Back to GroEL-Assisted Protein Folding: GroES Binding-Induced Displacement of Denatured Proteins from GroEL to Bulk Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Proteins
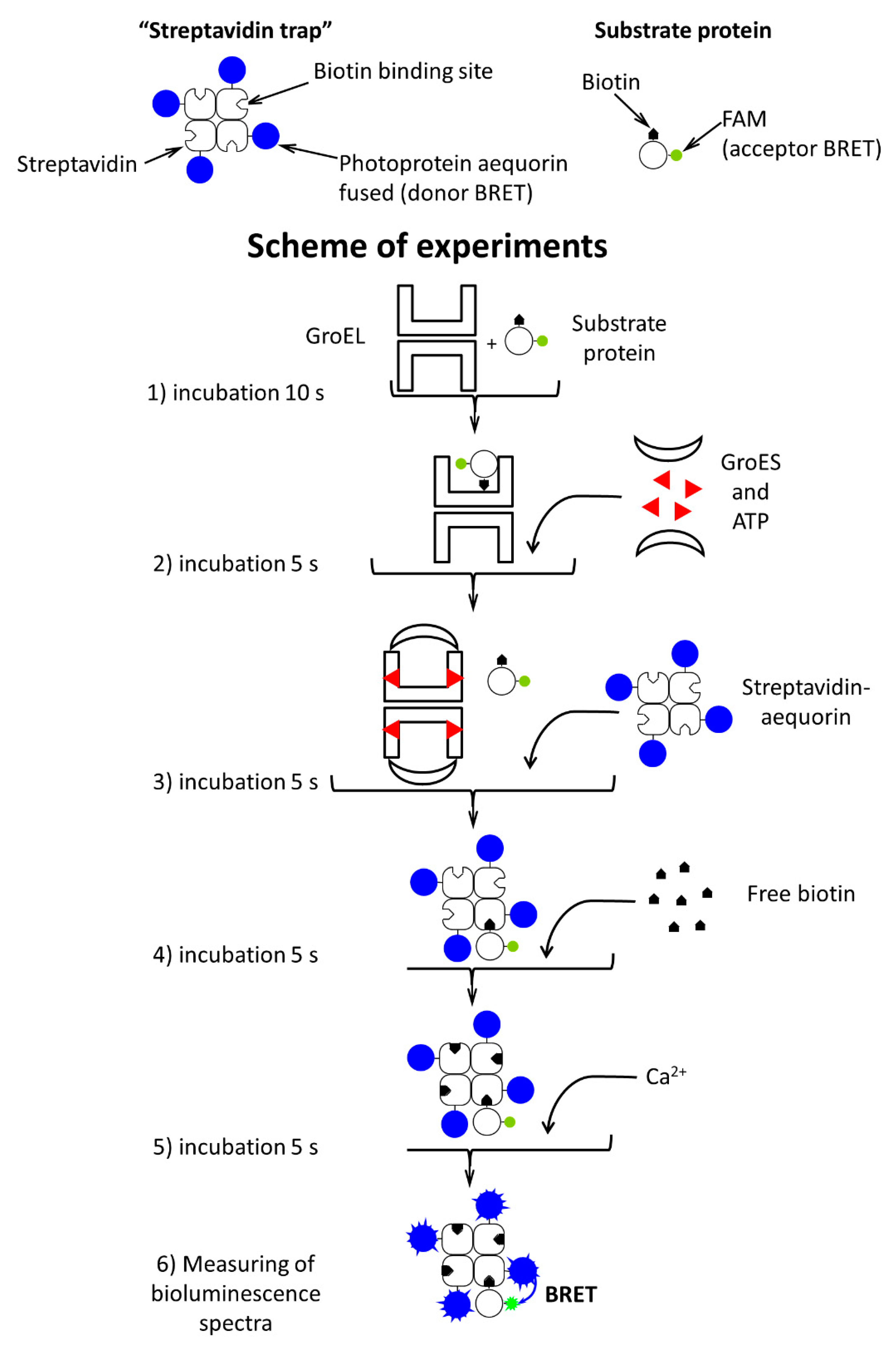
2.3. Methods
3. Results
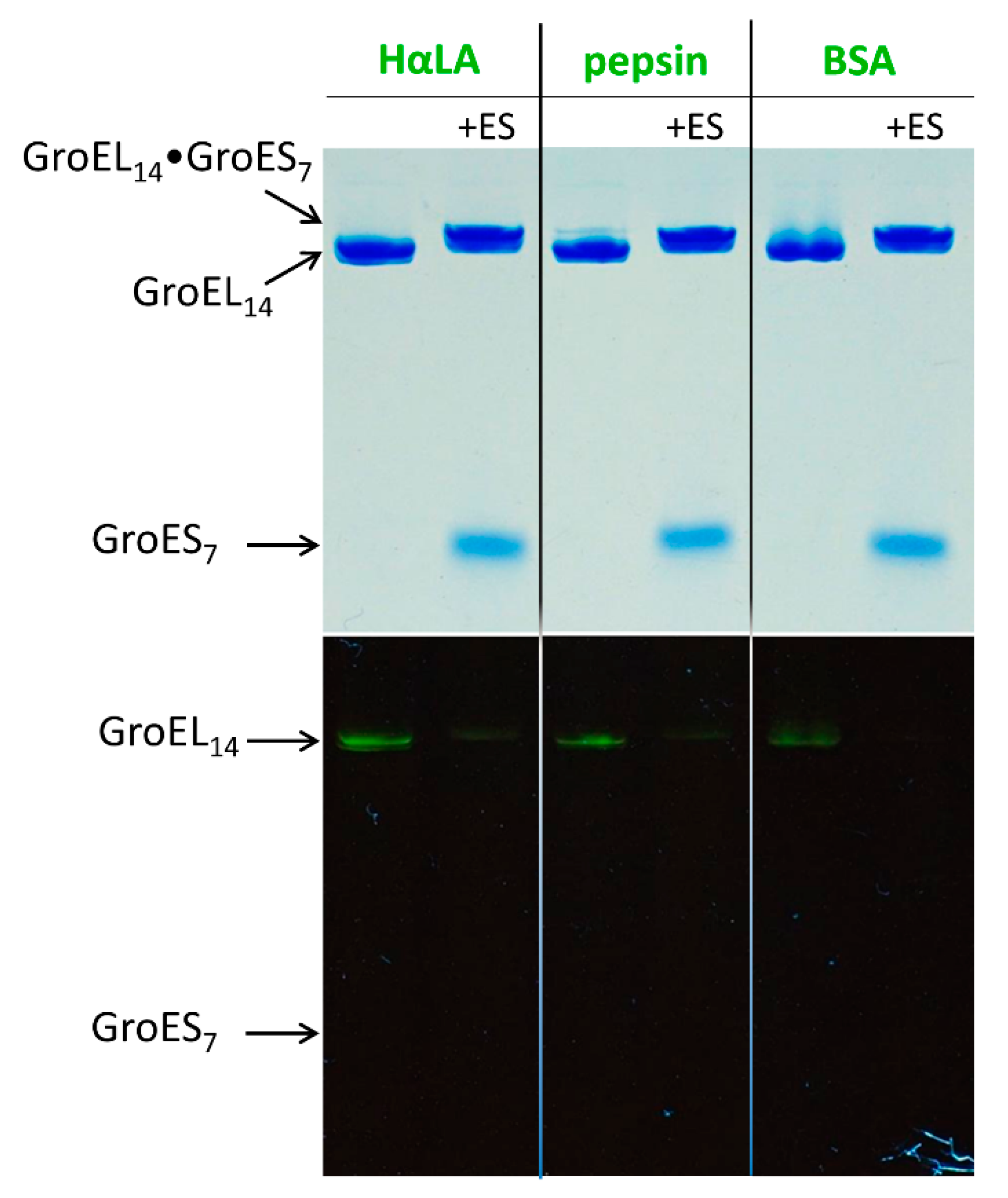
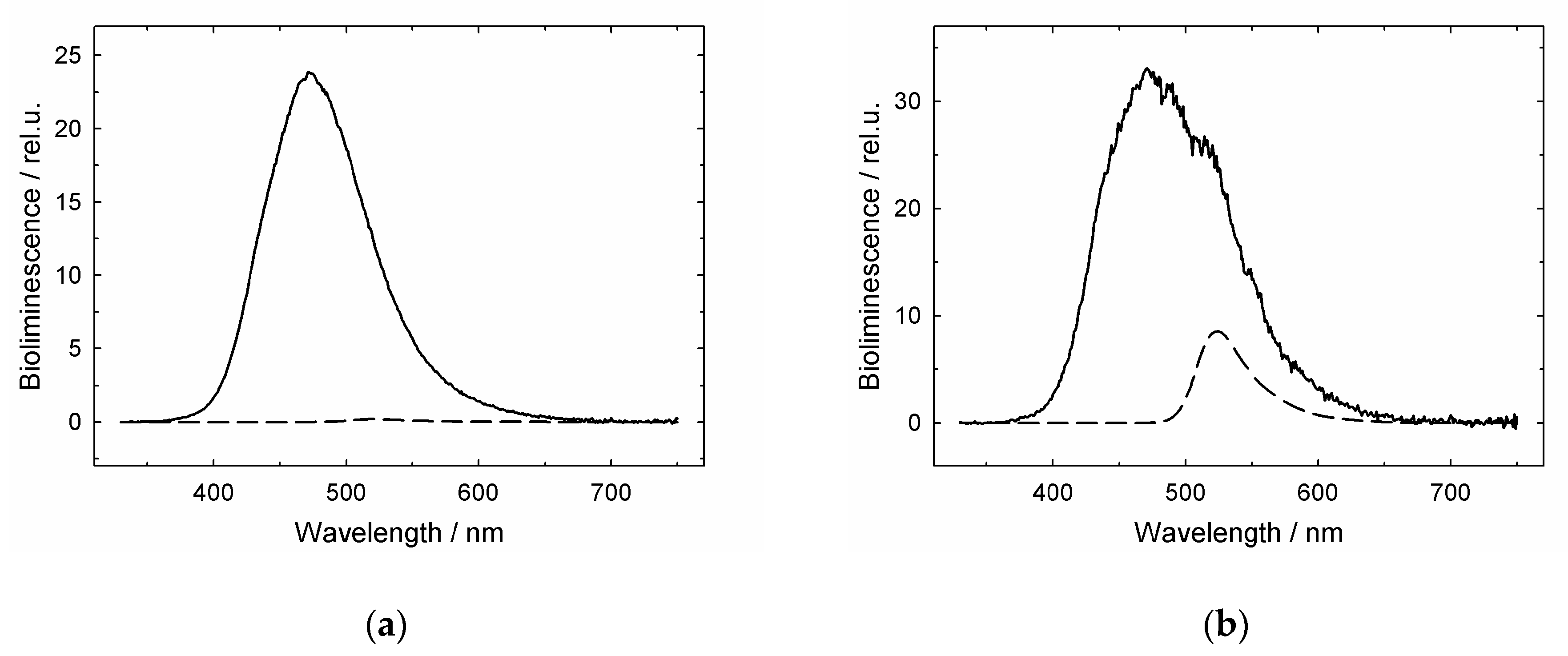
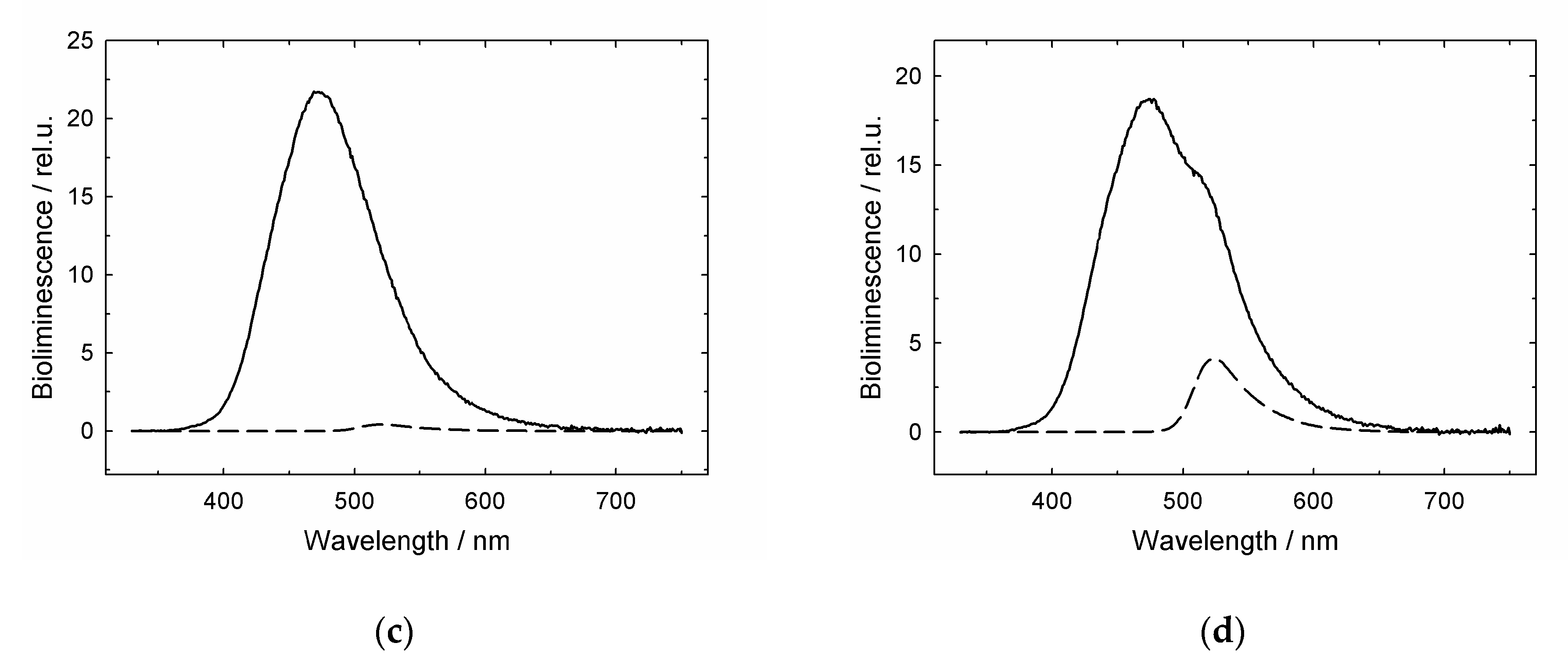
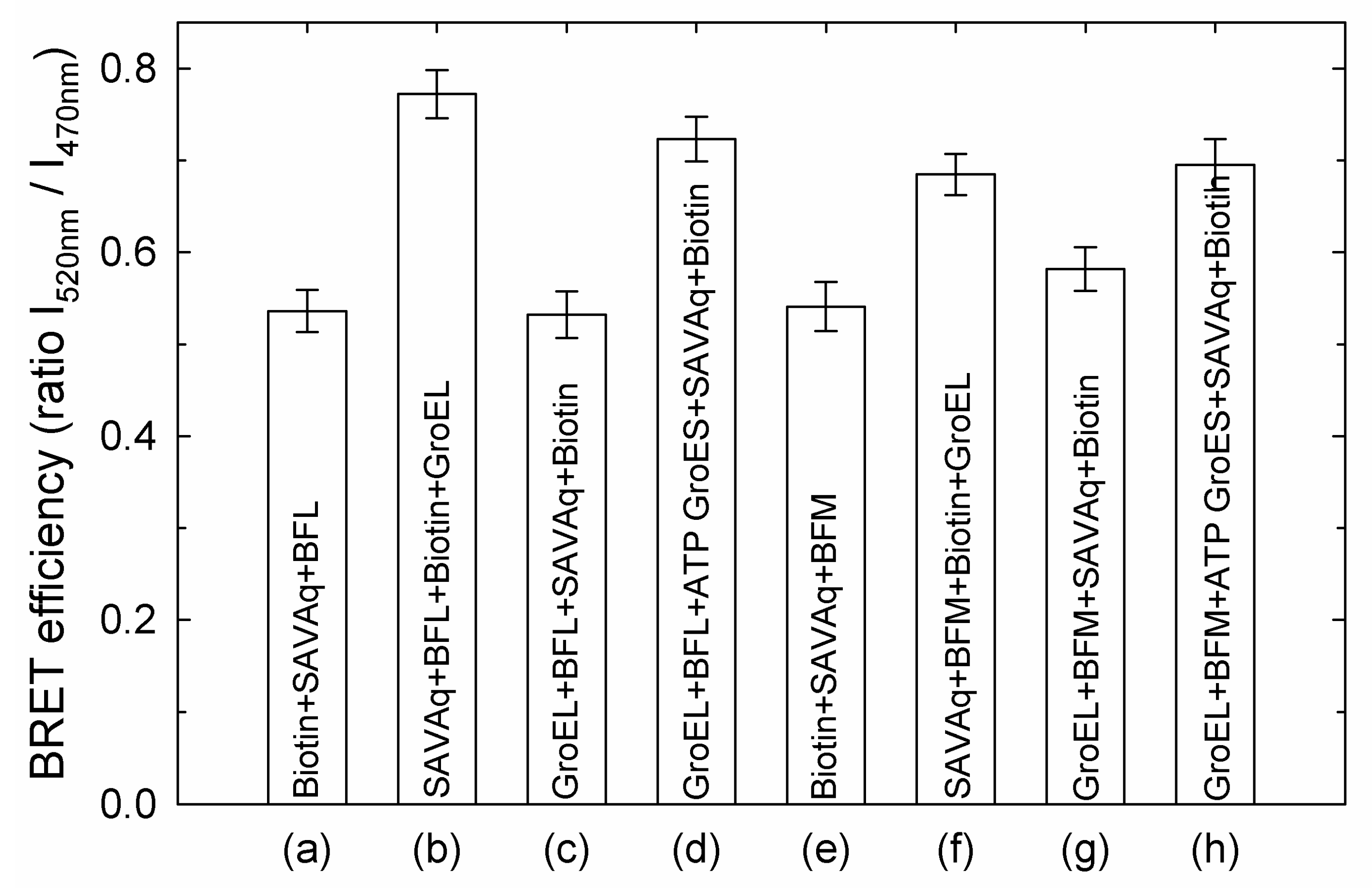
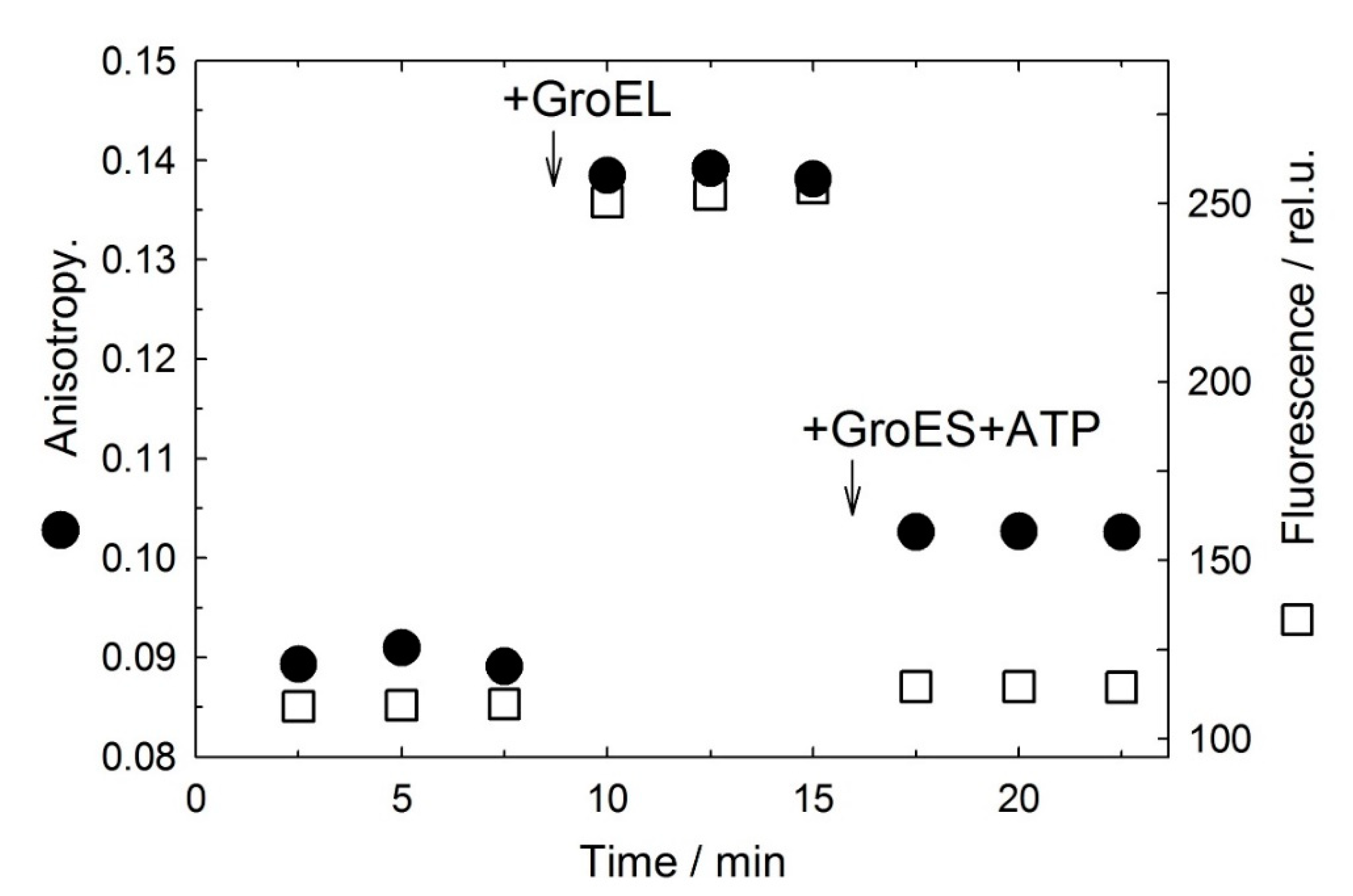
3.1. GroES Essentially Decreases the GroEL Affinity for Denatured Proteins
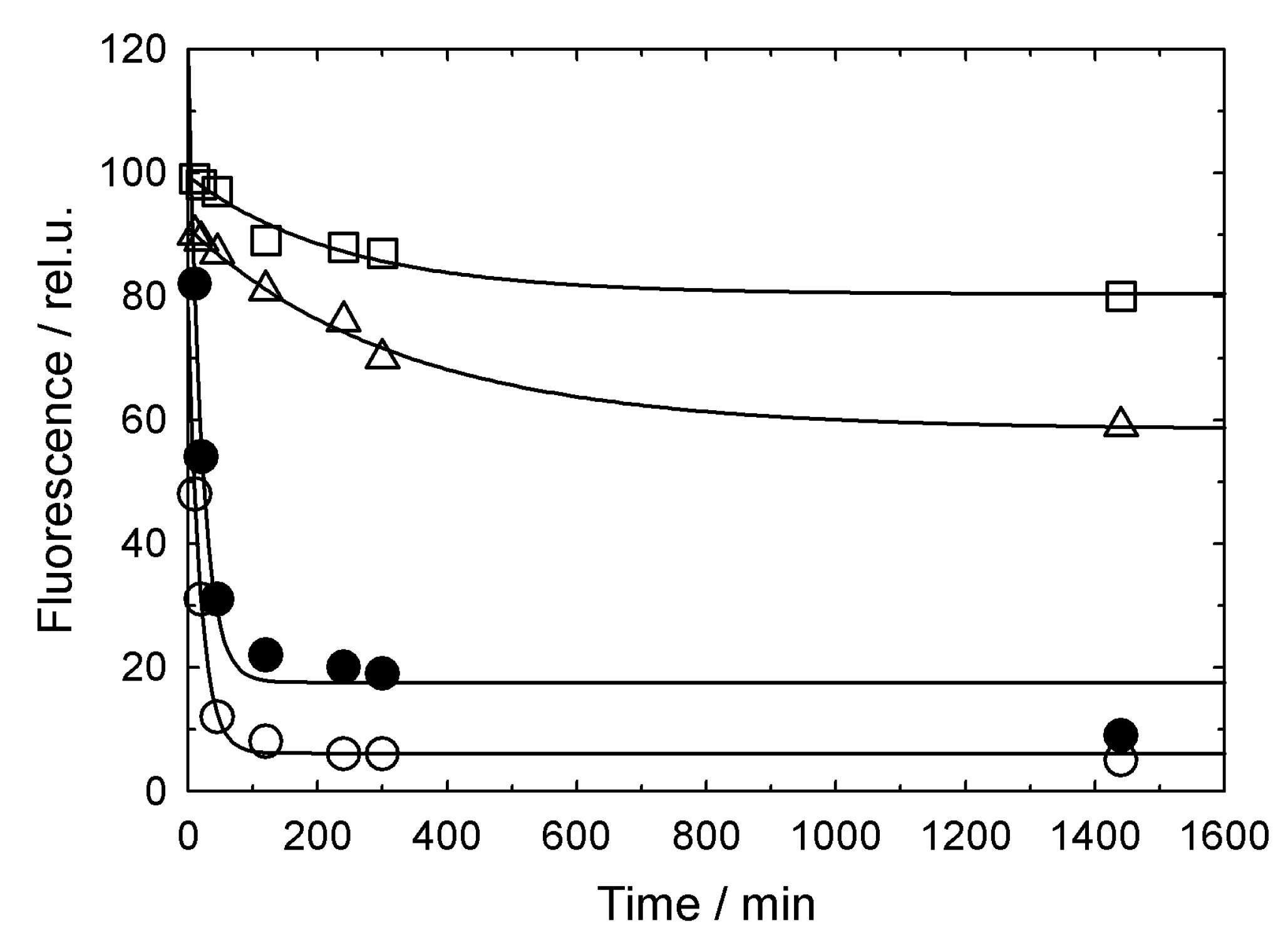
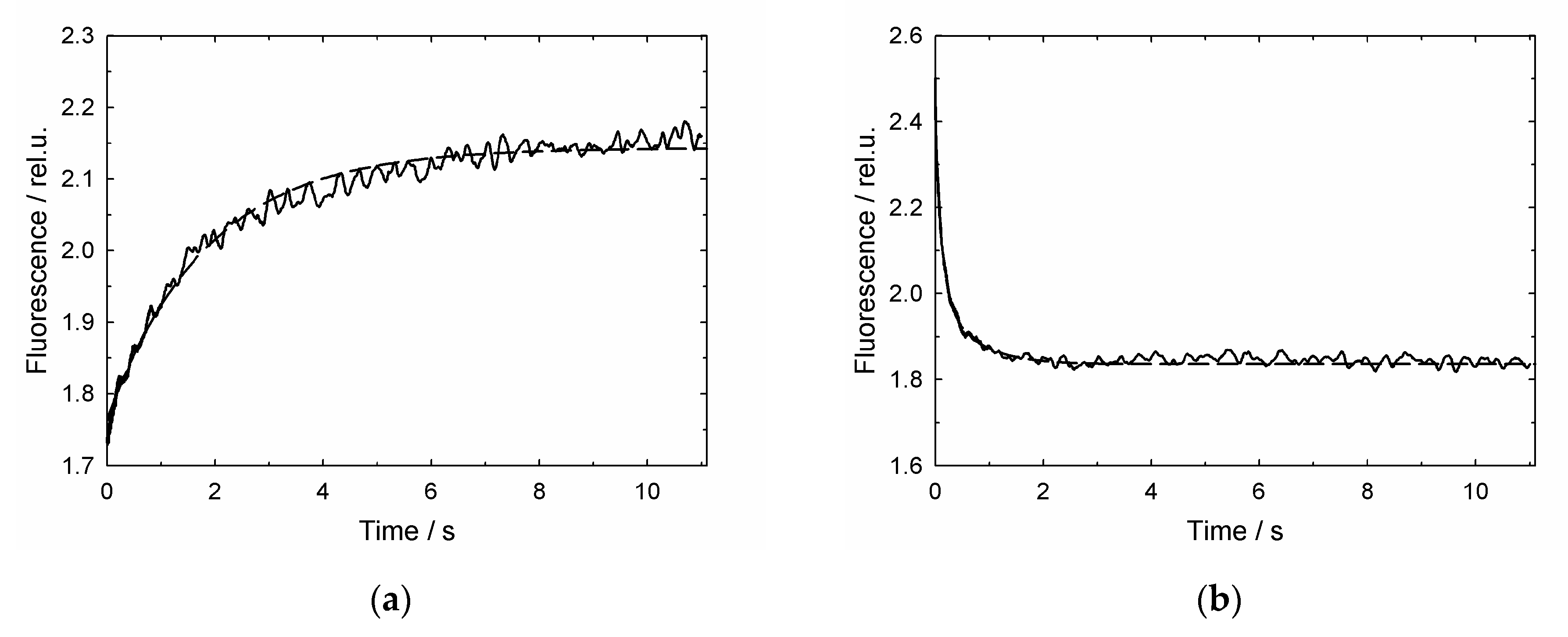
3.2. GroES-Assisted Dissociation of Denatured Proteins from the GroEL Surface Takes Much Less Time than the ATPase Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B.; Semisotnov, G.V. The mechanism of protein folding. In Conformations and Forces in Protein Folding; Nail, B.T., Dill, K.A., Eds.; AAAS: Washington, DC, USA, 1991; pp. 155–168. [Google Scholar]
- Seckler, R.; Jaenicke, R. Protein folding and protein refolding. FASEB J. 1992, 6, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Proteins as molecular chaperones. Nature 1987, 328, 378–379. [Google Scholar] [CrossRef]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Katsuno, M. Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights. Biomedicines 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.L.; Fenton, W.A.; Chapman, E.; Farr, G.W. Two families of chaperonin: Physiology and mechanism. Annu. Rev. Cell Dev. Biol. 2007, 23, 115–145. [Google Scholar] [CrossRef]
- Chen, S.; Roseman, A.M.; Hunter, A.S.; Wood, S.P.; Burston, S.G.; Ranson, N.A.; Clarke, A.R.; Saibil, H.R. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature 1994, 371, 261–264. [Google Scholar] [CrossRef]
- Harris, J.R.; Plückthun, A.; Zahn, R. Transmission Electron Microscopy of GroEL, GroES, and the Symmetrical GroEL/ES Complex. J. Struct. Biol. 1994, 112, 216–230. [Google Scholar] [CrossRef]
- Braig, K.; Otwinowski, Z.; Hegde, R.; Boisvert, D.C.; Joachimiak, A.; Horwich, A.L.; Sigler, P.B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature 1994, 371, 578–586. [Google Scholar] [CrossRef]
- Braig, K.; Adams, P.D.; Brünger, A.T. Conformational variability in the refined structure of the chaperonin groel at 2.8 Â resolution. Nat. Struct. Biol. 1995, 2, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, D.C.; Wang, J.; Otwinowski, Z.; Horwich, A.L.; Sigler, P.B. The 2.4 Å crystal structure of the bacterial chaperonin GroEL complexed with ATPγS. Nat. Struct. Biol. 1996, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Horwich, A.L.; Sigler, P.B. