Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows
Abstract
1. Introduction
2. Results
2.1. Sow Performance and Body Condition
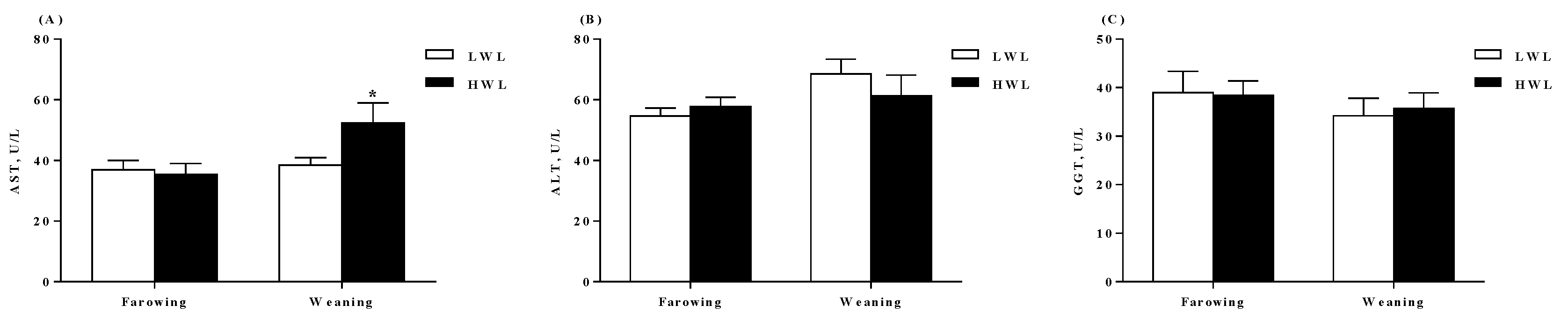
2.2. Plasma Biochemical Profiles, Colostrum and Milk Composition
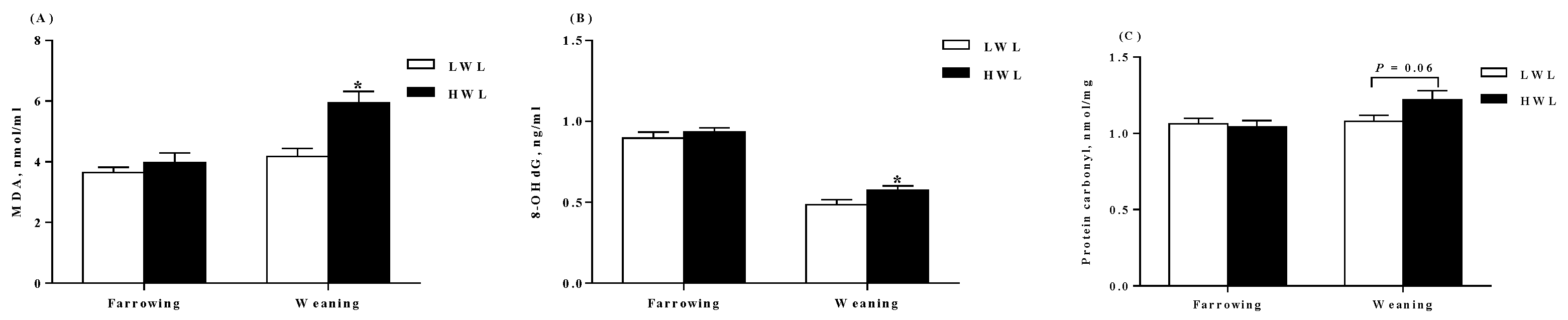
2.3. Plasma Antioxidant Parameters
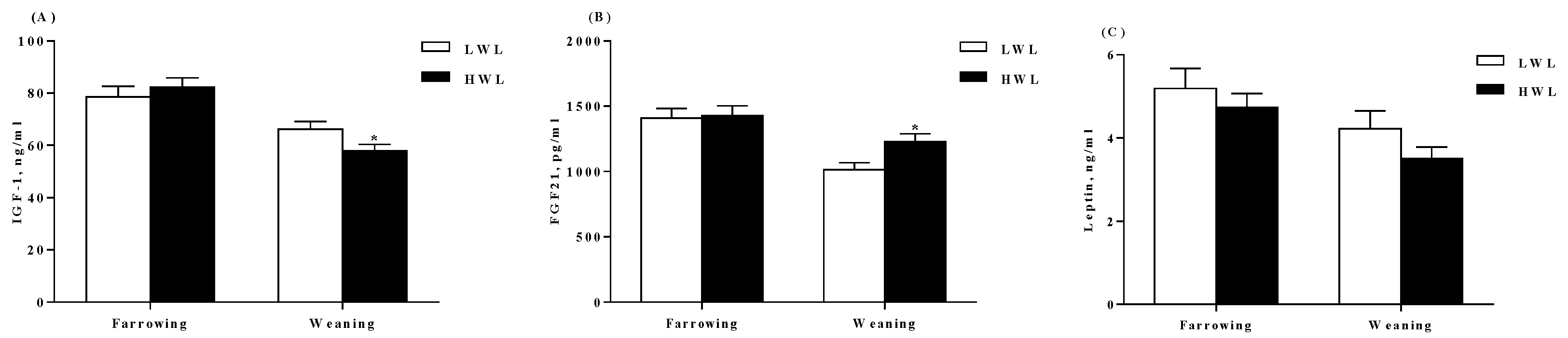
2.4. Plasma Hormone Determination
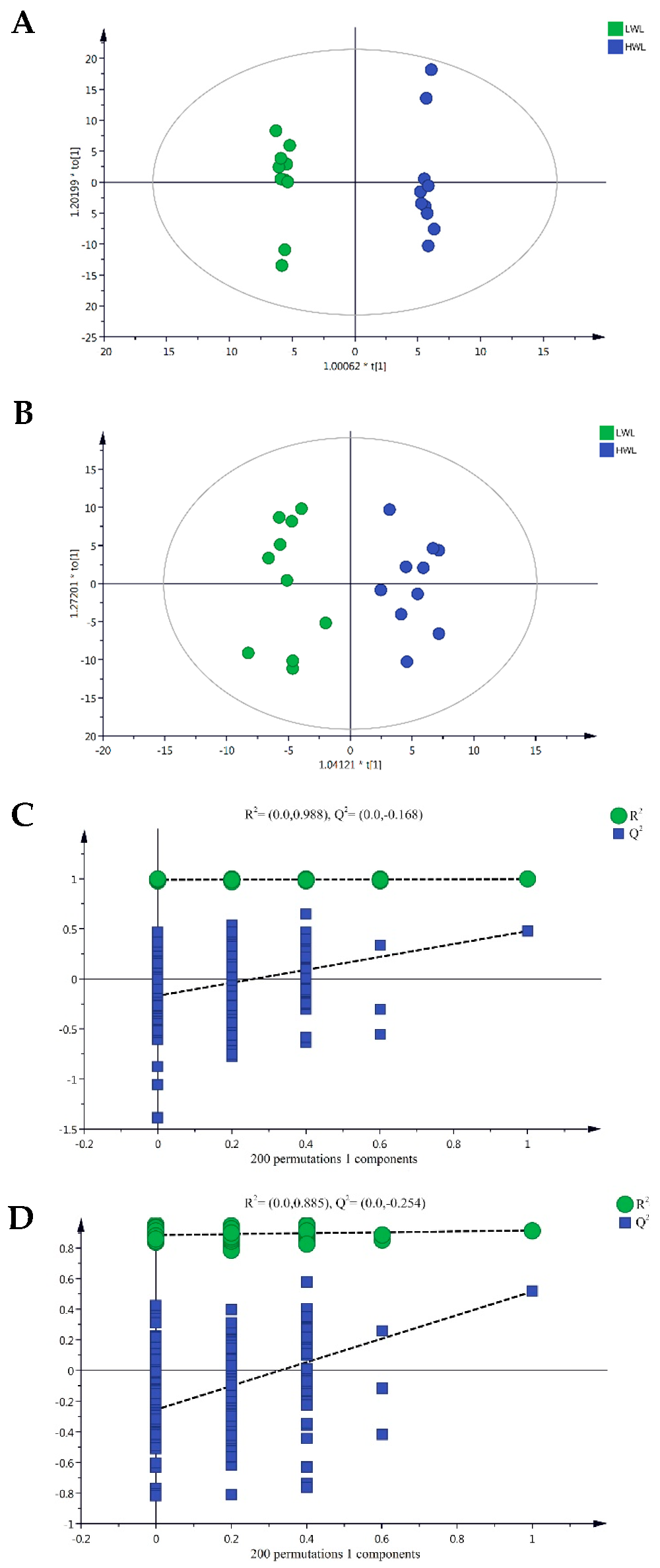
2.5. Plasma Metabolic Profiling Based on Ultrahigh-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry
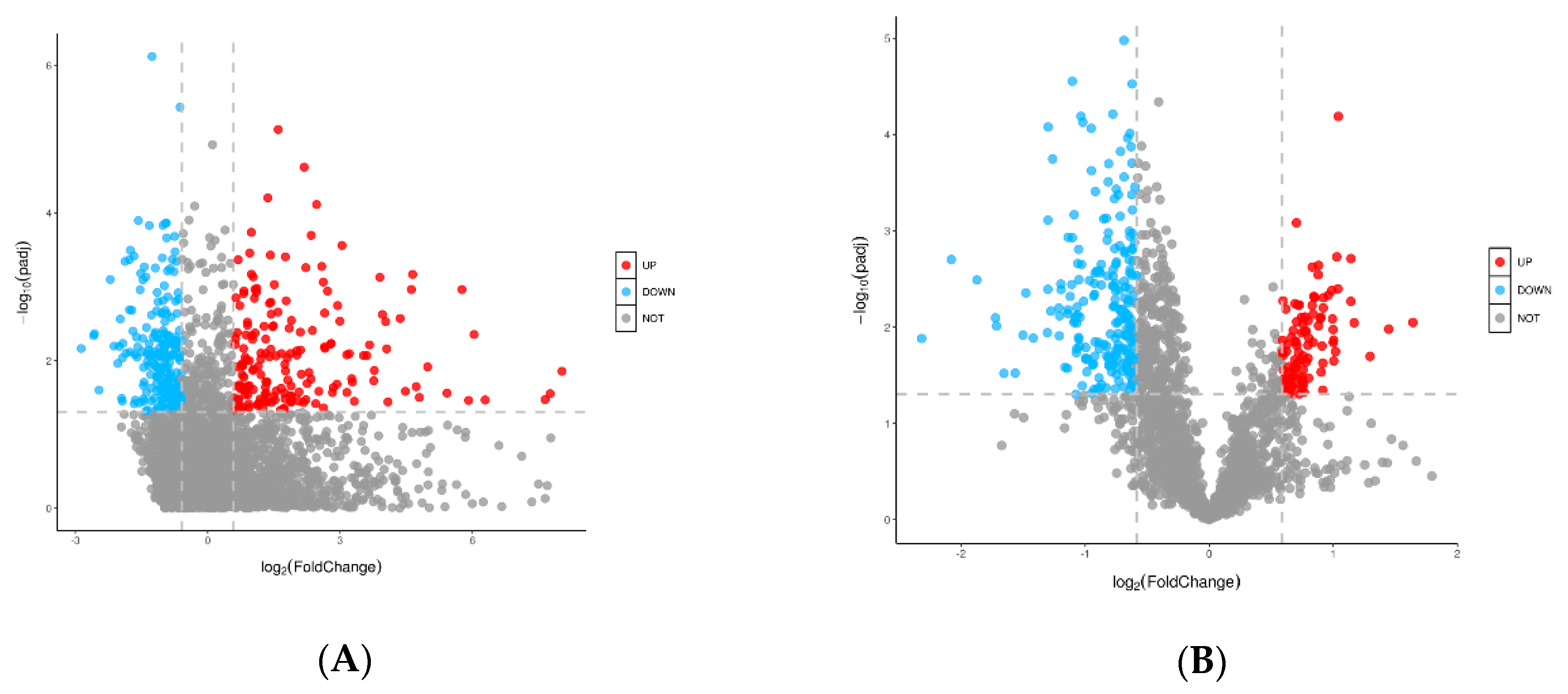
2.6. Identification of Different Metabolites
2.7. Integration of Key Metabolic Pathways
3. Discussion
3.1. Reproductive Performance and Plasma Hormones
3.2. Milk Composition
3.3. Oxidative Status
3.4. Amino Acids Metabolism-Related
3.5. Fat Metabolism-Related
3.6. Metabolic Dysfunction of Nucleotides, Bile Acids, and Vitamins
4. Materials and Methods
4.1. Animals, Diets, and Experiment Design
4.2. Sampling and Data Collection
4.3. Determination of Colostrum and Milk Composition
4.4. Measurement of Antioxidant Parameters in the Plasma
4.5. Measurement of Biochemical Parameters and Hormones in the Plasma
4.6. Metabolomics Based on Ultra-High-Performance Liquid Chromatography Time-of-Flight/Mass Spectrometry
4.7. Data Processing and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eissen, J.J.; Apeldoorn, E.J.; Kanis, E.; Verstegen, M.W.; de Greef, K.H. The importance of a high feed intake during lactation of primiparous sows nursing large litters. J. Anim. Sci. 2003, 81, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, Y.; Dial, G.D.; Pettigrew, J.E.; King, V.L. Feed intake pattern during lactation and subsequent reproductive performance of sows. J. Anim. Sci. 1996, 74, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.Y.; Bilkei, G. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 2005, 88, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Strathe, A.V.; Bruun, T.S.; Hansen, C.F. Sows with high milk production had both a high feed intake and high body mobilization. Animal 2017, 11, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, A.C.; Bernardi, M.L.; Bortolozzo, F.P.; Wentz, I. Body reserve mobilization during lactation in first parity sows and its effect on second litter size. Livest. Sci. 2010, 132, 165–172. [Google Scholar] [CrossRef]
- Prunier, A.; Quesnel, H. Influence of the nutritional status on ovarian development in female pigs. Anim. Reprod. Sci. 2000, 60–61, 185–197. [Google Scholar] [CrossRef]
