Abstract
The members of Gentiana genus are widely distributed in the Caucasus region where they are used as phytoremedies, but they still have not been studied for their chemical composition and bioactivity. High-performance liquid chromatography with diode array and electrospray triple quadrupole mass detection (HPLC-DAD-ESI-QQQ-MS) was used to investigate metabolites of herb and roots of six gentians (Gentiana asclepiadea, G. cruciata, G. gelida, G. paradoxa, G. pneumonanthe, G. septemfida) grown in the Caucasus. In total, 137 compounds were found including three carbohydrates, 71 iridoid glycosides (mostly loganic acid), loganin, swertiamarin, gentiopicroside and sweroside derivatives, 40 flavones C-, O-, C,O-glycosides (such as luteolin, apigenin, chrysoeriol, and acacetin derivatives), two phenolic O-glycosides, five hydroxycinnamates, eight xanthones, and seven triterpene glycosides. Most of these compounds were identified in gentian samples for the first time. Quantitative differences were found in levels of seven iridoid glycosides, nine glycosylflavones, and two xanthones obtained by HPLC-DAD assay. The gentian extracts were evaluated for their radical-scavenging properties against DPPH and superoxide anion radicals, lipid peroxidation inhibition, and α-amylase/α-glycosidase inhibition. The herb extracts showed higher activity than root extracts. Positive correlations were found between the content of quantified phenolics and antioxidant and digestive enzymes inhibiting activity. The findings presented in our work suggest that the Caucasian gentians are a good source of bioactive phytocompounds with antioxidant and antidiabetic potential.
1. Introduction
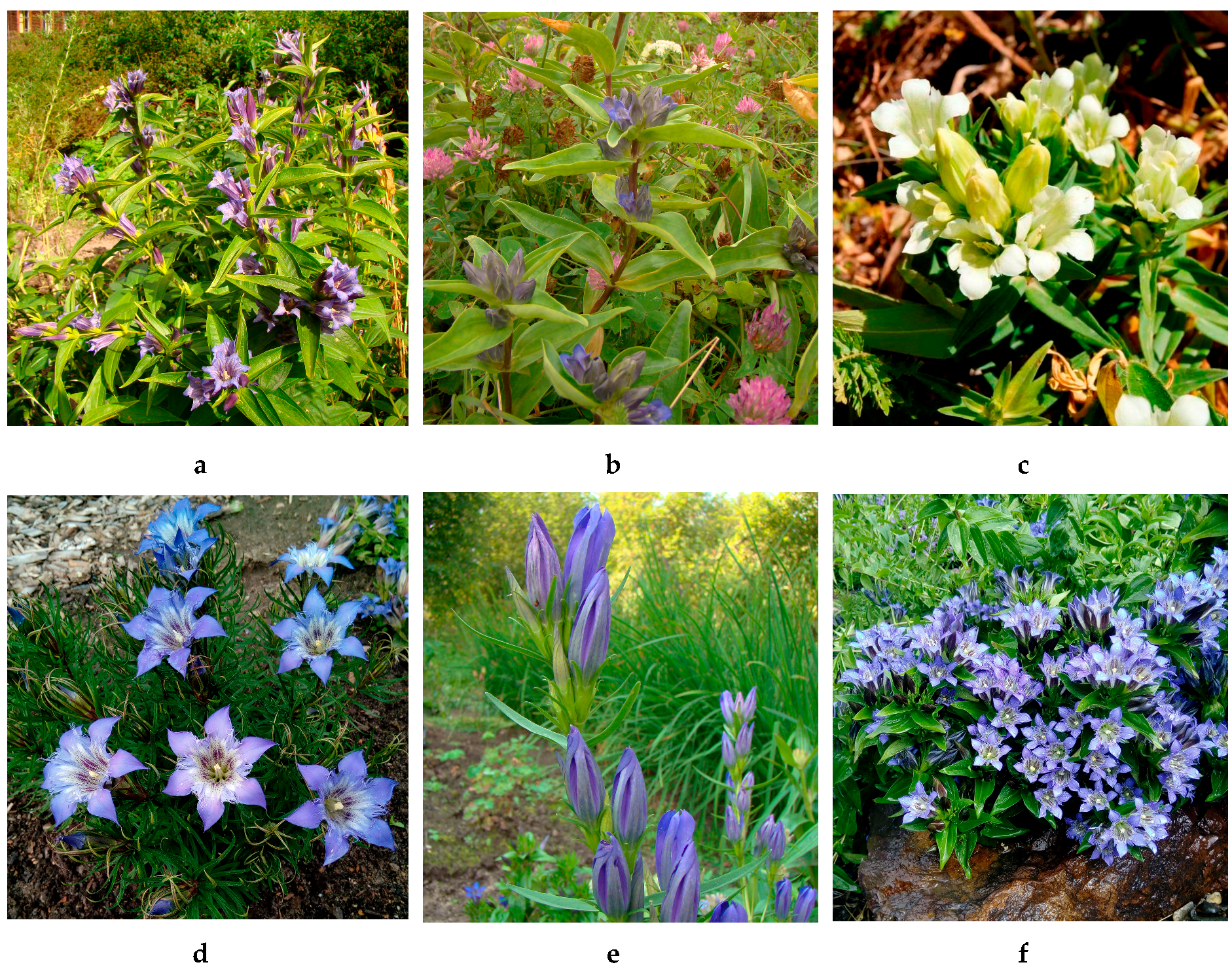
Gentiana L. is a cosmopolitan gentianaceous genus involving about 360 species with a wide distribution in both hemispheres [1]. The diversity of forms and the broad range of ecological tolerance has allowed the gentians to adapt to the various natural conditions. As a rich phytogeographic region, the Caucasus was no exception and this region demonstrated the presence of about 20 species [2]. Many gentians were used in the traditional medical systems of the Caucasus native peoples. However, the latest published paper about medical plants of Caucasus often overlooked aspects of the gentians’ application in traditional medical practice despite their wide use by native peoples [3,4,5,6]. The known ethnopharmacological data on uses of the gentians as medical plants are various, and the plants have demonstrated a wide spectrum of pathology treatments (Supplementary Materials, Table S1). The most frequently used Gentiana species in the Caucasus region are G. asclepiadea (willow gentian), G. cruciata (star gentian), G. gelida (cold gentian), G. paradoxa (peculiar gentian), G. pneumonanthe (marsh gentian), and G. septemfida (crested gentian) (Figure 1). There are usual therapeutic recommendations for these species such as for appetizers, antipyretics, and antidiabetics. [7,8]. The various native peoples of Caucasus region also used mentioned species as remedies against hepatitis, anaemia, stomach pain, malaria, haemorrhoid, tuberculosis, bronchitis, and pneumonia [9,10,11].
