Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Ethanolic Extract of M. Chamomilla
2.3. Characterization of M. Chamomilla Extract Volatile Compounds
2.4. Animals
2.5. Induction of PCOS and Hypothyroidism
2.6. Study Groups
2.7. Assessment of Percent Body Weight (% BW) Increase
2.8. Sampling
2.9. Assessment of Thyroid Gland Weight (Thy W)
2.10. Assessment of PCOS Induction
2.11. Assessment of Thyroid Function Markers
2.12. Assessment of Histopathological Alteration Using Light and Electron Microscope
2.13. Assessment of Caspase-3 and Proliferating Cell Nuclear Antigen (PCNA)Immunoexpression
2.14. Assessment of Oxidative Stress/Antioxidant Measures
2.15. Statistical Study
3. Results
3.1. Volatile Constituents of M. Chamomilla Extract
3.2. Proof of PCOS Induction
3.3. Effect of M. Chamomilla Extract and Metformin on Body and Thyroid Gland Weight
3.4. Effect of M. Chamomilla and Metformin on PCOS-Associated Thyroid Dysfunction
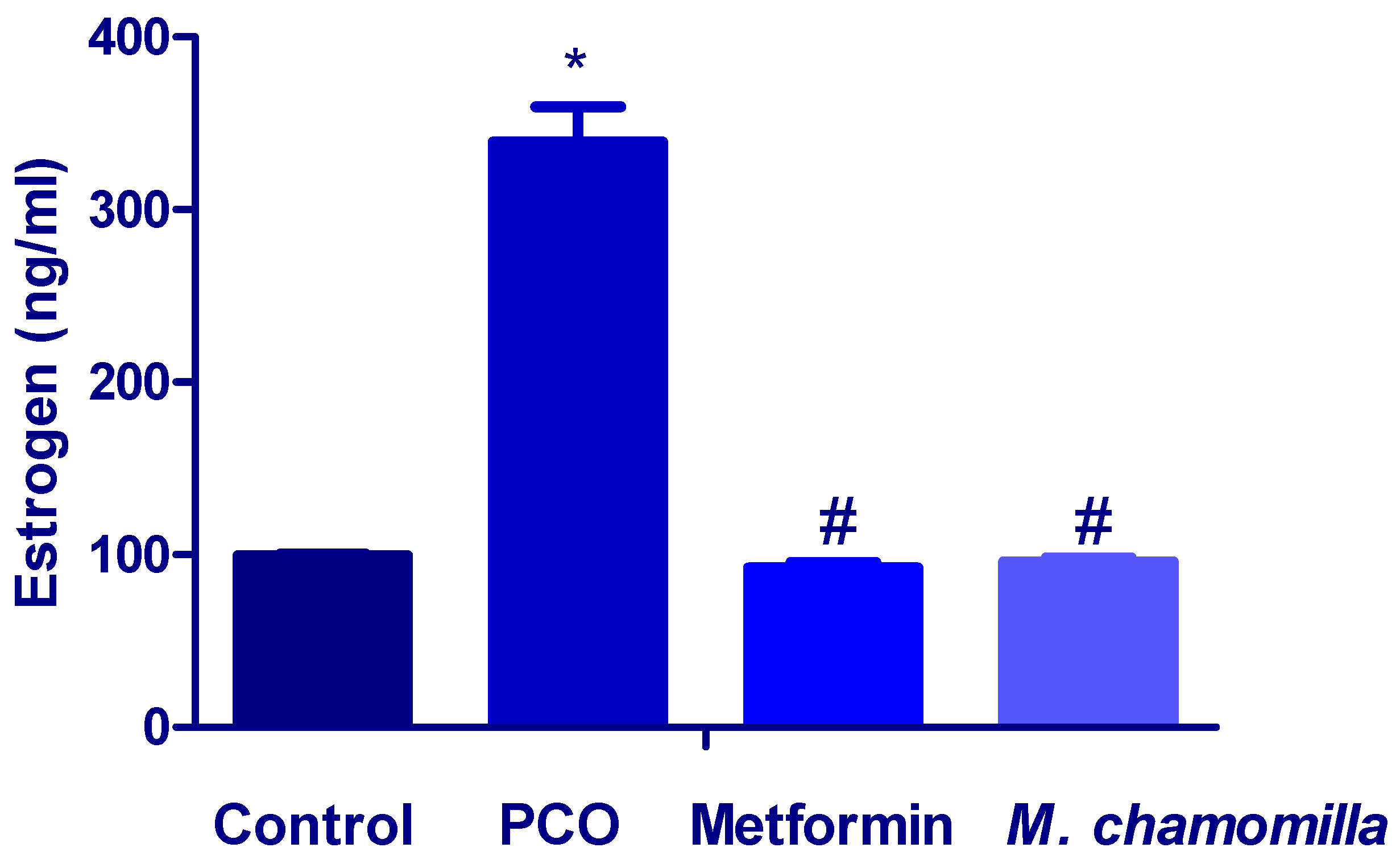
3.5. Effect of M. Chamomilla and Metformin on PCOS-Associated Increased Estrogen Level
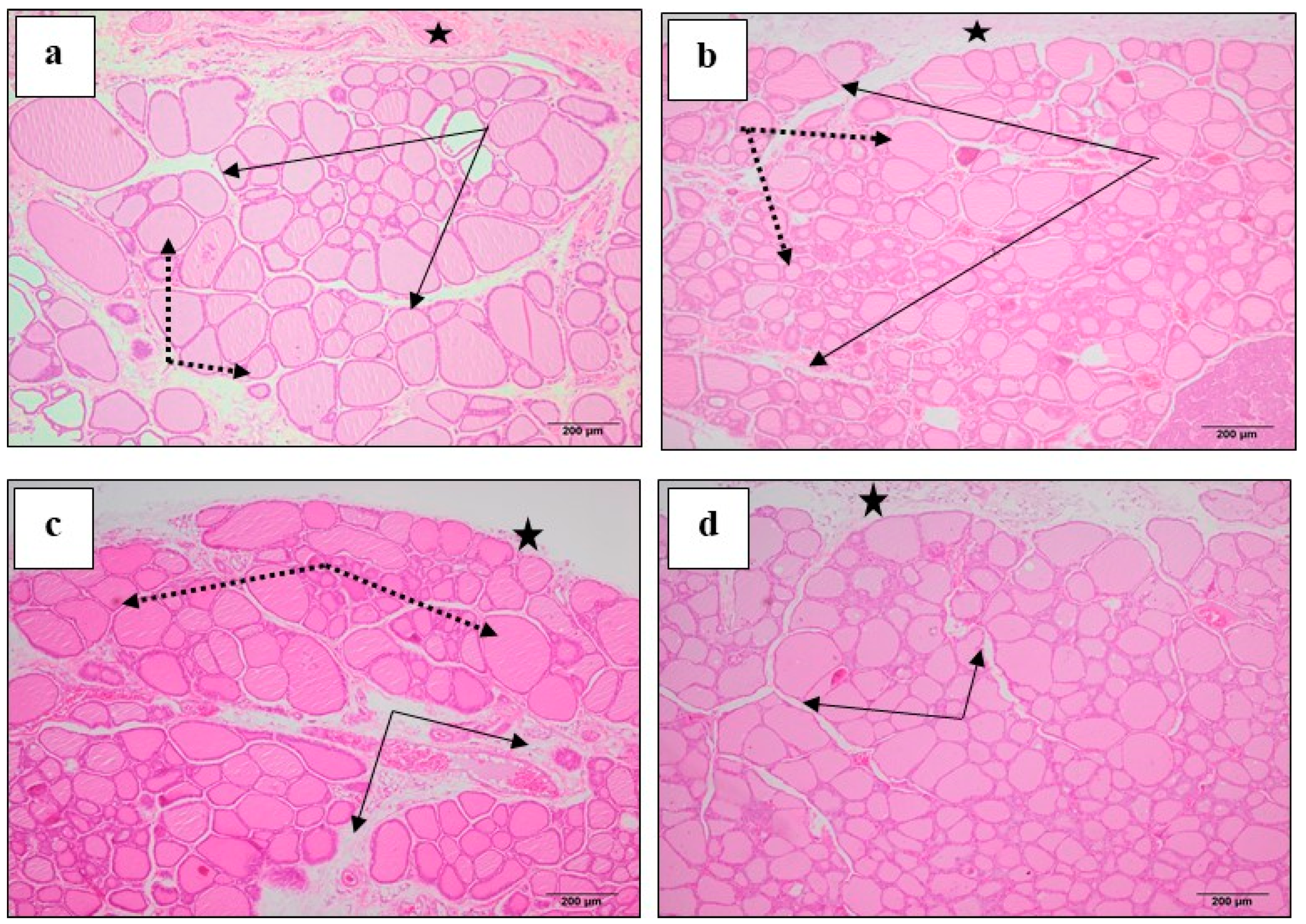
3.6. Effect of M. Chamomilla and Metformin on PCOS-Associated Thyroid Gland Histopathological Changes (H & E)
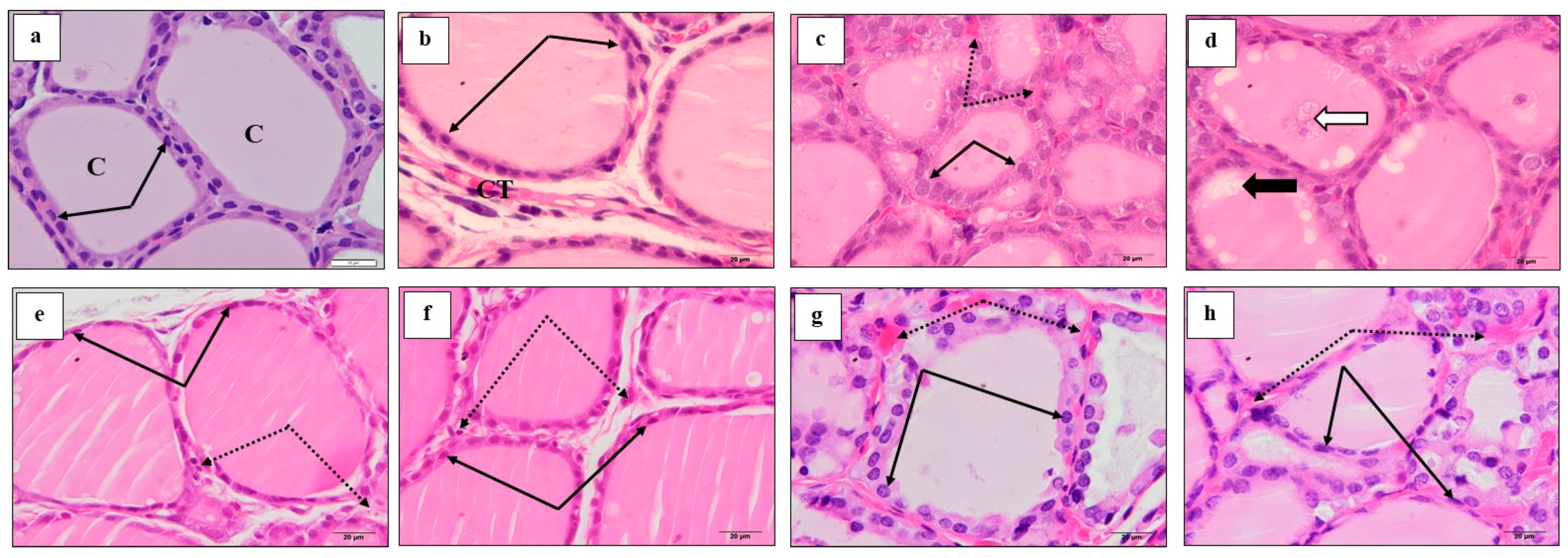
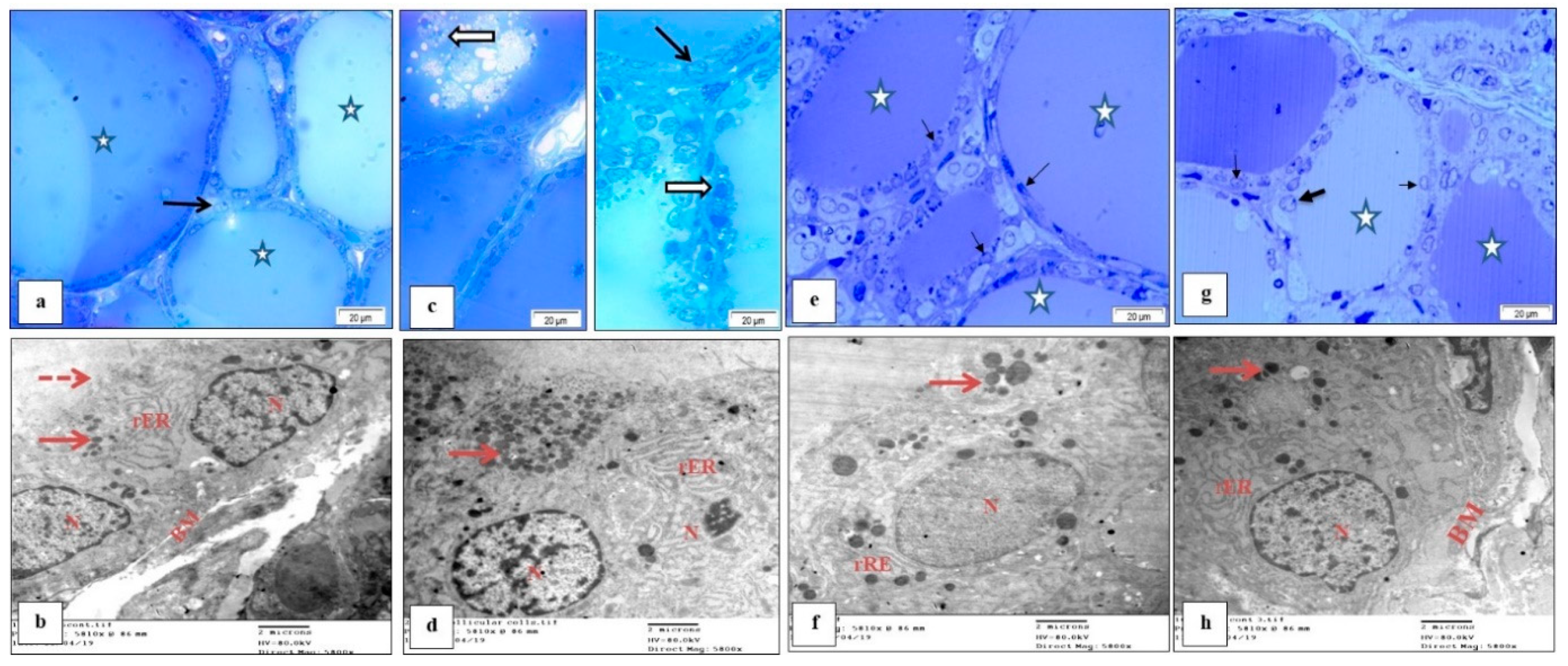
3.7. Effect of M. Chamomilla and Metformin on PCOS-Associated Thyroid Gland Ultrastructure Changes (Semithin Sections (Toluidine Blue) and Ultrathin Sections)
