Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Extraction
2.3. Mice
2.4. Induction of AD-Like Skin Lesions in Balb/c Mice by Oxazolone and ASE Application
2.5. Induction of AD-Like Skin Lesions in SKH-1 Hairless Mice by DNCB and ASE Application
2.6. Histopathological Evaluation of Skin Lesions
2.7. Determination of Serum IgE and IL-4 Concentration
2.8. HPLC Analysis of ASE
2.9. Cells and Cell Culture
2.10. Determination of Gene Expression
2.11. Determination of β-Hexosaminidase Release
2.12. Determination of β-Hexosaminidase Release
2.13. Statistical Analysis
3. Results and Discussion
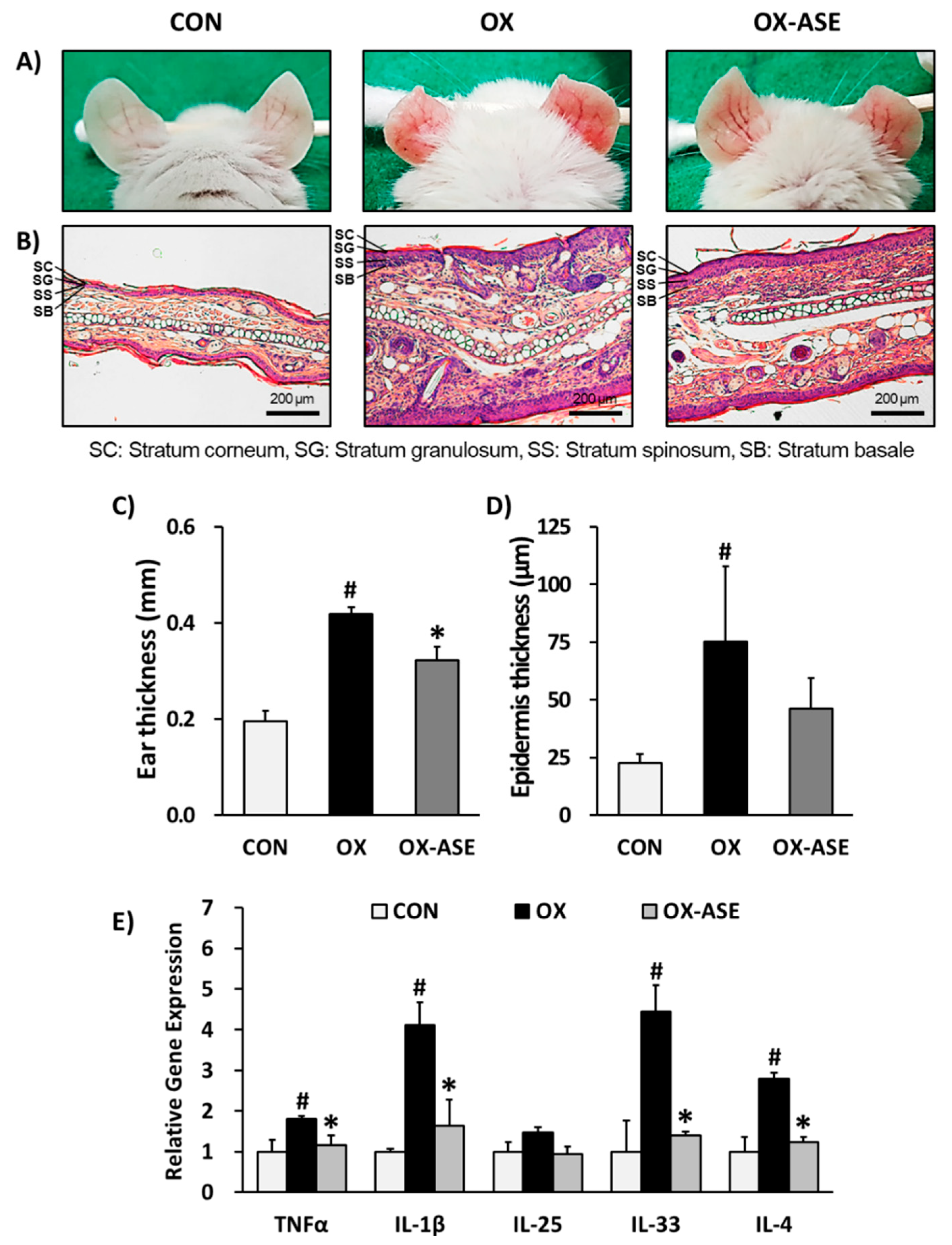
3.1. Effects of ASE Application on Oxazolone-Induced AD-Like Skin Lesions Mice
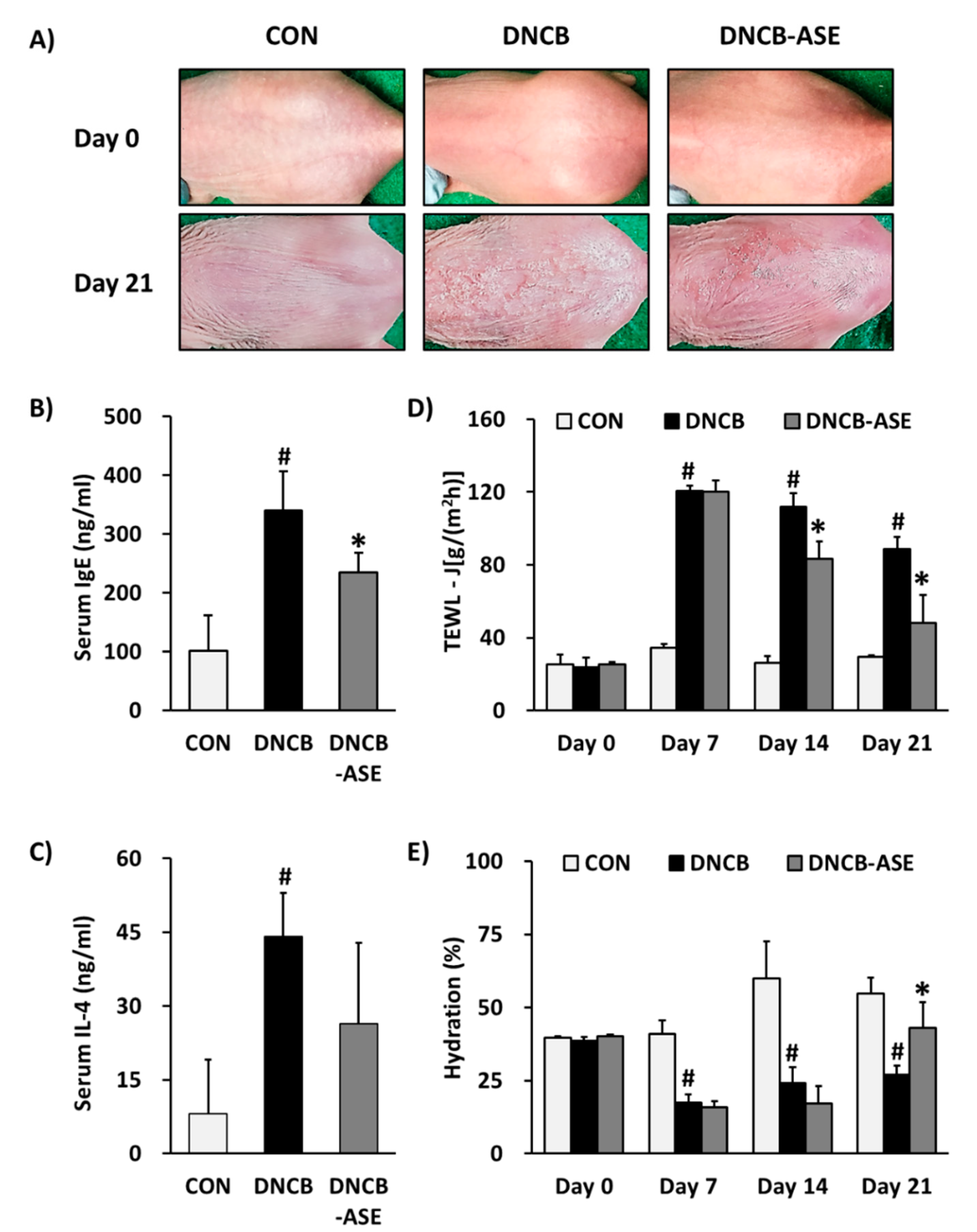
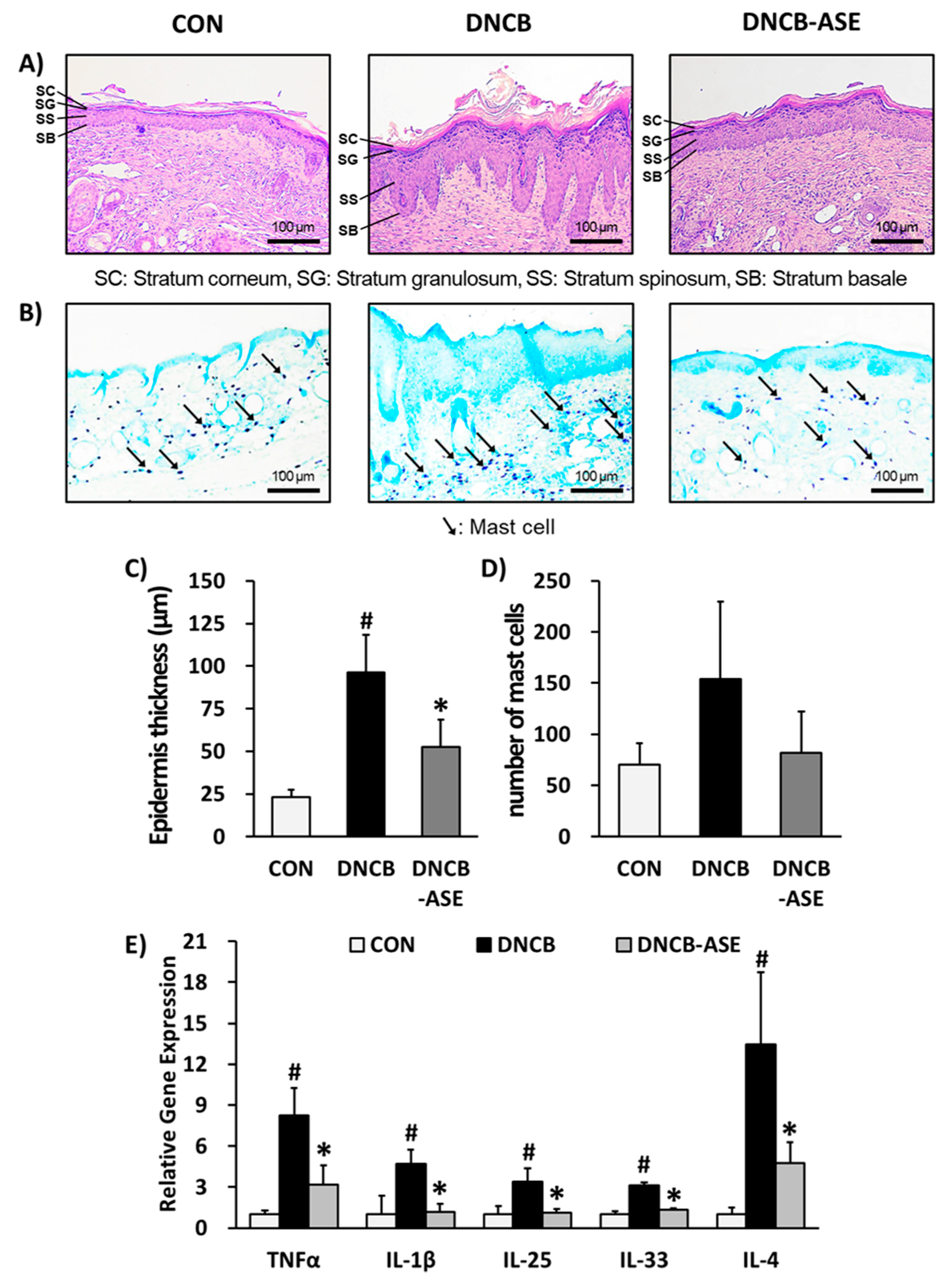
3.2. Effects of ASE Application on DNCB-Induced AD-Like Skin Lesions Mice
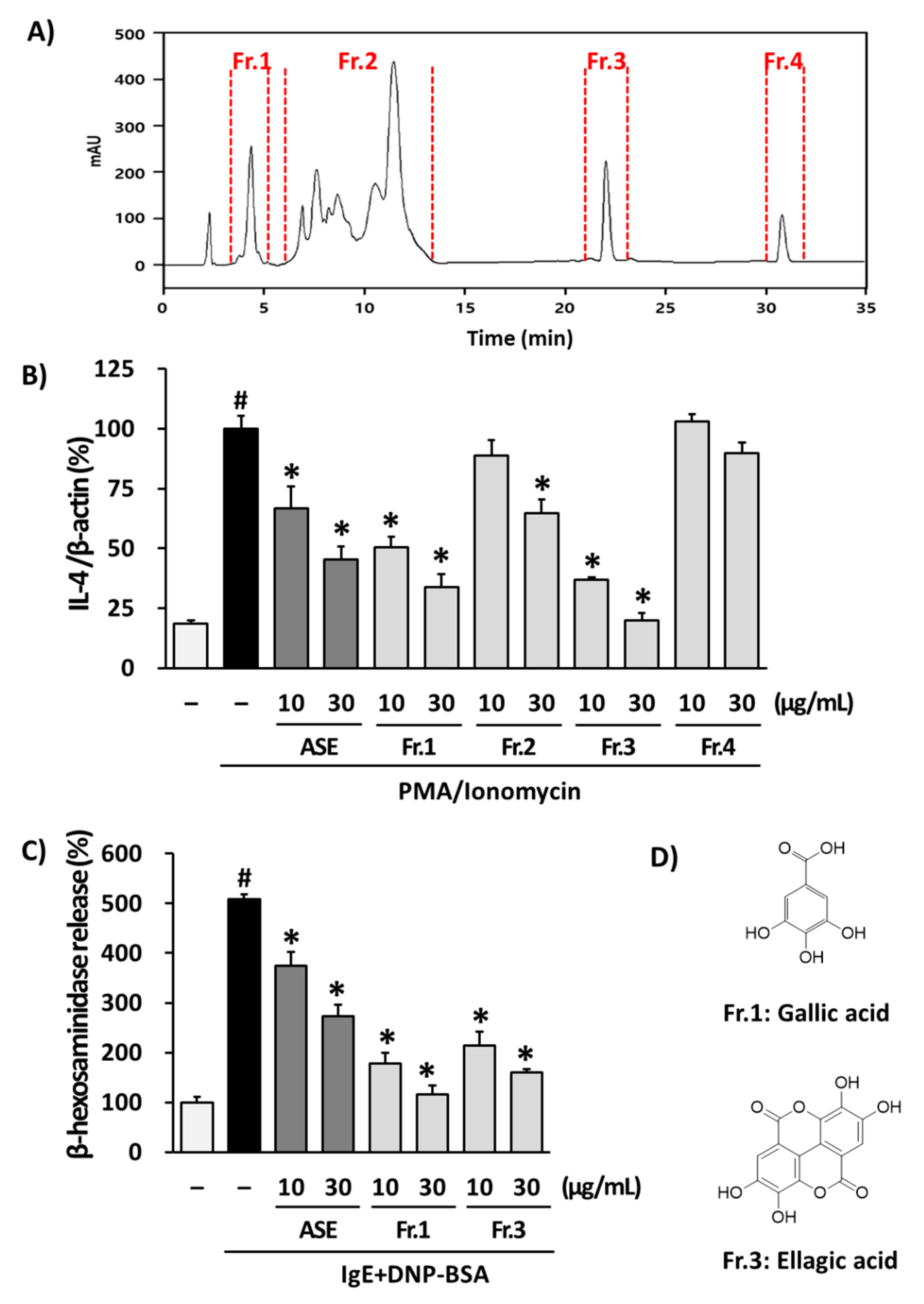
3.3. Isolation of Phytochemicals from ASE and Their Effects in RBL-2H3 Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanifin, J.M.; Tofte, S.J. Update on therapy of atopic dermatitis. J. Allergy Clin. Immunol. 1999, 104, S123–S125. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A. Mast cells and inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Agnihothri, R.; McGirt, L.Y.; Bankova, L.G.; Beck, L.A. Atopic dermatitis: A disease caused by innate immune defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Atopic dermatitis: Common extrinsic and enigmatic intrinsic types. In Immunology of the Skin; Springer: Tokyo, Japan, 2016; pp. 339–358. [Google Scholar]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Ruhr, J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef]