Figure 1.
Caucasian gentians studied in present work: Gentiana asclepiadea (a), G. cruciata (b), G. gelida (c), G. paradoxa (d), G. pneumonanthe (e), G. septemfida (f).
Some chemical aspects of five species (G. asclepiadea, G. cruciata, G. gelida, G. pneumonanthe, G. septemfida) were shown previously, but G. paradoxa is still an unstudied species. The known data include information about flavonoids (flavones only) in four species [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], xanthones (mangiferin, gentisin and its glycosides) in three species [15,16,17,18,20,26,27], iridoid glycosides in five species [18,19,22,27,28,29,30,31,32], monoterpenes in G. pneumonanthe [32], and triterpenes and naphthodipyranodione in G. asclepiadea [32,33]. In total, thirty-nine compounds were found in five gentians with most diverse classes of flavonoids and iridoid glycosides (Table S2). We also establish some facts about pharmacological uses of G. asclepiadea and G. cruciate, and the spectrum of gentians’ bioactivity includes cytotoxic [34], antimicrobial [35], antigenotoxic [36], antioxidant [37], anticholinesterase [19], hepatoprotective [38], and antibiofilm potential [39]. Based on this short review, it is obvious that Caucasian gentians should receive more attention as sources of phytocompounds and bioactive plant remedies.
It is particularly significant that high-performance liquid chromatography (HPLC) profiling with diode array detection and/or mass detection was performed only for two species, (G. asclepiadea [37], G. cruciata [22]), although in limited manner. Therefore, a comprehensive and comparative study is needed for a clear understanding of the chemo-diversity of Caucasian gentians. The antioxidant studies of gentians are for the same reason and also essential to identify antioxidant principles of plants. The information about antidiabetic activity of selected gentians is still unknown; so, it would be useful to know their potential against key enzymes of carbohydrate metabolism such as α-amylase and α-glycosidase [40]. The aim of present paper was to profile soluble metabolites of six gentian herbs and roots using high-performance liquid chromatography with diode array and electrospray triple quadrupole mass detection (HPLC-DAD-ESI-QQQ-MS) techniques and to quantify selected flavonoids, xanthones and iridoid glycosides in gentian plants. In this paper, we also make a comparative study of antioxidant activity and the digestive enzyme inhibition potential of gentian extracts, and we found active compounds which were the bioactive principles of the gentians.