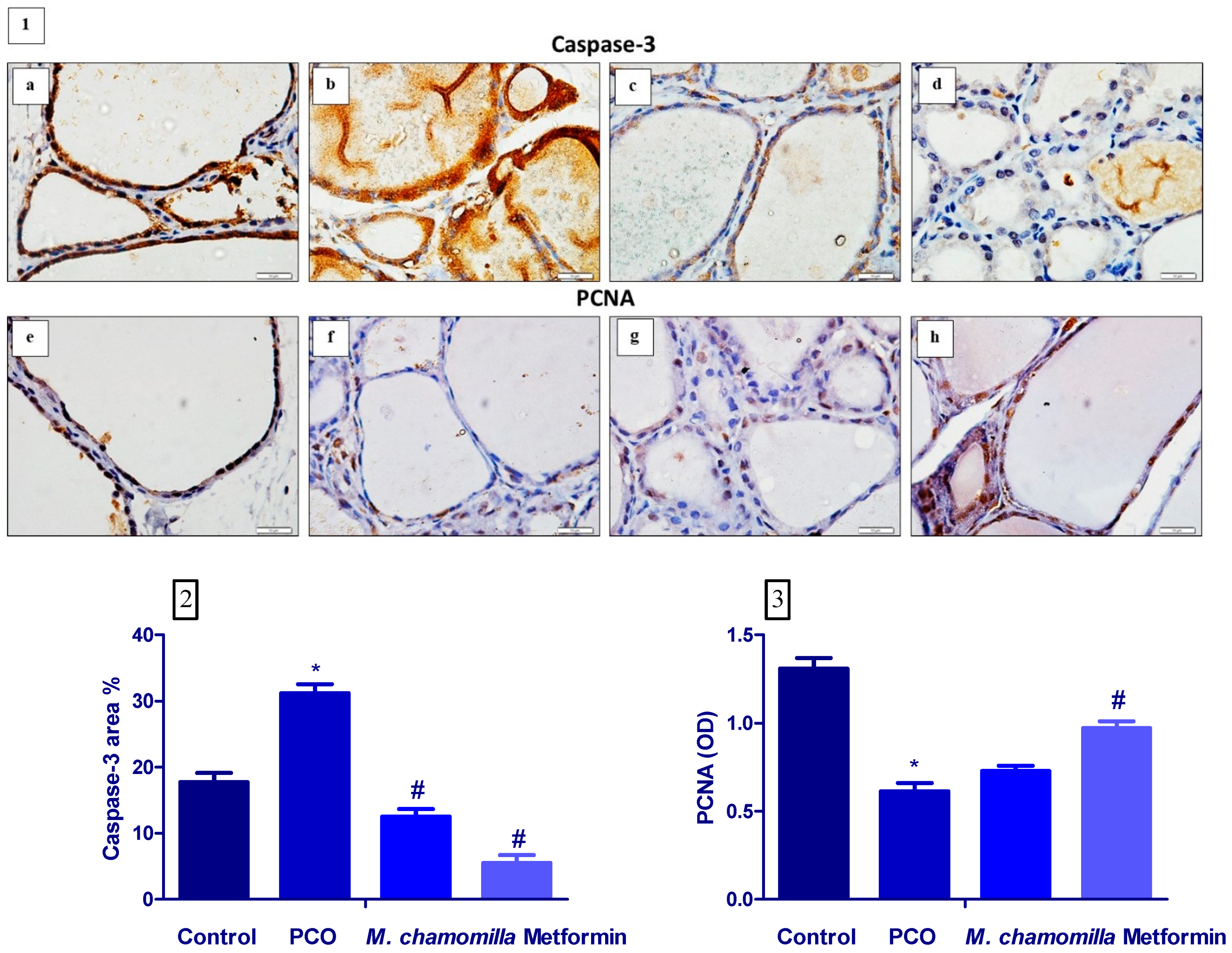
3.8. Effect of M. Chamomilla and Metformin on PCOS-Associated Thyroid Gland Caspase-3 and PCNA Immunoexpression
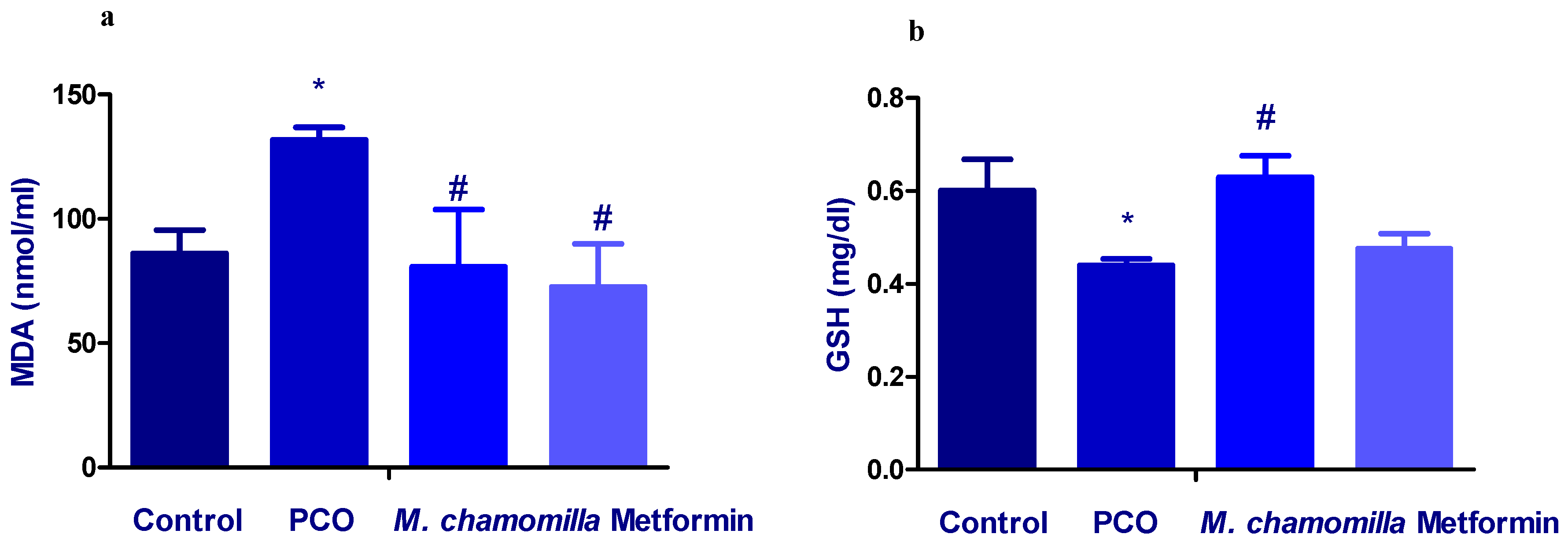
3.9. Effect of M. Chamomilla and Metformin on PCOS-Associated Oxidative Stress Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Teng, W.; Shan, Z.; Patil-Sisodia, K.; Cooper, D.S. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 228–237. [Google Scholar] [CrossRef]
- Cho, M.K. Thyroid dysfunction and subfertility. Clin. Ex. Reproductive Med. 2015, 42, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Barišić, T.; Mandić, V.; Vasilj, A.; Tiric, D. Higher levels of thyrotropin in pregnancy and adverse pregnancy outcomes. J. Maternal-Fetal Neonatal Med. 2019, 32, 2883–2888. [Google Scholar]
- Yang, J.; Liu, Y.; Liu, H.; Zheng, H.; Li, X.; Zhu, L.; Wang, Z. Associations of maternal iodine status and thyroid function with adverse pregnancy outcomes in Henan Province of China. J. Trace Elem. Med. Biol. 2018, 47, 104–110. [Google Scholar] [CrossRef]
- Ganie, M.A.; Laway, T.A.; Wani, M.A.; Zargar, S.; Nisar, F.; Ahamed, M.L.K.; Ahmed, S. Association of subclinical hypothyroidism and phenotype, insulin resistance, and lipid parameters in young women with polycystic ovary syndrome. Fertil. Sterility 2011, 95, 2039–2043. [Google Scholar] [CrossRef]
