero, P.; Amlaiky, N.; Maroteaux, L.; Hen, R.; Borrelli, E. Differential expression of the mouse D2 dopamine receptor isoforms. FEBS Lett. 1991, 278, 239–243. [Google Scholar] [CrossRef]
- Sedaghat, K.; Nantel, M.F.; Ginsberg, S.; Lalonde, V.; Tiberi, M. Molecular characterization of dopamine D2 receptor isoforms tagged with green fluorescent protein. Mol. Biotechnol. 2006, 34, 1–14. [Google Scholar] [CrossRef]
- Prou, D.; Gu, W.J.; Le Crom, S.; Vincent, J.D.; Salamero, J.; Vernier, P. Intracellular retention of the two isoforms of the D(2) dopamine receptor promotes endoplasmic reticulum disruption. J. Cell Sci. 2001, 114 Pt 19, 3517–3527. [Google Scholar]
- Usiello, A.; Baik, J.H.; Rouge-Pont, F.; Picetti, R.; Dierich, A.; LeMeur, M.; Piazza, P.V.; Borrelli, E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000, 408, 199–203. [Google Scholar] [CrossRef]
- Radl, D.; Chiacchiaretta, M.; Lewis, R.G.; Brami-Cherrier, K.; Arcuri, L.; Borrelli, E. Differential regulation of striatal motor behavior and related cellular responses by dopamine D2L and D2S isoforms. Proc. Natl. Acad. Sci. USA 2018, 115, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.G.; Condon, A.F.; Radl, D.; Borrelli, E.; Williams, J.T.; Neve, K.A. Cocaine-induced adaptation of dopamine D2S, but not D2L autoreceptors. eLife 2017, 6, e31924. [Google Scholar] [CrossRef]
- Margeta-Mitrovic, M.; Jan, Y.N.; Jan, L.Y. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Li, P.; Snyder, G.L.; Vanover, K.E. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403. [Google Scholar] [CrossRef]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef]
- Martire, G.; Mottola, G.; Pascale, M.C.; Malagolini, N.; Turrini, I.; Serafini-Cessi, F.; Jackson, M.R.; Bonatti, S. Different fate of a single reporter protein containing KDEL or KKXX targeting signals stably expressed in mammalian cells. J. Biol. Chem. 1996, 271, 3541–3547. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Miller, E. Protein sorting at the ER-Golgi interface. J. Cell Biol. 2016, 215, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Mrzljak, L.; Gutierrez, A.; de la Calle, A.; Goldman-Rakic, P.S. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 7731–7736. [Google Scholar] [CrossRef]
- Lopez-Aranda, M.F.; Acevedo, M.J.; Gutierrez, A.; Koulen, P.; Khan, Z.U. Role of a Galphai2 protein splice variant in the formation of an intracellular dopamine D2 receptor pool. J. Cell Sci. 2007, 120 Pt 13, 2171–2178. [Google Scholar] [CrossRef]
- Macey, T.A.; Gurevich, V.V.; Neve, K.A. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol. Pharmacol. 2004, 66, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.Y.; Varghese, G.; Chung, H.T.; Trogadis, J.; Seeman, P.; O’Dowd, B.F.; George, S.R. Resistance of the dopamine D2L receptor to desensitization accompanies the up-regulation of receptors on to the surface of Sf9 cells. Endocrinology 1997, 138, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, K.; Clark, S.D.; Cox, M.J.; Oak, J.N.; Liu, F.; Van Tol, H.H. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J. Biol. Chem. 2005, 280, 19350–19357. [Google Scholar] [CrossRef] [PubMed]
- Achour, L.; Labbe-Jullie, C.; Scott, M.G.; Marullo, S. An escort for GPCRs: Implications for regulation of receptor density at the cell surface. Trends Pharmacol. Sci. 2008, 29, 528–535. [Google Scholar] [CrossRef]
- Cho, D.I.; Min, C.; Jung, K.S.; Cheong, S.Y.; Zheng, M.; Cheong, S.J.; Oak, M.H.; Cheong, J.H.; Lee, B.K.; Kim, K.M. The N-terminal region of the dopamine D2 receptor, a rhodopsin-like GPCR, regulates correct integration into the plasma membrane and endocytic routes. Br. J. Pharmacol. 2012, 166, 659–675. [Google Scholar] [CrossRef]
- Fishburn, C.S.; Elazar, Z.; Fuchs, S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J. Biol. Chem. 1995, 270, 29819–29824. [Google Scholar]
