Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Sample Pre-Treatment
2.3. Liquid Chromatography (LC)
2.4. Mass Spectrometry
2.5. Statistical Analysis
2.6. Sensory Test
3. Results
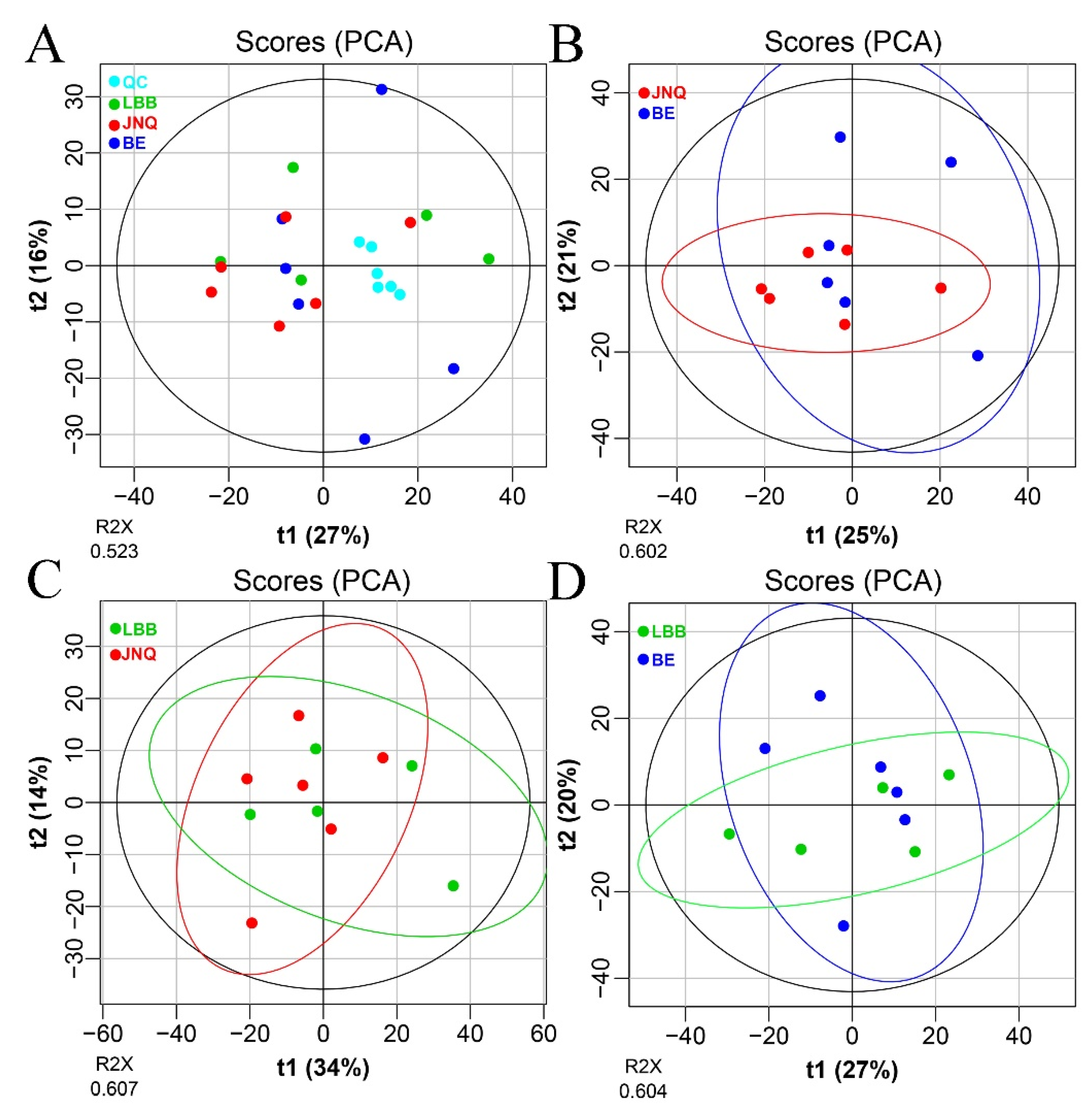
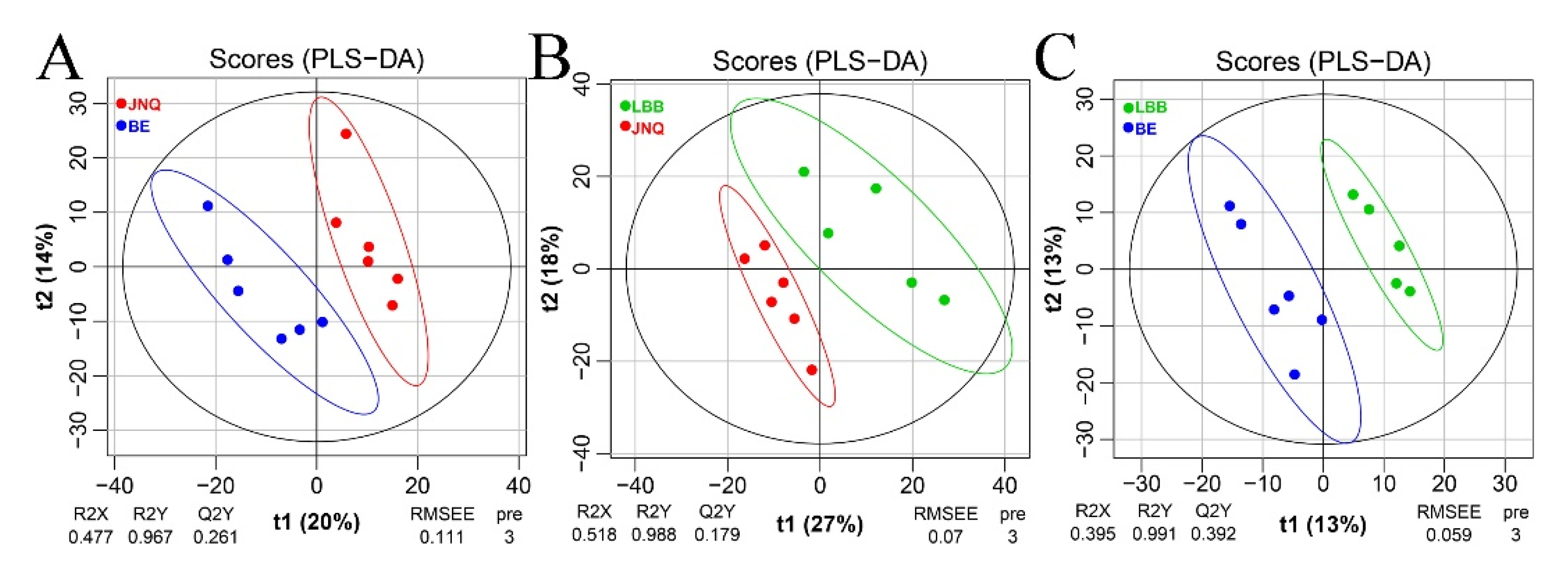
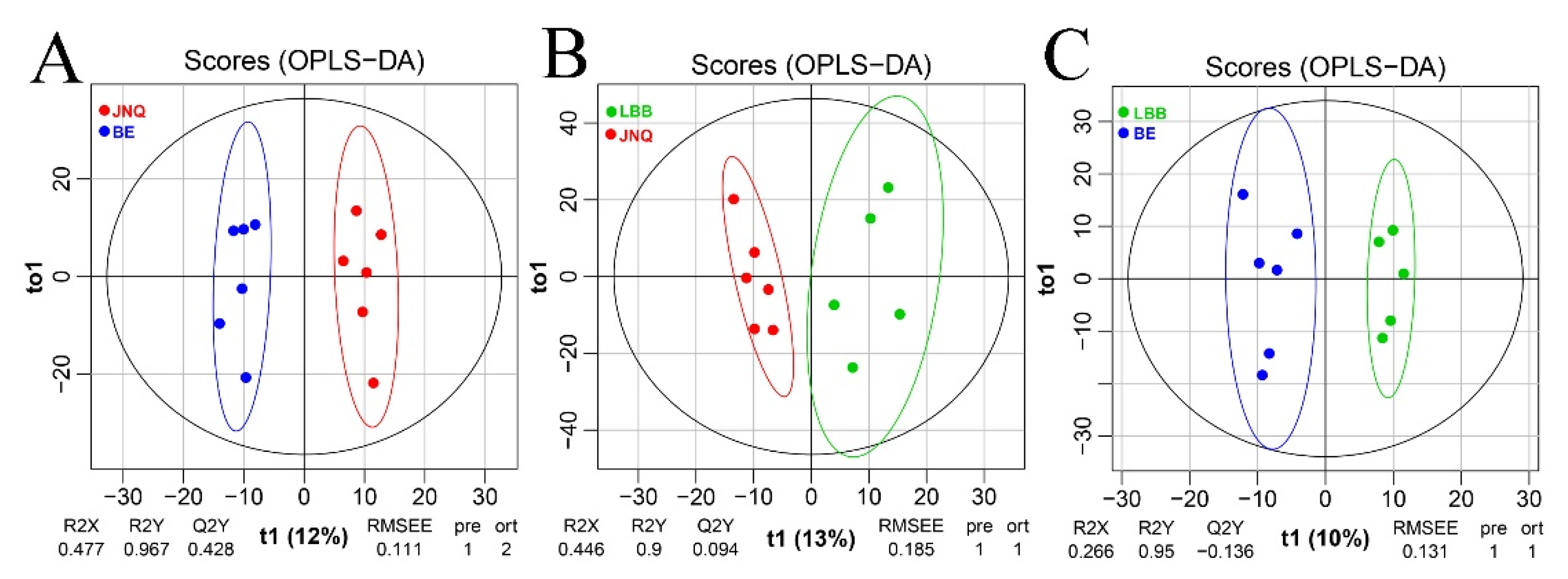
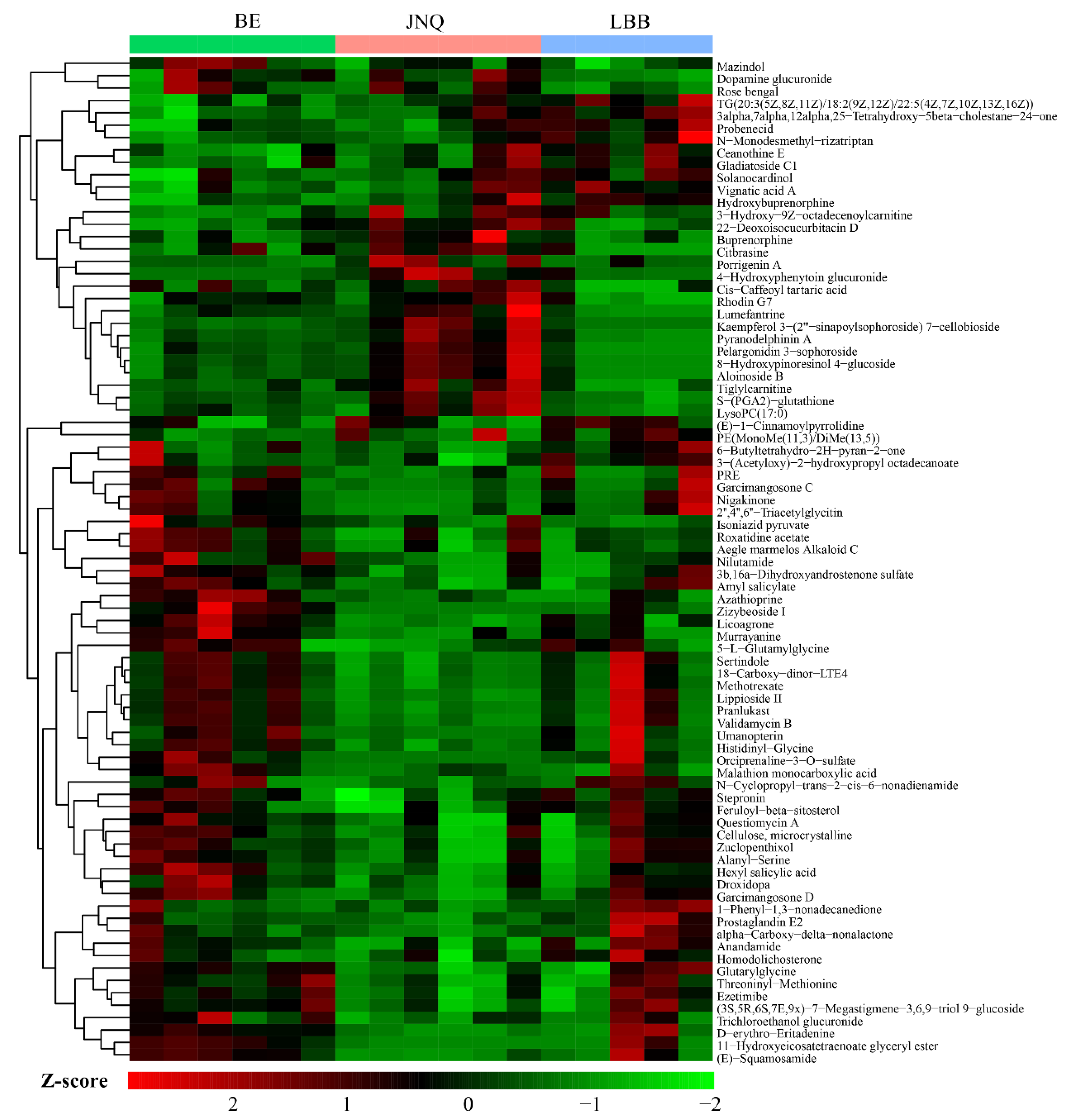
3.1. Metabolic Profiling of the Goat Samples
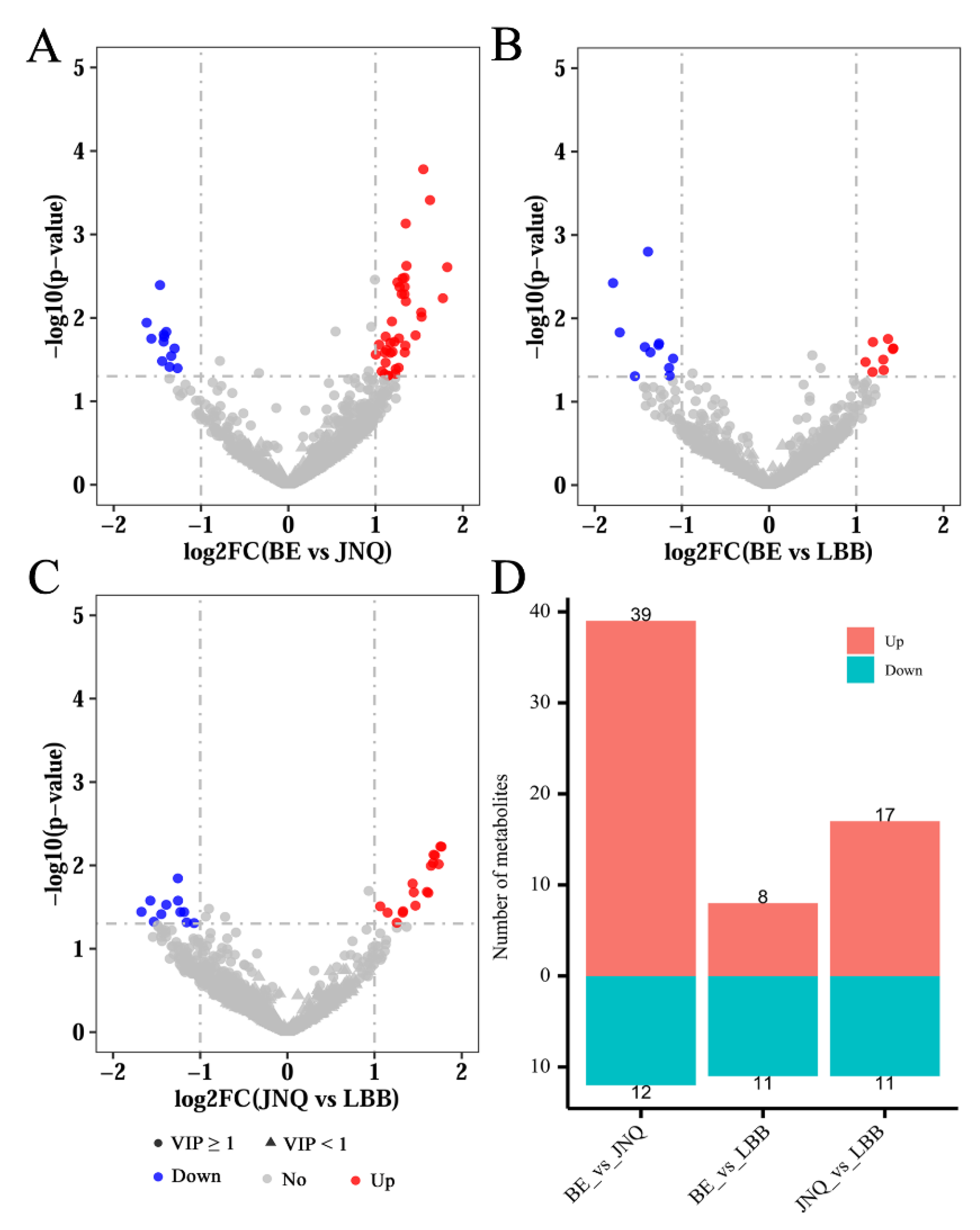
3.2. Identification of Differential Metabolites
3.3. Sensory Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Watkins, P.J.; Frank, D.; Singh, T.K.; Young, O.A.; Warner, R.D. Sheepmeat Flavor and the Effect of Different Feeding Systems: A Review. J. Agric. Food Chem. 2013, 61, 3561–3579. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R.; Sameen, A. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Nedvidek, W.; Ledl, F.; Fischer, P. Detection of 5-hydroxymethyl-2-methyl-3(2H)- furanone and of α-dicarbonyl compounds in reaction mixtures of hexoses and pentoses with different amines. Zeitschrift F R Lebensmittel-Untersuchung Und-Forschung 1992, 194, 222–228. [Google Scholar] [CrossRef]
- Cho, I.H.; Lee, S.; Jun, H.-R.; Roh, H.-J.; Kim, Y.-S. Comparison of volatile Maillard reaction products from tagatose and other reducing sugars with amino acids. Food Sci. Biotechnol. 2010, 19, 431–438. [Google Scholar] [CrossRef]
- Verzera, A.; Condurso, C.; Ziino, M.; Romeo, V.; Todaro, M.; Conte, F.; Dima, G. Free fatty acids and other volatile compounds for the characterisation of “Vastedda della valle del Belìce” cheese Acidos grasos libres y otros constituyentes volátiles para la caracterización de queso “Vastedda della vella del Belìce”. CyTA—J. Food 2010, 8, 237–243. [Google Scholar] [CrossRef]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. An overview. In Volatile Sulfur Compounds in Food; Qian, M.C., Fan, X., Mahattanatawee, K., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Chaper 1. [Google Scholar]
