NMR Based Metabolomic Analysis of Health Promoting Phytochemicals in Lentils
Abstract
1. Introduction
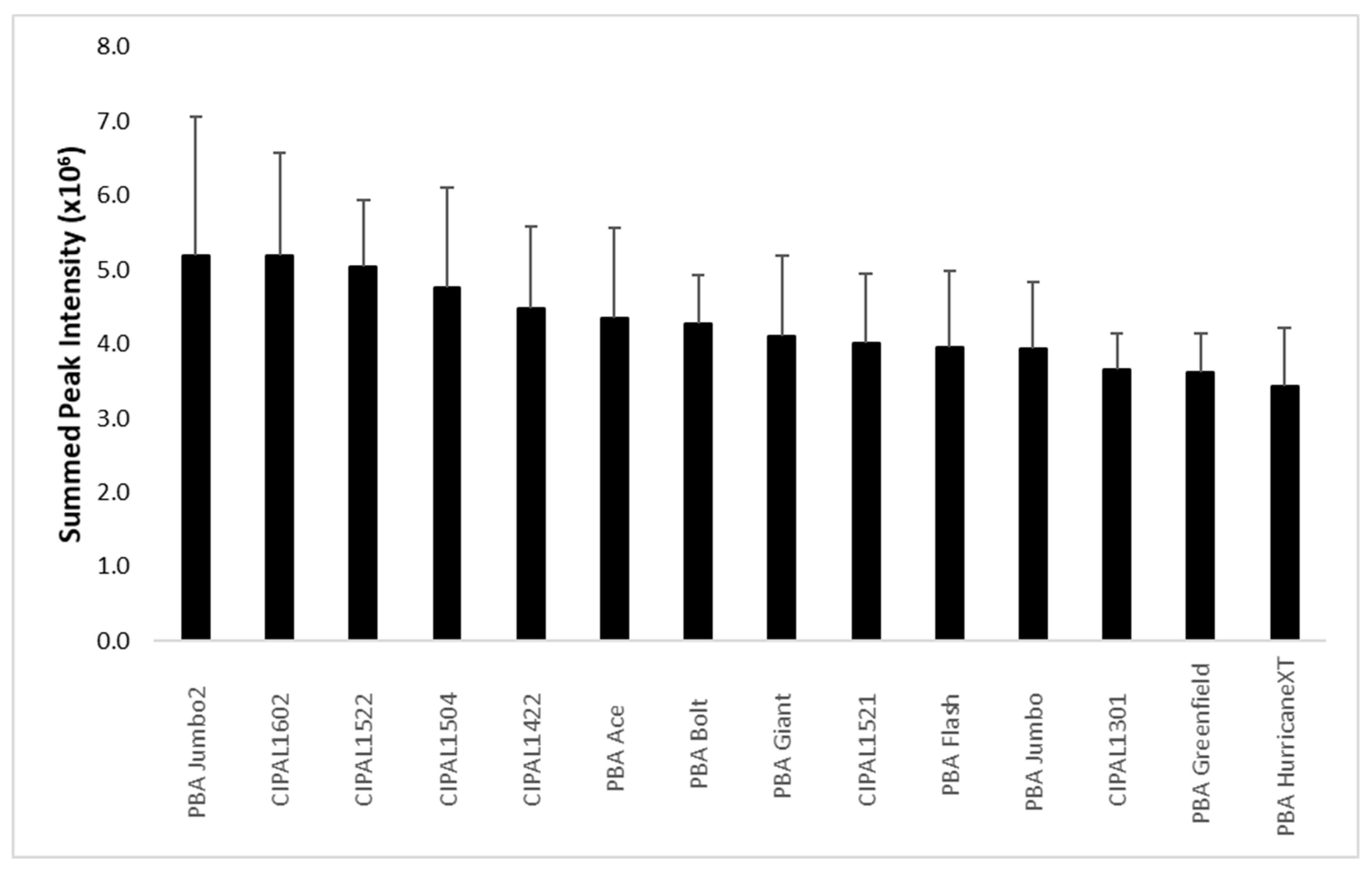
2. Results
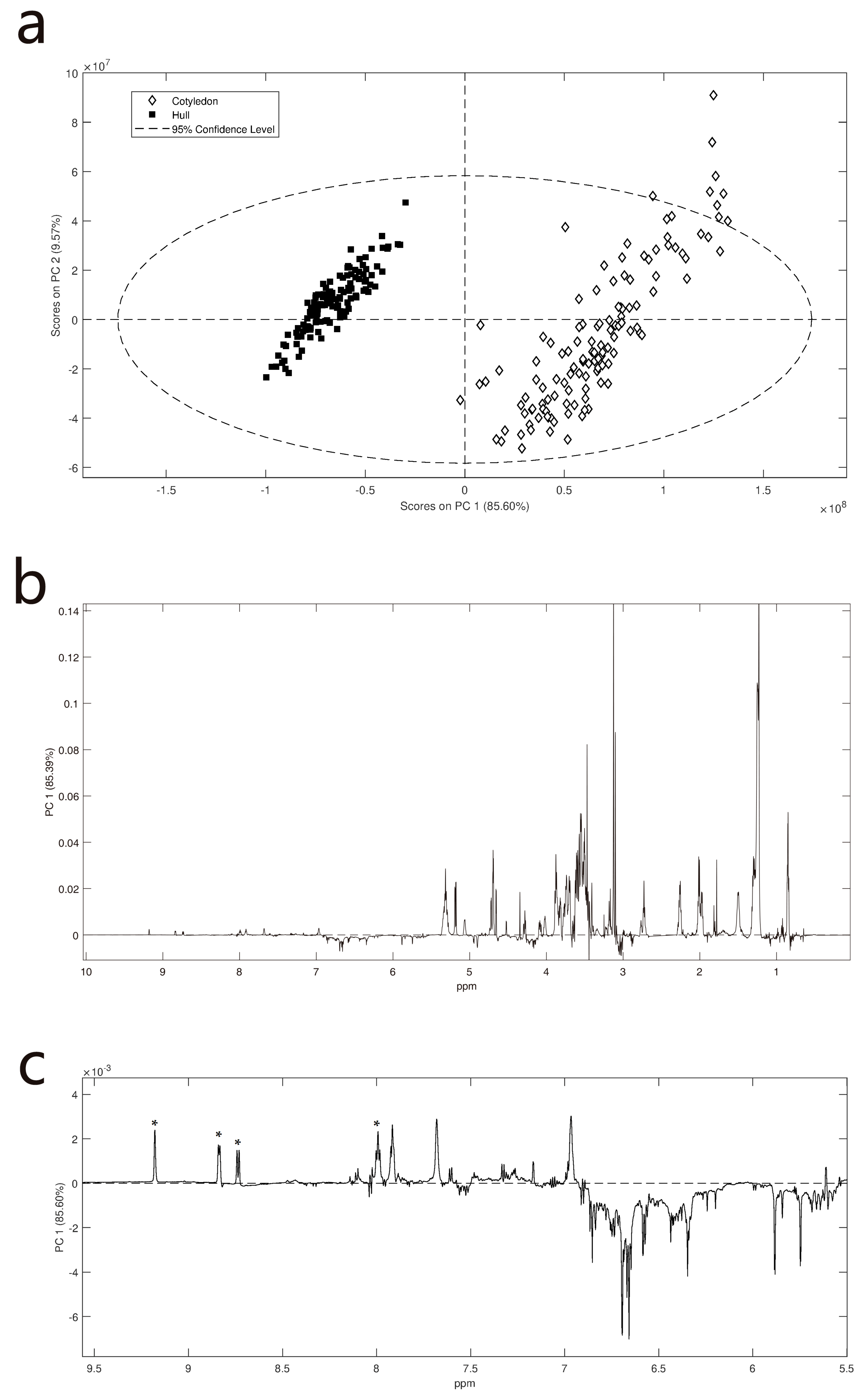
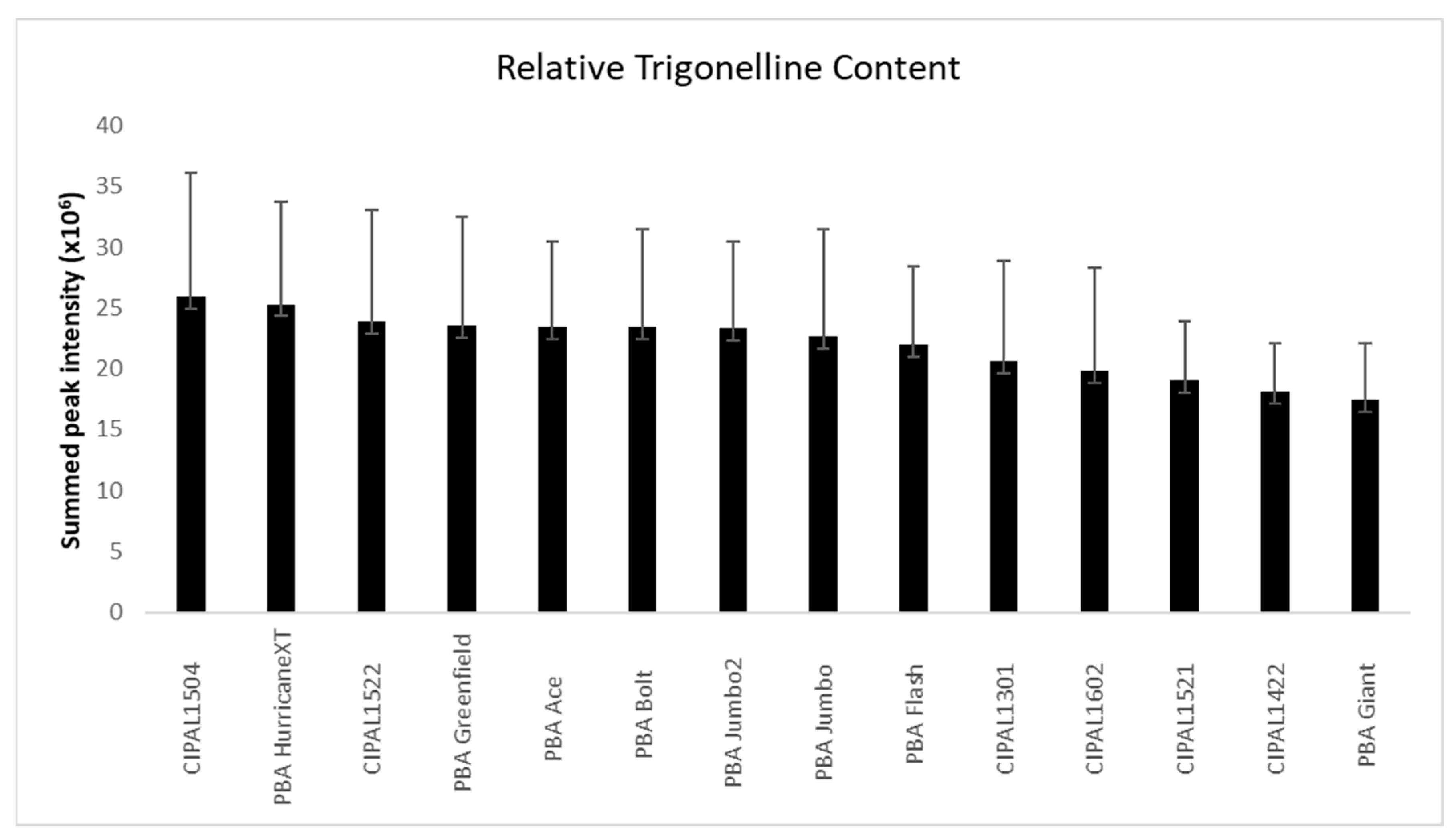
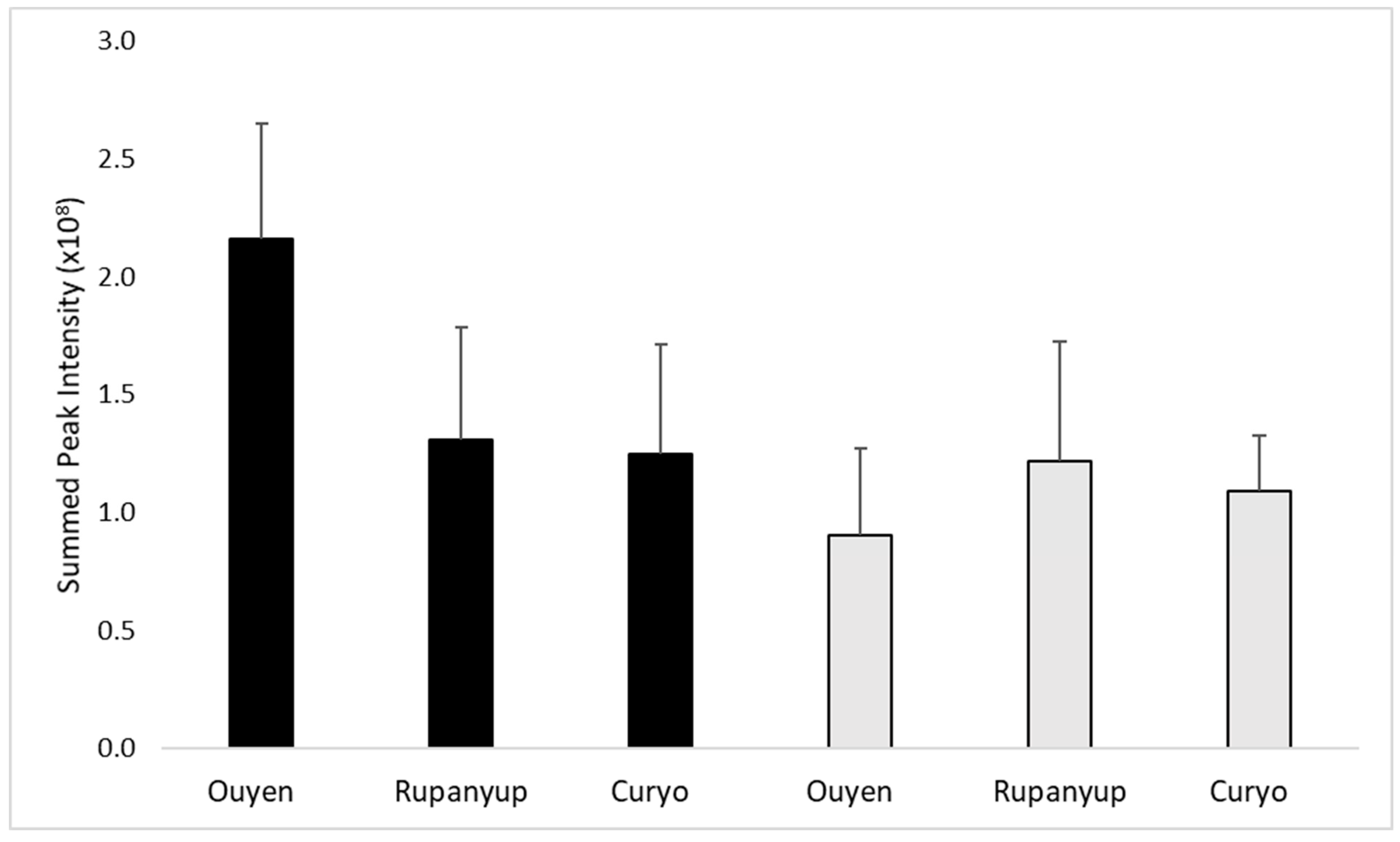
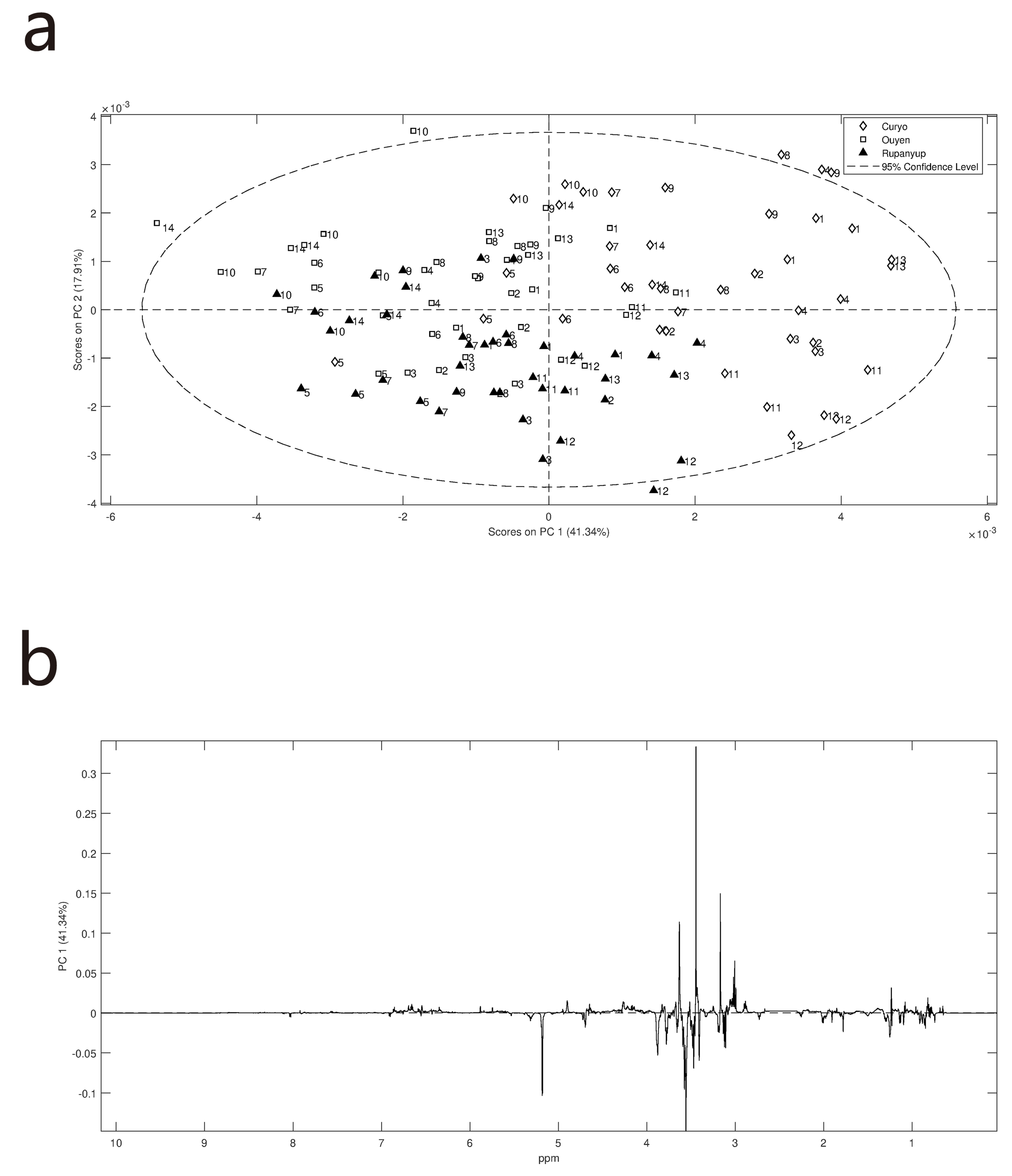
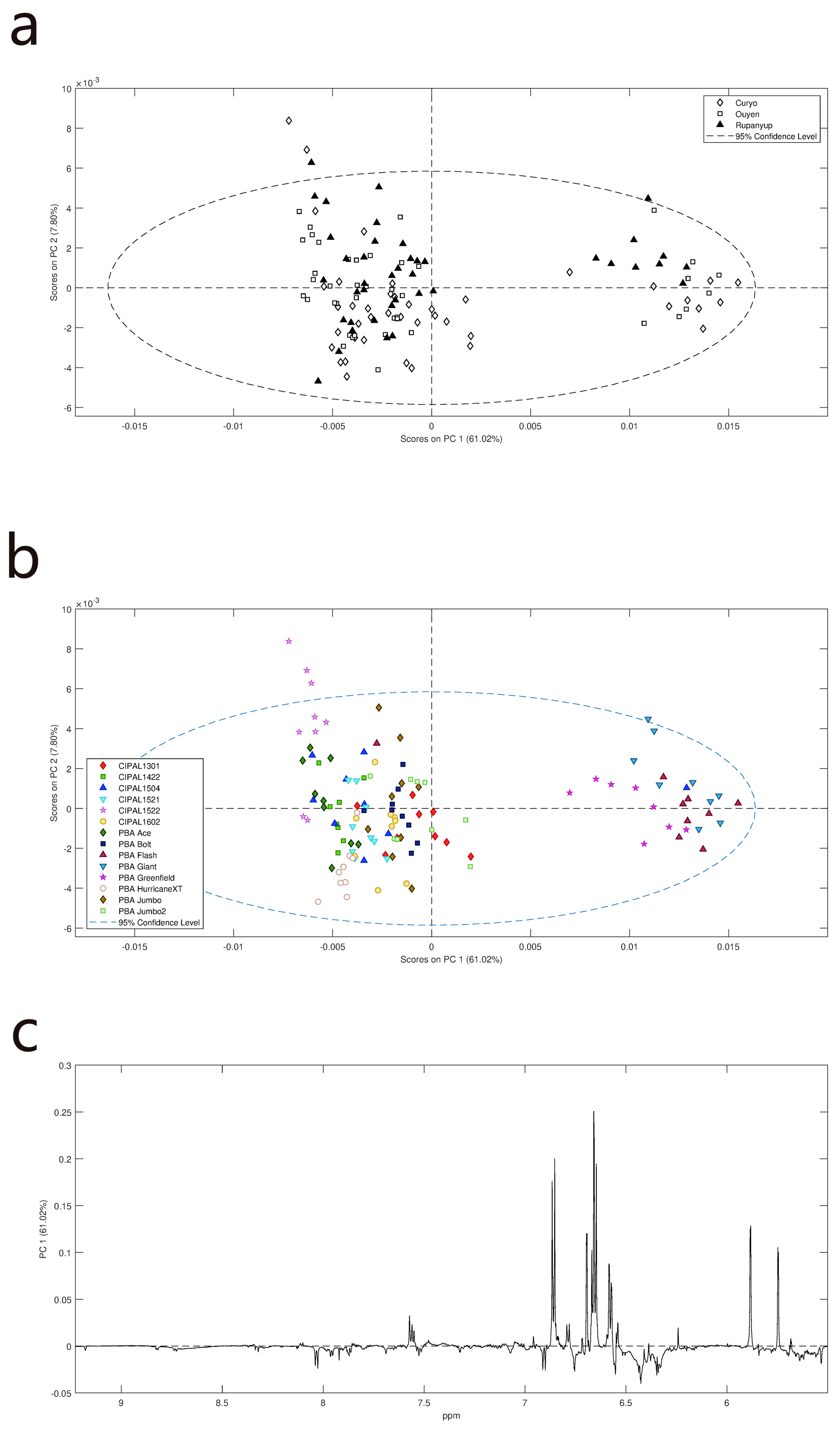
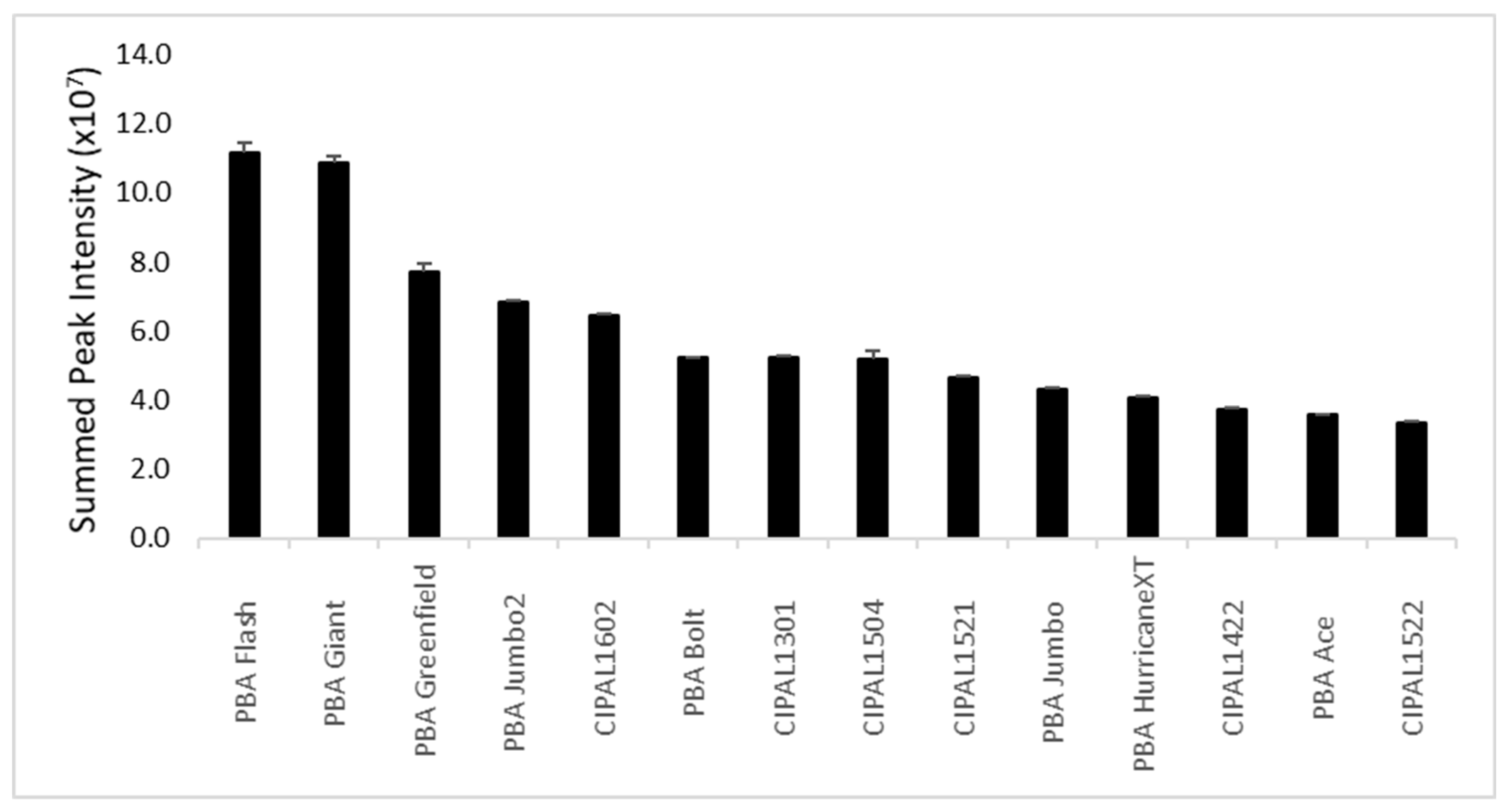
2.1. Lentil Cotyledon
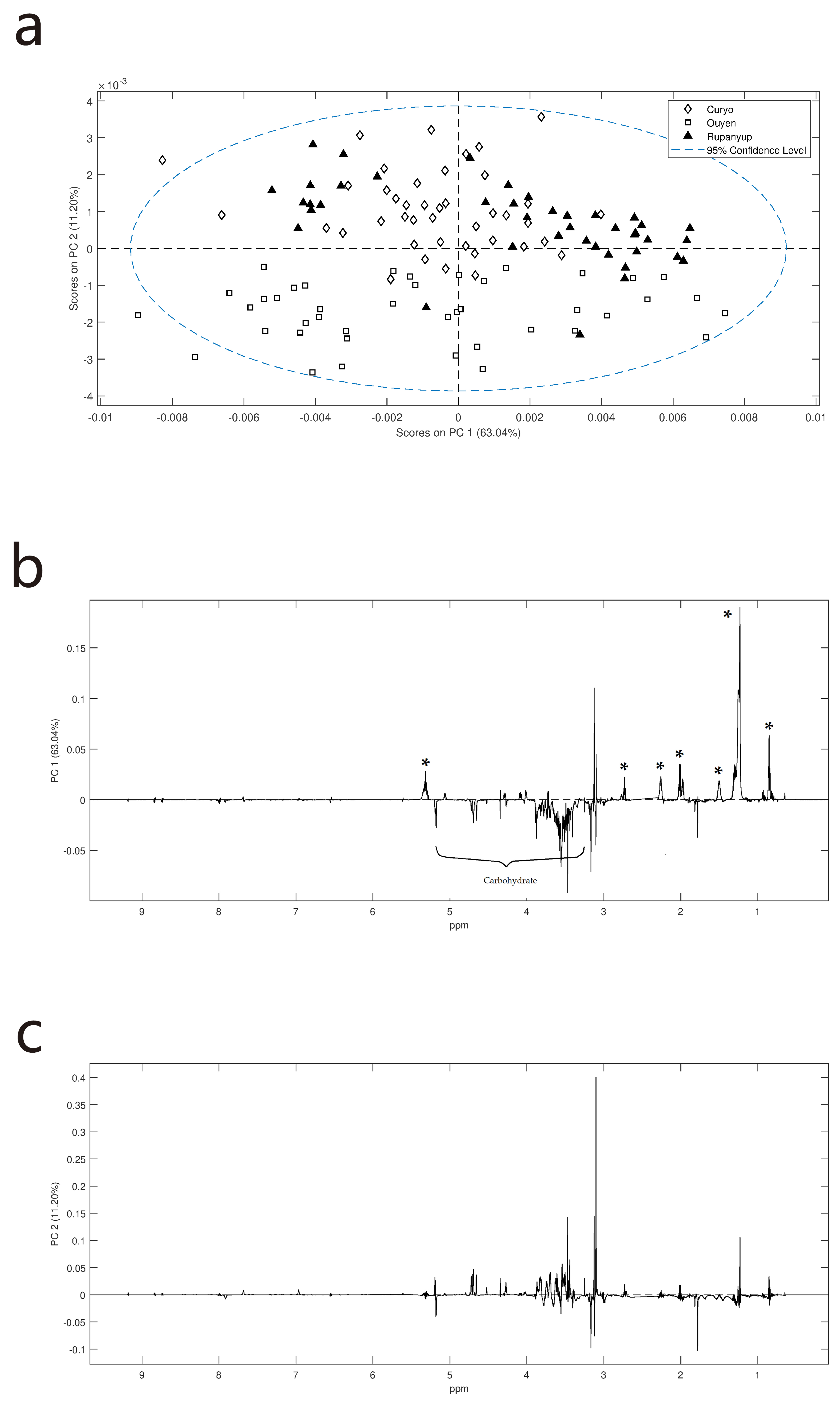
2.2. Lentil Hull
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Sample Preparation for NMR
4.4. NMR Spectroscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Williams, R.B.; Gutekunst, W.R.; Joyner, P.M.; Duan, W.; Li, Q.; Ross, C.A.; Williams, T.D.; Cichewicz, R.H. Bioactivity profiling with parallel mass Spectrometry reveals an assemblage of green tea metabolites affording protection against human huntingtin and alpha-synuclein toxicity. J. Agric. Food Chem. 2007, 55, 9450–9456. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Cherednichenko, G.; Ward, C.W.; Padilla, I.T.; Cabrales, E.; Lopez, J.R.; Eltit, J.M.; Allen, P.D.; Pessah, I.N. Green tea catechins are potent sensitizers of ryanodine receptor type 1 (RyR1). Biochem. Pharmacol. 