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature 1997, 388, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.F.; Weaver, A.J.; Landry, S.J.; Gierasch, L.; Deisenhofer, J. The crystal structure of the GroES co-chaperonin at 2.8 Å resolution. Nature 1996, 379, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, M.; da Silva, A.C.R.; Martin, J.; Erdjument-Bromage, H.; Tempst, P.; Hartl, F.U. Protein folding in the central cavity of the GroEL–GroES chaperonin complex. Nature 1996, 379, 420–426. [Google Scholar] [CrossRef]
- Hayer-Hartl, M.; Bracher, A.; Hartl, F.U. The GroEL–GroES Chaperonin Machine: A Nano-Cage for Protein Folding. Trends Biochem. Sci. 2016, 41, 62–76. [Google Scholar] [CrossRef]
- Shtilerman, M.; Lorimer, G.H.; Englander, S.W. Chaperonin Function: Folding by Forced Unfolding. Science 1999, 284, 822. [Google Scholar] [CrossRef]
- Horwich, A.L.; Farr, G.W.; Fenton, W.A. GroEL−GroES-Mediated Protein Folding. Chem. Rev. 2006, 106, 1917–1930. [Google Scholar] [CrossRef]
- Lin, Z.; Madan, D.; Rye, H.S. GroEL stimulates protein folding through forced unfolding. Nat. Struct. Mol. Biol. 2008, 15, 303–311. [Google Scholar] [CrossRef]