- Grunewald, S.; Reilander, H.; Michel, H. In vivo reconstitution of dopamine D2S receptor-mediated G protein activation in baculovirus-infected insect cells: Preferred coupling to Gi1 versus Gi2. Biochemistry 1996, 35, 15162–15173. [Google Scholar] [CrossRef]
- Senogles, S.E. The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase. A study with site-directed mutant Gi alpha proteins. J. Biol. Chem. 1994, 269, 23120–23127. [Google Scholar]
- Choi, D.S.; Wang, D.; Tolbert, L.; Sadee, W. Basal signaling activity of human dopamine D2L receptor demonstrated with an ecdysone-inducible mammalian expression system. J. Neurosci. Methods 2000, 94, 217–225. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Sasaoka, T.; Tonegawa, S.; Kung, M.P.; Sankoorikal, E.B. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 8305–8314. [Google Scholar] [CrossRef]
- Welter, M.; Vallone, D.; Samad, T.A.; Meziane, H.; Usiello, A.; Borrelli, E. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc. Natl. Acad. Sci. USA 2007, 104, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hranilovic, D.; Fetsko, L.A.; Bucan, M.; Wang, Y. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol. Psychiatry 2002, 7, 1075–1082. [Google Scholar] [CrossRef]
- Dong, C.; Filipeanu, C.M.; Duvernay, M.T.; Wu, G. Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 2007, 1768, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Doly, S.; Shirvani, H.; Gata, G.; Meye, F.J.; Emerit, M.B.; Enslen, H.; Achour, L.; Pardo-Lopez, L.; Yang, S.K.; Armand, V.; et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol. Psychiatry 2016, 21, 480–490. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, G. Mechanisms of the anterograde trafficking of GPCRs: Regulation of AT1R transport by interacting proteins and motifs. Traffic 2019, 20, 110–120. [Google Scholar] [CrossRef]
- Milligan, G. The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr. Opin. Pharmacol. 2010, 10, 23–29. [Google Scholar] [CrossRef]
- Wu, G. Regulation of post-Golgi traffic of G protein-coupled receptors. Sub-Cell. Biochem. 2012, 63, 83–95. [Google Scholar]
- Spang, A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013391. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Nilsson, T.; Peterson, P.A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990, 9, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Pelham, H.R. A C-terminal signal prevents secretion of luminal ER proteins. Cell 1987, 48, 899–907. [Google Scholar] [CrossRef]
- Jin, H.; Komita, M.; Aoe, T. The Role of BiP Retrieval by the KDEL Receptor in the Early Secretory Pathway and its Effect on Protein Quality Control and Neurodegeneration. Front. Mol. Neurosci. 2017, 10, 222. [Google Scholar] [CrossRef]
- Rothman, J.E.; Wieland, F.T. Protein sorting by transport vesicles. Science 1996, 272, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Huber, I.; Rotman, M.; Cassel, D. The ARF1 GTPase-activating protein: Zinc finger motif and Golgi complex localization. Science 1995, 270, 1999–2002. [Google Scholar] [CrossRef]
- Aoe, T.; Huber, I.; Vasudevan, C.; Watkins, S.C.; Romero, G.; Cassel, D.; Hsu, V.W. The KDEL receptor regulates a GTPase-activating protein for ADP-ribosylation factor 1 by interacting with its non-catalytic domain. J. Biol. Chem. 1999, 274, 20545–20549. [Google Scholar] [CrossRef]
- Boehmler, W.; Obrecht-Pflumio, S.; Canfield, V.; Thisse, C.; Thisse, B.; Levenson, R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 230, 481–493. [Google Scholar] [CrossRef]
- Kokubun, H.; Jin, H.; Aoe, T. Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex. Int. J. Mol. Sci. 2019, 20, 5614. [Google Scholar] [CrossRef]
- Cabrera, M.; Muniz, M.; Hidalgo, J.; Vega, L.; Martin, M.E.; Velasco, A. The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol. Biol. Cell 2003, 14, 4114–4125. [Google Scholar] [CrossRef]