- Poulsen, L.; Bindslev-Jensen, C.; Diamant, M.; Hansen, M.; Jepsen, K.; Reimert, C.; Bendtzen, K. Biomolecular regulation of the IgE immune response III. Cytokine profiles in atopic dermatitis, inhalant allergy and non-allergic donors. Cytokine 1996, 8, 651–657. [Google Scholar] [CrossRef]
- Martins, J.C.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 7, CD009864. [Google Scholar] [CrossRef]
- Huang, X.; Xu, B. Efficacy and safety of tacrolimus versus pimecrolimus for the treatment of atopic dermatitis in children: A network meta-analysis. Dermatology 2015, 231, 41–49. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Charman, C.; Morris, A.; Williams, H. Topical corticosteroid phobia in patients with atopic eczema. Br. J. Dermatol. 2000, 142, 931–936. [Google Scholar] [CrossRef]
- Nghiem, P.; Pearson, G.; Langley, R.G. Tacrolimus and pimecrolimus: From clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J. Am. Acad. Dermatol. 2002, 46, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Cooper, K.D.; Ho, V.C.; Kang, S.; Krafchik, B.R.; Margolis, D.J.; Schachner, L.A.; Sidbury, R.; Whitmore, S.E.; Sieck, C.K. Guidelines of care for atopic dermatitis. J. Am. Acad. Dermatol. 2004, 50, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, D.A. Use of Acorns for Food in California: Past, Present, Future. In Proceedings of the Symposium on Multiple-use Management of California’s Hardwoods, San Luis Obispo, CA, USA, 12–14 November 1986; pp. 453–458. [Google Scholar]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind. Crop. Prod. 2013, 45, 114–120. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Triterpenes isolated from acorns of Quercus serrata var. brevipetiolata exert anti-inflammatory activity. Ind. Crop. Prod. 2016, 91, 302–309. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Anti-inflammatory oleanolic triterpenes from Chinese Acorns. Molecules 2016, 21, 669. [Google Scholar] [CrossRef]
- Sheu, S.-Y.; Tsuang, Y.-H.; Hsu, F.-L.; Lu, F.-J.; Chiang, H.-C. Superoxide Anion Scavenge Effect of Quercs glauca Thunb. in Whole Blood of Patients with Ankylosing Spondylitis. Am. J. Chin. Med. 1997, 25, 307–315. [Google Scholar] [CrossRef]
- Yarani, R.; Mansouri, K.; Mohammadi-Motlagh, H.R.; Mahnam, A.; Emami Aleagha, M.S. In Vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm. Biol. 2013, 51, 361–368. [Google Scholar] [CrossRef]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.; Costa, P.; Bahia, M. Protective effect of Castanea sativa and Quercus robur leaf extracts against oxygen and nitrogen reactive species. J. Photochem. Photobiol. B Biol. 2008, 91, 87–95. [Google Scholar] [CrossRef]
- Basri, D.F.; Fan, S. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J. Pharmacol. 2005, 37, 26. [Google Scholar] [CrossRef]
- Güllüce, M.; Adıgüzel, A.; Öğütçü, H.; Şengül, M.; Karaman, I.; Şahin, F. Antimicrobial effects of Quercus ilex L. extract. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 208–211. [Google Scholar]
- Vinha, A.F.; Barreira, J.C.; Costa, A.S.; Oliveira, M.B.P. A new age for Quercus spp. fruits: Review on nutritional and phytochemical composition and related biological activities of acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef]