- Priya, S.; Sharma, S.K.; Sood, V.; Mattoo, R.U.H.; Finka, A.; Azem, A.; De Los Rios, P.; Goloubinoff, P. GroEL and CCT are catalytic unfoldases mediating out-of-cage polypeptide refolding without ATP. Proc. Natl. Acad. Sci. USA 2013, 110, 7199–7204. [Google Scholar] [CrossRef]
- Horwich, A.L.; Fenton, W.A. Chaperonin-mediated protein folding: Using a central cavity to kinetically assist polypeptide chain folding. Q. Rev. Biophys. 2009, 42, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Libich, D.S.; Tugarinov, V.; Clore, G.M. Intrinsic unfoldase/foldase activity of the chaperonin GroEL directly demonstrated using multinuclear relaxation-based NMR. Proc. Natl. Acad. Sci. USA 2015, 112, 8817–8823. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, N.Y.; Marchenkov, V.V.; Semisotnov, G.V.; Finkelstein, A.V. Strict experimental evidence that apo-chaperonin GroEL does not accelerate protein folding, although it does accelerate one of its steps. Proc. Natl. Acad. Sci. USA 2015, 112, E6831–E6832. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Buckle, A.M.; Perrett, S.; Johnson, C.M.; Corrales, F.J.; Golbik, R.; Fersht, A.R. Chaperone activity and structure of monomeric polypeptide binding domains of GroEL. Proc. Natl. Acad. Sci. USA 1996, 93, 15024–15029. [Google Scholar] [CrossRef]