- Jackson, L.P.; Lewis, M.; Kent, H.M.; Edeling, M.A.; Evans, P.R.; Duden, R.; Owen, D.J. Molecular basis for recognition of dilysine trafficking motifs by COPI. Dev. Cell 2012, 23, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Letourneur, F.; Gaynor, E.C.; Hennecke, S.; Demolliere, C.; Duden, R.; Emr, S.D.; Riezman, H.; Cosson, P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 1994, 79, 1199–1207. [Google Scholar] [CrossRef]
- Vrecl, M.; Anderson, L.; Hanyaloglu, A.; McGregor, A.M.; Groarke, A.D.; Milligan, G.; Taylor, P.L.; Eidne, K.A. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: Effect of beta-arrestin on internalization kinetics. Mol. Endocrinol. 1998, 12, 1818–1829. [Google Scholar] [PubMed]
- Pagano, A.; Rovelli, G.; Mosbacher, J.; Lohmann, T.; Duthey, B.; Stauffer, D.; Ristig, D.; Schuler, V.; Meigel, I.; Lampert, C.; et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 1189–1202. [Google Scholar] [CrossRef]
- Kubale, V.; Pogacnik, A.; Schwartz, W.T.; Vrecl, M. Seven transmembrane receptors (7TM) in the view of dimerization and experimental methods to study their dimerization and cross-talk. Slov. Vet. Res. 2008, 45, 89–102. [Google Scholar]
- Kuner, R.; Kohr, G.; Grunewald, S.; Eisenhardt, G.; Bach, A.; Kornau, H.C. Role of heteromer formation in GABAB receptor function. Science 1999, 283, 74–77. [Google Scholar] [CrossRef]
- Filipeanu, C.M.; Pullikuth, A.K.; Guidry, J.J. Molecular determinants of the human alpha2C-adrenergic receptor temperature-sensitive intracellular traffic. Mol. Pharmacol. 2015, 87, 792–802. [Google Scholar] [CrossRef]
- Nasu-Nishimura, Y.; Hurtado, D.; Braud, S.; Tang, T.T.; Isaac, J.T.; Roche, K.W. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 7014–7021. [Google Scholar] [CrossRef]
- Hermosilla, R.; Schulein, R. Sorting functions of the individual cytoplasmic domains of the G protein-coupled vasopressin V(2) receptor in Madin Darby canine kidney epithelial cells. Mol. Pharmacol. 2001, 60, 1031–1039. [Google Scholar] [CrossRef]
- Kubale, V.; Blagotinsek, K.; Nohr, J.; Eidne, K.A.; Vrecl, M. The Conserved Arginine Cluster in the Insert of the Third Cytoplasmic Loop of the Long Form of the D(2) Dopamine Receptor (D2L-R) Acts as an Intracellular Retention Signal. Int. J. Mol. Sci. 2016, 17, 1152. [Google Scholar] [CrossRef]
- Shioda, N.; Takeuchi, Y.; Fukunaga, K. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: Proteins interacting with the third cytoplasmic loop of dopamine D2 and D3 receptors. J. Pharmacol. Sci. 2010, 114, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Ferre, S.; Cordomi, A.; Moreno, E.; Mallol, J.; Casado, V.; Cortes, A.; Hoffmann, H.; Ortiz, J.; Canela, E.I.; et al. Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J. Biol. Chem. 2010, 285, 27346–27359. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, S.; Faron-Gorecka, A.; Dobrucki, J.; Polit, A.; Dziedzicka-Wasylewska, M. Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J. 2009, 276, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, S.; Polit, A.; Kedracka-Krok, S.; Wedzony, K.; Mackowiak, M.; Dziedzicka-Wasylewska, M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Likhite, N.; Jackson, C.A.; Liang, M.S.; Krzyzanowski, M.C.; Lei, P.; Wood, J.F.; Birkaya, B.; Michaels, K.L.; Andreadis, S.T.; Clark, S.D.; et al. The protein arginine methyltransferase PRMT5 promotes D2-like dopamine receptor signaling. Sci. Signal. 2015, 8, ra115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gouw, M.; Michael, S.; Diakogianni, A.; Valverde, J.A.; Bukirova, D.; Calyseva, J.; Palopoli, N.; Davey, N.E.; Chemes, L.B.; et al. ELM-the eukaryotic linear motif resource in 2020. Nucleic Acids Res. 2020, 48, D296–D306. [Google Scholar] [CrossRef]
- Duvernay, M.T.; Filipeanu, C.M.; Wu, G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell. Signal. 2005, 17, 1457–1465. [Google Scholar] [CrossRef]
- Bermak, J.C.; Li, M.; Bullock, C.; Zhou, Q.Y. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat. Cell Biol. 2001, 3, 492–498. [Google Scholar] [CrossRef]
