Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts
Abstract
1. Introduction
2. Results
2.1. Analytical Methods for Gingko Terpene Lactones and Flavonols
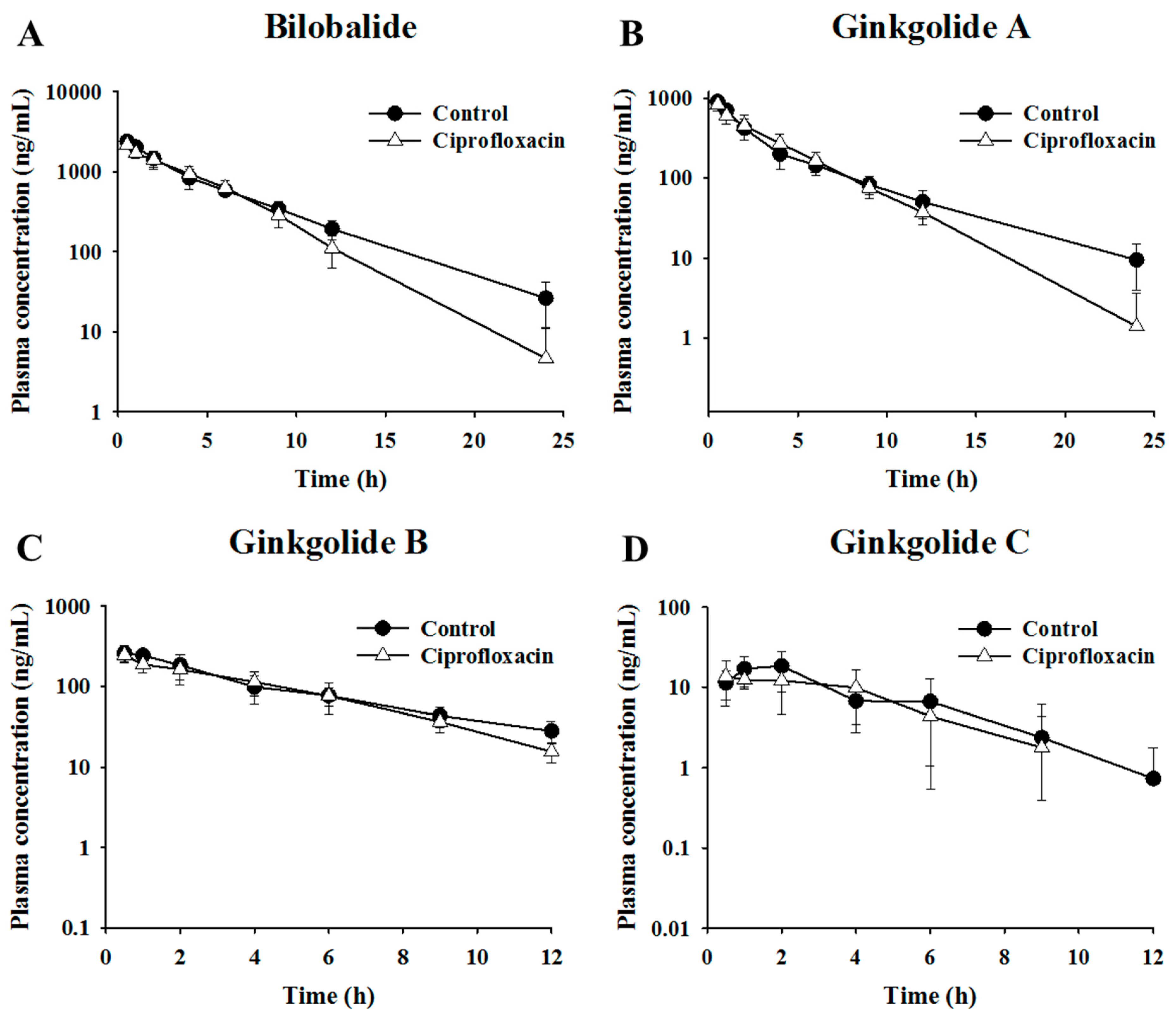
2.2. Pharmacokinetic Assessment of Terpene Lactones in Control and Antibacterial-Treated Mice
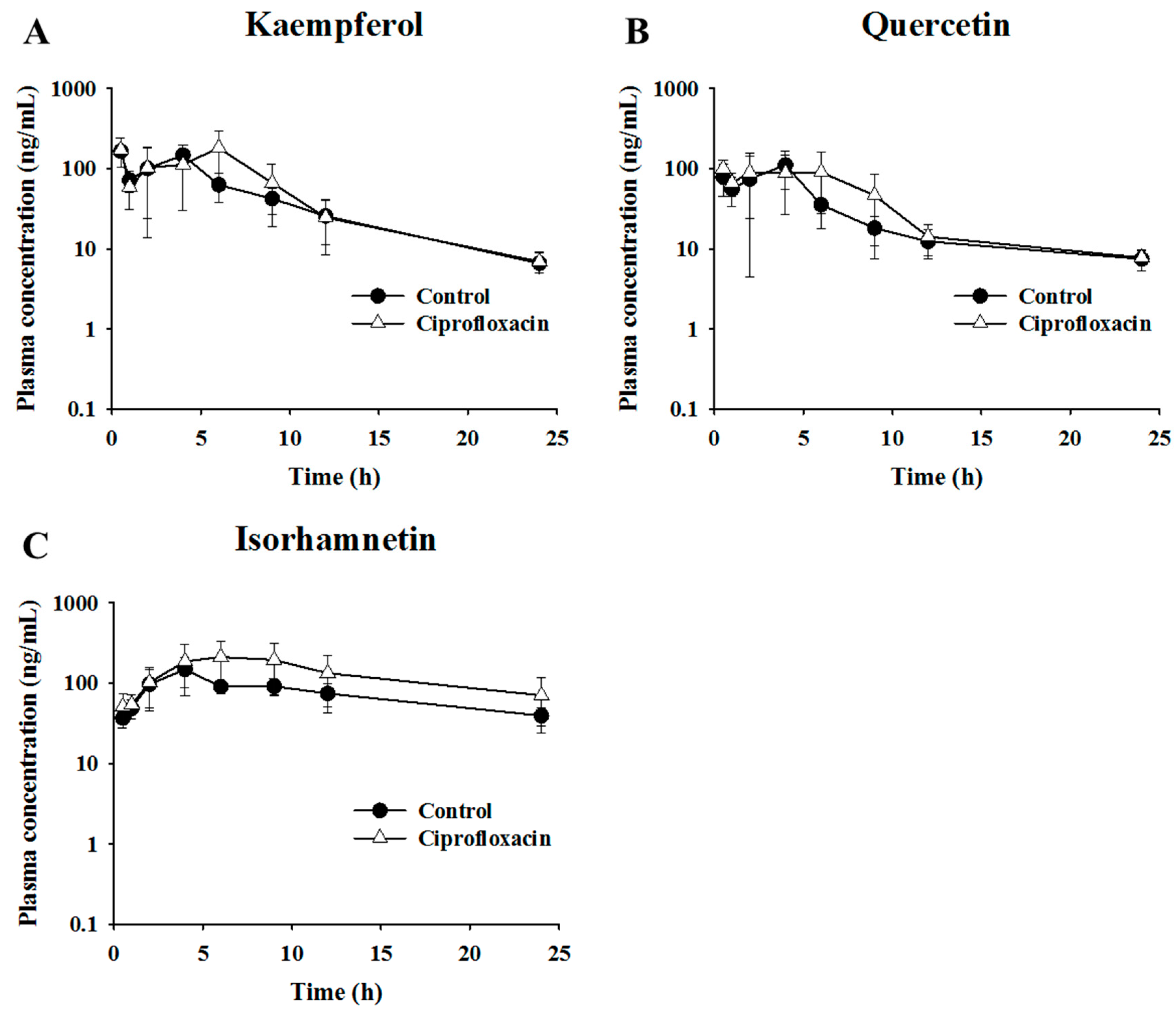
2.3. Pharmacokinetic Assessment of Flavonols in Control and Antibacterial-Treated Mice
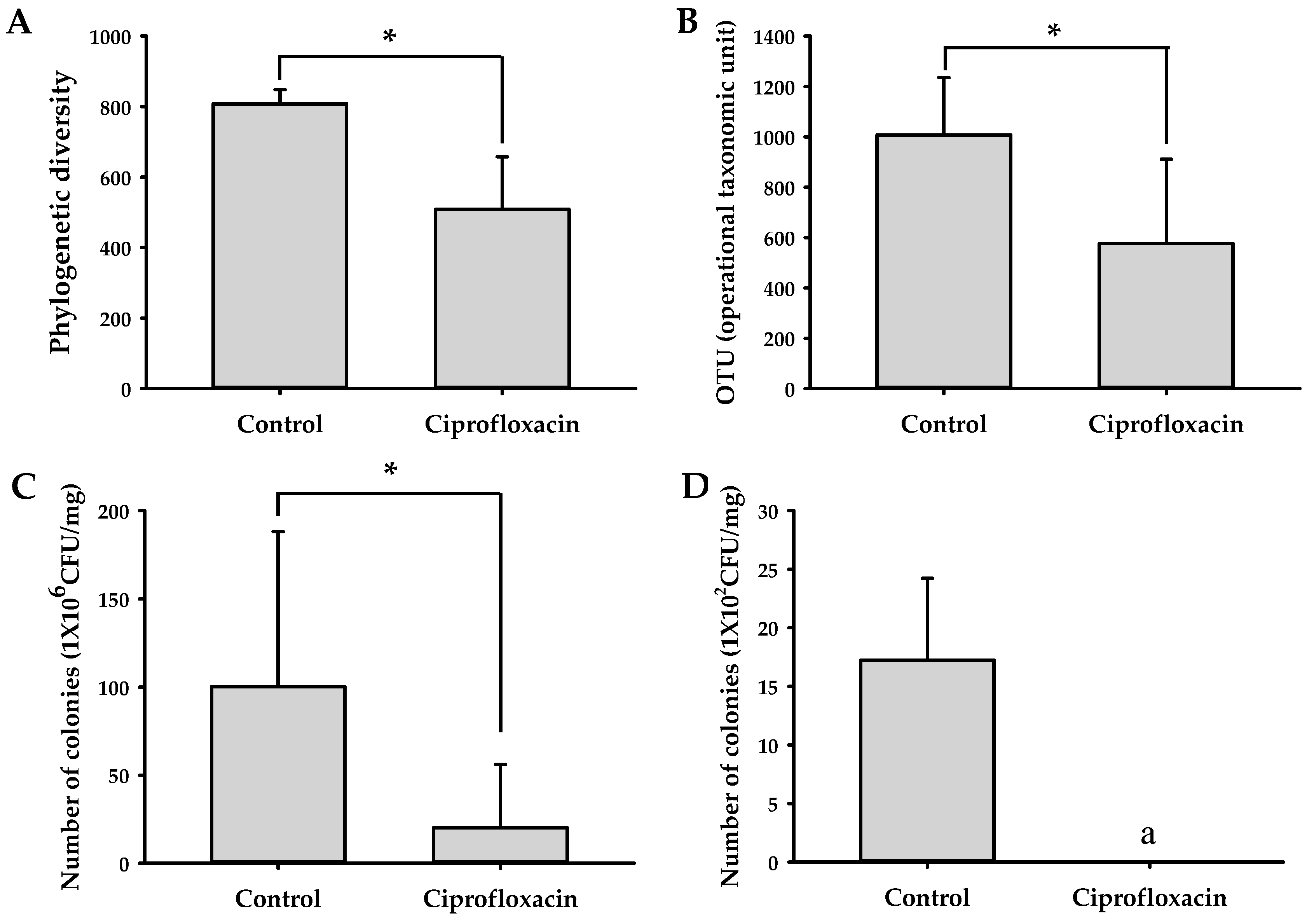
2.4. Effects of Ciprofloxacin on the Composition of Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Ginkgo Leaf Extract
4.3. Animal Experiments
4.4. Blood Sample Preparation
4.5. LC-MS/MS Conditions
4.6. Preparation of Stock Solutions and Calibration Standards
4.7. Culture and Pyrosequencing of Gut Microbiota
4.8. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hun Lee, J.; Shu, L.; Fuentes, F.; Su, Z.Y.; Tony Kong, A.N. Cancer chemoprevention by traditional chinese herbal medicine and dietary phytochemicals: Targeting nrf2-mediated oxidative stress/anti-inflammatory responses, epigenetics, and cancer stem cells. J. Tradit. Complement. Med. 2013, 3, 69–79. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.K.; Papavassiliou, A.G.; Stefanadis, C. