Effects of Sowing Season on Agronomic Traits and Fatty Acid Metabolic Profiling in Three Brassica napus L. Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Near Infrared Reflectance Spectroscopy
2.3. Assay of FA Composition
2.4. Statistical Analysis
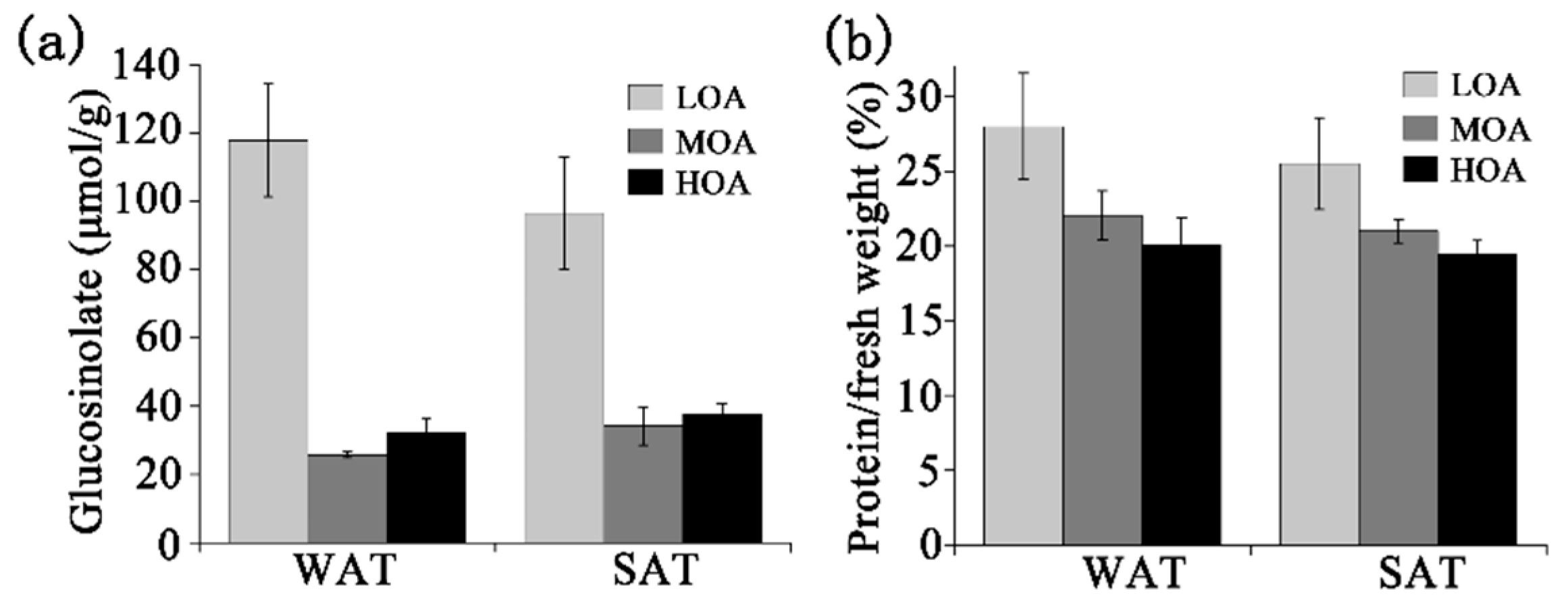
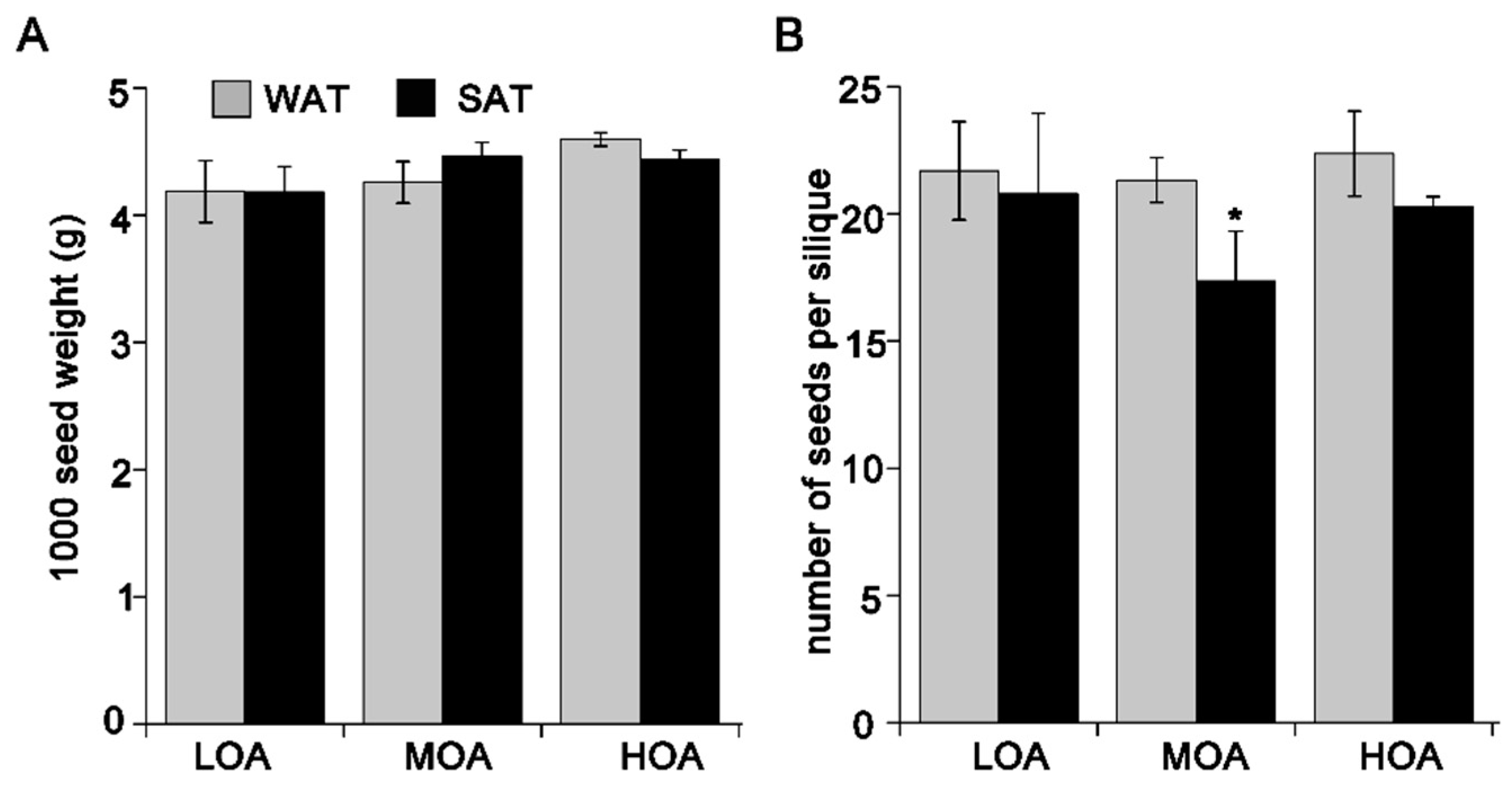
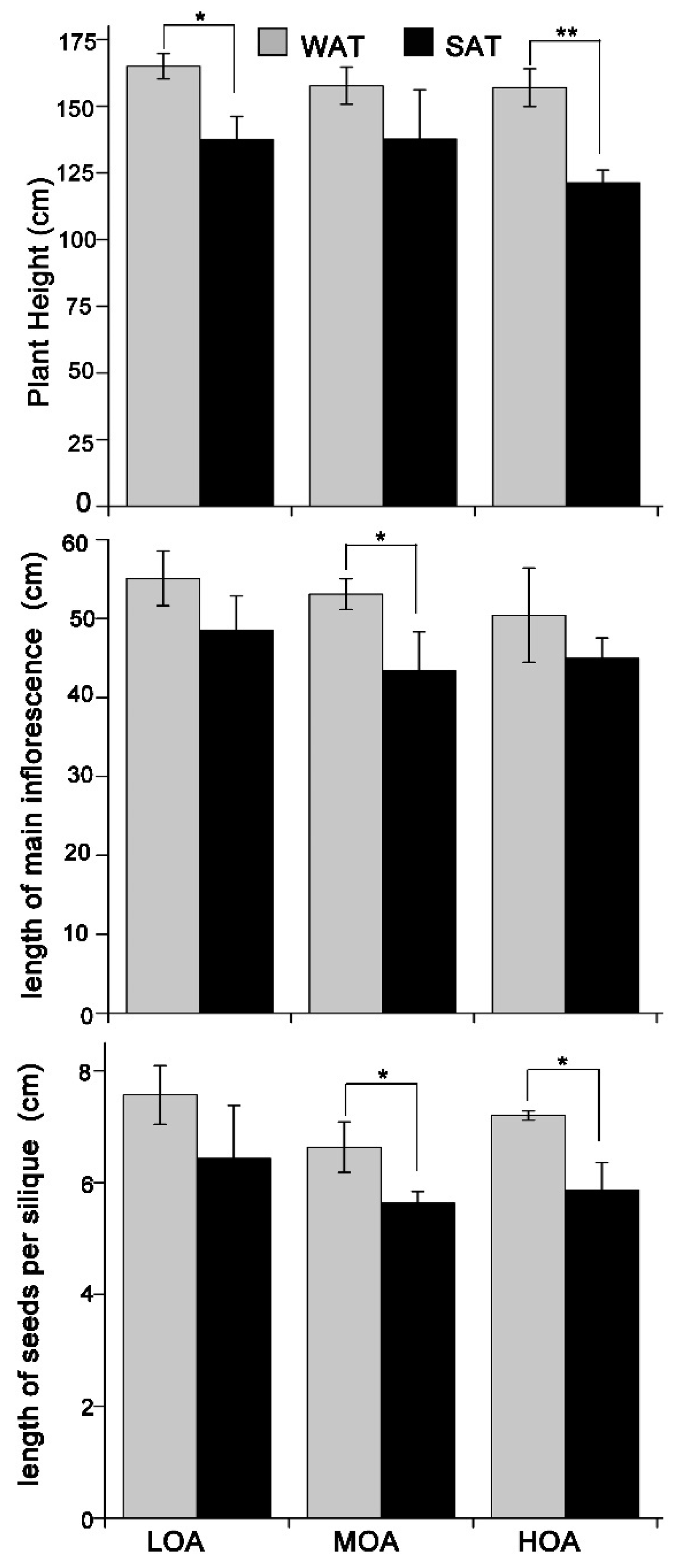
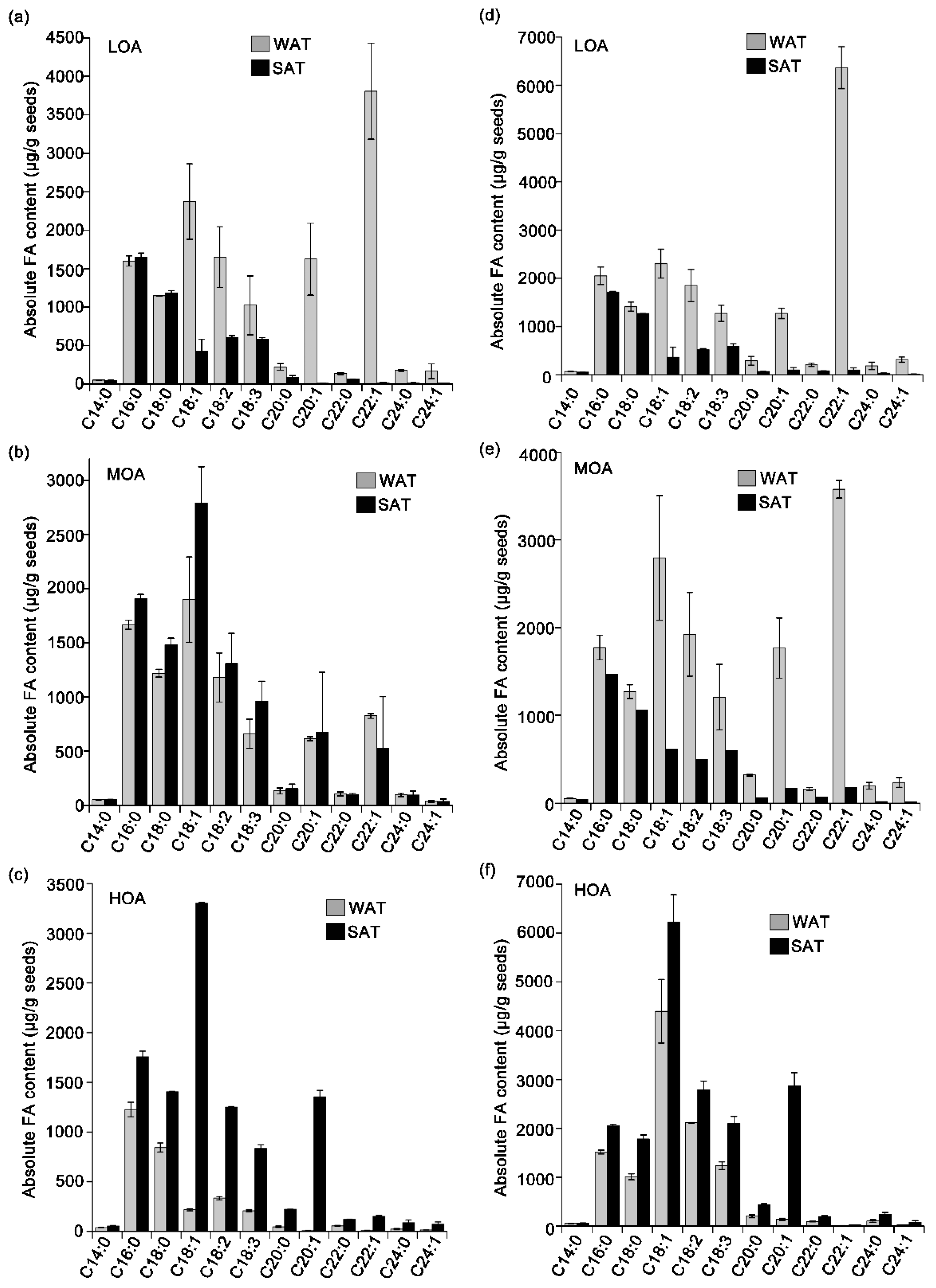
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Palmieri, N.; Forleo, M.B.; Suardi, A.; Coaloa, D.; Pari, L. Rapeseed for energy production: Environmental impacts and cultivation methods. Biomass Bioenergy 2014, 69, 1–11. [Google Scholar] [CrossRef]
- Supit, I.; Diepen, C.A.V.; Wit, A.J.W.D.; Kabat, P.; Baruth, B.; Ludwig, F. Recent changes in the climatic yield potential of various crops in Europe. Agric. Syst. 2010, 103, 683–694. [Google Scholar] [CrossRef]
- Heitman, A.J.; Castillo, M.S.; Smyth, T.J.; Crozier, C.R. Stem, Leaf, and Panicle Yield and Nutrient Content of Biomass and Sweet Sorghum. Agron. J. 2018, 110, 1659. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, B.; Liu, W.Z.; Li, H.; Wang, L.; Wang, B.; Deng, M.; Liang, W.; Deyholos, M.K.; Jiang, Y. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L.). BMC Plant Biol. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Degenhart, D.F.; Kondra, Z.P. Relationships between seed yield and growth characters, yield components, seed quality of summer-type oilseed rape (Brassica napus L.). Euphytica 1984, 33, 885–889. [Google Scholar] [CrossRef]