- Kay, A.B. Calcitonin gene-related peptide–and vascular endothelial growth factor–positive inflammatory cells in late-phase allergic skin reactions in atopic subjects. J. Allergy Clin. Immunol. 2011, 127, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, N.-J.; Bong, S.-K.; Jegal, J.; Park, S.-A.; Kim, S.-N.; Yang, M.H. Ameliorative effects of Juniperus rigida fruit on oxazolone-and 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2018, 214, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.-C.; Park, N.-J.; Bong, S.-K.; Lee, S.; Jegal, H.; Jin, L.T.; Kim, S.M.; Kim, Y.K.; Kim, S.-N. Eupatilin, an activator of PPARα, inhibits the development of oxazolone-induced atopic dermatitis symptoms in Balb/c mice. Biochem. Biophys. Res. Commun. 2018, 496, 508–514. [Google Scholar] [CrossRef]
- Huang, F.; Yamaki, K.; Tong, X.; Fu, L.; Zhang, R.; Cai, Y.; Yanagisawa, R.; Inoue, K.-I.; Takano, H.; Yoshino, S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int. Immunopharmacol. 2008, 8, 502–507. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T.; Leung, D.Y. Immune mechanisms leading to atopic dermatitis. J. Allergy Clin. Immunol. 2003, 112, S128–S139. [Google Scholar] [CrossRef]
- Shirakawa, T.; Deichmann, K.A.; Izuhara, K.; Mao, X.-Q.; Adra, C.N.; Hopkin, J.M. Atopy and asthma: Genetic variants of IL-4 and IL-13 signalling. Immunol. Today 2000, 21, 60–64. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.-K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Maggi, E.; Del Prete, G.; Tiri, A.; Macchia, D.; Parronchi, P.; Ricci, M.; Romagnani, S. T cell clones providing helper function for IgE synthesis release soluble factor (s) that induce IgE production in human B cells: Possible role for interleukin 4 (IL-4). Clin. Exp. Immunol. 1988, 73, 57. [Google Scholar] [PubMed]