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Wang, L.; Ravichandran, V.; Yin, Y.; Yin, J.; Zhang, Y. Natural Products from Mammalian Gut Microbiota. Trends Biotechnol. 2019, 37, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, X.; Tong, Q.; Zhou, X.; Chen, J.; Xiong, W.; Fang, J.; Wang, W.; Shi, C. Interactions of Dihydromyricetin, a Flavonoid from Vine Tea (Ampelopsis grossedentata) with Gut Microbiota. J. Food Sci. 2018, 83, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, W.; Yang, H.; Wang, D.; Ling, W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J. Food Sci. 2016, 81, H3026–H3034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and Black Tea Phenolics: Bioavailability, Transformation by Colonic Microbiota, and Modulation of Colonic Microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yu, Y.; Zheng, X.; Wu, J.; Li, Y.; Wu, X.; Du, Q.; Yin, X. Comparative investigation of in vitro biotransformation of 14 components in Ginkgo biloba extract in normal, diabetes and diabetic nephropathy rat intestinal bacteria matrix. J. Pharm. Biomed. Anal. 2014, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Li, Y.J.; Wu, X.W.; Wang, S.R.; Chen, K.; Zheng, X.X.; Du, Q.; Tang, D.Q. Simultaneous determination of baicalin, oroxylin A-7-O-glucuronide and wogonoside in rat plasma by UPLC-DAD and its application in pharmacokinetics of pure baicalin, Radix Scutellariae and Yinhuang granule. Biomed. Chromatogr. 2015, 29, 1819–1825. [Google Scholar] [CrossRef]
