The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity
Abstract
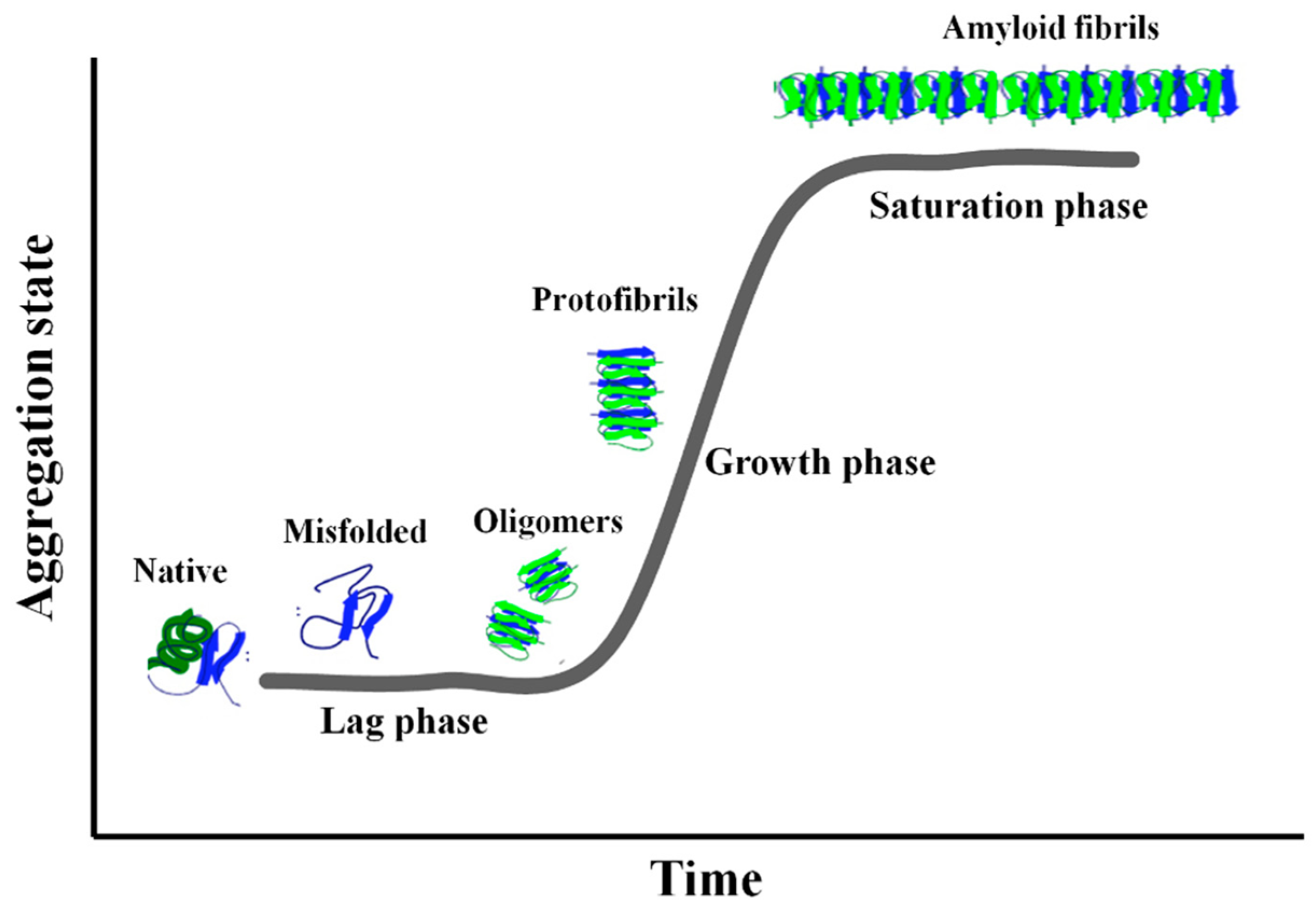
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Size Exclusion Chromatography (SEC) Characterization
2.4. Liposome Permeabilization
2.5. Circular Dichroism (CD) Spectroscopy
2.6. Energy Filtered-Transmission Electron Microscopy (EF-TEM)
2.7. Neurotoxicity Experiments
2.8. Dynamic Light Scattering
2.9. Statistical Analysis
3. Results and Discussion
3.1. The sCT Solutions
3.2. Functional Investigation on the Interaction of sCT with Liposomes and Neurons
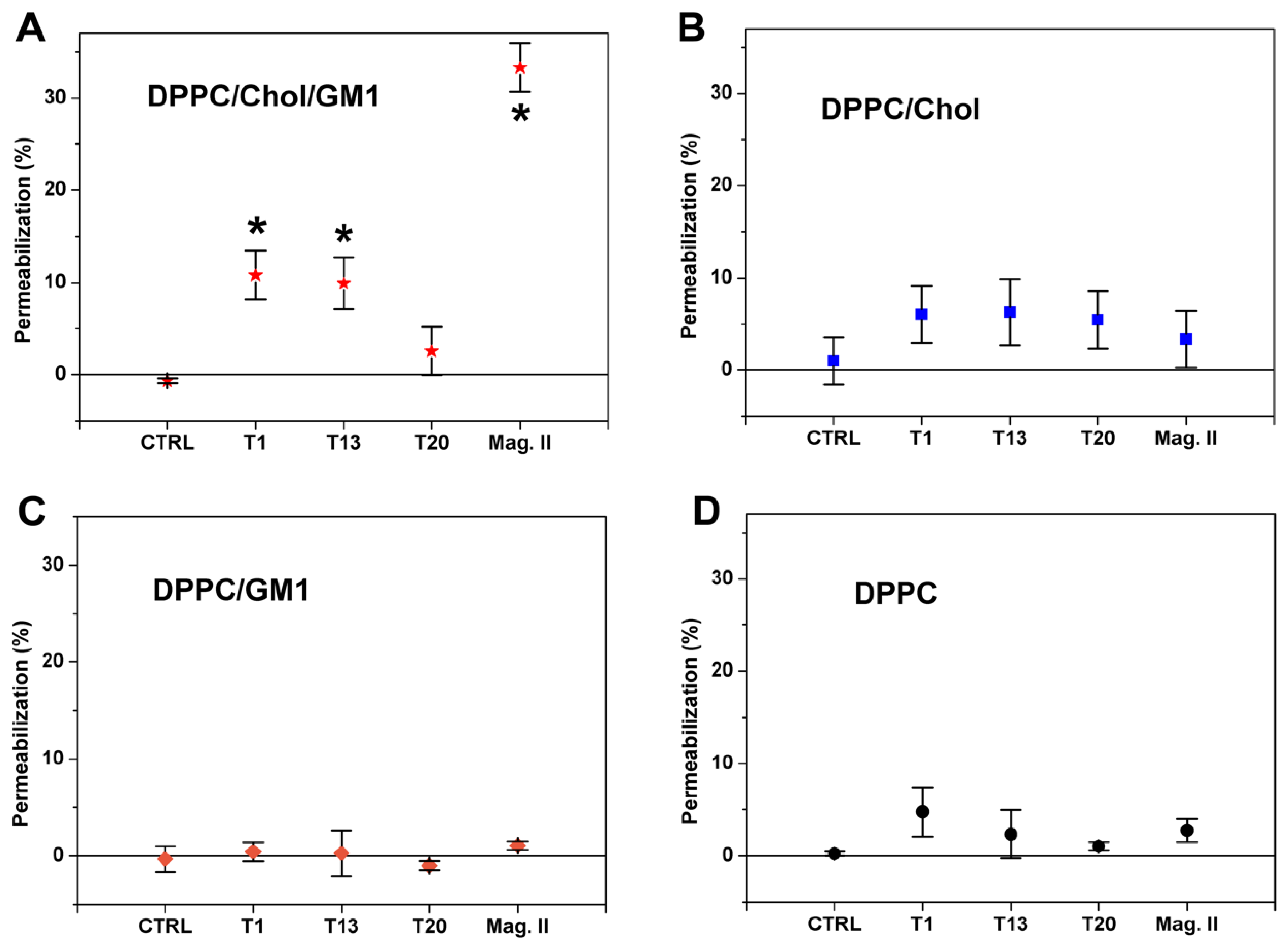
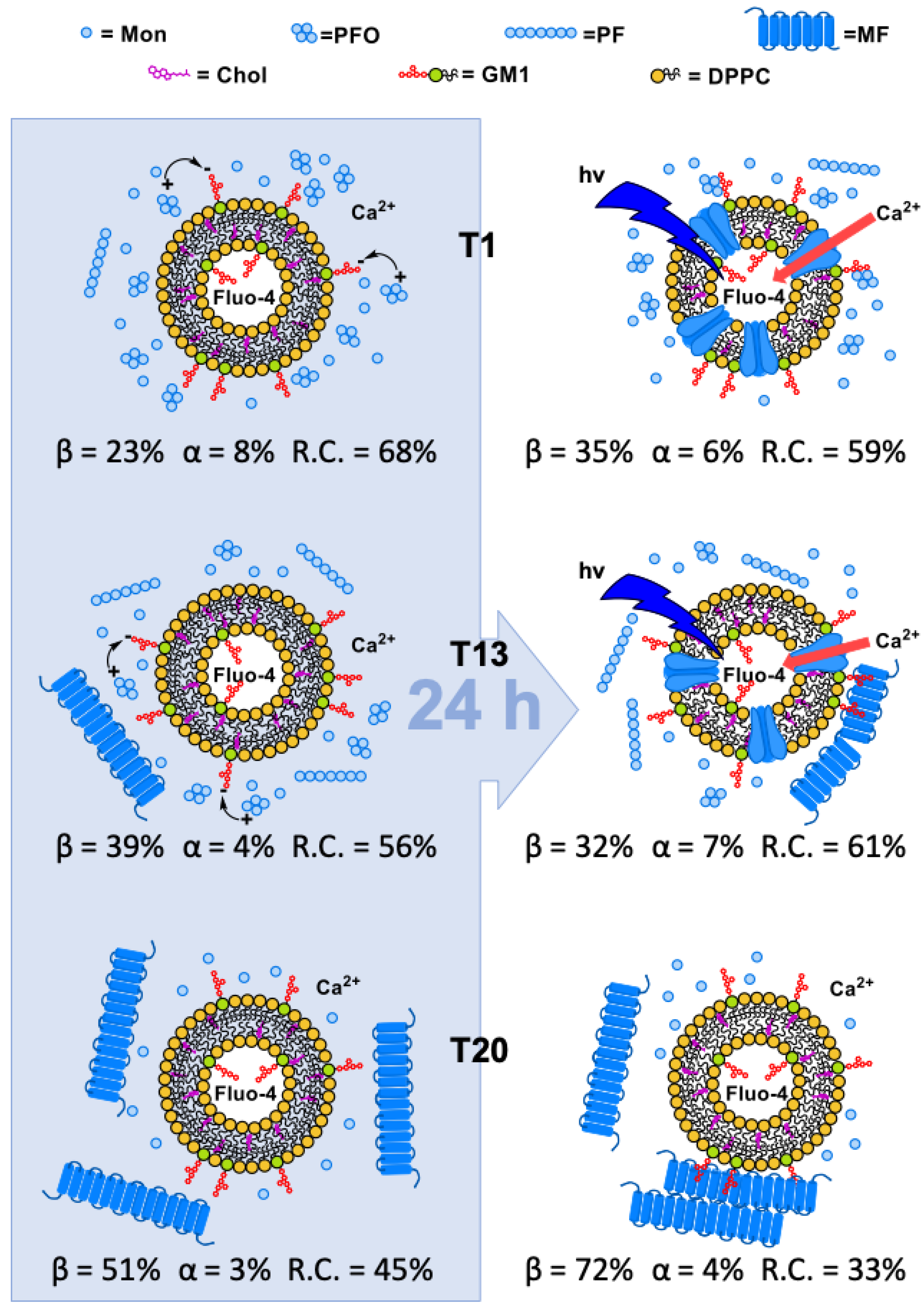
3.2.1. Ca2+-Influx in Liposomes
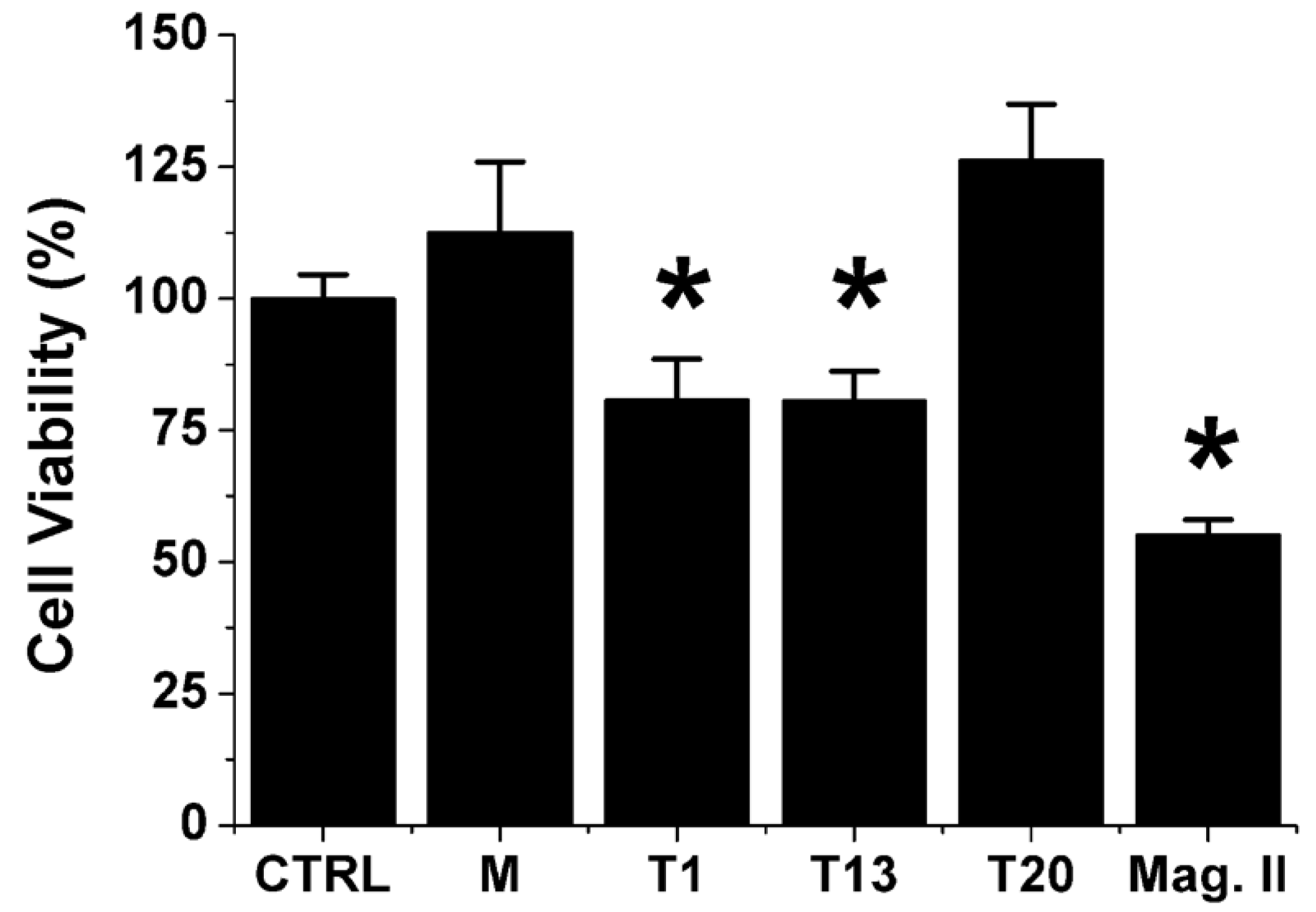
3.2.2. Neurotoxicity
3.3. Structural Investigation on the Interaction of sCT with Liposomes Mimicking “Lipid-Rafts”
3.3.1. Protein Conformation Evolution by CD Spectroscopy
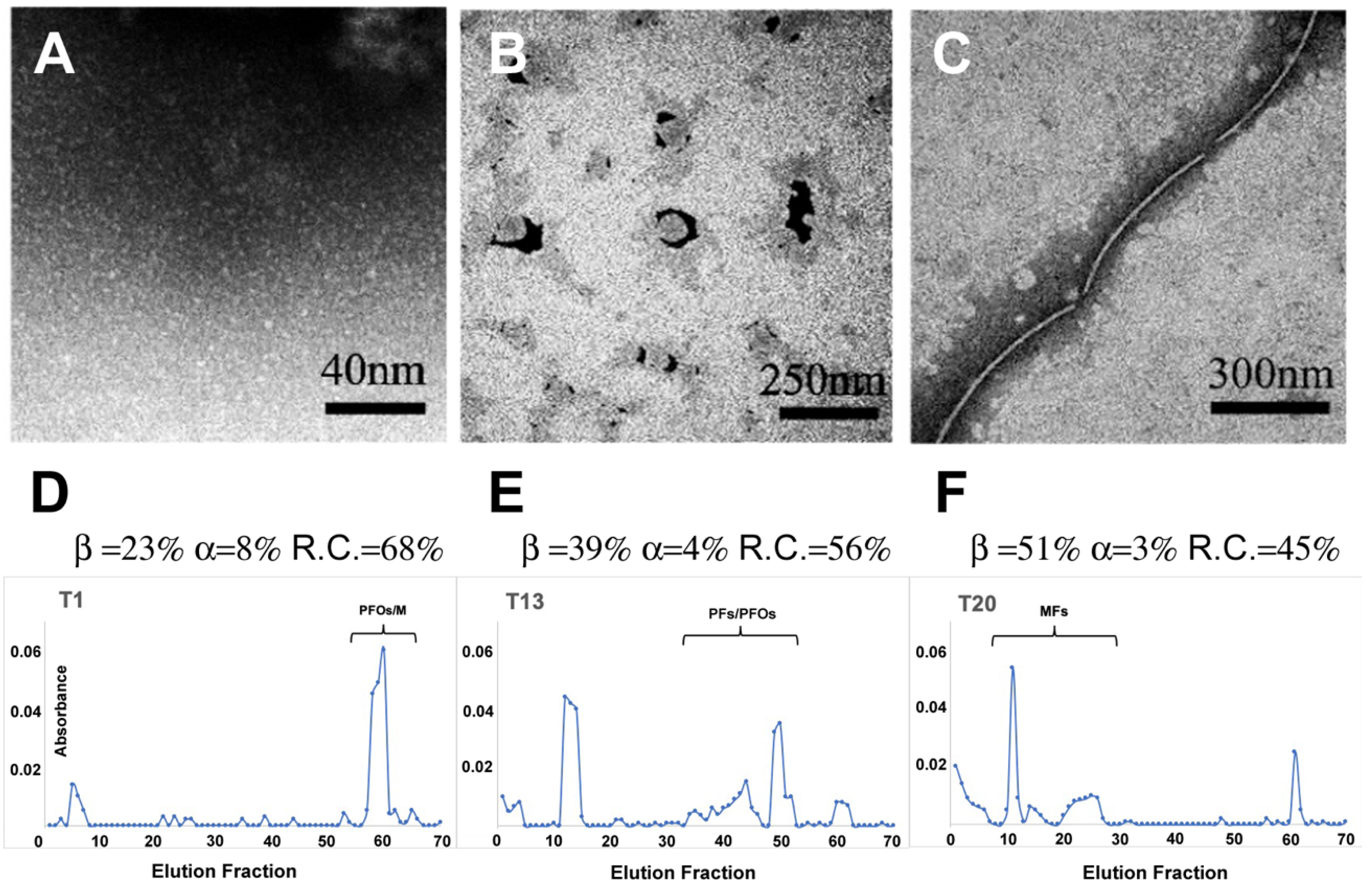
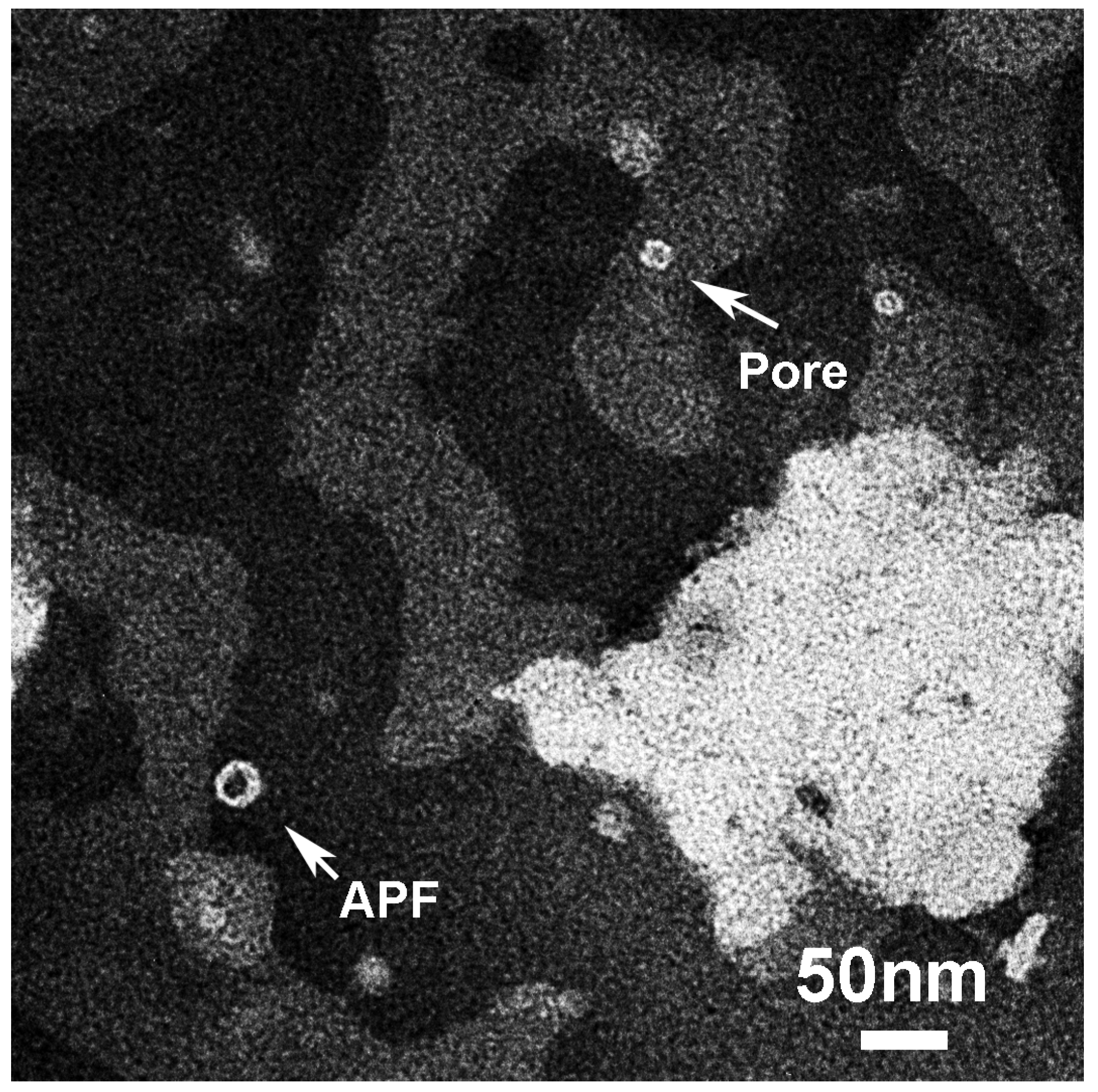
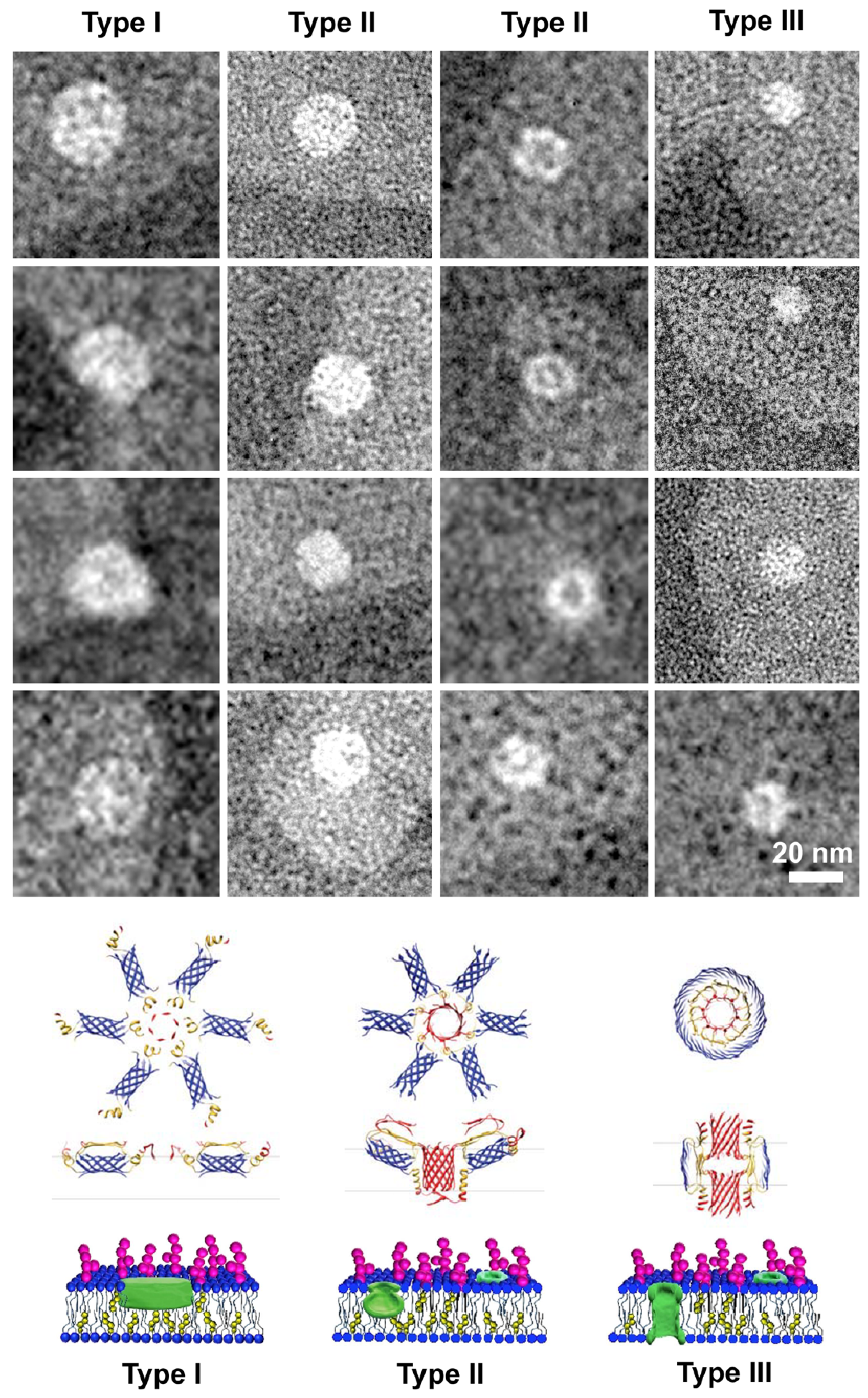
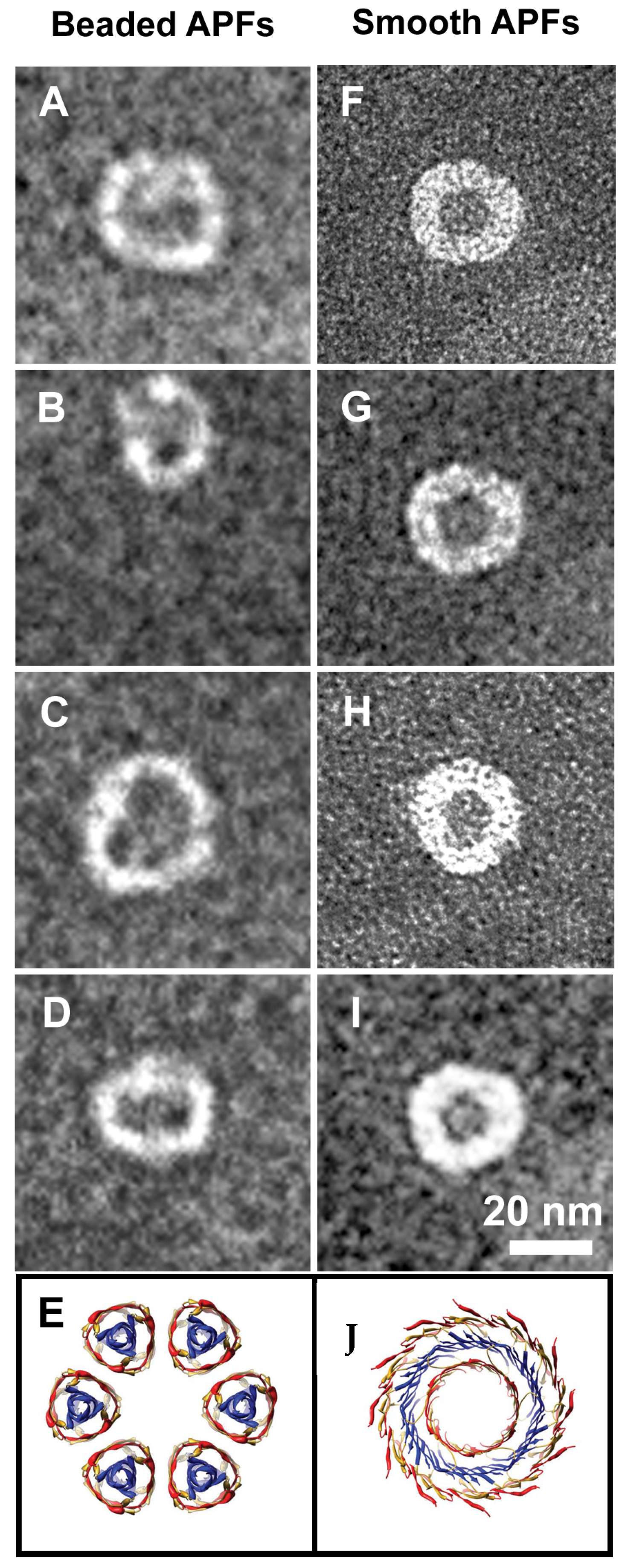
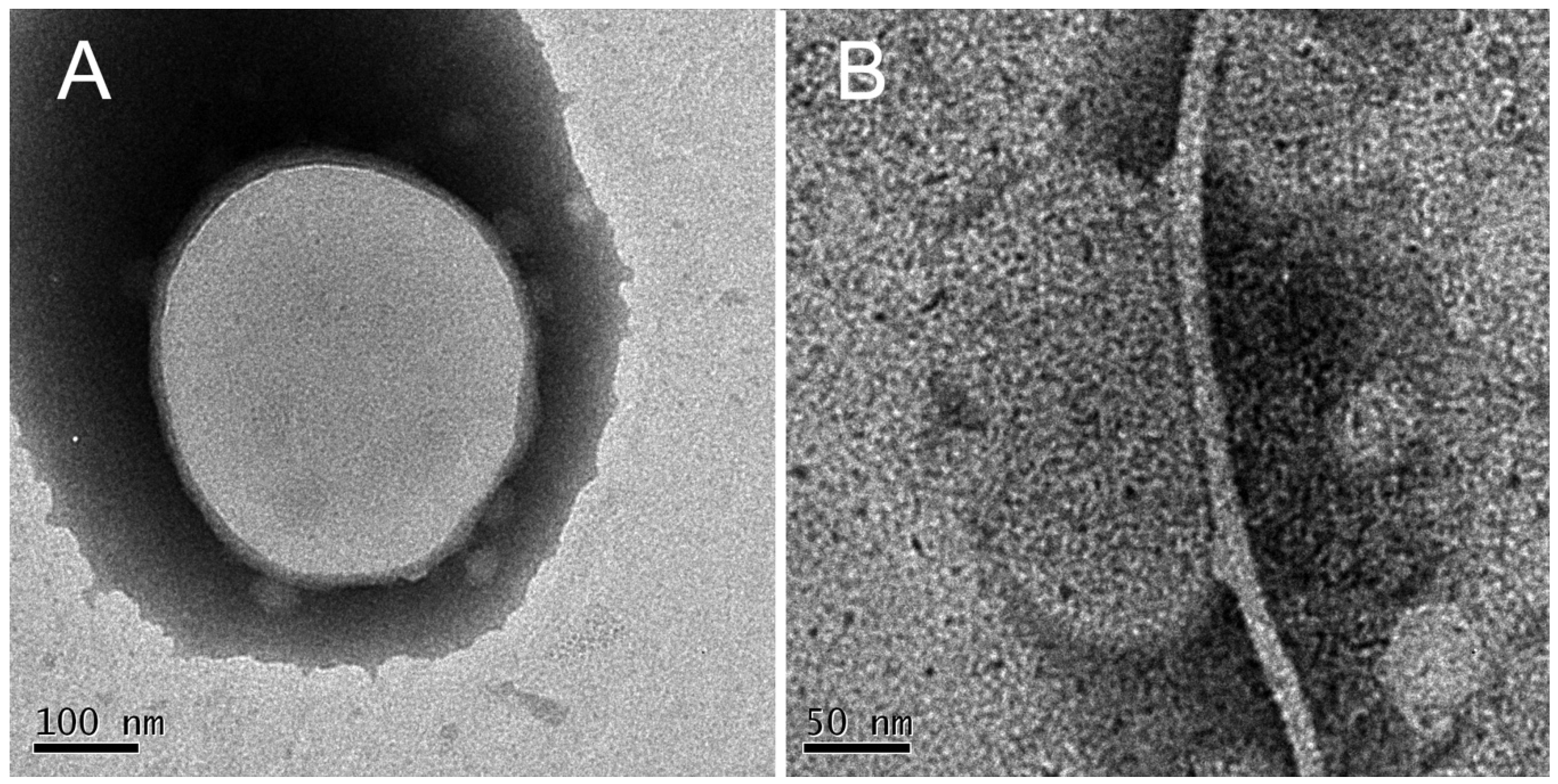
3.3.2. Liposome Morphological Evolution by EF-TEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation List
References
- Schnabel, J. Protein folding: The dark side of proteins. Nature 2010, 464, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M. Protein misfolding and aggregation: New examples in medicine and biology of the dark side of the protein world. Biochim. Biophys. Acta 2004, 1739, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Knowles, T.P.J.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Macchia, G.; Paradisi, S.; Frank, C.; Camerini, S.; Chistolini, P.; Gaudiano, M.C.; Petrucci, T.C.; Malchiodi-Albedi, F. Native metastable prefibrillar oligomers are the most neurotoxic species among amyloid aggregates. Biochim. Biophys. Acta—Mol. Basis Dis. 2014, 1842, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Irace, G.; Sirangelo, I. The effect of glycosaminoglycans (GAGs) on amyloid aggregation and toxicity. Molecules 2015, 20, 2510–2528. [Google Scholar] [CrossRef]
