Insights on Salt Tolerance of Two Endemic Limonium Species from Spain
Abstract
1. Introduction
2. Results
2.1. Seed Germination
2.2. Growth Performance under Salt Stress Conditions
2.3. Ion Accumulation
2.4. Statistical Analysis of the Differences in Growth Parameters, Photosynthetic Pigments and Ion Accumulation between the Two Species
2.5. Differences in the Metabolite Profiles of the Two Species in the Absence of Stress
2.6. Changes in Metabolites Relative Contents in Response to Salt Stress
2.7. Statistical Analysis of the Untargeted Metabolomics Results
3. Discussion
3.1. Relative Salt Tolerance of Limonium Albuferae and L. Dufourii
3.2. Main Salt Tolerance Mechanisms in the Investigated Species
3.3. Relevance of the Obtained Results for Conservation Strategies of the Two Endemic Limonium Species
4. Materials and Methods
4.1. Seed Germination
4.2. Plant Growth and Salt Treatments
4.3. Photosynthetic Pigments
4.4. Ion Content Measurements
4.5. Primary Metabolite Extraction and Metabolite Profiling Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flowers, T.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agravaal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Kubitzki, K. Plumbaginaceae. In Flowering Plants. Dichotyledons; Kubitzki, I.K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 2, pp. 523–530. [Google Scholar]
- Koutroumpa, K.; Theodoridis, S.; Warren, B.H.; Jiménez, A.; Celep, F.; Doğan, M.; Romeiras, M.M.; Santos-Guerra, A.; Fernández-Palacios, J.M.; Caujapé-Castells, J.; et al. An expanded molecular phylogeny of Plumbaginaceae, with emphasis on Limonium (sea lavanders): Taxonomic implications and biogeographic considerations. Ecol. Evol. 2018, 8, 12397–12424. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.D.; Heywood, V.H.; Hamilton, A.C. Centres of Plant Diversity, Volume 1: Europe, Africa, South West Asia and the Middle East; IUCN, Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Médail, F.; Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Aedo, C.; Medina, L.; Fernández-Albert, M. Species richness and endemicity in the Spanish vascular flora. Nord. J. Bot. 2013, 31, 478–488. [Google Scholar] [CrossRef]
- Moreno, J.C. Red List of Spanish Vascular Flora; Ministerio de Medio Ambiente, Rural y Marino: Madrid, Spain, 2008. [Google Scholar]
- Pignatti, S. Limonium Mill. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 38–50. [Google Scholar]
- Greuter, W.; Burdet, H.M.; Long, G. Med–Checklist. 4. Dicotyledones (Lauraceae–Rhamnaceae); Conservatoire et Jardin botaniques de la Ville de Genéve: Ginebra, Switzerland, 1989. [Google Scholar]
- Lledó, M.D.; Crespo, M.B.; Fay, M.F.; Chase, M.V. Molecular phylogenetics of Limonium and related genera (Plumbaginaceae): Biogeographical and systematic implications. Am. J. Bot. 2005, 92, 1189–1198. [Google Scholar] [CrossRef]
- Domina, G. Plumbaginaceae Juss. Euro+Med Plantbase–the Information Resource for Euro–Mediterranean Plant Diversity. 2011. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 15 June 2019).
- Hassler, M. World Plants: Synonymic Checklist of the Vascular Plants of the World (Version November 2018). In Species 2000 & ITIS Catalogue of Life; Roskov, Y., Ower, G., Orell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bougoin, T., DeWalt, R.E., Decock, W., Nieukerken, E., et al., Eds.; Species 2000; Naturalis: Leiden, The Netherlands, 2018; ISSN 2405-8858. Available online: www.catalogueoflife.org/col (accessed on 25 March 2019).
- Erben, M. Limonium Mill. In Flora Iberica; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Soriano, C., Eds.; Real Jardín Botánico, C.S.I.C.: Madrid, Spain, 1993; Volume 3, pp. 2–143. [Google Scholar]
- IUCN/SSC. IUCN Red List Categories and Criteria; Version 3.1; IUCN: Cambridge, UK, 2001. [Google Scholar]
- Crespo, M.B.; Lledó, M.D. El Género Limonium en la Comunidad Valenciana; Conselleria de Medi Ambient, Generalitat Valenciana: Valencia, Spain, 1998; p. 118. [Google Scholar]
- Laguna, E. (Ed.) Flora Rara, Endémica o Amenazada de la Comunidad Valenciana; Generalitat Valenciana: Valencia, Spain, 1998. [Google Scholar]
- Decreto 70/2009, de 22 de mayo, del Consell, por el que se crea y regula el Catálogo Valenciano de Especies de Flora Amenazadas y se regulan medidas adicionales de conservación. D. Of. de la Comunitat Valencia. 2009, 6021, 20143–20162.