- Kang, M.J.; Ko, G.S.; Oh, D.G.; Kim, J.S.; Noh, K.; Kang, W.; Yoon, W.K.; Kim, H.C.; Jeong, H.G.; Jeong, T.C. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch. Pharm. Res. 2014, 37, 371–378. [Google Scholar] [CrossRef]
- Kim, K.A.; Yoo, H.H.; Gu, W.; Yu, D.H.; Jin, M.J.; Choi, H.L.; Yuan, K.; Guerin-Deremaux, L.; Kim, D.H. Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng. J. Ginseng Res. 2014, 38, 203–207. [Google Scholar] [CrossRef]
- Kim, K.A.; Yoo, H.H.; Gu, W.; Yu, D.H.; Jin, M.J.; Choi, H.L.; Yuan, K.; Guerin-Deremaux, L.; Kim, D.H. A prebiotic fiber increases the formation and subsequent absorption of compound K following oral administration of ginseng in rats. J. Ginseng Res. 2015, 39, 183–187. [Google Scholar] [CrossRef]
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb 761, in isolated ischemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Bang, J.H.; Lee, J.; Han, J.S.; Baik, T.G.; Jeon, W.K. Ginkgo biloba L. extract protects against chronic cerebral hypoperfusion by modulating neuroinflammation and the cholinergic system. Phytomedicine 2016, 23, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Kim, Y.; Kim, K.S.; Jang, H.J. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 2017, 88, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jin, W.; Ao, M.; Zhai, S.; Xu, H.; Yu, L. Evaluation of the anti-inflammatory properties of the active constituents in Ginkgo biloba for the treatment of pulmonary diseases. Food Funct. 2019, 10, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, L.; Xu, F.; Sun, Y.; Du, F.; Ma, X.; Zhong, C.; Li, X.; Wang, F.; Zhang, N.; et al. Systemic and cerebral exposure to and pharmacokinetics of flavonols and terpene lactones after dosing standardized Ginkgo biloba leaf extracts to rats via different routes of administration. Br. J. Pharmacol. 2013, 170, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Dudley, E.; Plummer, S.; Tang, J.; Newton, R.P.; Brenton, A.G. Quantitative determination of major active components in Ginkgo biloba dietary supplements by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Stromgaard, K.; Nakanishi, K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew. Chem. Int. Ed. Engl. 2004, 43, 1640–1658. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Aa, L.; Fei, F.; Tan, Z.; Aa, J.; Wang, G.; Liu, C. The pharmacokinetics study of ginkgolide A, B and the effect of food on bioavailability after oral administration of ginkgolide extracts in beagle dogs. Biomed. Chromatogr. 2018, 32, E4212. [Google Scholar] [CrossRef]