- Celik, C.; Abali, R.; Tasdemir, N.; Guzel, S.; Yuksel, A.; Aksu, E.; Yılmaz, M. Is subclinical hypothyroidism contributing dyslipidemia and insulin resistance in women with polycystic ovary syndrome? Gynecol. Endocrinol. 2012, 28, 615–618. [Google Scholar] [CrossRef]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef]
- Moran, L.J.; Norman, R.J.; Teede, H.J. Metabolic risk in PCOS: Phenotype and adiposity impact. Trends Endocrinol. Metab. 2015, 26, 136–143. [Google Scholar] [CrossRef]
- Ding, X.; Yang, L.; Tang, R.; Chen, Q.; Pan, J.; Yang, H.; Chen, Z.; Chen, X.; Mu, L. Subclinical Hypothyroidism in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Duran, C.; Basaran, M.; Kutlu, O.; Kucukaydin, Z.; Bakdik, S.; Burnik, F.S.; Aslan, U.; Erdem, S.S.; Ecirli, S. Frequency of nodular goiter and autoimmune thyroid disease in patients with polycystic ovary syndrome. Endocrine 2015, 49, 464–469. [Google Scholar] [CrossRef]
- Alzahrani, A.A.; Alahmadi, A.A.; Ali, S.S.; Alahmadi, B.A.; Arab, R.A.; Wahman, L.F.; El-Shitany, N.A. Biochemical and histological evidence of thyroid gland dysfunction in estradiol valerate model of the polycystic ovary in Wistar rats. Biochem. Biophys. Res. Commun. 2019, 514, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B. Rat models of polycystic ovary syndrome. In Sourcebook of Models for Biomedical Research; Michael Conn, P., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 405–410. [Google Scholar]