- Kirkitadze, M.D.; Bitan, G.; Teplow, D.B. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J. Neurosci. Res. 2002, 69, 567–577. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science (80-) 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Kayed, R.; Lasagna-Reeves, C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimers Dis. 2013, 33 (Suppl. 1), S67–S78. [Google Scholar] [CrossRef]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Pensalfini, A.; Margol, L.; Sokolov, Y.; Sarsoza, F.; Head, E.; Hall, J.; Glabe, C. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J. Biol. Chem. 2009, 284, 4230–4237. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Condron, M.M.; Teplow, D.B. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc. Natl. Acad. Sci. USA 2009, 106, 14745–14750. [Google Scholar] [CrossRef] [PubMed]
- Campioni, S.; Mannini, B.; Zampagni, M.; Pensalfini, A.; Parrini, C.; Evangelisti, E.; Relini, A.; Stefani, M.; Dobson, C.M.; Cecchi, C.; et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 2010, 6, 140–147. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef]
- Salay, L.C.; Qi, W.; Keshet, B.; Tamm, L.K.; Fernandez, E.J. Membrane interactions of a self-assembling model peptide that mimics the self-association, structure and toxicity of Abeta (1-40). Biochim. Biophys. Acta 2009, 1788, 1714–1721. [Google Scholar] [CrossRef]
- Glabe, C.G. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging 2006, 27, 570–575. [Google Scholar] [CrossRef]
- Schubert, D.; Behl, C.; Lesley, R.; Brack, A.; Dargusch, R.; Sagara, Y.; Kimura, H. Amyloid peptides are toxic via a common oxidative mechanism. Proc. Natl. Acad. Sci. USA 1995, 92, 1989–1993. [Google Scholar] [CrossRef]