2010, 80, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Dickerhof, N.; Magon, N.J.; Tyndall, J.D.A.; Kettle, A.J.; Hampton, M.B. Potent inhibition of macrophage migration inhibitory factor (MIF) by myeloperoxidase-dependent oxidation of epicatechins. Biochem. J. 2014, 462, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, M.; Wei, Y.; Wang, C.; Shen, L. A review on the structure-activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42. [Google Scholar] [CrossRef]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on alpha-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Wood, J.; Wang, F.; Zhou, Z.; Blanchard, C.; Strappe, P. Extracts of common pulses demonstrate potent in vitro anti-adipogenic properties. Int. J. Food Sci. Technol. 2016, 51, 1327–1337. [Google Scholar] [CrossRef]
- Getek, M.; Czech, N.; Muc-Wierzgon, M.; Grochowska-Niedworok, E.; Kokot, T.; Nowakowska-Zajdel, E. The Active Role of Leguminous Plant Components in Type 2 Diabetes. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef]
- Anderson, J.W.; Major, A.W. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. Br. J. Nutr. 2002, 88, S263–S271. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Tahir, M.; Vandenberg, A.; Chibbar, R.N. Influence of environment on seed soluble carbohydrates in selected lentil cultivars. J. Food Compost. Anal. 2011, 24, 596–602. [Google Scholar] [CrossRef]
- Peterbauer, T.; Lahuta, L.B.; Blochl, A.; Mucha, J.; Jones, D.A.; Hedley, C.L.; Gorecki, R.J.; Richter, A. Analysis of the raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol. 2001, 127, 1764–1772. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, B.; Chung, J.O.; Hwang, J.A.; Lee, S.J.; Lee, C.H.; Hong, Y.S. Geographical and Climatic Dependencies of Green Tea (Camellia sinensis) Metabolites: A 1H NMR-Based Metabolomics Study. J. Agric. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef]
- Huo, Y.; Kamal, G.M.; Wang, J.; Liu, H.; Zhang, G.; Hu, Z.; Anwar, F.; Du, H. 1H NMR-based metabolomics for discrimination of rice from different geographical origins of China. J. Cereal Sci. 2017, 76, 243–252. [Google Scholar] [CrossRef]