- Aguilella, A.; Fos, S.; Laguna, E. (Eds.) Catálogo Valenciano de Especies de Flora Amenazadas; Generalitat Valenciana: Valencia, Spain, 2010. [Google Scholar]
- Mateo, G.; Crespo, M.B. Claves Ilustradas de la Flora Valenciana. Monografías de Flora Montiberica; Jolube: Jaca, Spain, 2014; Volume 6. [Google Scholar]
- Crespo, M.B.; Laguna, E. Nuevas localidades de Limonium dufourii (Girard) O. Kuntze (Plumbaginaceae). Anal. Jard. Bot. Madr. 1993, 51, 154–155. [Google Scholar]
- Laguna, E.; Aguilella, A.; Carretero, J.L.; Crespo, M.B.; Figuerola, R.; Mateo, G. Libro de la Flora Vascular Rara, Endémica o Amenazada de la Comunidad Valenciana; Generalitat Valenciana: Valencia, Spain, 1994. [Google Scholar]
- Anonymous. Orden 6/2013, de 25 de marzo, de la Conselleria de Infraestructuras, Territorio y Medio Ambiente, por la que se modifican los listados valencianos de especies protegidas de flora y fauna. D. Of. de la Comunitat Valencia. 2013, 6996, 8682–8690. [Google Scholar]
- Ferrer-Gallego, P.P.; Roselló, R.; Rosato, M.; Rosselló, J.A.; Laguna, E. Limonium albuferae (Plumbaginaceae), a new polyploid species from the Eastern Iberian Peninsula. Phytotaxa 2016, 252, 114–122. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution 1996, 20, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; González-Candelas, F. Analysis of population genetic structure and variability using RAPD markers in the endemic and endangered Limonium dufourii (Plumbaginaceae). Mol. Ecol. 1997, 6, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Servicio de Vida Silvestre. Limonium dufourii. Banco de Datos de Biodiversidad de la Comunidad Valenciana. Available online: https://bdb.gva.es/bancodedatos/censos/descargaCensos.asp?id=12995&nombre=Limonium&dufourii (accessed on 30 July 2019).
- Palacios, C.; Kresovich, S.; González-Candelas, F. A population genetic study of the endangered plant speices Limonium dufourii (Plumbaginaceae) based on amplified fragment length polymorphism (AFLP). Mol. Ecol. 1999, 8, 645–657. [Google Scholar] [CrossRef]
- Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Figueroa, M.E. Nucleation and facilitation in salt pans in Mediterranean salt marshes. J. Veg. Sci. 2001, 12, 761–770. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Waisel, Y. Biology of Halophytes; Academic Press: New York, NY, USA; London, UK, 1972. [Google Scholar]