- Guentert, M.; Bruening, J.; Emberger, R.; Koepsel, M.; Kuhn, W.; Thielmann, T.; Werkhoff, P. Identification and formation of some selected sulfur-containing flavor compounds in various meat model systems. J. Agric. Food Chem. 1990, 38, 2027–2041. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [CrossRef]
- Mabuchi, R.; Ishimaru, A.; Tanaka, M.; Kawaguchi, O.; Tanimoto, S. Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue. Metabolites 2018, 9, 1. [Google Scholar] [CrossRef]
- Aepli, M.; Finger, R. Determinants of sheep and goat meat consumption in Switzerland. Agric. Food Econ. 2013, 1, 11. [Google Scholar] [CrossRef][Green Version]
- Carlucci, A.; Girolami, A.; Napolitano, F.; Monteleone, E. Sensory evaluation of young goat meat. Meat Sci. 1998, 50, 131–136. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cao, R.; Lü, X.; Zhao, S.; Fekete, A.; Huang, Q.; Schmitt-Kopplin, P.; Wang, Y.; Xu, Z.; et al. Serum 27-nor-5β-Cholestane-3,7,12,24,25 Pentol Glucuronide Discovered by Metabolomics as Potential Diagnostic Biomarker for Epithelium Ovarian Cancer. J. Proteome Res. 2011, 10, 2625–2632. [Google Scholar] [CrossRef]
- Chambers, M.C.; MacLean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Palla, P.; Armano, G. RFmarkerDetector: Multivariate Analysis of Metabolomics Data using Random Forests; Version 1.0.1; R Package, 2016; Available online: https://cran.r project.org/bin/windows/contrib/3.6/RFmarkerDetector_1.0.1.zip (accessed on 5 September 2019).
- Hajjes, H.A.; de Sain-van der Velden, M.G.M.; Prinsen, H.C.M.T.; Willems, A.P.; van der Ham, M.; Gerrits, J.; Jans, J.J.M. Aspartylglycosamine is a biomarker for NGLY1-CDDG, a congenital disorder of deglycosylation. Mol. Genet. Metab. 2019, 127, 368–372. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal component analysis for special types of data. In Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 338–372. [Google Scholar]