- Nelson, M.N.; Rajasekaran, R.; Smith, A.; Chen, S.; Beeck, C.P.; Siddique, K.H.; Cowling, W.A. Quantitative trait loci for thermal time to flowering and photoperiod responsiveness discovered in summer annual-type. Brassica napus L. PLoS ONE 2014, 9, e102611. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Pellet, D.; Monney, C.; Herrera, J.M.; Rougier, M.; Baux, A. Fatty acids composition of oilseed rape genotypes as affected by solar radiation and temperature. Field Crops Res. 2017, 212, 165–174. [Google Scholar] [CrossRef]
- Vannozzi, G.P. Variability of Seed Fatty Acid Composition to Growing Degree-Days in High Oleic Acid Sunflower Genotypes: Helia. Helia 2015, 38, 61–78. [Google Scholar]
- Li, W.; Jiang, W.; Zhao, H.; Vyvadilova, M.; Stamm, M.; Hu, S. Genetic Diversity of Rapeseed Accessions from Different Geographic Locations Revealed by Expressed Sequence Tag-Simple Sequence Repeat and Random Amplified Polymorphic DNA Markers. Crop Sci. 2012, 52, 201. [Google Scholar] [CrossRef]
- Saleh, M. The Effect of Air Temperature and Thermoperiod on the Quantity and Quality of Matricaria chamomttla L. Oil; Veenman: Rotterdam, The Netherlands, 1970. [Google Scholar]
- Vuković, J. Influence of drying temperature on the quality of rapeseed seeds, oil quantity and content of free fatty acids. Ph.D. Thesis, Agronomski fakultet, Sveučilište U Zagrebu, Zagreb, Croatia, December 2012. [Google Scholar]
- Namazkar, S.; Stockmarr, A.; Frenck, G.; Egsgaard, H.; Terkelsen, T.; Mikkelsen, T.; Ingvordsen, C.H.; Jørgensen, R.B. Concurrent elevation of CO2, O3 and temperature severely affects oil quality and quantity in rapeseed. J. Exp. Bot. 2016, 67, 4117–4125. [Google Scholar] [CrossRef]
- Michiyama, H.; Arikuni, M.; Hirano, T.; Hayashi, H. Influence of Day Length before and after the Start of Anthesis on the Growth, Flowering and Seed-Setting in Common Buckwheat (Fagopyrum esculentum Moench). Plant Prod. Sci. 2003, 6, 235–242. [Google Scholar] [CrossRef]