- Hoving, L.L.; Soede, N.M.; Feitsma, H.; Kemp, B. Lactation Weight Loss in Primiparous Sows: Consequences for Embryo Survival and Progesterone and Relations with Metabolic Profiles. Reprod. Domest. Anim. 2012, 47, 1009–1016. [Google Scholar] [CrossRef]
- Wientjes, J.G.; Soede, N.M.; Knol, E.F.; van den Brand, H.; Kemp, B. Piglet birth weight and litter uniformity: Effects of weaning-to-pregnancy interval and body condition changes in sows of different parities and crossbred lines. J. Anim. Sci. 2013, 91, 2099–2107. [Google Scholar] [CrossRef]
- Quesnel, H.; Rodriguez-Martinez, H.; Vallet, J.; Ziecik, A. Nutritional and lactational effects on follicular development in the pig. Control Pig Reprod. VIII 2009, 8, 121–134. [Google Scholar]
- Ramsay, T.G.; Stoll, M.J.; Shannon, A.E.; Blomberg, L.A. Metabolomic analysis of longissimus from underperforming piglets relative to piglets with normal preweaning growth. J. Anim. Sci. Biotechnol. 2018, 9, 36. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Ming, D.; Wang, J.; Li, Z.; Ma, X.; Wang, J.; van Milgen, J.; Wang, F. Integrative analysis of indirect calorimetry and metabolomics profiling reveals alterations in energy metabolism between fed and fasted pigs. J. Anim. Sci. Biotechnol. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress—Clinical correlates of oxidative stress in the Framingham Study. Arterioscl. Throm. Vas. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Morrow, J.D. Is oxidant stress a connection between obesity and atherosclerosis? Arterioscl. Throm. Vas. 2003, 23, 368–370. [Google Scholar] [CrossRef]
- Rojkittikhun, T.; Einarsson, S.; Uvnas-Moberg, K.; Edqvist, L.E. Body weight loss during lactation in relation to energy and protein metabolism in standard-fed primiparous sows. Zentralbl. Veterinarmed. A 1993, 40, 249–257. [Google Scholar] [CrossRef]
- Vinsky, M.D.; Novak, S.; Dixon, W.T.; Dyck, M.K.; Foxcroft, G.R. Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development. Reprod. Fertil. Dev. 2006, 18, 347–355. [Google Scholar] [CrossRef]
- Kemp, B.; Soede, N.M. Should weaning be the start of the reproductive cycle in hyper-prolific sows? A physiological view. Reprod. Domest. Anim. 2012, 47, 320–326. [Google Scholar] [CrossRef]
- Hulten, F.; Valros, A.; Rundgren, M.; Einarsson, S. Reproductive endocrinology and postweaning performance in the multiparous sow. Part 1. Influence of metabolic status during lactation. Theriogenology 2002, 58, 1503–1517. [Google Scholar] [CrossRef]
- Mosnier, E.; Etienne, M.; Ramaekers, P.; Père, M.C. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest. Sci. 2010, 127, 127–136. [Google Scholar] [CrossRef]