- Pérez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Homles, E.; Trygg, J. OPLS discriminant anaylsis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Fasae, O.A.; Adu, I.F.; Aina, A.B.J.; Dipeolu, M.A. Growth performance, carcass characteristics and meat sensory evaluation of West African dwarf sheep fed varing levels of maize and cassava hay. Trop. Anim. Health Prod. 2011, 43, 503–510. [Google Scholar] [CrossRef]
- Miró, M. Cucurbitacins and their pharmacological effects. Phytother. Res. 1995, 9, 159–168. [Google Scholar] [CrossRef]
- Mahler, S.V.; Smith, K.S.; Berridge, K.C. Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances “Liking” of a Sweet Reward. Neuropsychopharmacology 2007, 32, 2267–2278. [Google Scholar] [CrossRef]
- Pacioni, G.; Rapino, C.; Zarivi, O.; Falconi, A.; Leonardi, M.; Battista, N.; Colafarina, S.; Sergi, M.; Bonfigli, A.; Miranda, M.; et al. Truffles contain endocannabinoid metabolic enzymes and anandamide. Phytochemistry 2015, 110, 104–110. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef]
- Resconi, V.; Escudero, A.; Campo, M. The Development of Aromas in Ruminant Meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef]
- Melton, S.L. Effects of feeds on flavor of red meat: A review. J. Anim. Sci. 1990, 68, 4421–4435. [Google Scholar] [CrossRef]
- Elmore, J.; Warren, H.; Mottram, D.; Scollan, N.; Enser, M.; Richardson, R.; Wood, J. A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed diets based on silage or concentrates. Meat Sci. 2004, 68, 27–33. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Joo, S.-T. Fatty Acid Profiles, Meat Quality, and Sensory Palatability of Grain-fed and Grass-fed Beef from Hanwoo, American, and Australian Crossbred Cattle. Food Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef]