- Flagella, Z.; Rotunno, T.; Tarantino, E.; Caterina, R.D.; Caro, A.D. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur. J. Agron. 2002, 17, 221–230. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical Characterization of Hempseed (Seed of Cannabis sativa L.) Oil from Eight Regions in China. J. Diet. Suppl. 2010, 7, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Omidi, H.; Tahmasebi, Z.; Naghdi Badi, H.A.; Torabi, H.; Miransari, M. Fatty acid composition of canola (Brassica napus L.), as affected by agronomical, genotypic and environmental parameters. C. R. Biol. 2010, 333, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.W.; Schäffler, A.; Rehart, S.; Lehr, A.; Müller-Ladner, U.; Neumann, E. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. 2013, 74, 303. [Google Scholar] [CrossRef]
- Qi, W.; Tinnenbroek-Capel, I.E.M.; Salentijn, E.M.J.; Zhao, Z.; Huang, B.; Cheng, J.; Shao, H.; Visser, R.G.F.; Krens, F.A.; van Loo, E.N. Genetically engineering Crambe abyssinica—A potentially high-value oil crop for salt land improvement. Land Degrad. Dev. 2018, 29, 1096–1106. [Google Scholar] [CrossRef]
- Tian, Z.P.; Tang, R.; You-Xiang, W.U.; Wang, T.Q. SSR Utilization in Yellow-seeded Brassica napus L. Guiyou 519 Hybrid Purity Test. Seed 2008, 27, 69–71. [Google Scholar]
- Daun, J.K.; Williams, P.C. Use of NIR spectroscopy to determine quality factors in harvest surveys of canola. In Proceedings of the 9th Int Rapeseed Congress, Cambridge, UK, 4–7 July 1995; pp. 864–866. [Google Scholar]
- Walters, E.H.; Stickland, N.C.; Loughna, P.T. The expression of the myogenic regulatory factors in denervated and normal muscles of different phenotypes. J. Muscle Res. Cell Motil. 2000, 21, 647–653. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Birch, A.N.E.; Hillman, J.R. Antinutritional compounds in the Brasi Analysis, biosynthesis, chemistry and dietary effects. J. Pomol. Hortic. Sci. 1998, 73, 1–18. [Google Scholar]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S219. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate Metabolites Required for an Arabidopsis Innate Immune Response. Science 2009, 323, 95. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K.; Onozawakomori, M.; Takahashi, F.; Asakura, M.; Bednarek, P.; Okuno, T.; Schulze-Lefert, P.; Takano, Y. Entry Mode–Dependent Function of an Indole Glucosinolate Pathway in Arabidopsis for Nonhost Resistance against Anthracnose Pathogens. Plant Cell 2010, 22, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J.; Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Ann. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.A.S.; Rodrigues, P.M.F. The effect of light and temperature on glucosinolate concentration in the leaves and roots of cabbage seedlings. J. Sci. Food Agric. 1998, 78, 208–212. [Google Scholar] [CrossRef]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in Crop Plants. Hortic. Rev. 1997, 19, 99–215. [Google Scholar]
- Inoue, N.; Ogasahara, S.; Hagiwara, M. Analysis of yielding process based on module concept in common buckwheat. Jpn. J. Crop Sci. 1999, 68, 34–35. [Google Scholar]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Sara, P.; Paul, R.; Herreros, J.M.; Tsolakis, A.; Dorado, M.P. The effect of biodiesel fatty acid composition on combustion and diesel; engine exhaust emissions. Fuel 2013, 104, 170–182. [Google Scholar]
- Koscielny, C.B.; Gardner, S.W.; Duncan, R.W. Impact of high temperature on heterosis and general combining ability in spring canola (Brassica napus L.). Field Crops Res. 2018, 221, 61–70. [Google Scholar] [CrossRef]