- Costermans, N.G.J.; Teerds, K.J.; Middelkoop, A.; Roelen, B.A.J.; Schoevers, E.J.; van Tol, H.T.A.; Laurenssen, B.; Koopmanschap, R.E.; Zhao, Y.; Blokland, M.; et al. Consequences of negative energy balance on follicular development and oocyte quality in primiparous sows. Biol. Reprod. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, K.M.; Giesy, S.L.; Harvatine, K.J.; Waldron, M.R.; Cheng, C.; Kharitonenkov, A.; Boisclair, Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 2011, 152, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Brown, S.A.; Le, P.T.; Wang, X.; DeMambro, V.; Horowitz, M.C.; MacDougald, O.; Baron, R.; Lotinun, S.; Karsenty, G.; et al. FGF21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology 2014, 155, 3516–3526. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Hua, L.; Feng, B.; Jiang, X.M.; Li, J.; Jiang, D.D.; Huang, X.H.; Zhu, Y.G.; Li, Z.; Yan, L.J.; et al. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. Ebiomedicine 2019, 41, 623–635. [Google Scholar] [CrossRef]
- Owen, B.M.; Bookout, A.L.; Ding, X.; Lin, V.Y.; Atkin, S.D.; Gautron, L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 2013, 19, 1153–1156. [Google Scholar] [CrossRef]
- Beyer, M.; Jentsch, W.; Kuhla, S.; Wittenburg, H.; Kreienbring, F.; Scholze, H.; Rudolph, P.E.; Metges, C.C. Effects of dietary energy intake during gestation and lactation on milk yield and composition of first, second and fourth parity sows. Arch. Anim. Nutr. 2007, 61, 452–468. [Google Scholar] [CrossRef]
- Noblet, J.; Etienne, M. Effect of energy level in lactating sows on yield and composition of milk and nutrient balance of piglets. J. Anim. Sci. 1986, 63, 1888–1896. [Google Scholar] [CrossRef]
- Revell, D.K.; Williams, I.H.; Mullan, B.P.; Ranford, J.L.; Smits, R.J. Body composition at farrowing and nutrition during lactation affect the performance of primiparous sows: II. Milk composition, milk yield, and pig growth. J. Anim. Sci. 1998, 76, 1738–1743. [Google Scholar] [CrossRef]
- Hu, L.; Peng, X.; Qin, L.; Wang, R.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Wu, D.; Che, L. Dietary nucleotides supplementation during the suckling period improves the antioxidative ability of neonates with intrauterine growth retardation when using a pig model. RSC Adv. 2018, 8, 16152–16160. [Google Scholar] [CrossRef]
- Cao, S.X.; Dhahbi, J.M.; Mote, P.L.; Spindler, S.R. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc. Natl. Acad. Sci. USA 2001, 98, 10630–10635. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Simm, A.; Brömme, H.J. Reactive oxygen species (ROS) and aging: Do we need them—can we measure them—Should we block them? Signal Transduct. 2005, 5, 115–125. [Google Scholar] [CrossRef]