- Kabbani, N.; Woll, M.P.; Nordman, J.C.; Levenson, R. Dopamine receptor interacting proteins: Targeting neuronal calcium sensor-1/D2 dopamine receptor interaction for antipsychotic drug development. Curr. Drug Targets 2012, 13, 72–79. [Google Scholar] [CrossRef]
- Sencanski, M.; Glisic, S.; Snajder, M.; Veljkovic, N.; Poklar Ulrih, N.; Mavri, J.; Vrecl, M. Computational design and characterization of nanobody-derived peptides that stabilize the active conformation of the beta2-adrenergic receptor (beta2-AR). Sci. Rep. 2019, 9, 16555. [Google Scholar] [CrossRef]
- Mandic, M.; Drinovec, L.; Glisic, S.; Veljkovic, N.; Nohr, J.; Vrecl, M. Demonstration of a direct interaction between beta2-adrenergic receptor and insulin receptor by BRET and bioinformatics. PLoS ONE 2014, 9, e112664. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Chu, X.P.; Mao, L.M.; Wang, M.; Lan, H.X.; Li, M.H.; Zhang, G.C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron 2006, 52, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fu, W.; Xie, J.; Luo, H.; Sells, S.F.; Geddes, J.W.; Bondada, V.; Rangnekar, V.M.; Mattson, M.P. Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat. Med. 1998, 4, 957–962. [Google Scholar] [CrossRef]
- Park, S.K.; Nguyen, M.D.; Fischer, A.; Luke, M.P.; Affar, E.B.; Dieffenbach, P.B.; Tseng, H.C.; Shi, Y.; Tsai, L.H. Par-4 links dopamine signaling and depression. Cell 2005, 122, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Binda, A.V.; Kabbani, N.; Lin, R.; Levenson, R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol. Pharmacol. 2002, 62, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Karpa, K.; Kabbani, N.; Goldman-Rakic, P.; Levenson, R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc. Natl. Acad. Sci. USA 2001, 98, 5258–5263. [Google Scholar] [CrossRef]
- Lee, F.J.; Pei, L.; Moszczynska, A.; Vukusic, B.; Fletcher, P.J.; Liu, F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007, 26, 2127–2136. [Google Scholar] [CrossRef]
- Navarro, G.; Aymerich, M.S.; Marcellino, D.; Cortes, A.; Casado, V.; Mallol, J.; Canela, E.I.; Agnati, L.; Woods, A.S.; Fuxe, K.; et al. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J. Biol. Chem. 2009, 284, 28058–28068. [Google Scholar] [CrossRef]
- Woods, A.S.; Marcellino, D.; Jackson, S.N.; Franco, R.; Ferre, S.; Agnati, L.F.; Fuxe, K. How calmodulin interacts with the adenosine A(2A) and the dopamine D(2) receptors. J. Proteome Res. 2008, 7, 3428–3434. [Google Scholar] [CrossRef]
- Hanson, P.I.; Otto, H.; Barton, N.; Jahn, R. The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J. Biol. Chem. 1995, 270, 16955–16961. [Google Scholar] [CrossRef]
- Zou, S.; Li, L.; Pei, L.; Vukusic, B.; Van Tol, H.H.; Lee, F.J.; Wan, Q.; Liu, F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.D.; Oxford, G.S.; Milgram, S.L. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J. Biol. Chem. 1999, 274, 19894–19900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Imachi, H.; Lyu, J.Y.; Fukunaga, K.; Sato, S.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Kikuchi, F.; Dong, T.; et al. Prolactin regulatory element-binding protein is involved in suppression of the adiponectin gene in vivo. J. Endocrinol. Investig. 2017, 40, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, M.Y.; Kim, T.H.; Min, D.K.; Yang, G.E.; Ahn, Y.H. Prolactin regulatory element-binding (PREB) protein regulates hepatic glucose homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Murao, K.; Imachi, H.; Li, J.; Nishiuchi, T.; Dobashi, H.; Hosomi, N.; Masugata, H.; Zhang, G.X.; Iwama, H.; et al. The transcription factor prolactin regulatory element-binding protein mediates prolactin transcription induced by thyrotropin-releasing hormone in GH3 cells. Endocrine 2010, 38, 53–59. [Google Scholar] [CrossRef]
