Infrared Spectral Analysis
The infrared analysis of the spectrum for
C1 DAM,
C2 DAM, and
C2v DAM conformers of DAM were analyzed over the 0–4000 cm
−1 range, and provide detailed insights into its molecular vibrations, particularly involving its two amine groups and central methylene bridge (
Figure 7). Detailed attention is given to two critical regions of the spectrum: 500–1000 cm
−1 and 2800–3400 cm
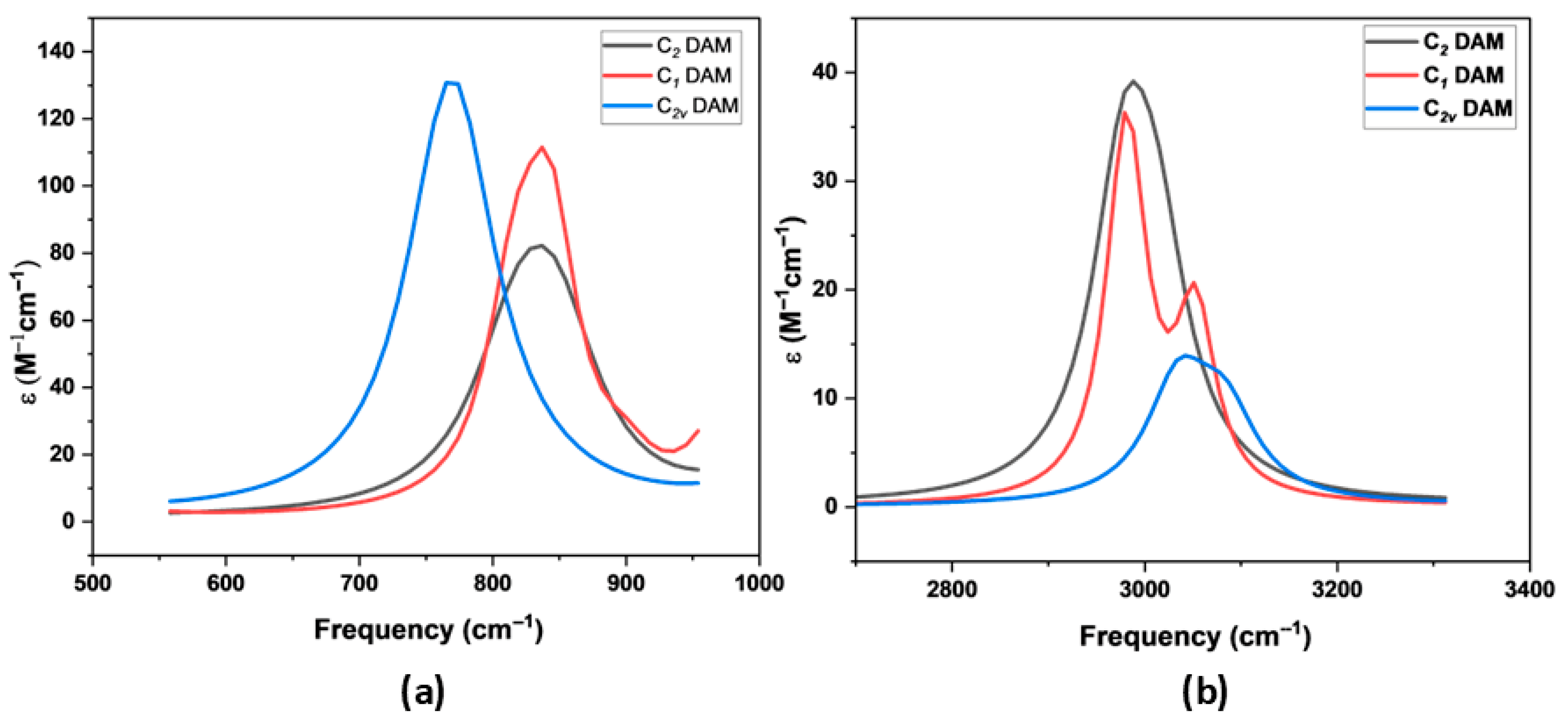
−1, where the most intense and diagnostic vibrational modes are observed. These zoomed-in views of both regions presented in
Figure 8, reveal two major peaks: a high-intensity band from symmetric NH
2 rocking and another from anti-symmetric N–C stretching, both serving as key vibrational markers for distinguishing between the conformers.