- Wood, J.; Richardson, R.; Nute, G.; Fisher, A.; Campo, M.M.; Kasapidou, E.; Sheard, P.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Juarez, M.; Polvillo, O.; Gómez, M.; Alcalde, M.; Romero, F.; Valera, M.; Alcalde, M. Breed effect on carcass and meat quality of foals slaughtered at 24months of age. Meat Sci. 2009, 83, 224–228. [Google Scholar] [CrossRef]
- Xie, X.; Meng, Q.; Cui, Z.; Ren, L. Effect of Cattle Breed on Meat Quality, Muscle Fiber Characteristics, Lipid Oxidation and Fatty Acids in China. Asian-Australas. J. Anim. Sci. 2012, 25, 824–831. [Google Scholar] [CrossRef]
- Dance, L.; Matthews, K.; Doran, O. Effect of breed on fatty acid composition and stearoyl-CoA desaturase protein expression in the Semimembranosus muscle and subcutaneous adipose tissue of cattle. Livest. Sci. 2009, 125, 291–297. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S. The role of lipid in the flavour of cooked beef. Dev. Food Sci. 2006, 43, 375–378. [Google Scholar]
- Park, Y.W.; Jeong, M.K.; Park, C.; Lee, J. Distribution of Triacylglycerols and Fatty Acids in Soybean Oil with Thermal Oxidation and Methylene Blue Photosensitization. J. Am. Oil Chem. Soc. 2010, 88, 373–380. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Role of bioactive carbonyl compounds on the conversion of asparagine into acrylamide during heating. Eur. Food Res. Technol. 2012, 235, 1093–1099. [Google Scholar] [CrossRef]
- Kerler, J.; Grosch, W. Odorants Contributing to Warmed-Over Flavor (WOF) of Refrigerated Cooked Beef. J. Food Sci. 1996, 61, 1271–1275. [Google Scholar] [CrossRef]
- Møller, J.K.; Hinrichsen, L.L.; Andersen, H.J. Formation of amino acid (l-leucine, l-phenylalanine) derived volatile flavour compounds by Moraxella phenylpyruvica and Staphylococcus xylosus in cured meat model systems. Int. J. Food Microbiol. 1998, 42, 101–117. [Google Scholar] [CrossRef]