- Jelodar, G.; Masoomi, S.; Rahmanifar, F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran. J. Basic Med. Sci. 2018, 21, 645–650. [Google Scholar] [PubMed]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Johari, H.; Sharifi, E.; Mardan, M.; Kafilzadeh, F.; Hemayatkhah, V.; Kargar, H.; Nikpoor, N. The effects of a hydroalcoholic extract of Matricaria chamomilla flower on the pituitary-gonadal axis and ovaries of rats. Int. J. Endocrinol. Metab. 2011, 9, 330–334. [Google Scholar] [CrossRef]
- Heidary, M.; Yazdanpanahi, Z.; Dabbaghmanesh, M.H.; Parsanezhad, M.E.; Emamghoreishi, M.; Akbarzadeh, M. Effect of chamomile capsule on lipid- and hormonal-related parameters among women of reproductive age with polycystic ovary syndrome. J. Res. Med. Sci. 2018, 23, 33. [Google Scholar]
- Zangeneh, F.Z.; Minaee, B.; Amirzargar, A.; Ahangarpour, A.; Mousavizadeh, K. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertility 2010, 11, 169–174. [Google Scholar]
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018, 9, 3743–3754. [Google Scholar] [CrossRef]
- Sobota, J.A.; Bäck, N.; Eipper, B.A.; Mains, R.E. Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J. Cell Sci. 2009, 122, 3542–3553. [Google Scholar] [CrossRef]
- Janssen, O.E.; Mehlmauer, N.; Hahn, S.; Offner, A.H.; Gartner, R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 2004, 150, 363–369. [Google Scholar] [CrossRef]
- Goyal, D.; Relia, P.; Sehra, A.; Khandelwal, D.; Dutta, D.; Jain, D.; Kalra, S. Prevalence of hypothyroidism and thyroid autoimmunity in polycystic ovarian syndrome patients: A North Indian study. Thyroid Res. Pract. 2019, 16, 55–59. [Google Scholar]