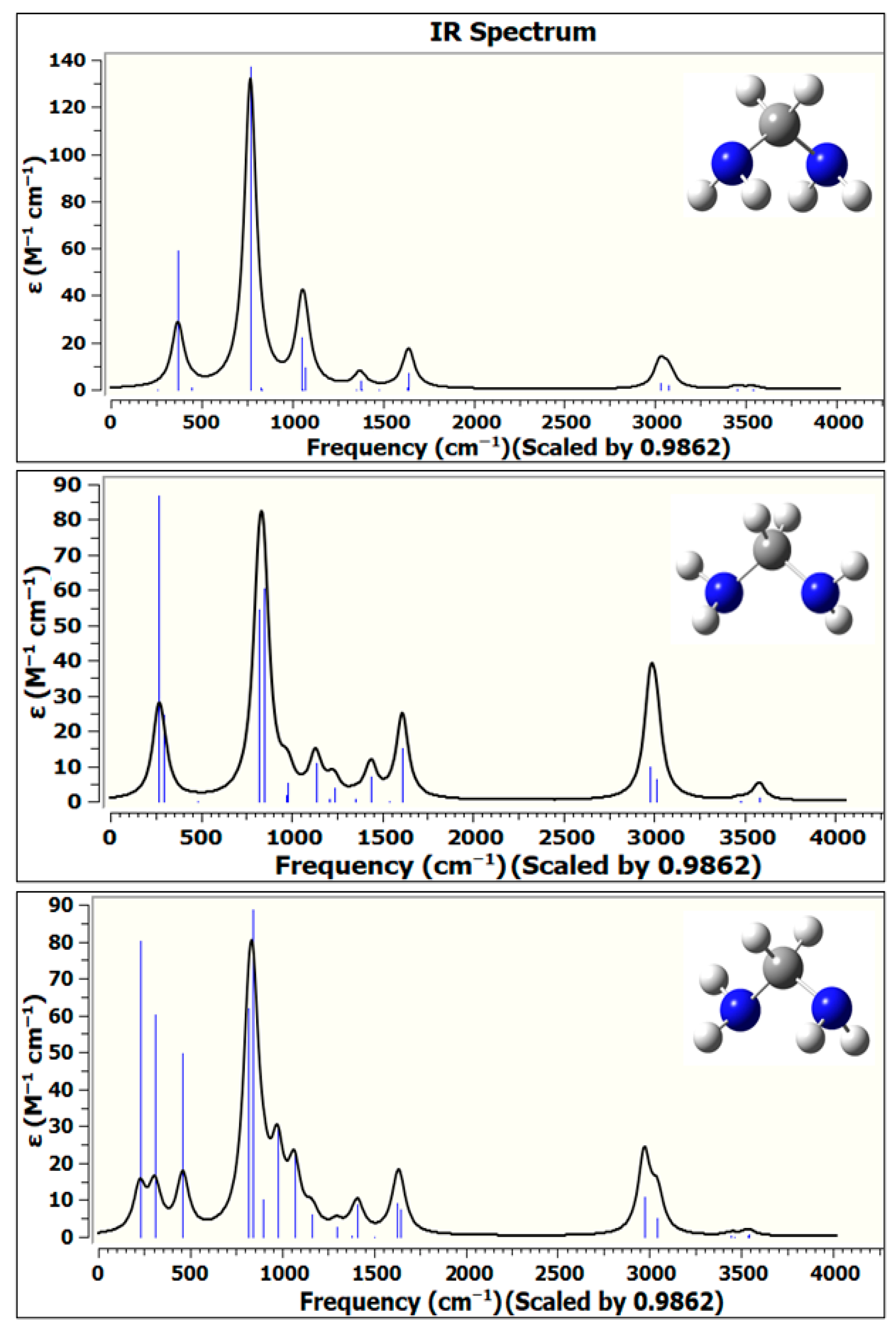
Table 7 presents the computed infrared vibrational frequencies and corresponding intensities and wavelengths for all three conformers. The data were computed using the B2PLYPD functional with the aug–cc–pVTZ basis set. Given the importance of accurately characterizing the vibrational modes of DAM, especially for spectroscopic identification and astrochemical applications, the infrared spectrum was generated with high precision. The spectral peaks were determined by Gaussian fitting of the computed IR absorption data, employing a full width at half maximum (FWHM) of 25 cm
−1.