- Yang, Y.X.; Heo, S.; Jin, Z.; Yun, J.H.; Choi, J.Y.; Yoon, S.Y.; Park, M.S.; Yang, B.K.; Chae, B.J. Effects of lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in primiparous and multiparous sows. Anim. Reprod. Sci. 2009, 112, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Stanstrup, J.; Thymann, T.; Sangild, P.T.; Dragsted, L.O. Progressive Changes in the Plasma Metabolome during Malnutrition in Juvenile Pigs. J. Proteome Res. 2016, 15, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Vanniekerk, B.D.H.; Reid, J.T.; Paladines, O.L.; Bensadoun, A. Urinary Creatinine as an Index of Body Composition. J. Nutr. 1963, 79, 463–473. [Google Scholar] [CrossRef]
- Balasse, E.O.; Fery, F. Ketone-Body Production and Disposal—Effects of Fasting, Diabetes, and Exercise. Diabetes Metab. Rev. 1989, 5, 247–270. [Google Scholar] [CrossRef]
- Ruderman, N.B. Muscle Amino-Acid Metabolism and Gluconeogenesis. Annu. Rev. Med. 1975, 26, 245–258. [Google Scholar] [CrossRef]
- Sanahuja, J.C.; Rio, M.E.; Lede, M.N. Decrease in Appetite and Biochemical Changes in Amino Acid Imbalance in the Rat. J. Nutr. 1965, 86, 424–432. [Google Scholar] [CrossRef]
- Hill, A.; Blundell, J. Role of amino acids in appetite control in man. In Amino Acid Availability and Brain Function in Health and Disease; Springer: Berlin/Heidelberg, Germany, 1988; pp. 239–248. [Google Scholar]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Rempel, L.A.; Miles, J.R.; Oliver, W.T.; Broeckling, C.D. Non-targeted Plasma Metabolome of Early and Late Lactation Gilts. Front. Mol. Biosci. 2016, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, C.B. An introduction to the nutrition and metabolism of choline. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xue, Y.; Seddik, H.-E.; Yin, Y.; Hu, F.; Mao, S. Dynamic Changes of Plasma Metabolome in Response to Severe Feed Restriction in Pregnant Ewes. Metabolites 2019, 9, 112. [Google Scholar] [CrossRef]
- Leroy, J.L.; Van Soom, A.; Opsomer, G.; Bols, P.E. The consequences of metabolic changes in high-yielding dairy cows on oocyte and embryo quality. Animal 2008, 2, 1120–1127. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Wellner, N.; Diep, T.A.; Janfelt, C.; Hansen, H.S. N-acylation of phosphatidylethanolamine and its biological functions in mammals. BBA Mol. Cell Biol. Lipids 2013, 1831, 652–662. [Google Scholar] [CrossRef]