- Fénichel, P.; Gobert, B.; Carré, Y.; Barbarino-Monnier, P.; Hiéronimus, S. Polycystic ovary syndrome in autoimmune disease. Lancet 1999, 353, 2210. [Google Scholar] [CrossRef]
- Reimand, K.; Talja, I.; Metsküla, K.; Kadastik, Ü.; Matt, K.; Uibo, R. Autoantibody studies of female patients with reproductive failure. J. Reproductive Immunol. 2001, 51, 167–176. [Google Scholar] [CrossRef]
- Chen, S.; Cho, M.; Karlsberg, K.; Zhou, D.; Yuan, Y.C. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J. Biol. Chem. 2004, 279, 48071–48078. [Google Scholar] [CrossRef] [PubMed]
- Brueggemeier, R.W.; Gu, X.; Mobley, J.A.; Joomprabutra, S.; Bhat, A.S.; Whetstone, J.L. Effects of Phytoestrogens and Synthetic Combinatorial Libraries on Aromatase, Estrogen Biosynthesis, and Metabolism. Ann. N. Y. Acad. Sci. 2001, 948, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, S.; Hagbjörk, A.L.; Ekman, S.; Fransson-Steen, R.; Terelius, Y. Metabolism of human cytochrome P450 marker substrates in mouse: A strain and gender comparison. Xenobiotica 2004, 34, 811–834. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab. Rev. 2016, 48, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Wang, C.; Hou, L.; Zheng, X.; Xu, Y.; Ding, L.; Pang, S. Metformin affects thyroid function in male rats. Oncotarget 2017, 8, 107589–107595. [Google Scholar] [CrossRef]