- Ungar, I.A. Ecophysiology of Vascular Halophytes; CRC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Khan, M.A. Halophyte seed germination: Success and pitfalls. In International Symposium on Optimum Resource Utilization in Salt Affected Ecosystems in Arid and Semi Arid Regions; Hegazi, A.M., El-Shaer, H.M., El-Demerdashe, S., Guirguis, R.A., Abdel Salam Metwally, A., Hasan, F.A., Khashaba, H.E., Eds.; Desert Research Centre: Cairo, Egypt, 2003; pp. 346–358. [Google Scholar]
- Khan, M.A.; Gul, B. Halophyte seed germination. In Ecophysiology of High Salinity Tolerant Plants; Khan, M., Weber, D., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 11–30. [Google Scholar]
- Martín-Vide, J.; López-Bustins, J.A. The Western Mediterranean Oscillation and rainfall in the Iberian Peninsula. Int. J. Climatol. 2006, 26, 1455–1475. [Google Scholar] [CrossRef]
- Del Río, S.; Herrero, L.; Fraile, R.; Penas, A. Spatial distribution of recent rainfall trends in Spain (1961–2006). Int. J. Climatol. 2011, 31, 656–667. [Google Scholar]
- Grieve, C.M.; Poss, J.A. Productivity and mineral nutrition of Limonium species irrigated with saline wastewaters. HortScience 2005, 40, 654–665. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.J.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lidón, A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef]
- Touchette, B.W.; Kneppers, M.K.; Eggert, M.C. Salt marsh plants: Biological overview and vulnerability to Climate Change. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M.S., Shabala, S., Fujita, M., Eds.; CAB International: Wallingford, UK, 2019; pp. 115–134. [Google Scholar]
- Houle, G.; Morel, L.; Reynolds, C.; Siégel, J. The effect of salinity on different developmental stages of an endemic annual plant, Aster laurentianus (Asteraceae). Am. J. Bot. 2001, 88, 62–67. [Google Scholar] [CrossRef]
- Giménez Luque, E.; Delgado-Fernández, I.; Gómez Mercado, F. Effect of salinity and temperature on seed germination in Limonium cossonianum. Botany 2013, 91, 12–16. [Google Scholar] [CrossRef]
- Delgado Fernández, I.C.; Giménez Luque, E.; Gómez Mercado, F.; Marrrero, J.M. Germination responses of Limonium insigne (Coss.) Kuntze to salinity and temperature. Pak. J. Bot. 2015, 47, 807–812. [Google Scholar]
- Delgado Fernández, I.C.; Giménez Luque, E.; GómezMercado, F.; Pedrosa, V. Influence of temperature and salinity on the germination of Limonium tabernense Erben from Tabernas Desert (Almería, SE Spain). Flora 2016, 218, 68–74. [Google Scholar] [CrossRef]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four Mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.A. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can. J. Bot. 2004, 82, 151–157. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos Naranjo, E.; Garzón, O.; Castillo, J.M.; Luque, T.; Figueroa, M.E. Effects of salinity on germination and seedling establishment of endangered Limonium emarginatum (Willd.) O. Kuntze. J. Coast. Res. 2008, 24, 201–205. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Naranjo, M.A.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Boscaiu, M.T.; Ballesteros, G.; Naranjo, M.A.; Vicente, O.; Boira, H. Responses to salt stress in Juncus acutus and J. maritimus during seed germination and vegetative plant growth. Plant Biosyst. 2011, 145, 770–777. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; Tian, Y.; Zhou, D. Influence of salinity and temperature on seed germination rate and the hydrotime model parameters for the halophyte, Chloris virgata, and the glycophyte, Digitaria sanguinalis. S. Afr. J. Bot. 2012, 78, 203–210. [Google Scholar] [CrossRef]
- Manzoor, S.; Hameed, A.; Khan, M.A.; Gul, B. Seed germination ecology of a medicinal halophyte Zygophyllum propinquum: Responses to abiotic factors. Flora 2017, 233, 163–170. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Keiffer, C.H.; Ungar, I.A. The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am. J. Bot. 1997, 84, 104–111. [Google Scholar] [CrossRef]
- Pujol, J.A.; Calvo, J.F.; Ramírez-Díaz, L. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann. Bot. 2000, 85, 279–286. [Google Scholar] [CrossRef]
- Al Hassan, M.; López-Gresa, M.P.; Boscaiu, M.; Vicente, O. Stress tolerance mechanisms in Juncus: Responses to salinity and drought in three Juncus species adapted to different natural environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Quart. Rev. Biol. 1986, 61, 313–335. [Google Scholar] [CrossRef]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct. Plant Biol. 2016, 43, 607–619. [Google Scholar]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidón, A.; Vicente, O. Soluble carbohydrates as osmolytes in several halophytes from a Mediterranean salt marsh. Not. Bot. Horti Agrobo. 2011, 39, 9–17. [Google Scholar] [CrossRef]
- Boscaiu, M.; Lull, C.; Llinares, J.; Vicente, O.; Boira, H. Proline as a biochemical marker in relation to the ecology of two halophytic Juncus species. J. Plant Ecol. 2013, 6, 177–186. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-invasive plants vs. halophytes in Mediterranean salt marshes: Stress tolerance mechanisms in two related species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef]