- Greig, F.H.; Kennedy, S.; Spickett, C.M. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic Biol Med. 2012, 52, 266–280. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Gamble, J. Acetyl-Coa Carboxylase—An Important Regulator of Fatty-Acid Oxidation in the Heart. Can. J. Physiol. Pharmacol. 1994, 72, 1101–1109. [Google Scholar] [CrossRef]
- Fu, Z.D.; Klaassen, C.D. Increased bile acids in enterohepatic circulation by short-term calorie restriction in male mice. Toxicol. Appl. Pharmacol. 2013, 273, 680–690. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Feng, C.; Bai, K.; Wang, A.; Ge, X.; Zhao, Y.; Zhang, L.; Wang, T. Effects of dimethylglycine sodium salt supplementation on growth performance, hepatic antioxidant capacity, and mitochondria-related gene expression in weanling piglets born with low birth weight. J. Anim. Sci. 2018, 96, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lou, B.S.; Hsu, F.L.; Hou, C.C. Use of urinary metabolomics to evaluate the effect of hyperuricemia on the kidney. Food Chem. Toxicol. 2014, 74, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Guo, C.; Hu, F.; Liu, J.; Mao, S. Hepatic Metabolic Profile Reveals the Adaptive Mechanisms of Ewes to Severe Undernutrition during Late Gestation. Metabolites 2018, 8, 85. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef]
- Che, L.; Hu, L.; Wu, C.; Xu, Q.; Zhou, Q.; Peng, X.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of increased energy and amino acid intake in late gestation on reproductive performance, milk composition, metabolic, and redox status of sows. J. Anim. Sci. 2019, 97, 2914–2926. [Google Scholar] [CrossRef]
- Hou, X.; Wang, T.; Ahmad, H.; Xu, Z. Ameliorative effect of ampelopsin on LPS-induced acute phase response in piglets. J. Funct. Foods 2017, 35, 489–498. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Zhu, Y.-F.; Cai, L.-S.; Wu, T.-X. Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 2008, 280, 136–139. [Google Scholar] [CrossRef]
- Pialoux, V.; Mounier, R.; Rock, E.; Mazur, A.; Schmitt, L.; Richalet, J.P.; Robach, P.; Coudert, J.; Fellmann, N. Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int. J. Sports Med. 2009, 30, 87–93. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Jia, H.X.; Shen, X.T.; Guan, Y.; Xu, M.M.; Tu, J.; Mo, M.; Xie, L.; Yuan, J.; Zhang, Z.; Cai, S.J.; et al. Predicting the pathological response to neoadjuvant chemoradiation using untargeted metabolomics in locally advanced rectal cancer. Radiother. Oncol. 2018, 128, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- METLIN. Available online: http://metlin.scripps.edu (accessed on 24 October 2019).