- Pappa, T.; Alevizaki, M. Metformin and Thyroid: An Update. Eur. Thyroid J. 2013, 2, 22–28. [Google Scholar] [CrossRef]
- Mohammadi, M. Oxidative stress and polycystic ovary syndrome: A brief review. Int. J. Preventive Med. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Macut, D.; Bjekić-Macut, J.; Savić-Radojević, A. Dyslipidemia and oxidative stress in PCOS. In Polycystic Ovary Syndrome; Karger Publishers: Basel, Switzerland, 2013; Volume 40, pp. 51–63. [Google Scholar]
- Bhaskaran, N.; Shukla, S.; Kanwal, R.; Srivastava, J.K.; Gupta, S. Induction of heme oxygenase-1 by chamomile protects murine macrophages against oxidative stress. Life Sci. 2012, 90, 1027–1033. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Mol. Cell. Pharmacol. 2009, 1, 138–147. [Google Scholar] [CrossRef]
- Ganzera, M.; Schneider, P.; Stuppner, H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006, 78, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Blazquez, M.A. Characterization of the Essential Oils from Commercial Chamomile Flowers and Chamomile Teabags by GC-MS Analysis. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1487–1491. [Google Scholar]
- Fırat, Z.; Demirci, F.; Demirci, B. Antioxidant activity of chamomile essential oil and main components. Nat. Volatiles Essent. Oils 2018, 5, 11–16. [Google Scholar]