- Andrés, A.; Cava, R.; Ruiz, J. Monitoring volatile compounds during dry-cured ham ripening by solid-phase microextraction coupled to a new direct-extraction device. J. Chromatogr. A 2002, 963, 83–88. [Google Scholar] [CrossRef]
- McAdam, K.; Enos, T.; Goss, C.; Kimpton, H.; Faizi, A.; Edwards, S.; Rodu, B. Analysis of coumarin and angelica lactones in smokeless tobacco products. Chem. Cent. J. 2018, 12, 142–157. [Google Scholar] [CrossRef]
- Gopu, V.; Shetty, P.H. Regulation of acylated homoserine lactones (AHLs) in beef by spice marination. J. Food Sci. Technol. 2016, 53, 2686–2694. [Google Scholar] [CrossRef]
- Inagaki, S.; Amano, Y.; Kumazawa, K. Identification and Characterization of Volatile Components Causing the Characteristic Flavor of Wagyu Beef (Japanese Black Cattle). J. Agric. Food Chem. 2017, 65, 8691–8695. [Google Scholar] [CrossRef]
- Specht, K.; Baltes, W. Identification of Volatile Flavor Compounds with High Aroma Values from Shallow-Fried Beef. J. Agric. Food Chem. 1994, 42, 2246–2253. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Busnelli, G. Alkaloids and the Bitter Taste. In Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Fattorusso, E., Taglialatela Scafati, O., Eds.; Wiley VCH: Weinheim, Germany, 2007; pp. 53–70. [Google Scholar]
- Górecka, D.; Lampart-Szczapa, E.; Janitz, W.; Sokolowska, B. Composition of fractional and functional properties of dietary fiber of lupines (L. luteus andL. albus). Nahrung/Food 2000, 44, 229–232. [Google Scholar] [CrossRef]
- Zdunczyk, Z.; Gruzauskas, R.; Juskiewicz, J.; Semaskaite, A.; Jankowski, J.; Godycka-Klos, I.; Alencikiene, G. Growth performance, gastrointestinal tract responses, and meat characteristics of broiler chickens fed a diet containing the natural alkaloid sanguinarine from Macleaya cordata. J. Appl. Poult. Res. 2010, 19, 393–400. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Contreras-Castillo, C.J.; Selani, M.M.; Nadai, A.P.; Mourão, G.B.; Gallo, C.R. Winery grape-residue extract: Effects on quality and sensory attributes of cooked chicken meat. Food Sci. Biotechnol. 2011, 20, 1257–1264. [Google Scholar] [CrossRef]