- MetaboAnalyst-Statistical, Functional and Integrative Analysis of Metabolomics Data. Available online: http://www.metaboanalyst.ca (accessed on 24 October 2019).
- Dourmad, J.; Etienne, M.; Noblet, J.; Causeur, D. Prediction de la composition chimique des truies reproductrices a partir du poids vif et de l’epaisseur de lard dorsal. J. Rech. Porc. Fr. 1997, 29, 255–262. [Google Scholar]


| Item | LWL | HWL | p-Value |
|---|---|---|---|
| Total born piglets, no. | 15.54 ± 0.98 | 15.80 ± 0.96 | 0.854 |
| Live born piglets, no. | 14.46 ± 0.79 | 15.00 ± 0.91 | 0.958 |
| Stillborn piglets, no. | 0.92 ± 0.31 | 0.60 ± 0.27 | 0.539 |
| Mummified fetuses, no. | 0.15 ± 0.10 | 0.20 ± 0.14 | 0.749 |
| Total born | |||
| Litter birth weight, kg | 20.97 ± 1.26 | 19.22 ± 1.40 | 0.378 |
| Mean piglet BW, kg | 1.37 ± 0.06 | 1.24 ± 0.07 | 0.332 |
| Live born | |||
| Litter birth weight, kg | 19.74 ± 1.00 | 18.59 ± 1.35 | 0.492 |
| Mean piglet BW, kg | 1.39 ± 0.06 | 1.25 ± 0.07 | 0.306 |
| Duration of farrowing, min | 234.38 ± 20.30 | 250.47 ± 24.47 | 0.617 |
| Placenta weight, kg | 4.00 ± 0.19 | 4.17 ± 0.35 | 0.695 |
| Litter size after cross-foster, no. | 11.93 ± 0.55 | 12.30 ± 0.23 | 0.535 |
| ADFI (Day 0–21), kg/day | 5.22 ± 0.09 | 4.96 ± 0.08 | 0.037 |
| Litter weight at weaning, kg | 60.70 ± 3.87 | 67.38 ± 7.47 | 0.478 |
| Piglet ADG (Day 0–21), g/day | 246 ± 10 | 233 ± 12 | 0.433 |
| Item | LWL | HWL | p-Value |
|---|---|---|---|
| Body weight at gestation 110 day, kg | 282.67 ± 7.62 | 283.76 ± 4.50 | 0.899 |
| At parturition | |||
| Body weight, kg | 259.13 ± 7.36 | 263.82 ± 4.16 | 0.572 |
| Back fat depth, mm | 18.40 ± 0.90 | 18.94 ± 0.66 | 0.626 |
| Fat mass, kg | 53.40 ± 2.37 | 54.53 ± 1.20 | 0.675 |
| Protein mass, kg | 40.40 ± 1.14 | 41.18 ± 0.76 | 0.567 |
| At weaning | |||
| Body weight, kg | 251.67 ± 6.88 | 230.41 ± 3.64 | 0.008 |
| Back fat depth, mm | 15.07 ± 0.87 | 13.41 ± 0.67 | 0.140 |
| Fat mass, kg | 47.07 ± 2.15 | 40.35 ± 1.45 | 0.013 |
| Protein mass, kg | 40.27 ± 1.11 | 37.06 ± 0.56 | 0.018 |
| Lactation BW loss, kg | 7.67 ± 1.94 | 33.41 ± 1.60 | <0.001 |
| Lactation back fat loss, mm | 3.50 ± 0.25 | 5.35 ± 0.77 | 0.033 |
| Lactation BW loss, % | 2.89 ± 0.74 | 12.65 ± 0.53 | <0.001 |
| Calculated sow fat mass | |||
| Lactation fat loss, kg | 6.29 ± 0.45 | 14.22 ± 1.05 | <0.001 |
| Lactation fat loss, % | 11.86 ± 0.75 | 26.10 ± 1.80 | <0.001 |
| Calculated sow protein mass | |||
| Lactation protein loss, kg | 0.14 ± 0.37 | 3.93 ± 0.39 | <0.001 |
| Lactation protein loss, % | 0.27 ± 0.90 | 9.44 ± 0.82 | <0.001 |
| WEI, h | 112.00 ± 6.49 | 132.71 ± 7.16 | 0.029 |
| Item | LWL | HWL | p-Value |
|---|---|---|---|
| Colostrum | |||
| Fat, % | 4.42 ± 0.38 | 4.65 ± 0.39 | 0.674 |
| Protein, % | 19.24 ± 0.95 | 19.85 ± 0.98 | 0.656 |
| Lactose, % | 2.71 ± 0.20 | 2.60 ± 0.21 | 0.702 |
| Total solids, % | 27.07 ± 0.97 | 27.79 ± 1.19 | 0.637 |
| Urea nitrogen, mg/dl | 28.00 ± 1.14 | 26.72 ± 1.94 | 0.573 |
| Milk | |||
| Fat, % | 8.14 ± 0.34 | 8.86 ± 0.74 | 0.379 |