- Jain, N.; Knowles, T.J.; Lund, P.A.; Chaudhuri, T.K. Minichaperone (GroEL191-345) mediated folding of MalZ proceeds by binding and release of native and functional intermediates. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 941–951. [Google Scholar] [CrossRef]
- Chatellier, J.; Hill, F.; Lund, P.A.; Fersht, A.R. In vivo activities of GroEL minichaperones. Proc. Natl. Acad. Sci. USA 1998, 95, 9861–9866. [Google Scholar] [CrossRef]
- Marchenkov, V.V.; Semisotnov, G.V. GroEL-assisted protein folding: Does it occur within the chaperonin inner cavity? Int. J. Mol. Sci. 2009, 10, 2066–2083. [Google Scholar] [CrossRef]
- Marchenkov, V.V.; Sokolovskii, I.V.; Kotova, N.V.; Galzitskaya, O.V.; Bochkareva, E.S.; Girshovich, A.S.; Semisotnov, G.V. The interaction of the GroEL chaperone with early kinetic intermediates of renaturing proteins inhibits the formation of their native structure. Biofizika 2004, 49, 987–994. [Google Scholar]
- Staniforth, R.A.; Burston, S.G.; Atkinson, T.; Clarke, A.R. Affinity of chaperonin-60 for a protein substrate and its modulation by nucleotides and chaperonin-10. Biochem. J. 1994, 300, 651–658. [Google Scholar] [CrossRef]
- Makio, T.; Takasu-Ishikawa, E.; Kuwajima, K. Nucleotide-induced transition of GroEL from the high-affinity to the low-affinity state for a target protein: Effects of ATP and ADP on the GroEL-affected refolding of α-lactalbumin. J. Mol. Biol. 2001, 312, 555–567. [Google Scholar] [CrossRef]