- Madrid-Garnbin, F.; Brunius, C.; Garcia-Aloy, M.; Estruel-Amades, S.; Landberg, R.; Andres-Lacueva, C. Untargeted H-1 NMR-Based Metabolomics Analysis of Urine and Serum Profiles after Consumption of Lentils, Chickpeas, and Beans: An Extended Meal Study To Discover Dietary Biomarkers of Pulses. J. Agric. Food Chem. 2018, 66, 6997–7005. [Google Scholar] [CrossRef]
- Tsopmo, A.; Muir, A.D. Chemical Profiling of Lentil (Lens culinaris Medik.) Cultivars and Isolation of Compounds. J. Agric. Food Chem. 2010, 58, 8715–8721. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddiquee, K.H.M.; Nayyar, H. Impact of heat stress during seed filling on seed quality and seed yield in lentil (Lens culinaris Medikus) genotypes. J. Sci. Food Agric. 2018, 98, 5134–5141. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- de Zwart, F.J.; Slow, S.; Payne, R.J.; Lever, M.; George, P.M.; Gerrard, J.A.; Chambers, S.T. Glycine betaine and glycine betaine analogues in common foods. Food Chem. 2003, 83, 197–204. [Google Scholar] [CrossRef]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Amino acids in seeds and seedlings of the genus Lens. Phytochemistry 2001, 58, 281–289. [Google Scholar] [CrossRef]