| Protein, % | 5.68 ± 0.22 | 5.85 ± 0.14 | 0.502 |
| Lactose, % | 5.35 ± 0.30 | 5.69 ± 0.20 | 0.342 |
| Total solids, % | 19.86 ± 0.73 | 21.10 ± 0.74 | 0.237 |
| Urea nitrogen, mg/dl | 24.77 ± 1.00 | 26.10 ± 1.34 | 0.429 |
| Item | LWL | HWL | p-Value |
|---|---|---|---|
| Farrowing | |||
| GLU, mmol/L | 3.65 ± 0.51 | 2.80 ± 0.43 | 0.207 |
| LAC, mmol/L | 2.24 ± 0.20 | 2.47 ± 0.15 | 0.368 |
| NEFA, μmol/L | 809.36 ± 71.18 | 848.00 ± 117.00 | 0.750 |
| TG, μmol/L | 251.15 ± 22.88 | 241.17 ± 32.25 | 0.732 |
| UREA, mmol/L | 3.48 ± 0.18 | 3.65 ± 0.21 | 0.537 |
| TP, g/L | 74.20 ± 2.44 | 73.26 ± 1.86 | 0.764 |
| ALB, g/L | 38.84 ± 1.00 | 38.58 ± 1.50 | 0.884 |
| Weaning | |||
| GLU, mmol/L | 4.15 ± 0.57 | 4.86 ± 0.63 | 0.200 |
| LAC, mmol/L | 2.45 ± 0.24 | 2.02 ± 0.19 | 0.210 |
| NEFA, μmol/L | 55.00 ± 8.11 | 80.55 ± 9.52 | 0.025 |
| TG, μmol/L | 342.64 ± 75.13 | 428.00 ± 76.32 | 0.450 |
| UREA, mmol/L | 7.02 ± 0.46 | 6.04 ± 0.77 | 0.148 |
| TP, g/L | 83.25 ± 1.70 | 80.66 ± 1.68 | 0.339 |
| ALB, g/L | 38.79 ± 0.90 | 38.51 ± 1.85 | 0.902 |
| Name | Ion | FC | p-Value | M-to-Z Ratio | RT (min) |
|---|---|---|---|---|---|
| Amino acid metabolism-related | |||||
| 3,4-Dihydroxybenzoate | [M − H]− | 2.22 | 0.001 | 153.0189 | 0.69 |
| 3-Phenylpropanoic acid | [M − H]− | 1.67 | 0.018 | 149.0606 | 2.24 |
| Betaine | [M + H]+ | 1.32 | 0.002 | 118.0856 | 8.58 |
| Creatine | [M + H]+ | 0.58 | 0.023 | 132.0760 | 11.06 |
| Creatinine | [M + H]+ | 1.23 | 0.017 | 114.0653 | 5.22 |
| Hippuric acid | [M + H]+ | 1.59 | 0.027 | 180.0647 | 6.28 |
| L-Carnosine | [M + H]+ | 1.69 | 0.001 | 227.1134 | 13.40 |
| L-Citrulline | [M + H]+ | 1.47 | 0.000 | 176.1024 | 12.46 |
| L-Phenylalanine | [M + H]+ | 1.28 | 0.048 | 166.0859 | 7.94 |
| L-Tyrosine | [M − H]− | 1.33 | 0.019 | 180.0667 | 9.38 |
| Salicylic acid | [M − H]− | 1.85 | 0.001 | 137.0240 | 1.01 |
| Succinate | [M − H]− | 1.39 | 0.003 | 117.0190 | 12.53 |
| Tyramine | [M + H − H2O]+ | 1.28 | 0.046 | 120.0800 | 7.93 |
| Hydroxyisocaproic acid | [M − H]− | 1.47 | 0.006 | 131.0710 | 3.45 |
| 2-Oxoadipic acid | [M − H2O − H]− | 1.04 | 0.088 | 141.0170 | 10.81 |
| L-Tryptophan | [M − H]− | 1.27 | 0.064 | 203.0827 | 7.91 |
| D-Proline | [M − H]− | 1.27 | 0.052 | 114.0559 | 9.93 |
| 3-Indolepropionic acid | [M + H]+ | 2.44 | 0.007 | 190.0854 | 3.22 |
| Fat metabolism-related | |||||
| Acetylcholine | [M + H]+ | 1.56 | 0.001 | 146.1169 | 11.96 |
| LysoPC [16:0] | [M + H]+ | 1.37 | 0.013 | 496.3382 | 5.80 |
| Propionic acid | [M + CH3COO]− | 1.96 | 0.030 | 133.0499 | 3.38 |
| Valeric acid | [M − H]− | 1.45 | 0.025 | 101.0604 | 3.29 |
| PC(18:1(9Z)/18:1(9Z)) | [M − H + 2Na]+ | 0.63 | 0.042 | 830.5659 | 1.41 |
| Acetylcarnitine | [M + H]+ | 1.25 | 0.079 | 204.1226 | 9.66 |
| Stearic acid | [M − H]− | 2.13 | 0.079 | 283.2644 | 1.68 |
| alpha-Linolenic acid | [M − H]− | 1.20 | 0.063 | 277.2172 | 1.25 |
| Nucleoside metabolism-related | |||||
| Adenosine | [M + H]+ | 0.49 | 0.000 | 268.1031 | 5.27 |
| Allantoin | [M + H]+ | 1.49 | 0.000 | 159.0505 | 5.63 |