- Belfiore, M.; Cariati, I.; Matteucci, A.; Gaddini, L.; Macchia, G.; Fioravanti, R.; Frank, C.; Tancredi, V.; D’Arcangelo, G.; Diociaiuti, M. Calcitonin native prefibrillar oligomers but not monomers induce membrane damage that triggers NMDA-mediated Ca2+-influx, LTP impairment and neurotoxicity. Sci. Rep. 2019, 9, 5144. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Lansbury, P.T., Jr. Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q. Rev. Biophys. 2006, 39, 167–201. [Google Scholar] [CrossRef]
- Montoya, M.; Gouaux, E. Beta-barrel membrane protein folding and structure viewed through the lens of alpha-hemolysin. Biochim. Biophys. Acta 2003, 1609, 19–27. [Google Scholar] [CrossRef]
- Di Scala, C.; Yahi, N.; Boutemeur, S.; Flores, A.; Rodriguez, L.; Chahinian, H.; Fantini, J. Common molecular mechanism of amyloid pore formation by Alzheimer’s β-amyloid peptide and α-synuclein. Sci. Rep. 2016, 6, 28781. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.H.; T Arce, F.; Gillman, A.L.; Jang, H.; Kagan, B.L.; Nussinov, R.; Yang, J.; Lal, R. Amyloid beta Ion Channels in a Membrane Comprising Brain Total Lipid Extracts. ACS Chem. Neurosci. 2017, 8, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Tempra, C.; Scollo, F.; Milardi, D.; La Rosa, C. Amyloid growth and membrane damage: Current themes and emerging perspectives from theory and experiments on Aβ and hIAPP. Biochim. Biophys. Acta—Biomembr. 2018, 1860, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Mendis, L.H.S.; Grey, A.C.; Faull, R.L.M.; Curtis, M.A. Hippocampal lipid differences in Alzheimer’s disease: A human brain study using matrix-assisted laser desorption/ionization-imaging mass spectrometry. Brain Behav. 2016, 6, e00517. [Google Scholar] [CrossRef]
- Alarcon, J.M.; Brito, J.A.; Hermosilla, T.; Atwater, I.; Mears, D.; Rojas, E. Ion channel formation by Alzheimer’s disease amyloid beta-peptide (Abeta40) in unilamellar liposomes is determined by anionic phospholipids. Peptides 2006, 27, 95–104. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Kasahara, K.; Sanai, Y. Possible roles of glycosphingolipids in lipid rafts. Biophys. Chem. 1999, 82, 121–127. [Google Scholar] [CrossRef]