- Jinap, S.; Iqbal, S.Z.; Talib, N.H.; Hasnol, N.D.S. Heterocyclic aromatic amines in deep fried lamb meat: The influence of spices marination and sensory quality. J. Food Sci. Technol. 2016, 53, 1411–1417. [Google Scholar]
- Tilahun, A.; Jambare, L.; Teshale, A.; Getachew, A. Review on chemical and drug residue in meat. World J. Agric. Sci. 2016, 12, 196–204. [Google Scholar]
- Schiffman, S.S.; Nagle, H.T. Effect of Environmental Pollutants on Taste and Smell. Otolaryngol. Neck Surg. 1992, 106, 693–700. [Google Scholar] [CrossRef]
- Dacasto, M.; Eeckhoutte, C.; Capolongoa, F.; Dupuy, J.; Carletti, M.; Nebbia, C.; Alvinerie, M.; Galtier, P. Effect of breed and gender on bovine liver cytochrome P450 3A (CYP3A) expression and inter-species comparison with other domestic ruminants. Veter Res. 2005, 36, 179–190. [Google Scholar] [CrossRef]
- Ammoun, I.; Encinas, T.; Veiga-Lopez, A.; Ros, J.; Contreras, I.; Gonzalez-Añover, P.; Cocero, M.; McNeilly, A.; Gonzalez-Bulnes, A. Effects of breed on kinetics of ovine FSH and ovarian response in superovulated sheep. Theriogenology 2006, 66, 896–905. [Google Scholar] [CrossRef]
| Constituent | % | Nutritional Value | - |
|---|---|---|---|
| Peanut hay | 58.26 | Dry matter/(kg·d−1) | 0.60 |
| Corn | 29.60 | Digestive energy/(MJ·d−1) | 6.29 |
| Bran | 4.21 | Protein/(g·d−1) | 64 |
| Soybean meal | 2.06 | Calcium/(g·d−1) | 4.6 |
| Cottonseed meal | 2.03 | Phosphorus/(g·d−1) | 3.0 |
| Calcium bicarbonate | 1.09 | Sodium/(g·d−1) | 3.1 |
| Sodium chloride | 0.55 | - | - |
| Sodium bicarbonate | 0.37 | - | - |
| 5% Premix (trace elements, multivitamins, monensin, ion balancer, growth factor) | 1.83 | - | - |
| - | BE | BE ± SEM | JNQ | JNQ ± SEM | LBB | LBB ± SEM |
|---|---|---|---|---|---|---|
| Odor of mutton | 6.5 | 0.21 | 4 | 0.17 | 4.8 | 0.11 |