A scaling factor of 0.9862 was applied to both the vibrational frequencies and the zero-point vibrational energies (ZPVEs). This scaling factor follows well-established computational protocols and is supported by the benchmarking results of Alecu et al. [
44], who recommended a universal scaling factor ratio of λ
harm/λ
ZPVE = 1.014 ± 0.002. This corresponds to an experimental correction ratio of approximately 0.9862 for ZPVE, ensuring that the computed vibrational data more accurately reflect true molecular behavior. This correction is consistent with and further substantiated by our computational methodology [
45].
The C2v DAM spectrum displays diverse vibrational modes across the low and high wavenumber regions. At the low frequency region, the peak at 254.6 cm−1 (39.26 μm) (very weak intensity) is assigned to rocking vibrations of hydrogen atoms on both NH2 groups, specifically an anti-symmetric out-of-plane bending (OPB) mode. Moving up the spectrum, a significant absorption at 369.7 cm−1 (27.05 µm) with high intensity (79 km mol−1) corresponds to a twisting motion of H atoms on the NH2 groups, accompanied by CH2 rocking, indicative of a symmetric NH2 out-of-plane bending. A moderate peak at 444.3 cm−1 (1 km mol−1, 22.50 µm) is associated with N–C–N wagging, which reflects the collective bending of the nitrogen atoms relative to the central carbon. At 769.2 cm−1 (2.99 µm), a strong and highly intense band with an intensity of 383 km mol−1 represents symmetric rocking of the NH2 groups, a key diagnostic feature of primary amines. The next significant region is near 828.7 cm−1 (12.07 µm), attributed to asymmetric N–C stretching, whereas 834.5 cm−1 (11.98 µm) is a result of CH2 rocking coupled with anti-symmetric twisting of the NH2 groups. In the mid-IR region, 1053.6 cm−1 (9.49 µm) shows a strong absorption (85 km mol−1) from symmetric N–C stretching, a fundamental skeletal vibration, followed by a weak mode at 1055.6 cm−1 that is linked to NH2 and CH2 twisting. Another notable skeletal vibration is the N–C–N symmetric stretching at 1072.7 cm−1 (9.32 µm) with a moderate intensity of 36 km mol−1, indicating structural stability along the backbone. Subsequent vibrations such as NH2 twisting at 1352.6 cm−1, CH2 in-plane rocking at 1374.5 cm−1, and a very weak symmetric H twist at 1383.3 cm−1 further highlight internal rearrangements. The H–C–H rocking at 1478.7 cm−1 and H–N–H anti-symmetric and symmetric bending modes at 1631.4 cm−1 and 1642.4 cm−1, respectively, demonstrate characteristic bending vibrations of the NH2 groups. In the high-frequency region, CH2 symmetric and asymmetric stretching modes appear at 3026.2 cm−1 (λ ≈ 3.30 µm) and 3074.5 cm−1 (λ ≈ 3.25 µm), showing moderate intensities. The NH2 symmetric and asymmetric N–H stretches, often termed as amino stretches, are observed prominently in the 2800–3500 cm−1 range, corresponding to wavelengths between 2.80 and 3.50 µm. Specifically, symmetric N–H stretches occur at 3450.8 cm−1 and 3454.8 cm−1, while asymmetric N–H stretches appear at 3538.4 cm−1 and 3543.2 cm−1, though with relatively lower intensities, which is typical for primary amines due to intermolecular hydrogen bonding and coupling effects.
To further validate our vibrational analysis, we compared the most important diagnostic IR modes of the
C2v DAM obtained in this work with those reported by Watrous et al. [
13]. The agreement is very good across the spectral region. For the NH
2 stretching vibrations, our scaled harmonic frequencies predict an asymmetric stretch near 3490 cm
−1 and a symmetric stretch near 3380 cm
−1, while Watrous et al. [
13] reported corresponding bands at 3482 and 3372 cm
−1, respectively (Δ < 10 cm
−1). The NH
2 scissoring mode is reproduced at 1615 cm
−1 in our work versus their value of 1625 cm
−1. Likewise, the CH
2 stretching region (2930–2985 cm
−1) differs by less than 15 cm
−1 from the previous values [
13], and the NH
2 wagging/twisting modes in the 700–900 cm
−1 region are reproduced within 5–10 cm
−1. Such close agreement between the two independent computations confirms the robustness of our vibrational assignments and provides confidence that the predicted spectrum is reliable for astrochemical identification.