- Zameer, S.; Najmi, A.K.; Vohora, D.; Akhtar, M. A review on therapeutic potentials of Trigonella foenum graecum (fenugreek) and its chemical constituents in neurological disorders: Complementary roles to its hypolipidemic, hypoglycemic, and antioxidant potential. Nutr. Neurosci. 2018, 21, 539–545. [Google Scholar] [CrossRef]
- Christodoulou, M.I.; Tchoumtchoua, J.; Skaltsounis, A.L.; Scorilas, A.; Halabalaki, M. Natural alkaloids intervening the insulin pathway: New hopes for anti-diabetic agents? Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Castaneda, R.; Rodriguez, I.; Nam, Y.H.; Hong, B.N.; Kang, T.H. Trigonelline promotes auditory function through nerve growth factor signaling on diabetic animal models. Phytomedicine 2017, 36, 128–136. [Google Scholar] [CrossRef]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018, 121, 283–296. [Google Scholar] [CrossRef]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline inhibits caspase 3 to protect beta cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef]
- Khalili, M.; Alavi, M.; Esmaeil-Jamaat, E.; Baluchnejadmojarad, T.; Roghani, M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int. Immunopharmacol. 2018, 61, 355–362. [Google Scholar] [CrossRef]
- Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Trigonelline protects hippocampus against intracerebral Abeta(1-40) as a model of Alzheimer’s disease in the rat: Insights into underlying mechanisms. Metab. Brain Dis. 2018. [Google Scholar] [CrossRef]
- Han, I.H.; Baik, B.K. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem. 2006, 83, 428–433. [Google Scholar] [CrossRef]
- Vdovin, A.D.; Kuliev, Z.A.; Abdullaev, N.D. 1H and 13C NMR spectroscopy in the study of flavan-3-ols, proanthocyanidins, and their derivatives. III. 13C nuclear magnetic resonance spectroscopy of flavan-3-ols and proanthocyanidins. Chem. Nat. Compd. 1998, 33, 417–437. [Google Scholar] [CrossRef]
- Okuyama, K.H.; Hiraga, Y.G.; Kurono, T.T. The Constituents of Osmunda spp. II. A New Flavonol Glycoside of Osmunda asiatica. Chem. Pharm. Bull. (Tokyo) 1978, 26, 3071–3074. [Google Scholar] [CrossRef]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Free amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumers. J. Agric. Food Chem. 2000, 48, 716–723. [Google Scholar] [CrossRef]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Nonprotein amino acids in edible lentil and garden pea seedlings. Amino Acids 2001, 20, 319–324. [Google Scholar] [CrossRef]
- Tramontano, W.A.; Jouve, D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar] [CrossRef]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant Activity and Phenolic Compositions of Lentil (Lens culinaris var. Morton) Extract and Its Fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Profiling the Phenolic Compounds of the Four Major Seed Coat Types and Their Relation to Color Genes in Lentil. J. Nat. Prod. 2017, 80, 1310–1317. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Mirali, M.; Ambrose, S.J.; Wood, S.A.; Vandenberg, A.; Purves, R.W. Development of a fast extraction method and optimization of liquid chromatography-mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J. Chrom. B 2014, 969, 149–161. [Google Scholar] [CrossRef]
- Hayden, H.L.; Rochfort, S.J.; Ezernieks, V.; Savin, K.W.; Mele, P.M. Metabolomics approaches for the discrimination of disease suppressive soils for Rhizoctonia solani AG8 in cereal crops using H-1 NMR and LC-MS. Sci. Total Environ. 2019, 651, 1627–1638. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochfort, S.; Vassiliadis, S.; Maharjan, P.; Brand, J.; Panozzo, J. NMR Based Metabolomic Analysis of Health Promoting Phytochemicals in Lentils. Metabolites 2019, 9, 168. https://doi.org/10.3390/metabo9080168
Rochfort S, Vassiliadis S, Maharjan P, Brand J, Panozzo J. NMR Based Metabolomic Analysis of Health Promoting Phytochemicals in Lentils. Metabolites. 2019; 9(8):168. https://doi.org/10.3390/metabo9080168
Chicago/Turabian StyleRochfort, Simone, Simone Vassiliadis, Pankaj Maharjan, Jason Brand, and Joe Panozzo. 2019. "NMR Based Metabolomic Analysis of Health Promoting Phytochemicals in Lentils" Metabolites 9, no. 8: 168. https://doi.org/10.3390/metabo9080168
APA StyleRochfort, S., Vassiliadis, S., Maharjan, P., Brand, J., & Panozzo, J. (2019). NMR Based Metabolomic Analysis of Health Promoting Phytochemicals in Lentils. Metabolites, 9(8), 168. https://doi.org/10.3390/metabo9080168