- Alarcón, J.J.; Morales, M.A.; Torrecillas, A.; Sánchez-Blanco, M.J. Growth, water relations and accumulation of organic and inorganic solutes in the halophytes Limonium latifolium cv. avignon and its interspecific hybrid Limonium caspia × Limonium latifolium cv. bettlaard during salt stress. J. Plant Physiol. 1999, 154, 795–801. [Google Scholar] [CrossRef]
- Morales, M.A.; Olmos, E.; Torrecillas, A.; Sánchez-Blanco, M.J.; Alarcó, J. Differences in water relations, leaf ion accumulation and excretion rates between cultivated and wild species of Limonium sp. grown in conditions of saline stress. Flora 2001, 196, 345–352. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M.; Poss, J.A. Salinity effects on emergence, survival, and ion accumulation of Limonium perezii. J. Plant Nutr. 2005, 28, 1243–1257. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Assaha, D.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium-and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Chen, M.; Sui, N.; Wang, B.S. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S. Afr. J. Bot. 2010, 76, 95–101. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Grieve, C. Accumulation of chiro-inositol and other nonstructural carbohydrates in Limonium species in response to saline irrigation waters. J. Am. Soc. Hortic. Sci. 2009, 134, 329–336. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2008, 132, 209–219. [Google Scholar] [CrossRef]
- Planchet, E.; Limami, A.M.; d’Mello, J.P. Amino acid synthesis under abiotic stress. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 262–276. ISBN 978-1-78064-263. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 25, 2520. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Califor. Agr. Exp. Stat. 1950, 32, 347. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Technical advance: Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
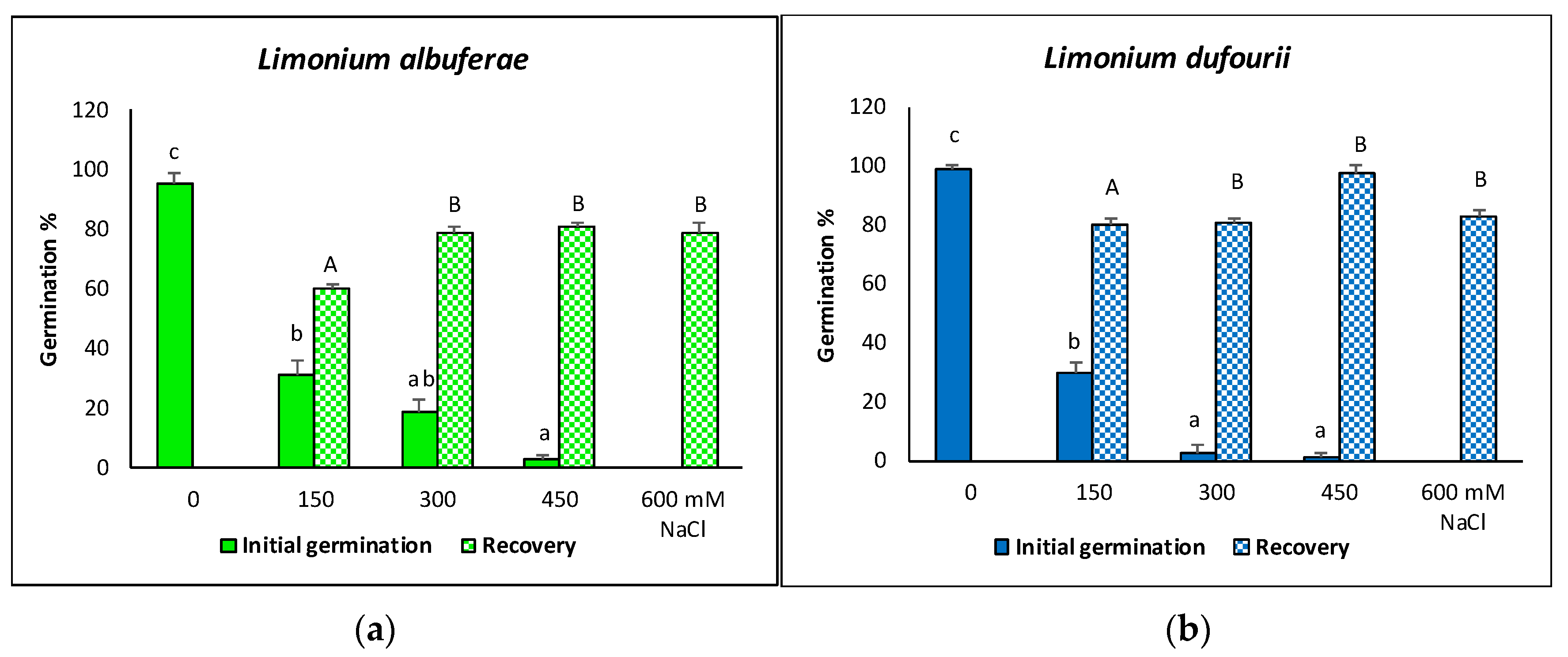


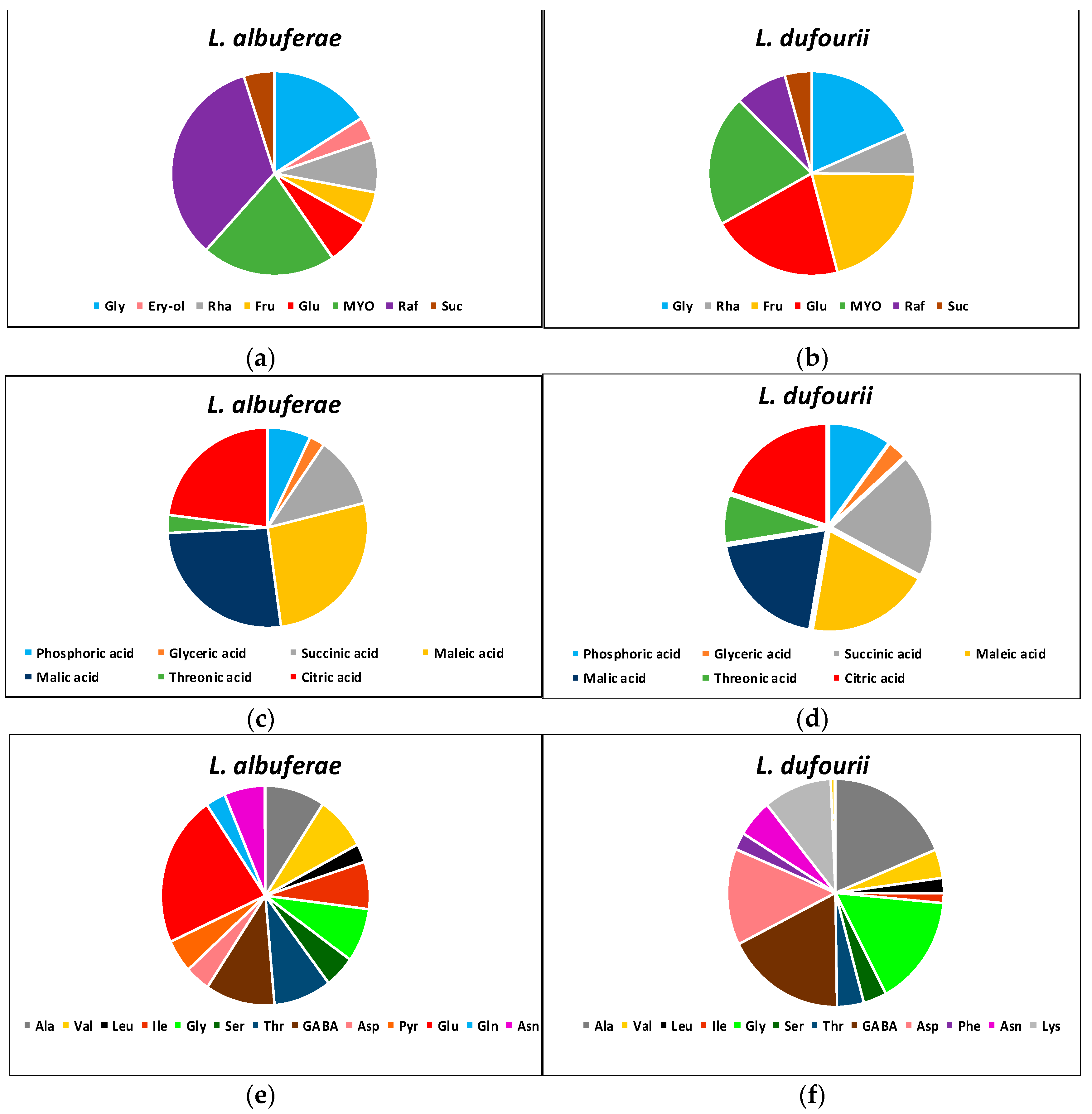
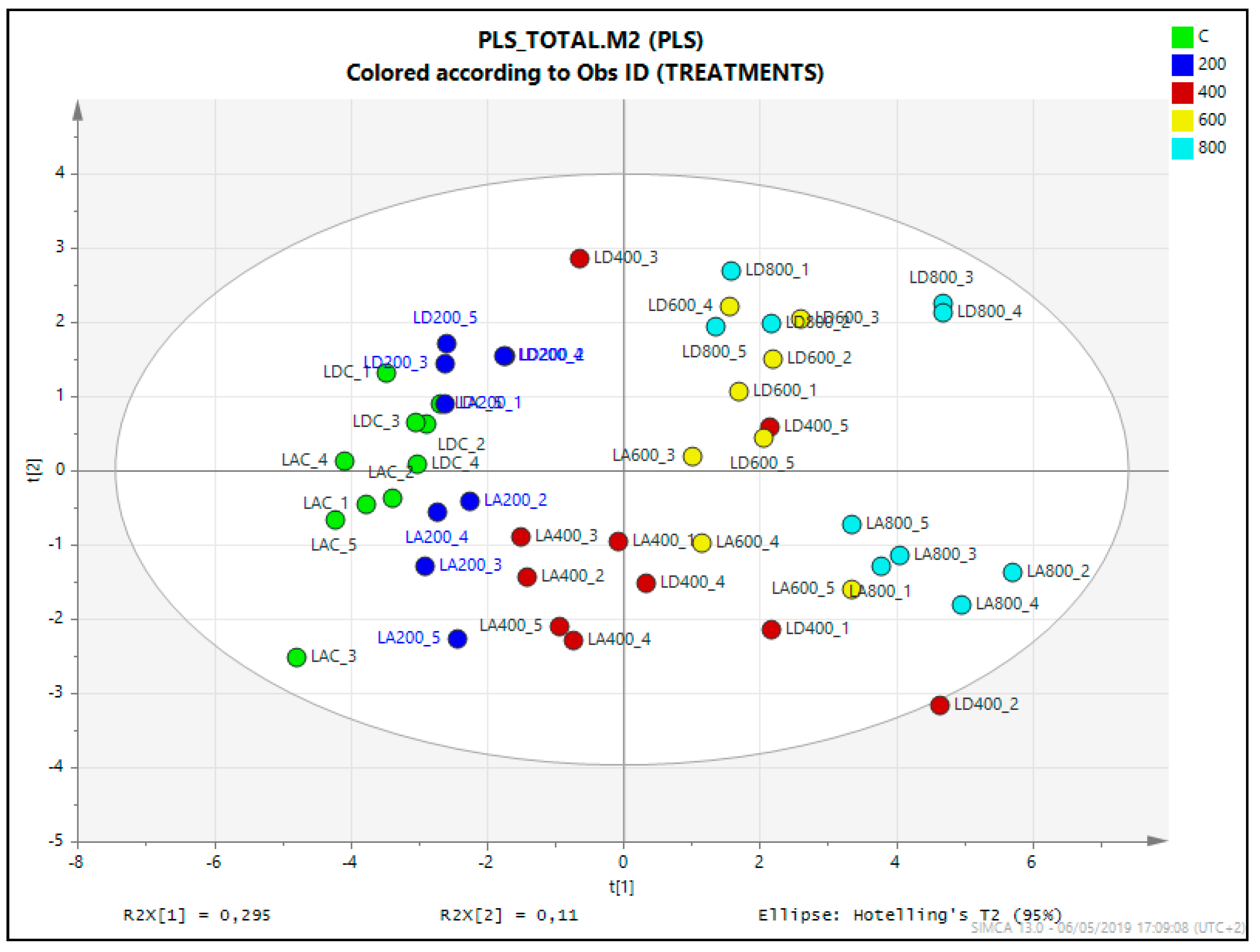
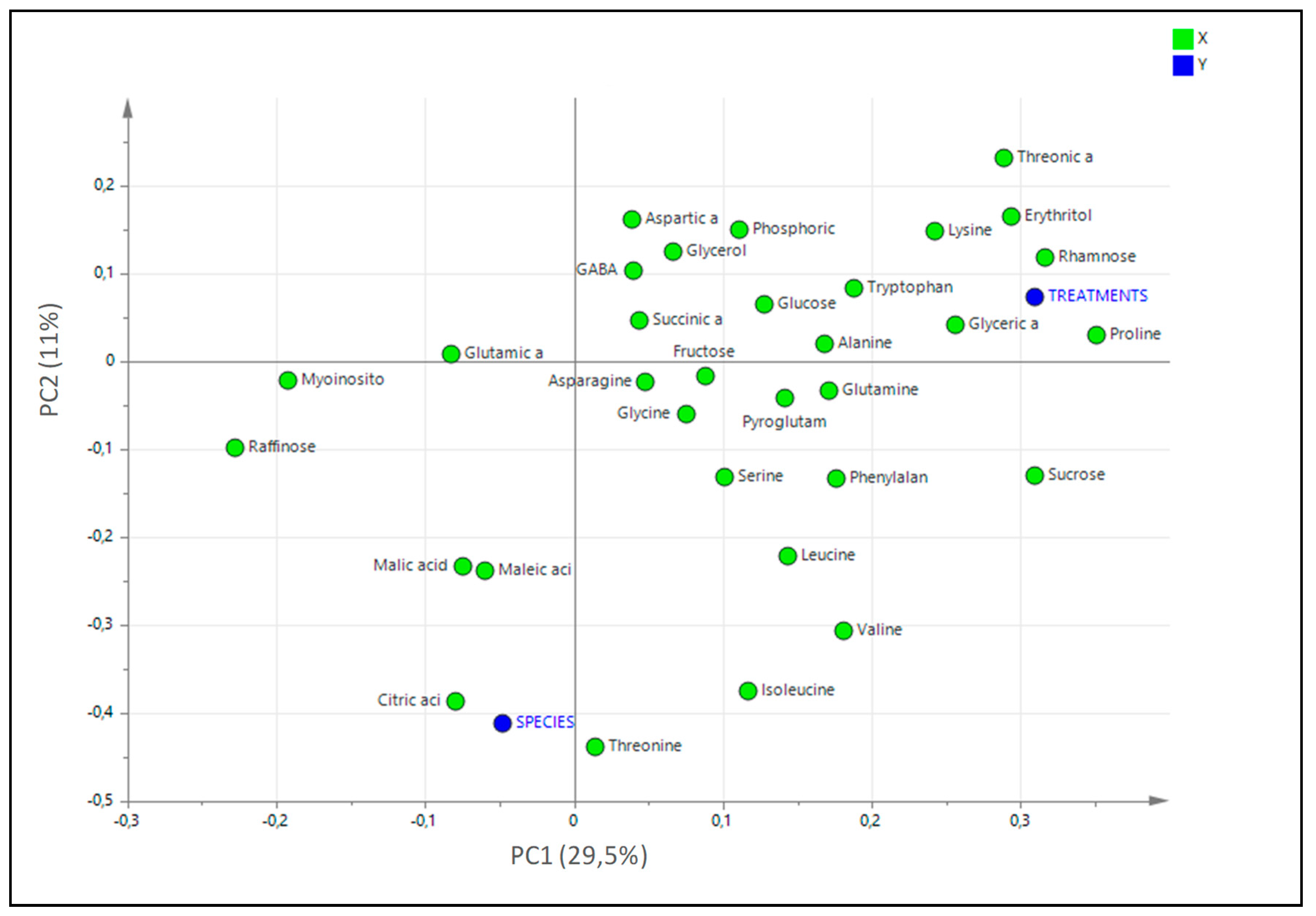
| NaCl (mM) | Limonium albuferae | Limonium dufourii | ||
|---|---|---|---|---|
| Initial Germination | Recovery | Initial Germination | Recovery | |
| 0 | 5.78 ± 0.33 a | - | 5.32 ± 0.38 a | - |
| 150 | 10.86 ± 0.78 b | 4.41 ± 0.15 ab | 11.99 ± 1.16 bc | 5.97 ± 0.45 b |
| 300 | 16.67 ± 0.59 c | 4.41 ± 0.20 ab | 6.50 ± 0.71 ab | 4.88 ± 042 ab |
| 450 | 20.00 ± 1.41 c | 4.51 ± 0.17 b | 13.00 c | 3.89 ± 0. 28 a |
| 600 | - | 3.73 ± 0.16 a | - | 3.97 ± 0.68 ab |
| Parameters | NaCl (mM) | L. albuferae | L. dufourii |
|---|---|---|---|
| Leaf Number | 0 | 13.4 ± 0.24 c | 24 ± 2.83 d |
| 200 | 12.4 ± 0.81 c | 20.4 ± 1.14 c | |