- Morishima-Kawashima, M.; Ihara, Y. The presence of amyloid beta-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 1998, 37, 15247–15253. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Iijima, K.; Yamamoto, N.; Yanagisawa, K.; Sato, T. Density of GM1 in nanoclusters is a critical factor in the formation of a spherical assembly of amyloid beta-protein on synaptic plasma membranes. Langmuir 2013, 29, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi-Albedi, F.; Paradisi, S.; Matteucci, A.; Frank, C.; Diociaiuti, M. Amyloid Oligomer Neurotoxicity, Calcium Dysregulation, and Lipid Rafts. Int. J. Alzheimers. Dis. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pralle, A.; Keller, P.; Florin, E.L.; Simons, K.; Horber, J.K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000, 148, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; Kuhl, T.L.; Kjaer, K.; Smith, G.S. Packing of ganglioside-phospholipid monolayers: An x-ray diffraction and reflectivity study. Biophys. J. 2001, 81, 2707–2715. [Google Scholar] [CrossRef]
- Fernandez-Perez, E.J.; Sepulveda, F.J.; Peoples, R.; Aguayo, L.G. Role of membrane GM1 on early neuronal membrane actions of Abeta during onset of Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. How do membranes initiate Alzheimer’s Disease? Formation of toxic amyloid fibrils by the amyloid beta-protein on ganglioside clusters. Acc. Chem. Res. 2014, 47, 2397–2404. [Google Scholar] [CrossRef]
- Kakio, A.; Nishimoto, S.; Kozutsumi, Y.; Matsuzaki, K. Formation of a membrane-active form of amyloid beta-protein in raft-like model membranes. Biochem. Biophys. Res. Commun. 2003, 303, 514–518. [Google Scholar] [CrossRef]
- Bucciantini, M.; Nosi, D.; Forzan, M.; Russo, E.; Calamai, M.; Pieri, L.; Formigli, L.; Quercioli, F.; Soria, S.; Pavone, F.; et al. Toxic effects of amyloid fibrils on cell membranes: The importance of ganglioside GM1. FASEB J. 2012, 26, 818–831. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamaguchi, T.; Fukunaga, S.; Hoshino, M.; Matsuzaki, K. Mechanism of amyloid beta-protein aggregation mediated by GM1 ganglioside clusters. Biochemistry 2011, 50, 6433–6440. [Google Scholar] [CrossRef]