The C2 DAM conformer exhibits prominent IR absorptions associated with various NH2 group motions and CH2/NC vibrations. The strongest IR active mode is the NH2 anti-symmetric wagging at 847.3 cm−1 with an intensity of 145 km mol−1, indicating a significant dipole change along this vibrational coordinate. Another intense mode is the symmetric NH2 rocking at 817.8 cm−1 (126 km mol−1), reinforcing the presence of strongly IR-active NH2 group dynamics in this geometry. At higher wavenumbers, the C–H stretching vibrations are observed near 2978.4 cm−1 (sym, intensity: 82 km mol−1) and 3013.1 cm−1 (anti-sym, 53 km mol−1), which are typical for aliphatic CH groups. The NH2 bending modes around 1611.3 cm−1 (anti-sym, 68 km mol−1) and 1613.7 cm−1 (sym, 2 km mol−1) further demonstrate significant out-of-plane deformation vibrations. A noteworthy feature is the N–C anti-symmetric stretch at 1132.7 cm−1 with high intensity (34 km mol−1), aiding potential identification. The CH2 in-plane rocking and wagging/twisting modes between 1234 and 1438 cm−1 show moderate intensities and suggest conformer-specific flexibility in methylene motion. The amino H stretching vibrations are found in the 3474–3579 cm−1 region with relatively weak intensities (maximum 9 km mol−1), consistent with free or weakly hydrogen-bonded NH2 groups.
The C1 DAM conformer, characterized by lower symmetry, shows slightly shifted but comparable vibrational modes with some distinctions in IR intensities, reflecting geometric variations. The NH2 anti-symmetric wagging at 842.7 cm−1 (148 km mol−1) is the most intense peak, similar to C2 DAM, but slightly red-shifted. The NH2 rocking (816.1 cm−1, 100 km mol−1) and N–C anti-symmetric stretching (1066.5 cm−1, 48 km mol−1) also remain significant, underscoring the NH2-related modes as dominant IR-active features in both conformers. Compared to C2 DAM, the N–C symmetric stretch at 899.1 cm−1 is weaker (18 km mol−1) but still observable. The CH2 wagging and rocking modes between 1296.3 and 1410.7 cm−1 show slight shifts and moderate intensities, hinting at conformational flexibility. The C–H stretching region (2972–3043 cm−1) shows strong absorptions with slightly lower intensity compared to C2 DAM (2972.1 cm−1, 64 km mol−1). NH bending modes appear at 1626.5 cm−1 (anti−sym, 29 km mol−1) and 1645.3 cm−1 (sym, 24 km mol−1), indicating vibrational coupling differences due to asymmetric geometry. The amino hydrogen stretching modes between 3444 and 3542 cm−1 are weak in intensity (all below 4 km mol−1), similar to C2 DAM, supporting the general IR inactivity of these high-frequency vibrations unless involved in strong interactions.
Compared to structurally analogous species such as methylamine (CH
3NH
2) and ethylenediamine (NH
2CH
2CH
2NH
2) [
46], DAM conformers exhibit enhanced vibrational complexity due to their geminal diamine structure, leading to multiple coupled NH
2 modes. The dominance of NH
2 out-of-plane deformations in the IR spectrum of DAM parallels trends observed in other interstellar amines, reaffirming the diagnostic significance of these vibrations. Specifically, high dipole moment changes during NH
2 wagging and CH
2 bending motions are consistent with efficient IR excitation and radiative decay pathways, which can be exploited for remote sensing in the gas phase.
Given the high IR intensities of wagging, rocking, and bending modes associated with NH2 and CH2 groups, particularly in the 600–1200 cm−1 region, all three conformers present spectroscopically observable fingerprints for gas-phase detection in laboratory simulations or astronomical environments. The presence of multiple vibrational modes with intensities exceeding 80 km mol−1 indicates strong dipole moment changes during vibration, which significantly enhances its detectability in both astronomical observations and atmospheric studies.