| 400 | 12 ± 0.77 c | 18.6 ± 2.19 bc | |
| 600 | 9.8 ± 0.58 b | 16.2 ± 3.56 b | |
| 800 | 7.4 ± 0.24 a | 12.4 ± 2.19 a | |
| Leaf FW* (% of control) | 0 | 100 ± 0.60 c | 100 ± 0.82 c |
| 200 | 106.27 ± 7.91 c | 46.94 ± 3.28 b | |
| 400 | 83.26 ± 6.42 b | 37.72 ± 1.50 ab | |
| 600 | 49.93 ± 2.55 a | 31.65 ± 7.59 a | |
| 800 | 36.86 ± 1.76 a | 29.84 ± 3.40 a | |
| Roots FW* (% of control) | 0 | 100 ± 0.18 a | 100 ± 0.38 a |
| 200 | 204.11 ± 36.73 b | 240.50 ± 29.61 c | |
| 400 | 187.98 ± 18.56 b | 214.38 ± 17.13 bc | |
| 600 | 141.50 ± 34.02 ab | 159.88 ± 35.61 ab | |
| 800 | 108.72 ± 13.96 a | 142.12 ± 14.15 a | |
| WC % in Leaves | 0 | 85.22 ± 0.54 e | 87 ± 0.30 c |
| 200 | 82.46 ± 0.10 d | 84.77± 0.32 c | |
| 400 | 79.45 ± 0.54 c | 79.50 ± 0.92 b | |
| 600 | 75.82 ± 0.5 b | 75.54 ± 2.55 a | |
| 800 | 72.45 ± 0.55 a | 76.04 ± 0.65 ab | |
| WC% in Roots | 0 | 77.73 ± 0.64 a | 83.75 ± 4.37 a |
| 200 | 79.14 ± 0.70 a | 78.04 ± 9.73 a | |
| 400 | 78.42 ± 0.57 a | 79.60 ± 5.83 a | |
| 600 | 77.96 ± 0.63 a | 72.81 ± 5.67 a | |
| 800 | 76.80 ± 0.36 a | 75.47 ± 6.36 a | |
| Chl a (mg g −1 DW) | 0 | 4.99 ± 0.69 a | 3.61 ± 0.62 b |
| 200 | 3.48 ± 0.13 a | 2.00 ± 0.30 a | |
| 400 | 2.35 ± 0.44 a | 1.96 ± 0.34 a | |
| 600 | 2.47 ± 0.32 a | 1.64 ± 0.26 a | |
| 800 | 3.22 ± 0.76 a | 1.64 ± 0.31 a | |
| Chl b (mg g −1 DW) | 0 | 3.56 ± 0.97 b | 2.06 ± 0.29 c |
| 200 | 1.50 ± 0.34 a | 1.42 ± 0.25 b | |
| 400 | 0.81 ± 0.21 a | 0.95 ± 0.13 ab | |
| 600 | 0.76 ± 0.16 a | 0.66 ± 0.13 a | |
| 800 | 1.00 ± 0.26 a | 0.65 ± 0.06 a | |
| Caro (mg g −1 DW) | 0 | 1.76 ± 0.52 b | 1.04 ± 0.12 c |
| 200 | 0.95 ± 0.30 a | 0.88 ± 0.07 bc | |
| 400 | 0.57 ± 0.09 a | 0.71 ± 0.06 ab | |
| 600 | 0.63 ± 0.15 a | 0.69 ± 0.08 ab | |
| 800 | 0.91 ± 0.23 a | 0.56 ± 0.04 a |
| Parameters | NaCl (mM) | Species | |
|---|---|---|---|
| L. albuferae | L. dufourii | ||
| Na+ in roots (µmol g−1 DW) | 0 | 150.26 ± 19.54 a | 325.60 ± 71.19 a |
| 200 | 861.19 ± 71.59 b | 1104.48 ± 108.85 b | |
| 400 | 1081.68 ± 87.38 b | 1693.58 ± 119.04 c | |
| 600 | 1169.40 ± 97.91 b | 1744.62 ± 119.59 c | |
| 800 | 2186.31 ± 272.50 c | 2575.18 ± 63.85 d | |
| Na+ in leaves (µmol g−1 DW) | 0 | 499.48 ± 31.68 a | 275.66 ± 68.10 a |
| 200 | 2170.93 ± 121.15 b | 1990.42 ± 426.87 b | |
| 400 | 2644.25 ± 70.25 c | 2989.64 ± 160.04 c | |
| 600 | 2845.45 ± 308.40 c | 3042.36 ± 199.10 c | |
| 800 | 3033.51 ± 115.87 c | 4271.14 ± 135.61 d | |
| Cl− in roots (µmol g−1 DW) | 0 | 110.208 ± 19.83 a | 123.51 ± 28.55 a |
| 200 | 766.74 ± 75.35 b | 646.08 ± 85.31 b | |