- Di Scala, C.; Troadec, J.D.; Lelievre, C.; Garmy, N.; Fantini, J.; Chahinian, H. Mechanism of cholesterol-assisted oligomeric channel formation by a short Alzheimer beta-amyloid peptide. J. Neurochem. 2014, 128, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pannuzzo, M. On the physiological/pathological link between Abeta peptide, cholesterol, calcium ions and membrane deformation: A molecular dynamics study. Biochim. Biophys. Acta 2016, 1858, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, Y.; Durell, S.R.; Anishkin, A.; Guy, H.R. Beta-barrel models of soluble amyloid beta oligomers and annular protofibrils. Proteins 2010, 78, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, Y.; Durell, S.; Arispe, N.; Guy, H.R. Models of membrane-bound Alzheimer’s Abeta peptide assemblies. Proteins 2010, 78, 3473–3487. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Gaudiano, M.C.; Malchiodi-Albedi, F. The Slowly Aggregating Salmon Calcitonin: A Useful Tool for the Study of the Amyloid Oligomers Structure and Activity. Int. J. Mol. Sci. 2011, 12, 9277–9295. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi-Albedi, F.; Contrusciere, V.; Raggi, C.; Fecchi, K.; Rainaldi, G.; Paradisi, S.; Matteucci, A.; Santini, M.T.; Sargiacomo, M.; Frank, C.; et al. Lipid raft disruption protects mature neurons against amyloid oligomer toxicity. Biochim. Biophys. Acta—Mol. Basis Dis. 2010, 1802, 406–415. [Google Scholar] [CrossRef]
- Diociaiuti, M.; Giordani, C.; Kamel, G.S.; Brasili, F.; Sennato, S.; Bombelli, C.; Meneses, K.Y.; Giraldo, M.A.; Bordi, F. Monosialoganglioside-GM1 triggers binding of the amyloid-protein salmon calcitonin to a Langmuir membrane model mimicking the occurrence of lipid-rafts. Biochem. Biophys. Rep. 2016, 8, 365–375. [Google Scholar] [CrossRef][Green Version]
- Brown, R.E. Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. J. Cell Sci. 1998, 111, 1–9. [Google Scholar]