- Yan-Yan, Z.; Li-Li, G.; Guo-Ming, S.; Rong, R.; Jing-Zhen, T. Determination of Ginkgolides A, B, C, J and Bilobalide in Plasma by LC-ESI (-)/MS/MS (QQQ) and its Application to the Pharmacokinetic Study of Ginkgo Biloba Extract in Rats. Drug Res. (Stuttg.) 2016, 66, 520–526. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The influence of antibiotics and dietary components on gut microbiota. Prz. Gastroenterol. 2018, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Kim, U.; Kim, I.S.; Kim, Y.; Kim, D.H.; Han, S.B.; Kim, D.H.; Kwon, O.S.; Yoo, H.H. Effects of gut microflora on pharmacokinetics of hesperidin: A study on non-antibiotic and pseudo-germ-free rats. J. Toxicol. Environ. Health A 2010, 73, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; Von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Yoo, H.H.; Kim, I.S.; Yoo, D.H.; Kim, D.H. Effects of orally administered antibiotics on the bioavailability of amlodipine: Gut microbiota-mediated drug interaction. J. Hypertens. 2016, 34, 156–162. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, E280. [Google Scholar] [CrossRef]
- Kim, I.S.; Yoo, D.H.; Jung, I.H.; Lim, S.; Jeong, J.J.; Kim, K.A.; Bae, O.N.; Yoo, H.H.; Kim, D.H. Reduced metabolic activity of gut microbiota by antibiotics can potentiate the antithrombotic effect of aspirin. Biochem. Pharmacol. 2016, 122, 72–79. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, M.S.; Jeong, J.J.; Lim, S.M.; Kim, I.S.; Yoo, H.H.; Kim, D.H. Effect of Probiotics on Pharmacokinetics of Orally Administered Acetaminophen in Mice. Drug Metab. Dispos. 2018, 46, 122–130. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Duan, J.; Xie, Y.; Luo, H.; Li, G.; Wu, T.; Zhang, T. Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it. Food Chem. Toxicol. 2014, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, W. Method of Preparation of An Extract from Gingo Biloba Leaves and Pharmaceuticals Containing the Extract. U.S. Patent Application No. 5,322,688, 21 June 1994. [Google Scholar]
- Gray, D.; LeVanseler, K.; Pan, M.D. Determination of flavonol aglycones in Ginkgo biloba dietary supplement crude materials and finished products by high-performance liquid chromatography: Single laboratory validation. J. AOAC. Int. 2005, 88, 692–702. [Google Scholar] [PubMed]
- Zun, H.; Yunan, Z.; Chao, W.; Weiyu, W.; Dongming, X. Acid Hydrolytic Method for determination of ginkgo biloba total flavonoids in rat plasma by HPLC for pharmacokinetic studies. Tsinghua Sci. Technol. 2010, 15, 452–459. [Google Scholar]