- Owen, C.; Parker, A.R.G. Omega-3 fatty acids and mood disorders. Am. J. Psychiatry 2006, 163, 2018–2019. [Google Scholar] [CrossRef]
- Khosla, P.; Fungwe, T.V. Conjugated linoleic acid: Effects on plasma lipids and cardiovascular function. Curr. Opin. Lipidol. 2001, 12, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Faintuch, J.; Horie, L.M.; Barbeiro, H.V.; Barbeiro, D.F.; Soriano, F.G.; Ishida, R.K.; Cecconello, I. Systemic inflammation in morbidly obese subjects: Response to oral supplementation with alpha-linolenic acid. Obes. Surg. 2007, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, M.; Chen, T.; Xuan, L.; Li, Z.; Du, X.; Zhou, L.; Zhang, G.; Jiang, L. TRANSPARENT TESTA2 regulates embryonic fatty acid biosynthesis by targeting FUSCA3 during the early developmental stage of Arabidopsis seeds. Plant J. 2014, 77, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Gu, J.; Deng, L.; Ren, L.; Hong, Y.; Lu, Q.; Chen, X.; Liang, X. Identification of the Candidate Proteins Related to Oleic Acid Accumulation during Peanut (Arachis hypogaea L.) Seed Development through Comparative Proteome Analysis. Int. J. Mol. Sci. 2018, 19, 1235. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
| Month | The SAT Site | The WAT Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The Maximum Temperature (°C) | The Minimum Temperature (°C) | The Maximum Temperature (°C) | The Minimum Temperature (°C) | |||||||||
| 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | |
| January | 10.42 | 10.65 | 7.33 | 1.35 | 4.01 | 2.16 | 9.49 | 10.55 | 9.62 | −0.68 | 1.21 | −0.17 |
| February | 8.98 | 11.45 | 11.39 | 2.52 | 4.61 | 2.25 | 12.40 | 12.35 | 7.23 | 0.41 | 2.06 | −0.56 |
| March | 14.28 | 14.69 | 15.33 | 7.12 | 8.07 | 7.95 | 17.88 | 19.38 | 17.35 | 4.72 | 5.64 | 4.67 |