- Tsurupa, G.P.; Ikura, T.; Makio, T.; Kuwajima, K. Refolding kinetics of staphylococcal nuclease and its mutants in the presence of the chaperonin GroEL. J. Mol. Biol. 1998, 277, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Buchner, J.; Schmidt, M.; Fuchs, M.; Jaenicke, R.; Rudolph, R.; Schmid, F.X.; Kiefhaber, T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry 1991, 30, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Gafni, A. Thermal switching between enhanced and arrested reactivation of bacterial glucose-6-phosphate dehydrogenase assisted by GroEL in the absence of ATP. J. Biol. Chem. 1993, 268, 21632–21636. [Google Scholar]
- Ayling, A.; Baneyx, F. Influence of the GroE molecular chaperone machine on the in vitro refolding of Escherichia coli beta-galactosidase. Protein Sci. 1996, 5, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, P.V.; Gatenby, A.A.; Lorimer, G.H. Purified chaperonin 60 (groEL) interacts with the nonnative states of a multitude of Escherichia coli proteins. Protein Sci. 1992, 1, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Grimm, R.; Donaldson, G.K.; Van Der Vies, S.M.; Schäfer, E.; Gatenby, A.A. Chaperonin-mediated reconstitution of the phytochrome photoreceptor. J. Biol. Chem. 1993, 268, 5220–5226. [Google Scholar]