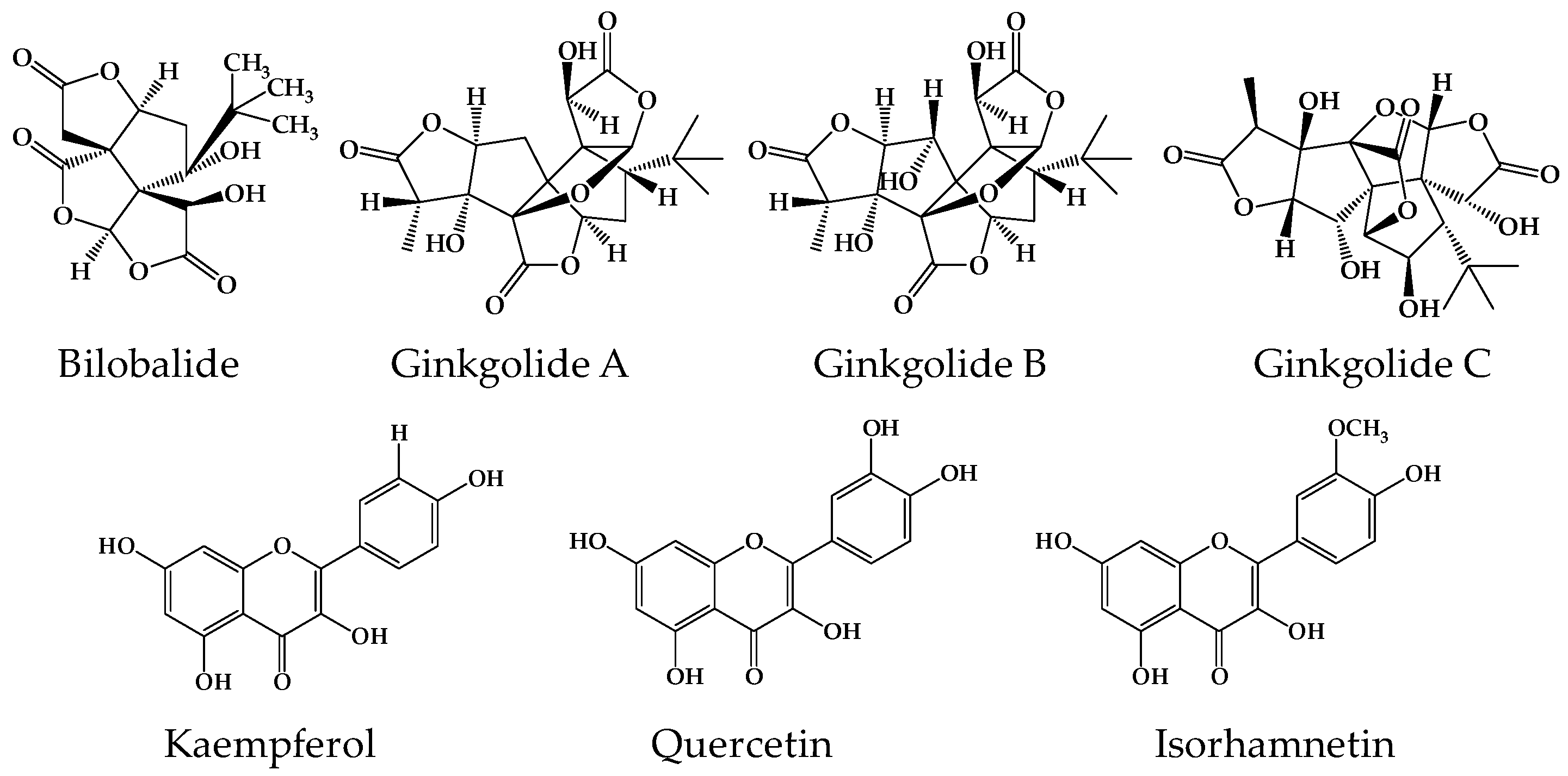
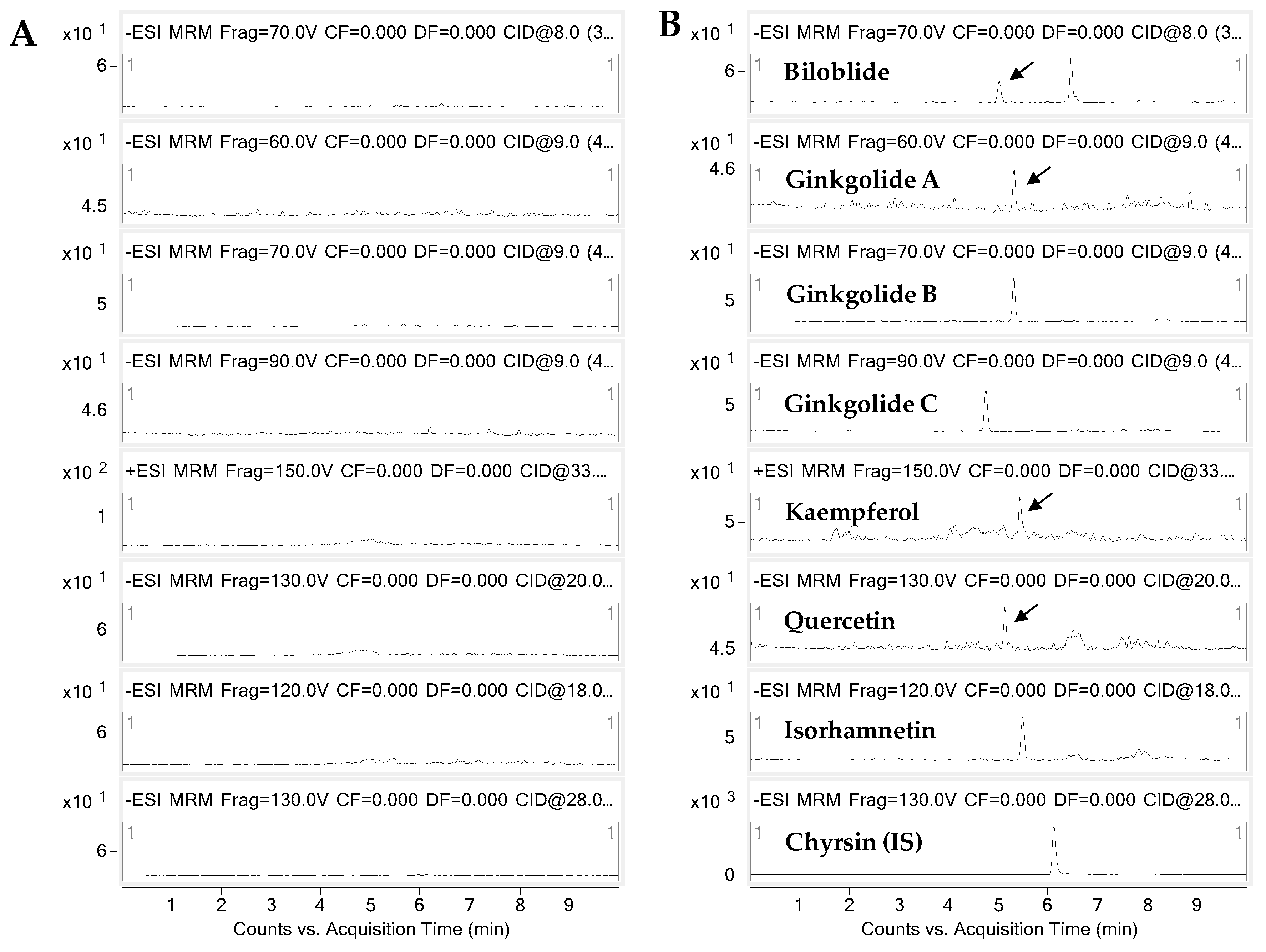
| Components | Linear Range (ng/mL) | Slope | Intercept | R | LOD a (ng/mL) | LOQ b (ng/mL) |
|---|---|---|---|---|---|---|
| Bilobalide | 5–3000 | 0.5043 | 0.0020 | 0.9952 | 2 | 5 |
| Ginkgolide A | 5–1000 | 0.0866 | 0.0006 | 0.9937 | 2 | 5 |
| Ginkgolide B | 5–1000 | 0.3158 | 0.0036 | 0.9990 | 2 | 5 |
| Ginkgolide C | 5–1000 | 0.2241 | 0.0032 | 0.9982 | 2 | 5 |
| Kaempferol | 5–3000 | 0.0070 | −0.0009 | 0.9963 | 3 | 5 |
| Quercetin | 5–3000 | 0.1918 | −0.0012 | 0.9928 | 2 | 5 |
| Isorhamnetin | 5–3000 | 0.6044 | −0.0010 | 0.9977 | 2 | 5 |
| Components | Control Group (n = 7) | Antibacterial-Treated Group (n = 7) | ||||
|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (ng/mL) | AUC (ng·h/mL) | Tmax (h) | Cmax (ng/mL) | AUC (ng·h/mL) | |
| Bilobalide | 0.5 ± 0.0 | 2388.6 ± 249.0 | 10692.7 ± 708.3 | 0.5 ± 0.0 | 2170.7 ± 251.2 | 9639.5 ± 945.2 |
| Ginkgolide A | 0.5 ± 0.0 | 902.6 ± 101.5 | 3047.8 ± 204.2 | 0.5 ± 0.0 | 831.7 ± 134.8 | 2950.3 ± 462.8 |
| Ginkgolide B | 0.7 ± 0.3 | 271.8 ± 52.8 | 1314.6 ± 321.8 | 0.5 ± 0.0 | 247.9 ± 43.8 | 1063.2 ± 213.5 |
| Ginkgolide C | 1.6 ± 0.6 | 20.4 ± 8.2 | 82.4 ± 40.2 | 1.6 ± 1.2 | 17.4 ± 6.9 | 63.7 ± 33.0 |