- Del Prete, G.; Maggi, E.; Parronchi, P.; Chretien, I.; Tiri, A.; Macchia, D.; Ricci, M.; Banchereau, J.; De Vries, J.; Romagnani, S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J. Immunol. 1988, 140, 4193–4198. [Google Scholar] [PubMed]
- Man, M.-Q.; Hatano, Y.; Lee, S.H.; Man, M.; Chang, S.; Feingold, K.R.; Leung, D.Y.; Holleran, W.; Uchida, Y.; Elias, P.M. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: Structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Investig. Dermatol. 2008, 128, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.; Arts, J.; Basketter, D.; English, J.; Diepgen, T.; Fuhlbrigge, R.; Gaspari, A. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kobayashi, T.; Nagao, K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 984.e1–990.e1. [Google Scholar] [CrossRef]
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into atopic dermatitis gained from genetically defined mouse models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef]
- Fujii, Y.; Takeuchi, H.; Sakuma, S.; Sengoku, T.; Takakura, S. Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Ski. Pharmacol. Physiol. 2009, 22, 240–247. [Google Scholar] [CrossRef]
- Iikura, M.; Yamaguchi, M.; Hirai, K.; Miyamasu, M.; Yamada, H.; Nakajima, T.; Fujisawa, T.; Ra, C.; Morita, Y.; Yamamoto, K. Regulation of surface FcεRI expression on human eosinophils by IL-4 and IgE. Int. Arch. Allergy Immunol. 2001, 124, 470–477. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.B.; Kang, S. Topical application of Chrysanthemum indicum L. Attenuates the development of atopic dermatitis-like skin lesions by suppressing serum IgE levels, IFN-γ, and IL-4 in Nc/Nga mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 821967. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; Olivry, T.; Carlotti, D.N.; International Task Force on Canine Atopic Dermatitis. Current evidence of skin barrier dysfunction in human and canine atopic dermatitis. Vet. Dermatol. 2011, 22, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.; Takaoka, R.; Rivitti, E.A.; Aoki, V. Atopic dermatitis: Correlation between non-damaged skin barrier function and disease activity. Int. J. Dermatol. 2012, 51, 672–676. [Google Scholar] [CrossRef]
- Liu, F.-T.; Goodarzi, H.; Chen, H.-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef]
- Jensen, J.-M.; Pfeiffer, S.; Witt, M.; Bräutigam, M.; Neumann, C.; Weichenthal, M.; Schwarz, T.; Fölster-Holst, R.; Proksch, E. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2009, 124, R19–R28. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorgan. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Jegal, J.; Park, N.-J.; Jo, B.-G.; Bong, S.-K.; Jegal, H.; Yang, M.; Kim, S.-N. Anti-Atopic Properties of Gracillin Isolated from Dioscorea quinqueloba on 2,4-Dinitrochlorobenzene-Induced Skin Lesions in Mice. Nutrients 2018, 10, 1205. [Google Scholar] [CrossRef]
- Jo, B.-G.; Park, N.-J.; Jegal, J.; Choi, S.; Lee, S.W.; Yi, L.W.; Kim, S.-N.; Yang, M.H. Stellera chamaejasme and its main compound luteolin 7-o-glucoside alleviates skin lesions in oxazolone-and 2,4-dinitrochlorobenzene-stimulated murine models of atopic dermatitis. Planta Med. 2019, 85, 583–590. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jun, C.-D.; Suk, K.; Choi, B.-J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.-Y.; Kim, D.-K.; Shin, T.-Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2005, 91, 123–131. [Google Scholar] [CrossRef]