- Stine, W.B., Jr.; Dahlgren, K.N.; Krafft, G.A.; LaDu, M.J. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J. Biol. Chem. 2003, 278, 11612–11622. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Weissig, V. Liposomes: A Practical Approach; Oxford University Press: Oxford, UK, 2003; ISBN 9780199636549. [Google Scholar]
- Duysens, L.N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim. Biophys. Acta 1956, 19, 1–12. [Google Scholar] [CrossRef]
- Wallace, B.A.; Mao, D. Circular dichroism analyses of membrane proteins: An examination of differential light scattering and absorption flattening effects in large membrane vesicles and membrane sheets. Anal. Biochem. 1984, 142, 317–328. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Scheffler, D. Routine preparation of air-dried negatively stained and unstained specimens on holey carbon support films: A review of applications. Micron 2002, 33, 461–480. [Google Scholar] [CrossRef]
- Egerton, R.F.R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope, 3rd ed.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Diociaiuti, M. Electron energy loss spectroscopy microanalysis and imaging in the transmission electron microscope: Example of biological applications. J. Electron. Spectros. Relat. Phenom. 2005, 143, 189–203. [Google Scholar] [CrossRef]
- De Vos, C.; Deriemaeker, L.; Finsy, R. Quantitative assessment of the conditioning of the inversion of quasi-elastic and static light scattering data for particle size distributions. Langmuir 1996, 12, 2630–2636. [Google Scholar] [CrossRef]
- Gaudiano, M.C.; Colone, M.; Bombelli, C.; Chistolini, P.; Valvo, L.; Diociaiuti, M. Early stages of salmon calcitonin aggregation: Effect induced by ageing and oxidation processes in water and in the presence of model membranes. Biochim. Biophys. Acta—Proteins Proteom. 2005, 1750, 134–145. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Monaco, I.; La Rosa, C.; Milardi, D. The active role of Ca2+ ions in Abeta-mediated membrane damage. Chem. Commun. 2018, 54, 3629–3631. [Google Scholar] [CrossRef]