| April | 20.75 | 21.43 | 21.66 | 13.75 | 11.95 | 13.61 | 20.99 | 18.41 | 20.77 | 10.00 | 9.11 | 8.99 |
| May | 22.13 | 24.19 | 24.04 | 14.38 | 16.42 | 15.73 | 21.70 | 22.46 | 21.88 | 11.42 | 12.81 | 11.89 |
| June | 24.70 | 24.76 | 27.37 | 18.93 | 19.52 | 19.84 | 21.87 | 22.50 | 23.54 | 14.79 | 14.97 | 14.66 |
| July | 27.26 | 26.56 | 29.36 | 19.88 | 18.87 | 20.99 | 22.61 | 22.48 | 24.52 | 15.37 | 14.63 | 16.62 |
| August | 27.35 | 25.93 | 28.86 | 19.13 | 19.02 | 20.19 | 22.88 | 21.24 | 24.73 | 15.38 | 14.75 | 15.91 |
| September | 26.61 | 23.16 | 25.94 | 18.27 | 17.35 | 16.85 | 22.51 | 19.70 | 19.42 | 14.70 | 13.69 | 13.55 |
| October | 22.42 | 21.90 | 22.30 | 13.83 | 13.69 | 15.16 | 19.02 | 18.92 | 19.74 | 10.62 | 10.47 | 11.86 |
| November | 14.46 | 18.58 | 15.61 | 9.72 | 11.89 | 9.16 | 12.71 | 18.85 | 16.91 | 6.08 | 7.08 | 7.01 |
| December | 10.39 | 9.87 | 12.78 | 3.73 | 4.70 | 5.63 | 7.95 | 9.94 | 12.04 | 0.64 | 2.69 | 3.04 |
| Annual | 19.14 | 19.43 | 20.16 | 11.88 | 12.51 | 12.46 | 17.67 | 18.07 | 18.14 | 8.62 | 9.09 | 8.96 |
| Mean Annual Humidity | Max. Temp (°C) | Min. Temp (°C) | Days Below 12 (°C) | Mean Shortwave Radiation (w·m−2) | |
|---|---|---|---|---|---|
| WAT | 2.41 | 30.90 | 0.86 | 78 | 156.10 |
| SAT | 4.64 | 29.40 | 9.28 | 5 | 307.73 |
| WAT | SAT | |
|---|---|---|
| Germination | 20 October | 8 May |
| Bud emergence (days) | 116 ± 5 | 76 ± 3 |
| First flower (days) | 138 ± 4 | 100 ± 2 |
| Last flower (days) | 176 ± 5 | 130 ± 5 |
| Maturity of seeds (days) | 201 ± 7 | 154 ± 8 |
| Days of reproduction | 92 ± 5 | 85 ± 4 |
| Days of growth | 256 ± 10 | 145 ± 8 |
| Year | Seed Yield in WAT (kg∙ha−1) | Year | Seed Yield in SAT (kg∙ha−1) | ||||
|---|---|---|---|---|---|---|---|
| LOA | MOA | HOA | LOA | MOA | HOA | ||