- Khayyal, M.T.; Kreuter, M.H.; Kemmler, M.; Altmann, P.; Abdel-Naby, D.H.; El-Ghazaly, M.A. Effect of a chamomile extract in protecting against radiation-induced intestinal mucositis. Phytotherapy Res. 2019, 33, 728–736. [Google Scholar] [CrossRef] [PubMed]
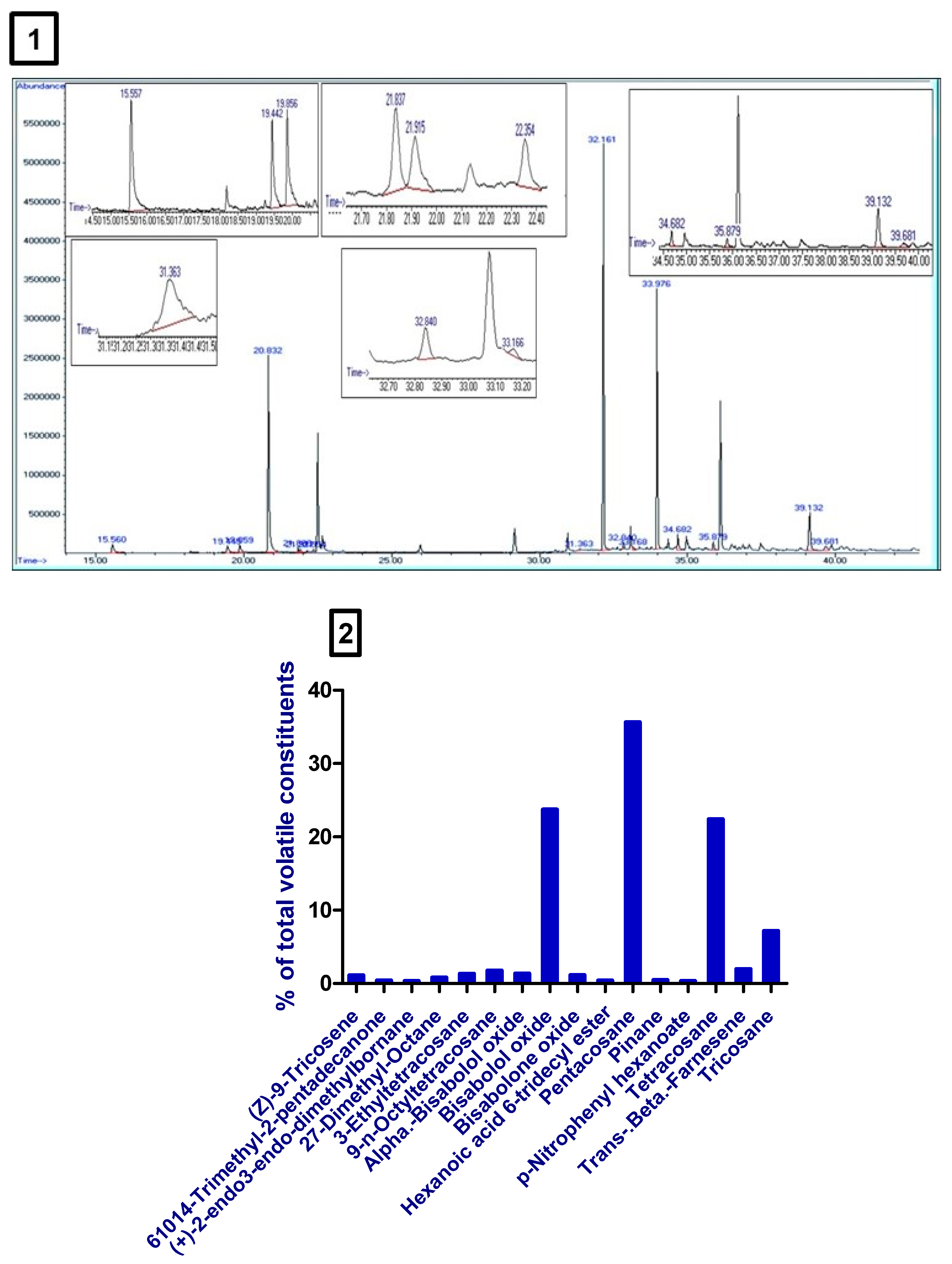

| Serial Number | Retention Time (min) | Compound Name | Relative Area (%) |
|---|---|---|---|
| 1 | 35.88 | (Z)-9-tricosene | 0.67 |
| 2 | 21.915 | 6,10,14-trimethyl-2-pentadecanone | 0.24 |
| 3 | 22.358 | (+)-2-endo,3-endo-dimethylbornane | 0.19 |
| 4 | 32.844 | 2,7-dimethyl-octane | 0.48 |
| 5 | 39.681 | 3-ethyltetracosane | 0.81 |
| 6 | 34.68 | 9-n-octyltetracosane | 1.08 |
| 7 | 19.444 | Alpha-bisabolol oxide | 0.84 |
| 8 | 20.831 | Bisabolol oxide | 14.69 |
| 9 | 19.858 | Bisabolone oxide | 0.70 |
| 10 | 31.363 | Hexanoic acid, 6-tridecyl ester | 0.23 |
| 11 | 32.162 | Pentacosane | 22.05 |
| 12 | 21.839 | Pinane | 0.27 |
| 13 | 33.17 | p-Nitrophenyl hexanoate | 0.19 |
| 14 | 33.98 | Tetracosane | 13.86 |
| 15 | 15.562 | Trans-beta-farnesene | 1.20 |
| 16 | 39.133 | Tricosane | 4.43 |
| % BW Increase | Thy W (mg) | |
|---|---|---|
| Control | 15.79 ± 2.1 | 4.14 ± 1.7 |
| PCOS | 14.34 ± 2.0 | 29.16 ± 2.2 a |
| M. chamomilla | 18.4 ± 2.0 | 32.05 ± 2.8 |
| Metformin | 10.68 ± 2.1 | 12.23 ± 4.3 b |
| Control | PCOS | M. chamomilla | Metformin | |
|---|---|---|---|---|
| TSH (μU/mL) | 0.012 ± 0.002 | 0.089 ± 0.027 a | 0.013 ± 0.003 b | 0.018 ± 0.005 b |
| T3 (pg/mL) | 2.68 ± 0.17 | 2.13 ± 0.12 a | 2.62 ± 0.14 b | 2.67 ± 0.11 b |