- Fraley, R.; Wilschut, J.; Duzgunes, N.; Smith, C.; Papahadjopoulos, D. Studies on the mechanism of membrane fusion: Role of phosphate in promoting calcium ion induced fusion of phospholipid vesicles. Biochemistry 1980, 19, 6021–6029. [Google Scholar] [CrossRef]
- Hallock, K.J.; Lee, D.K.; Ramamoorthy, A. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys. J. 2003, 84, 3052–3060. [Google Scholar] [CrossRef]
- Hasan, M.; Karal, M.A.S.; Levadnyy, V.; Yamazaki, M. Mechanism of Initial Stage of Pore Formation Induced by Antimicrobial Peptide Magainin 2. Langmuir 2018, 34, 3349–3362. [Google Scholar] [CrossRef] [PubMed]
- Meleleo, D.; Sblano, C. Influence of cholesterol on human calcitonin channel formation. Possible role of sterol as molecular chaperone. AIMS Biophys. 2019, 6, 23–38. [Google Scholar] [CrossRef]
- Diociaiuti, M.; Polzi, L.Z.; Valvo, L.; Malchiodi-Albedi, F.; Bombelli, C.; Gaudiano, M.C. Calcitonin Forms Oligomeric Pore-Like Structures in Lipid Membranes. Biophys. J. 2006, 91, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, V.; Brocca, P.; Motta, S.; Messa, M.; Colombo, L.; Salmona, M.; Fragneto, G.; Cantù, L.; Del Favero, E. Amyloidβ Peptides in interaction with raft-mime model membranes: A neutron reflectivity insight. Sci. Rep. 2016, 6, 20997. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Mukhopadhyay, C. Binding, Conformational Transition and Dimerization of Amyloid-β Peptide on GM1-Containing Ternary Membrane: Insights from Molecular Dynamics Simulation. PLoS ONE 2013, 8, e71308. [Google Scholar] [CrossRef] [PubMed]
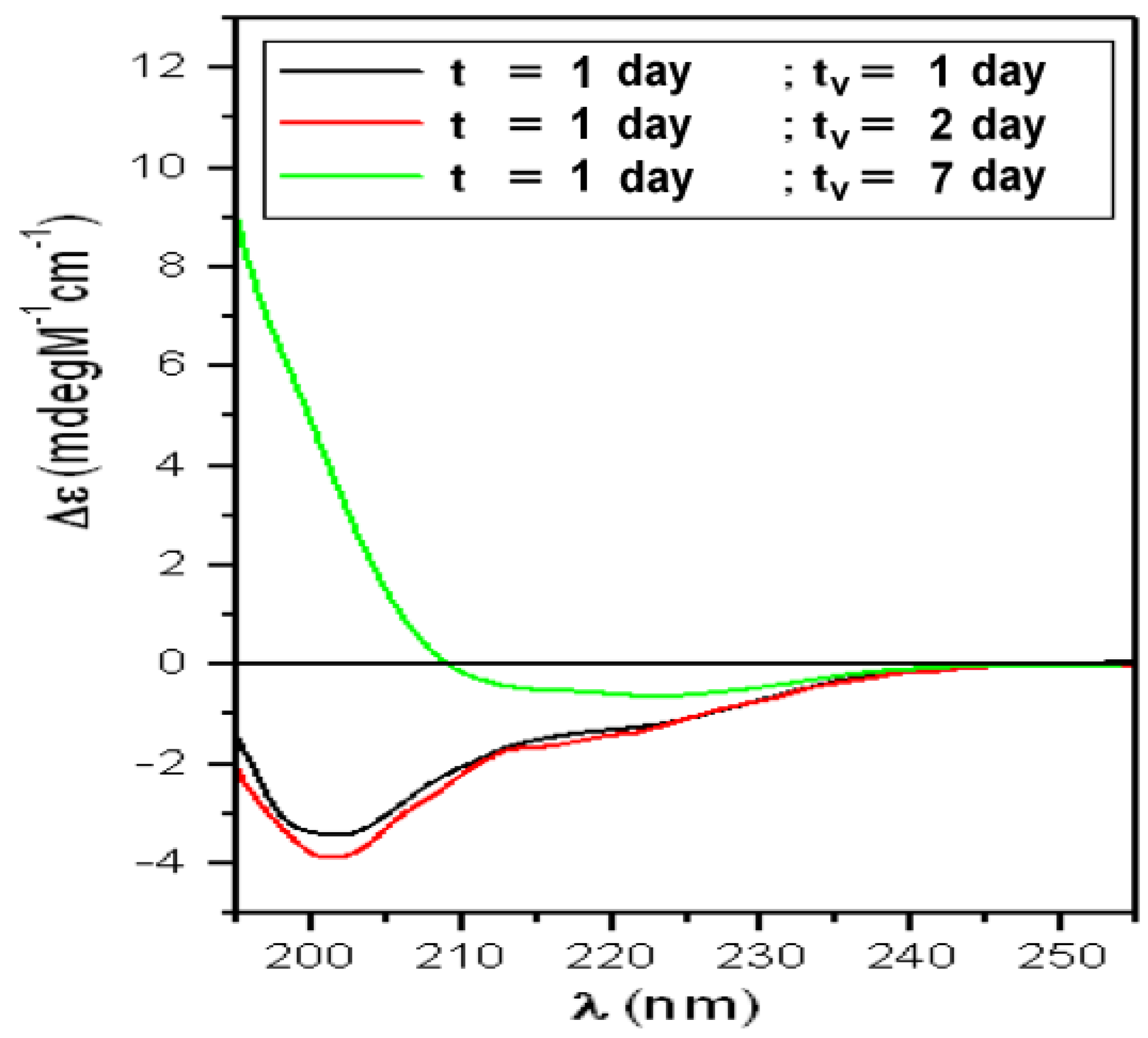
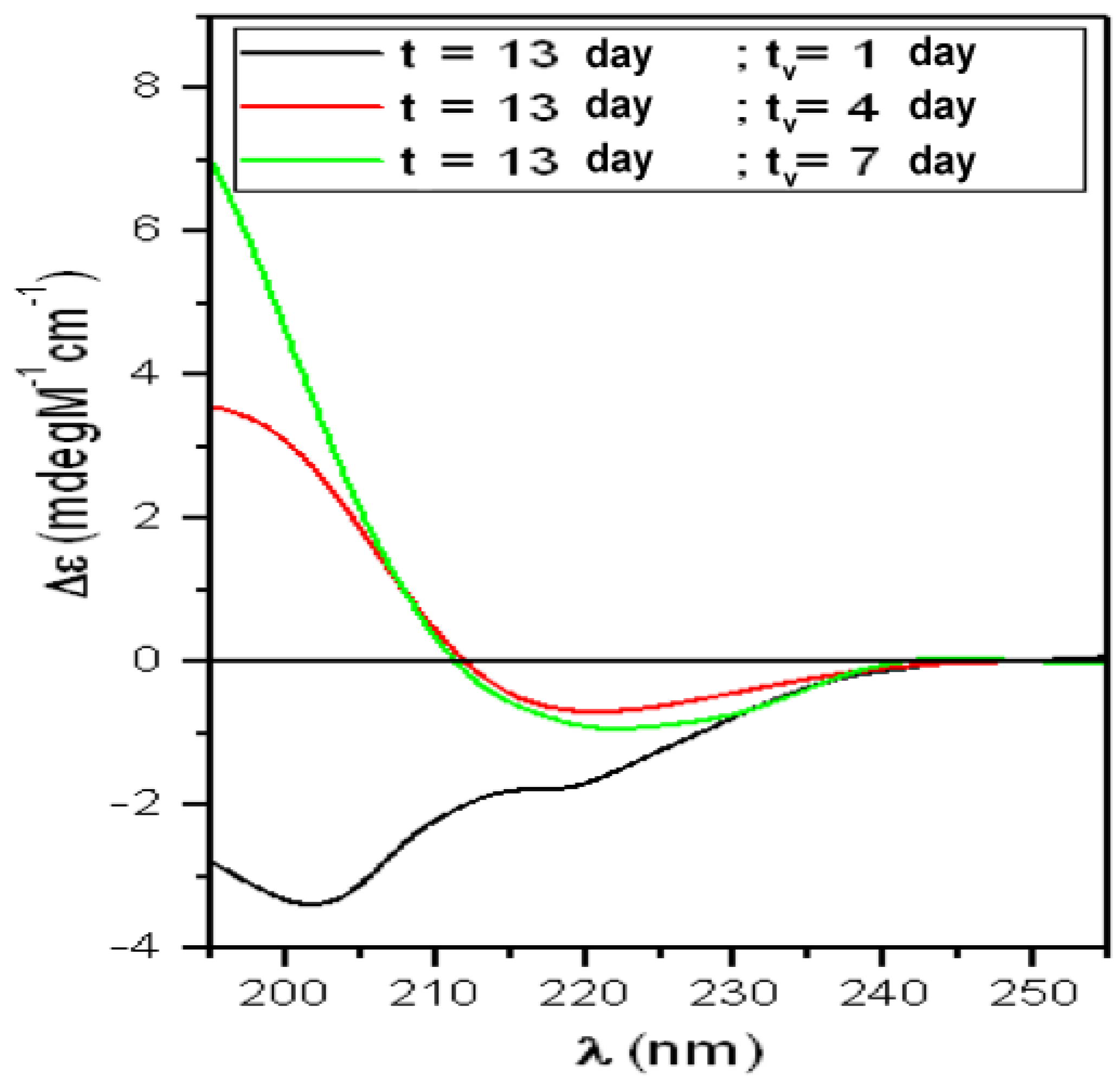
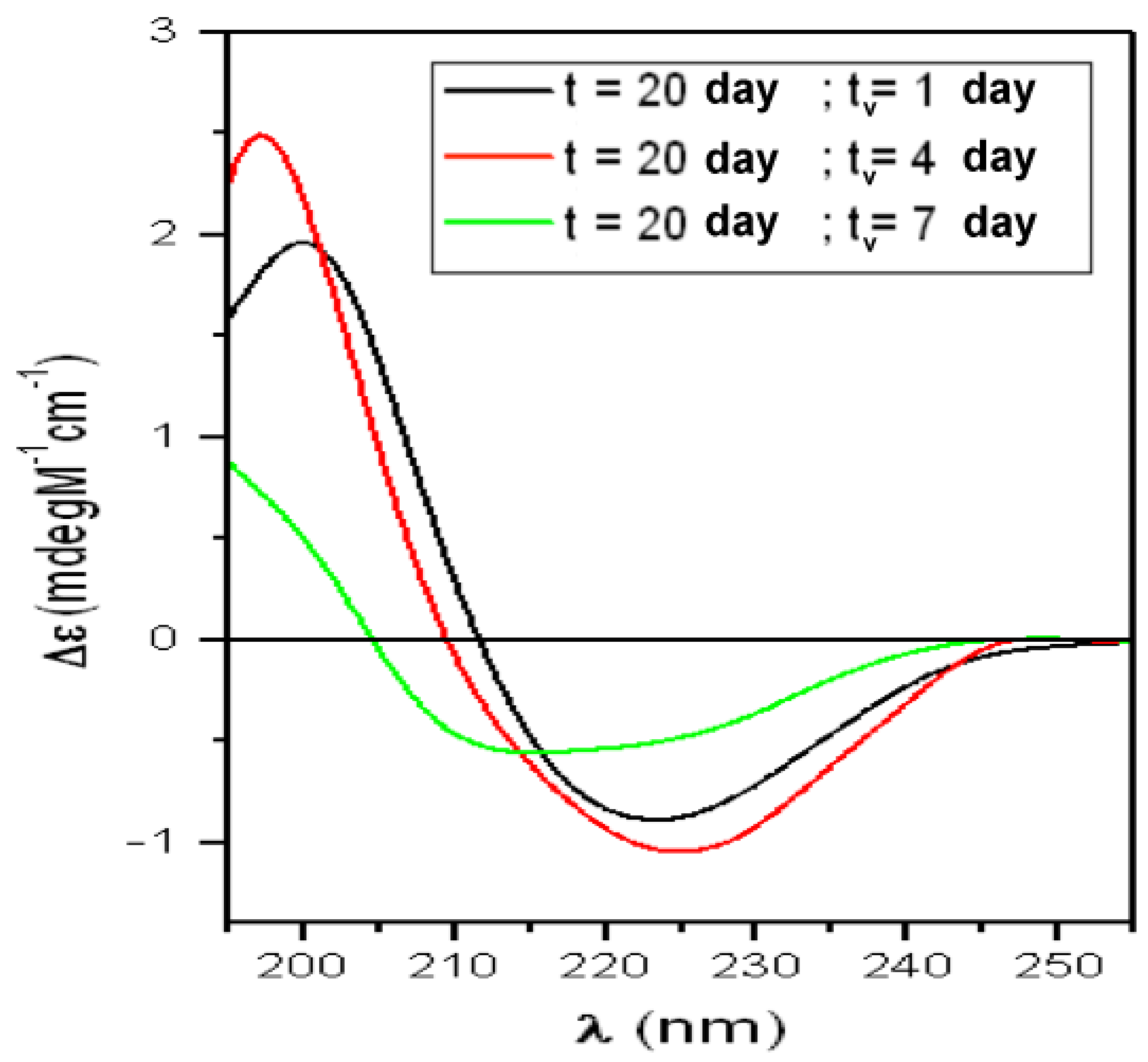
| t (Day) | α-Helix (%) | β-Sheet (%) | Turns (%) | Random-Coil (%) | NRMSD |
|---|---|---|---|---|---|
| 0 | 6 | 15 | 11 | 69 | 0.021 |
| 1 | 8 | 13 | 10 | 68 | 0.025 |
| 9 | 4 | 19 | 14 | 61 | 0.026 |
| 15 | 4 | 24 | 15 | 56 | 0.031 |