| 400 | 1025.84 ± 83.21 c | 892.34 ± 55.12 c | |
| 600 | 1383.95 ± 72.81 d | 1120.20 ± 83.55 d | |
| 800 | 1575.37 ± 55.61 d | 1473.89 ± 111.90 e | |
| Cl− in leaves (µmol g−1 DW) | 0 | 437.95 ± 32.76 a | 671.27 ± 31.86 a |
| 200 | 1120.56 ± 67.86 b | 1530.25 ± 74.81 b | |
| 400 | 1451.80 ± 35.61 c | 1723.05 ± 32.51 b | |
| 600 | 1508.57 ± 137.70 c | 1746.18 ± 110.38 b | |
| 800 | 1689.12 ± 65.28 c | 2472.19 ± 75.76 c | |
| K+ in roots (µmol g−1 DW) | 0 | 180.38 ± 31.32 a | 149.00 ± 18.31 b |
| 200 | 222.38 ± 26.56 a | 93.85 ± 24.03 ab | |
| 400 | 172.02 ± 26.04 a | 64.62 ± 12.72 a | |
| 600 | 177.11 ± 11.67 a | 78.94 ± 17.08 a | |
| 800 | 170.94 ± 16.49 a | 146.49 ± 9.15 b | |
| K+ in leaves (µmol g−1 DW) | 0 | 607.93 ± 31.41 b | 774.63 ± 78.57 b |
| 200 | 410.06 ± 25.32 a | 490.56 ± 30.28 a | |
| 400 | 458.93 ± 19.28 a | 494.16 ± 38.64 a | |
| 600 | 436.83 ± 35.94 a | 533.84 ± 81.54 a | |
| 800 | 414.70 ± 6.06 a | 444.43 ± 32.07 a | |
| Ca2+ in roots (µmol g−1 DW) | 0 | 30.16 ± 2.14 a | 43.00 ± 9.44 a |
| 200 | 106.50 ± 9.37 b | 84.50 ± 8.96 b | |
| 400 | 121.91 ± 13.30 bc | 130.32 ± 5.31 c | |
| 600 | 145.20 ± 14.16 c | 129.29 ± 3.73 c | |
| 800 | 191.83 ± 5.00 d | 176.31 ± 4.11 d | |
| Ca2+ in leaves (µmol g−1 DW) | 0 | 249.57 ± 13.90 a | 210.18 ± 14.96 a |
| 200 | 305.30 ± 15.32 ab | 322.30 ± 24.60 b | |
| 400 | 360.52 ± 18.77 b | 394.36 ± 23.20 b | |
| 600 | 302.40 ± 30.14 ab | 341.84 ± 10.12 b | |
| 800 | 353.95 ± 19.94 b | 349.03 ± 38.56 b | |
| Parameter | A (Species) | B (Treatment) | C (Organ) | A × B Interaction | A × C Interaction | B × C Interaction | A × B × C Interaction |
|---|---|---|---|---|---|---|---|
| Leaf (number) | 0.000 *** | 0.498 | - | 0.004 ** | - | - | - |
| FW | 0.000 *** | 0.007 ** | 0.000 *** | 0.002 ** | 0.000 *** | 0.081 ns | 0.032 * |
| WC% | 0.001 ** | 0.631 ns | 0.407 ns | 0.471 ns | 0.382 ns | 0.586 ns | 0.466 ns |
| Chl a | 0.062 ns | 0.030 * | - | 0.533 ns | - | - | - |
| Chl b | 0.000 *** | 0.180 ns | - | 0.350 ns | - | - | - |
| Caro | 0.002 ** | 0.098 ns | - | 0.006 ** | - | - | - |
| Na+ | 0.000 *** | 0.000 *** | 0.000 *** | 0.010 ** | 0.000 *** | 0.481 ns | 0.039 * |
| K+ | 0.000 *** | 0.899 ns | 0.242 ns | 0.241 ns | 0.000 *** | 0.000 *** | 0.465 ns |
| Cl− | 0.000 *** | 0.000 *** | 0.000 *** | 0.039 * | 0.018 * | 0.000 *** | 0.026 * |
| Ca2+ | 0.000 *** | 0.862 ns | 0.000 *** | 0.088 ns | 0.010 * | 0.326 ns | 0.259 ns |
| Group of Compounds | Parameter | S | T | S × T |
|---|---|---|---|---|
| Carbohydrates | Erythritol | 0.0099 ** | 0.0000 *** | 0.0419 ** |
| Fructose | 0.0000 *** | 0.0408 ** | 0.0660 * | |