| T4 (ng/dL) | 2.07 ± 0.10 | 1.59 ± 0.02 a | 1.96 ± 0.12 b | 2.38 ± 0.16 b |
| Control | PCOS | M. chamomilla | Metformin | |
|---|---|---|---|---|
| GPx (mU/mL) | 23.0 ± 1.7 | 14.9 ± 1.7 a | 26.1 ± 2.5 b | 23.0 ± 2.5 b |
| CAT (U/mL) | 321 ± 35 | 148 ± 1.8 a | 292 ± 1.2 b | 248 ± 3.2 b |
| SOD (U/mL) | 345 ± 19 | 335 ± 7.2 | 344 ± 28 | 327 ± 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahmadi, A.A.; Alzahrani, A.A.; Ali, S.S.; Alahmadi, B.A.; Arab, R.A.; El-Shitany, N.A.E.-A. Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism. Biomolecules 2020, 10, 88. https://doi.org/10.3390/biom10010088
Alahmadi AA, Alzahrani AA, Ali SS, Alahmadi BA, Arab RA, El-Shitany NAE-A. Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism. Biomolecules. 2020; 10(1):88. https://doi.org/10.3390/biom10010088
Chicago/Turabian StyleAlahmadi, Ahlam Abdulaziz, Areej Ali Alzahrani, Soad Shaker Ali, Bassam Abdulaziz Alahmadi, Rana Ali Arab, and Nagla Abd El-Aziz El-Shitany. 2020. "Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism" Biomolecules 10, no. 1: 88. https://doi.org/10.3390/biom10010088
APA StyleAlahmadi, A. A., Alzahrani, A. A., Ali, S. S., Alahmadi, B. A., Arab, R. A., & El-Shitany, N. A. E.-A. (2020). Both Matricaria chamomilla and Metformin Extract Improved the Function and Histological Structure of Thyroid Gland in Polycystic Ovary Syndrome Rats through Antioxidant Mechanism. Biomolecules, 10(1), 88. https://doi.org/10.3390/biom10010088