- Zhang, W.; Murao, K.; Imachi, H.; Iwama, H.; Chen, K.; Fei, Z.; Zhang, X.; Ishida, T.; Tamiya, T. Suppression of prolactin expression by cabergoline requires prolactin regulatory element-binding protein (PREB) in GH3 cells. Horm. Metab. Res. 2010, 42, 557–561. [Google Scholar] [CrossRef]
- Wimalasena, D.S.; Wimalasena, K. Kinetic evidence for channeling of dopamine between monoamine transporter and membranous dopamine-beta-monooxygenase in chromaffin granule ghosts. J. Biol. Chem. 2004, 279, 15298–15304. [Google Scholar] [CrossRef]
- Mochida, G.H.; Mahajnah, M.; Hill, A.D.; Basel-Vanagaite, L.; Gleason, D.; Hill, R.S.; Bodell, A.; Crosier, M.; Straussberg, R.; Walsh, C.A. A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am. J. Hum. Genet. 2009, 85, 897–902. [Google Scholar] [CrossRef]
- Shioda, N. Dopamine D2L receptor-interacting proteins regulate dopaminergic signaling. J. Pharmacol. Sci. 2017, 135, 51–54. [Google Scholar] [CrossRef]
- Shioda, N.; Yamamoto, Y.; Watanabe, M.; Binas, B.; Owada, Y.; Fukunaga, K. Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 3146–3155. [Google Scholar] [CrossRef]
- Yabuki, Y.; Takahata, I.; Matsuo, K.; Owada, Y.; Fukunaga, K. Ramelteon Improves Post-traumatic Stress Disorder-Like Behaviors Exhibited by Fatty Acid-Binding Protein 3 Null Mice. Mol. Neurobiol. 2018, 55, 3577–3591. [Google Scholar] [CrossRef]
- Brismar, H.; Asghar, M.; Carey, R.M.; Greengard, P.; Aperia, A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc. Natl. Acad. Sci. USA 1998, 95, 5573–5578. [Google Scholar] [CrossRef]
- Holtback, U.; Brismar, H.; DiBona, G.F.; Fu, M.; Greengard, P.; Aperia, A. Receptor recruitment: A mechanism for interactions between G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.M.; Liu, Y.; Vidensky, S.; Maier, S.; Jung, E.; Farhan, H.; Robinson, M.B.; Sitte, H.H.; Rothstein, J.D. The endoplasmic reticulum exit of glutamate transporter is regulated by the inducible mammalian Yip6b/GTRAP3-18 protein. J. Biol. Chem. 2008, 283, 6175–6183. [Google Scholar] [CrossRef] [PubMed]
- Dupre, D.J.; Robitaille, M.; Richer, M.; Ethier, N.; Mamarbachi, A.M.; Hebert, T.E. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J. Biol. Chem. 2007, 282, 13703–13715. [Google Scholar] [CrossRef]
- Bowers, M.S.; McFarland, K.; Lake, R.W.; Peterson, Y.K.; Lapish, C.C.; Gregory, M.L.; Lanier, S.M.; Kalivas, P.W. Activator of G protein signaling 3: A gatekeeper of cocaine sensitization and drug seeking. Neuron 2004, 42, 269–281. [Google Scholar] [CrossRef]
- Rassu, M.; Del Giudice, M.G.; Sanna, S.; Taymans, J.M.; Morari, M.; Brugnoli, A.; Frassineti, M.; Masala, A.; Esposito, S.; Galioto, M.; et al. Role of LRRK2 in the regulation of dopamine receptor trafficking. PLoS ONE 2017, 12, e0179082. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, M.; Arinami, T.; Toru, M. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: Ser311Cys polymorphisms of the dopamine D2-receptor gene and schizophrenia. J. Pharmacol. Sci. 2010, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Guiramand, J.; Montmayeur, J.P.; Ceraline, J.; Bhatia, M.; Borrelli, E. Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. J. Biol. Chem. 1995, 270, 7354–7358. [Google Scholar] [CrossRef]
- Kaiser, R.; Hofer, A.; Grapengiesser, A.; Gasser, T.; Kupsch, A.; Roots, I.; Brockmoller, J. L -dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 2003, 60, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.; Urbanek, M.; Guenther, D.; Robin, R.; Long, J.C. Linkage and association of a functional DRD2 variant [Ser311Cys] and DRD2 markers to alcoholism, substance abuse and schizophrenia in Southwestern American Indians. Am. J. Med. Genet. 1997, 74, 386–394. [Google Scholar] [CrossRef]
- Senogles, S.E.; Heimert, T.L.; Odife, E.R.; Quasney, M.W. A region of the third intracellular loop of the short form of the D2 dopamine receptor dictates Gi coupling specificity. J. Biol. Chem. 2004, 279, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).