| Glucose | 0.6060 ns | 0.0082 ** | 0.2290 ns | |
| Glycerol | 0.1200 ns | 0.4000 ns | 0.2700 ns | |
| Myoinositol | 0.5321 ns | 0.0109 * | 0.8501 ns | |
| Raffinose | 0.2707 ns | 0.0000 *** | 0.0097 ** | |
| Rhamnose | 0.0191 * | 0.0000 *** | 0.6961 ns | |
| Sucrose | 0.0240 * | 0.0000 *** | 0.0650 ns | |
| Inorganic Acids | Phosphoric acid | 0.3124 ns | 0.4720 ns | 0.2133 ns |
| Organic Acids | Citric acid | 0.0044 ** | 0.0907 ns | 0.0303 * |
| Glyceric acid | 0.3516 ns | 0.0000 *** | 0.0210 * | |
| Maleic acid | 0.2020 ns | 0.0840 ns | 0.3300 ns | |
| Malic acid | 0.0737 ns | 0.0533 ns | 0.0201 * | |
| Succinic acid | 0.6010 ns | 0.5586 ns | 0.0064 ** | |
| Threonic acid | 0.0018 ** | 0.0000 *** | 0.0754 ns | |
| Amino Acids | Alanine | 0.6222 ns | 0.0034 ** | 0.2083 ns |
| Asparagine | 0.9672 ns | 0.0322 * | 0.0053 ** | |
| Aspartic acid | 0.1198 ns | 0.0064 ** | 0.0039 ** | |
| GABA | 0.2916 ns | 0.4754 ns | 0.1937 ns | |
| Glutamic acid | 0.0000 *** | 0.5378 ns | 0.5780 ns | |
| Glutamine | 0.0000 *** | 0.0000 *** | 0.0000 *** | |
| Glycine | 0.7987 ns | 0.0669 ns | 0.0084 ** | |
| Isoleucine | 0.0115 * | 0.0009 *** | 0.0343 * | |
| Leucine | 0.4357 ns | 0.0024 ** | 0.0102 * | |
| Lysine | 0.0335 * | 0.0001 *** | 0.0975 ns | |
| Phenylalanine | 0.9134 ns | 0.0000 *** | 0.0000 *** | |
| Proline | 0.2250 ns | 0.0000 *** | 0.0353 * | |
| Pyroglutamic acid | 0.0000 *** | 0.0003 ** | 0.0003 ** | |
| Serine | 0.6344 ns | 0.0006 *** | 0.1424 ns | |
| Threonine | 0.0002 ** | 0.0000 *** | 0.0564 ns | |
| Tryptophan | 0.1810 ns | 0.0170 * | 0.0353 * | |
| Valine | 0.0422 * | 0.0000 *** | 0.0106 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites 2019, 9, 294. https://doi.org/10.3390/metabo9120294
González-Orenga S, Ferrer-Gallego PP, Laguna E, López-Gresa MP, Donat-Torres MP, Verdeguer M, Vicente O, Boscaiu M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites. 2019; 9(12):294. https://doi.org/10.3390/metabo9120294
Chicago/Turabian StyleGonzález-Orenga, Sara, P. Pablo Ferrer-Gallego, Emilio Laguna, M. Pilar López-Gresa, Maria P. Donat-Torres, Mercedes Verdeguer, Oscar Vicente, and Monica Boscaiu. 2019. "Insights on Salt Tolerance of Two Endemic Limonium Species from Spain" Metabolites 9, no. 12: 294. https://doi.org/10.3390/metabo9120294
APA StyleGonzález-Orenga, S., Ferrer-Gallego, P. P., Laguna, E., López-Gresa, M. P., Donat-Torres, M. P., Verdeguer, M., Vicente, O., & Boscaiu, M. (2019). Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites, 9(12), 294. https://doi.org/10.3390/metabo9120294