| 2014.10–2015.05 | 1812.89 ± 62.79 | 1571.36 ± 18.26 | 1808.01 ± 25.04 | 2014.05–2014.10 | 1437.16 ± 89.91 | 1537.21 ± 58.86 | 1400.55 ± 32.95 |
| 2015.10–2016.05 | 1810.50 ± 56.17 | 1791.00 ± 64.35 | 1693.38 ± 30.25 | 2015.03–2015.10 | 1317.62 ± 89.9 | 1478.66 ± 27.78 | 1500.60 ± 74.11 |
| 2016.10–2017.05 | 1854.28 ± 10.89 | 1683.60 ± 10.62 | 2039.85 ± 22.75 | 2016.05–2016.10 | 1484.13 ± 58.19 | 1446.92 ± 50.43 | 1352.37 ± 14.03 |
| Mean | 1825.89 ± 24.62 | 1681.99 ± 109.83 | 1847.08 ± 176.51 | Mean | 1412.08 ± 176.51 ** | 1487.60 ± 45.80 * | 1417.84 ± 75.61 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, L.; Qiu, G.; Wang, T.; Liu, C.; Yang, Y.; Feng, B.; Chen, C.; Zhang, W.; Liu, Z. Effects of Sowing Season on Agronomic Traits and Fatty Acid Metabolic Profiling in Three Brassica napus L. Cultivars. Metabolites 2019, 9, 37. https://doi.org/10.3390/metabo9020037
Li X, Wu L, Qiu G, Wang T, Liu C, Yang Y, Feng B, Chen C, Zhang W, Liu Z. Effects of Sowing Season on Agronomic Traits and Fatty Acid Metabolic Profiling in Three Brassica napus L. Cultivars. Metabolites. 2019; 9(2):37. https://doi.org/10.3390/metabo9020037
Chicago/Turabian StyleLi, Xiaoyi, Lintao Wu, Guoliang Qiu, Tao Wang, Chunhong Liu, Yongming Yang, Bin Feng, Cun Chen, Wei Zhang, and Zhibin Liu. 2019. "Effects of Sowing Season on Agronomic Traits and Fatty Acid Metabolic Profiling in Three Brassica napus L. Cultivars" Metabolites 9, no. 2: 37. https://doi.org/10.3390/metabo9020037
APA StyleLi, X., Wu, L., Qiu, G., Wang, T., Liu, C., Yang, Y., Feng, B., Chen, C., Zhang, W., & Liu, Z. (2019). Effects of Sowing Season on Agronomic Traits and Fatty Acid Metabolic Profiling in Three Brassica napus L. Cultivars. Metabolites, 9(2), 37. https://doi.org/10.3390/metabo9020037