| Fresh fragrance | 7.5 | 0.25 | 7.9 | 0.22 | 6.3 | 0.13 |
| Sweet taste | 5.8 | 0.14 | 4.2 | 0.16 | 3.7 | 0.08 |
| Bitterness | 1.2 | 0.03 | 0.5 | 0.01 | 1.5 | 0.05 |
| Compound | Taste | Classification | BJ P Value | JL P Value | BL P Value |
|---|---|---|---|---|---|
| Pyranodelphinin A | Fruit aroma | antioxidants | 0.02 * | 0.01 ** | 0.33 |
| LysoPC(17:0) | Help to form an aromatic odor and silky taste during chewing. | lipids | 0.07 | 0.02 * | 0.03 * |
| Roxatidine acetate | Bitter | other 2 | 0.09 | 0.97 | 0.03 * |
| Gladiatoside C1 | Bitter | antioxidants | 0.23 | 0.49 | 0.05 * |
| Stepronin | An unpleasant sense of anaesthesia | other 1 | 0.04 * | 0.03 * | 0.96 |
| Cellulose, microcrystalline | Unsavory | other 1 | 0.05 * | 0.42 | 0.30 |
| 18-Carboxy-dinor-LTE4 | No taste, related to inflammation | lipids | 0.00 ** | 0.13 | 0.76 |
| 22-Deoxoisocucurbitacin D | Unable to retrieve odor-related literature | ketone | 0.00 ** | 0.05 * | 0.69 |
| Sertindole | Extremely mild indole odor is a jasmine flower odor, mild cumin odor, moderate foot odor, and severe fecal odor. | other 1 | 0.01 ** | 0.08 | 0.93 |
| Methotrexate | Sweet | alkaloids | 0.00 ** | 0.11 | 0.83 |
| Ceanothine E | Bitter | alkaloids | 0.17 | 0.47 | 0.02 * |
| 8-Hydroxypinoresinol 4-glucoside | Sweet | other 2 | 0.02 * | 0.01 ** | 0.16 |
| Feruloyl-beta-sitosterol | Aroma | other 1 | 0.12 | 0.04 * | 0.61 |
| 1-Phenyl-1,3-nonadecanedione | Meat aroma | ketone | 0.35 | 0.04 * | 0.14 |
| S-(PGA2)-glutathione | Unsavory | lipids | 0.03 * | 0.01 ** | 0.06 |
| 2’’,4’’,6’’-Triacetylglycitin | Umami | lactone | 0.02 * | 0.09 | 0.84 |
| (E)-1-Cinnamoylpyrrolidine | Ammonia flavor | alkaloids | 0.54 | 0.20 | 0.04 * |
| Pranlukast | Bitter | other 2 | 0.00 ** | 0.12 | 0.83 |
| Vignatic acid A | A faint fishy smell | other 2 | 0.26 | 0.33 | 0.02 * |
| (3S,5R,6S,7E,9x)-7-Megastigmene-3,6,9-triol 9-glucoside | Bitter | other 2 | 0.00 ** | 0.26 | 0.57 |