- Kroes, B.V.; Van den Berg, A.; Van Ufford, H.Q.; Van Dijk, H.; Labadie, R. Anti-inflammatory activity of gallic acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yan, G.H. Ellagic Acid attenuates immunoglobulin E-mediated allergic response in mast cells. Biol. Pharm. Bull. 2009, 32, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Fontanari, C.; Borducchi, É.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharmacol. 2008, 580, 262–270. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jegal, H.; Bong, S.-K.; Yoon, K.-N.; Park, N.-J.; Shin, M.-S.; Yang, M.H.; Kim, Y.K.; Kim, S.-N. Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals. Biomolecules 2020, 10, 57. https://doi.org/10.3390/biom10010057
Lee S, Jegal H, Bong S-K, Yoon K-N, Park N-J, Shin M-S, Yang MH, Kim YK, Kim S-N. Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals. Biomolecules. 2020; 10(1):57. https://doi.org/10.3390/biom10010057
Chicago/Turabian StyleLee, Sullim, Hyun Jegal, Sim-Kyu Bong, Kyeong-No Yoon, No-June Park, Myoung-Sook Shin, Min Hye Yang, Yong Kee Kim, and Su-Nam Kim. 2020. "Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals" Biomolecules 10, no. 1: 57. https://doi.org/10.3390/biom10010057
APA StyleLee, S., Jegal, H., Bong, S.-K., Yoon, K.-N., Park, N.-J., Shin, M.-S., Yang, M. H., Kim, Y. K., & Kim, S.-N. (2020). Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals. Biomolecules, 10(1), 57. https://doi.org/10.3390/biom10010057