| 21 | 3 | 32 | 19 | 45 | 0.054 |
| T1 | α-Helix = 8% | Total β-Structures = 23% | Random-Coil = 68% | ||
|---|---|---|---|---|---|
| t = 1 day “Lag-phase” | |||||
| tv (day) | α-Helix (%) | β-sheet (%) | Turns (%) | Random-Coil (%) | NRMSD |
| 1 | 6 | 20 | 15 | 59 | 0.024 |
| 2 | 6 | 20 | 13 | 60 | 0.018 |
| 7 | 3 | 53 | 14 | 30 | 0.005 |
| T13 | α-Helix = 4% | Total β-Structures = 39% | Random-Coil = 56% | ||
|---|---|---|---|---|---|
| t = 13 days“Growing-Phase” | |||||
| tv (day) | α-Helix (%) | β-Sheet (%) | Turns (%) | Random-Coil (%) | NRMSD |
| 1 | 7 | 18 | 14 | 61 | 0.018 |
| 4 | 1 | 48 | 22 | 28 | 0.014 |
| 7 | 6 | 47 | 23 | 24 | 0.004 |
| T20 | α-Helix = 3% | Total β-Structures = 51% | Random-Coil = 45% | ||
|---|---|---|---|---|---|
| t = 20 days “Saturation-Phase” | |||||
| tv (day) | α-Helix (%) | β-sheet (%) | Turns (%) | Random-Coil (%) | NRMSD |
| 1 | 4 | 48 | 24 | 33 | 0.019 |
| 4 | 4 | 33 | 19 | 43 | 0.004 |
| 7 | 7 | 32 | 22 | 40 | 0.064 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diociaiuti, M.; Bombelli, C.; Zanetti-Polzi, L.; Belfiore, M.; Fioravanti, R.; Macchia, G.; Giordani, C. The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity. Biomolecules 2020, 10, 58. https://doi.org/10.3390/biom10010058
Diociaiuti M, Bombelli C, Zanetti-Polzi L, Belfiore M, Fioravanti R, Macchia G, Giordani C. The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity. Biomolecules. 2020; 10(1):58. https://doi.org/10.3390/biom10010058
Chicago/Turabian StyleDiociaiuti, Marco, Cecilia Bombelli, Laura Zanetti-Polzi, Marcello Belfiore, Raoul Fioravanti, Gianfranco Macchia, and Cristiano Giordani. 2020. "The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity" Biomolecules 10, no. 1: 58. https://doi.org/10.3390/biom10010058
APA StyleDiociaiuti, M., Bombelli, C., Zanetti-Polzi, L., Belfiore, M., Fioravanti, R., Macchia, G., & Giordani, C. (2020). The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity. Biomolecules, 10(1), 58. https://doi.org/10.3390/biom10010058