| Trichloroethanol glucuronide | Related to animal odor excretion, urine composition | other 2 | 0.05 * | 0.40 | 0.35 |
| Garcimangosone D | Unable to retrieve odor-related literature | antioxidants | 0.04 * | 0.05 * | 0.75 |
| Histidinyl-Glycine | Bitter-Sweet | other 2 | 0.00 ** | 0.18 | 0.68 |
| Aegle marmelos Alkaloid C | Supposed to be bitter | alkaloids | 0.07 | 0.94 | 0.02 * |
| Alanyl-Serine | Sweet | other 2 | 0.03 * | 0.28 | 0.58 |
| 4-Hydroxyphenytoin glucuronide | Unable to retrieve odor-related literature | other 2 | 0.02 * | 0.04 * | 0.41 |
| Pelargonidin 3-sophoroside | Bitter | antioxidants | 0.02 * | 0.01 ** | 0.09 |
| Aloinoside B | Aloe Vera Fragrance | antioxidants | 0.02 * | 0.01 ** | 0.32 |
| 3-Hydroxy-9Z-octadecenoylcarnitine | Fishy but aromatic | lipids | 0.04 * | 0.38 | 0.07 |
| Homodolichosterone | Unable to retrieve odor-related literature | ketone | 0.32 | 0.03 * | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Sun, B.; Hu, P.; Zhou, M.; Sun, S.; Du, P.; Ru, Y.; Suvorov, A.; Li, Y.; Liu, Y.; et al. Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat. Metabolites 2019, 9, 176. https://doi.org/10.3390/metabo9090176
Wang W, Sun B, Hu P, Zhou M, Sun S, Du P, Ru Y, Suvorov A, Li Y, Liu Y, et al. Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat. Metabolites. 2019; 9(9):176. https://doi.org/10.3390/metabo9090176
Chicago/Turabian StyleWang, Weiting, Bei Sun, Peng Hu, Meng Zhou, Sujun Sun, Pengfei Du, Yi Ru, Alexander Suvorov, Yongsheng Li, Yaobo Liu, and et al. 2019. "Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat" Metabolites 9, no. 9: 176. https://doi.org/10.3390/metabo9090176
APA StyleWang, W., Sun, B., Hu, P., Zhou, M., Sun, S., Du, P., Ru, Y., Suvorov, A., Li, Y., Liu, Y., & Wang, S. (2019). Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat. Metabolites, 9(9), 176. https://doi.org/10.3390/metabo9090176