| Components | Control Group (n = 7) | Antibacterial-Treated Group (n = 7) | ||||
|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (ng/mL) | AUC (ng h/mL) | Tmax (h) | Cmax (ng/mL) | AUC (ng·h/mL) | |
| Kaempferol | 1.9 ± 1.6 | 191.6 ± 42.1 | 1103.3 ± 341.1 | 2.8 ± 2.3 | 235.3 ± 71.7 | 1397.0 ± 509.0 |
| Quercetin | 2.4 ± 1.6 | 140.9 ± 48.4 | 773.1 ± 210.3 | 3.8 ± 1.9 | 165.1 ± 40.9 | 934.0 ± 275.1 |
| Isorhamnetin | 3.7 ± 0.8 | 156.0 ± 56.4 | 1798.4 ± 497.5 | 5.3 ± 2.1 | 268.1 ± 102.8 | 3143.7 ± 1613.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.S.; Kim, J.-K.; Kim, D.-H.; Yoo, H.H. Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts. Metabolites 2019, 9, 132. https://doi.org/10.3390/metabo9070132
Choi MS, Kim J-K, Kim D-H, Yoo HH. Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts. Metabolites. 2019; 9(7):132. https://doi.org/10.3390/metabo9070132
Chicago/Turabian StyleChoi, Min Sun, Jeon-Kyung Kim, Dong-Hyun Kim, and Hye Hyun Yoo. 2019. "Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts" Metabolites 9, no. 7: 132. https://doi.org/10.3390/metabo9070132
APA StyleChoi, M. S., Kim, J.-K., Kim, D.-H., & Yoo, H. H. (2019). Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts. Metabolites, 9(7), 132. https://doi.org/10.3390/metabo9070132