- Lissin, N.M.; Venyaminov, S.Y.; Girshovich, A.S. (Mg-ATP)-dependent self-assembly of molecular chaperone GroEL. Nature 1990, 348, 339–342. [Google Scholar] [CrossRef]
- Ryabova, N.; Marchenkov, V.; Kotova, N.; Semisotnov, G. Chaperonin GroEL reassembly: An effect of protein ligands and solvent composition. Biomolecules 2014, 4, 458–473. [Google Scholar] [CrossRef]
- Hayer-Hartl, M.K.; Hartl, F.U. A comment on: “The aromatic amino acid content of the bacterial chaperone protein groEL (cpn60): Evidence for the presence of a single tryptophan”, by N.C. Price, S.M. Kelly, S. Wood and A. auf der Mauer (1991) FEBS Lett. 292, 9–12. FEBS Lett. 1993, 320, 83–84, discussion 85. [Google Scholar] [CrossRef]
- Gorokhovatsky, A.Y.; Rudenko, N.V.; Marchenkov, V.V.; Skosyrev, V.S.; Arzhanov, M.A.; Burkhardt, N.; Zakharov, M.V.; Semisotnov, G.V.; Vinokurov, L.M.; Alakhov, Y.B. Homogeneous assay for biotin based on Aequorea victoria bioluminescence resonance energy transfer system. Anal. Biochem. 2003, 313, 68–75. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Vasilenko, K.S.; Kirkitadze, M.D.; Kotova, N.V.; Motuz, L.P.; Korotkov, K.V.; Semisotnov, G.V.; Alakhov, I.B. Biosynthesis and conformational state of 17-kDa and 27-kDa N-terminal fragments of elongation factor EF-2 in solution. Bioorg. Khim. 1996, 22, 489–502. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenkov, V.; Gorokhovatsky, A.; Marchenko, N.; Ivashina, T.; Semisotnov, G. Back to GroEL-Assisted Protein Folding: GroES Binding-Induced Displacement of Denatured Proteins from GroEL to Bulk Solution. Biomolecules 2020, 10, 162. https://doi.org/10.3390/biom10010162
Marchenkov V, Gorokhovatsky A, Marchenko N, Ivashina T, Semisotnov G. Back to GroEL-Assisted Protein Folding: GroES Binding-Induced Displacement of Denatured Proteins from GroEL to Bulk Solution. Biomolecules. 2020; 10(1):162. https://doi.org/10.3390/biom10010162
Chicago/Turabian StyleMarchenkov, Victor, Andrey Gorokhovatsky, Natalia Marchenko, Tanya Ivashina, and Gennady Semisotnov. 2020. "Back to GroEL-Assisted Protein Folding: GroES Binding-Induced Displacement of Denatured Proteins from GroEL to Bulk Solution" Biomolecules 10, no. 1: 162. https://doi.org/10.3390/biom10010162
APA StyleMarchenkov, V., Gorokhovatsky, A., Marchenko, N., Ivashina, T., & Semisotnov, G. (2020). Back to GroEL-Assisted Protein Folding: GroES Binding-Induced Displacement of Denatured Proteins from GroEL to Bulk Solution. Biomolecules, 10(1), 162. https://doi.org/10.3390/biom10010162