| Thymidine | [M − H]− | 2.00 | 0.023 | 241.0832 | 3.03 |
| Hypoxanthine | [M − H]− | 1.22 | 0.069 | 135.0310 | 5.20 |
| Vitamin metabolism-related | |||||
| N1-Methyl-2-pyridone-5-carboxamide | [M − H]− | 1.43 | 0.016 | 151.0509 | 2.47 |
| Nicotinamide | [M + H]+ | 1.89 | 0.014 | 123.0543 | 1.54 |
| 4-Pyridoxic acid | [M + H]+ | 1.61 | 0.002 | 184.0597 | 1.11 |
| L-Gulonic gamma-lactone | [M − H]− | 0.69 | 0.041 | 177.0405 | 4.33 |
| Anthranilic acid (Vitamin L1) | [M + H]+ | 1.37 | 0.075 | 138.0542 | 9.18 |
| Bile acid metabolism-related | |||||
| Cholic acid | [M + H − H2O]+ | 3.70 | 0.003 | 391.2834 | 3.46 |
| Chenodeoxycholate | [M − H]− | 2.22 | 0.027 | 391.2854 | 4.85 |
| Glycochenodeoxycholate | [M + H]+ | 2.08 | 0.013 | 450.3206 | 6.88 |
| Glycocholic acid | [M − H]− | 1.89 | 0.028 | 464.3015 | 7.75 |
| Others | |||||
| 1-Methylxanthine | [M + K − 2H]− | 1.61 | 0.036 | 203.0018 | 0.66 |
| D-Mannose | [M + NH4]+ | 0.79 | 0.042 | 198.0964 | 9.49 |
| Salicyluric acid | [M − H]− | 2.70 | 0.014 | 194.0457 | 6.98 |
| D-Quinovose | [M + CH3COO]− | 1.67 | 0.022 | 223.0823 | 6.61 |
| Pyrocatechol | [M − H]− | 1.79 | 0.032 | 109.0289 | 0.65 |
| Trimethylamine N-oxide | [M + H]+ | 2.44 | 0.004 | 76.0751 | 10.41 |
| Constituent | % | Nutritional Value | % |
|---|---|---|---|
| Corn | 59.86 | Metabolizable energy, Mcal/kg | 3.41 |
| Soybean meal | 17.68 | Crude protein | 17.20 |
| Fish meal | 2.00 | Lysine | 1.00 |
| Extruded soybean | 6.00 | Methionine | 0.27 |
| Wheat | 3.59 | Threonine | 0.63 |
| Soybean hull | 5.00 | Tryptophan | 0.18 |
| Limestone | 0.87 | Neutral detergent fiber | 11.00 |
| Calcium hydrophosphate | 0.93 | Calcium | 0.90 |
| Vitamin and mineral mix 1 | 0.63 | Available phosphorus | 0.45 |
| Salt | 0.40 | Crude fiber | 4.10 |
| Soybean oil | 2.00 | Ether extract | 6.10 |
| l-Lysine HCl (98.5%) | 0.25 | - | - |
| l-Threonine (98.5%) | 0.09 | - | - |
| dl-Methionine (99%) | 0.05 | - | - |
| Choline chloride (50%) | 0.15 | - | - |
| Potassium chloride | 0.50 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Che, L.; Wu, C.; Curtasu, M.V.; Wu, F.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; et al. Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows. Metabolites 2019, 9, 295. https://doi.org/10.3390/metabo9120295
Hu L, Che L, Wu C, Curtasu MV, Wu F, Fang Z, Lin Y, Xu S, Feng B, Li J, et al. Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows. Metabolites. 2019; 9(12):295. https://doi.org/10.3390/metabo9120295
Chicago/Turabian StyleHu, Liang, Lianqiang Che, Chen Wu, Mihai Victor Curtasu, Fali Wu, Zhengfeng Fang, Yan Lin, Shengyu Xu, Bin Feng, Jian Li, and et al. 2019. "Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows" Metabolites 9, no. 12: 295. https://doi.org/10.3390/metabo9120295
APA StyleHu, L., Che, L., Wu, C., Curtasu, M. V., Wu, F., Fang, Z., Lin, Y., Xu, S., Feng, B., Li, J., Zhuo, Y., Theil, P. K., & Wu, D. (2019). Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows. Metabolites, 9(12), 295. https://doi.org/10.3390/metabo9120295