2. Results and Discussion
2.1. Liquid Chromatography Mass Spectrometric (LC-MS) Metabolite Profiling of Six Caucasian Gentians: Chemodiversity of Herbs and Roots
An assay based on high-performance liquid chromatography with diode array and electrospray triple quadrupole mass detection (HPLC-DAD-ESI-QQQ-MS) was used to profile soluble metabolites of the herbs and roots of Gentiana asclepiadea, G. cruciata, G. gelida, G. paradoxa, G. pneumonanthe, and G. septemfida collected in Caucasus. A comparison of the ultraviolet (UV) spectra, mass spectral with daughter fragmentation (MSn) data, and retention times (Figure S1) with reference compounds (Figure S2) and literature data were used for identification of compounds (Table S3). The chromatograms demonstrated the presence of 137 compounds in six gentian herbs and roots (Table 1).

Table 1.
Compounds 1–137 found in the herb and roots of six Caucasian Gentiana species.
2.1.1. Carbohydrates
Highly hydrophilic components of gentian herbs and roots eluted with retention times 2.78–3.17 min were carbohydrates such as hexose (4; m/z 179 [M–H]–), O-hexosyl-hexose (2; m/z 341 [M–H]–), and O-hexosyl-O-hexosyl-hexose (3; m/z 503 [M–H]–). Typical monosaccharides of gentians are glucose and fructose, disaccharides – gentiobiose and saccharose, and trisaccharides – gentianose and gentiotriose [41]. The limitation of the RP-HPLC assay used is poor separation of isomeric carbohydrates. Therefore, an additional study is needed to clarify the carbohydrate profile of the gentians.
2.1.2. Iridoid Glycosides
Iridoid glycosides were the most diverse group of metabolites and it includes 71 compounds. The following types of iridoid glycosides were detected in studied gentians.
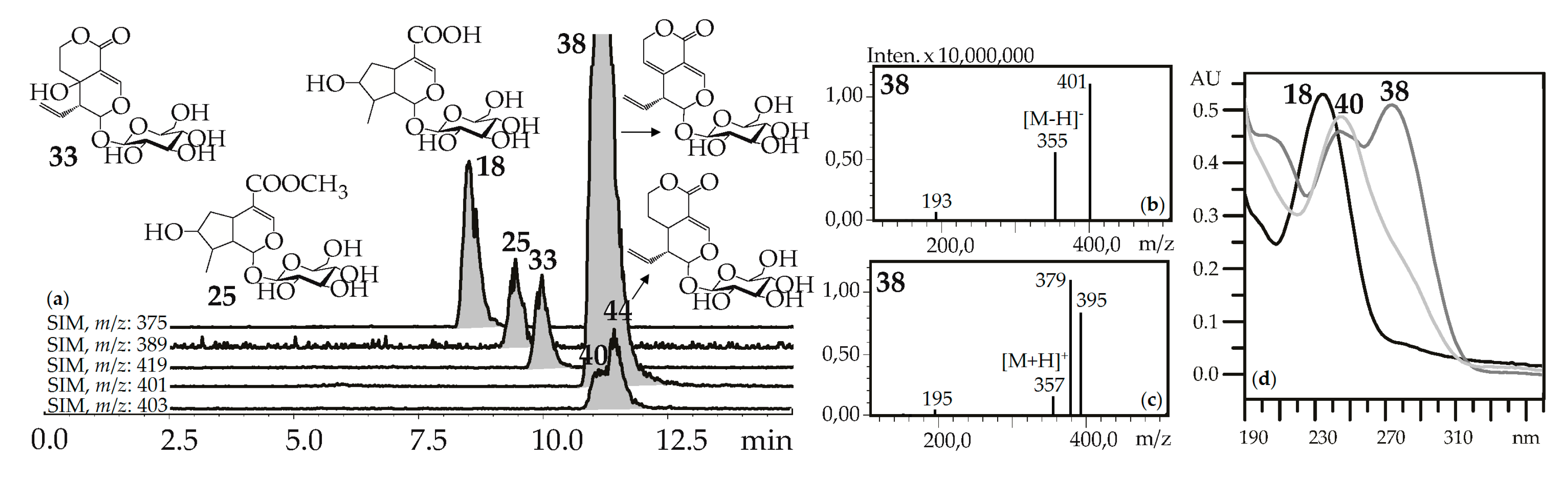
“Usual” iridoid glycosides consisted of iridoid aglycone and a hexose (glucose) fragment with typical mass spectrometric patterns in negative ionization mode that included an intense signal for the deprotonated ion [M–H]– and/or an ion-adduct [(M–H)+HCOOH]– and weak signal for the dehexosylated fragment [(M–H)–Hex]− (Figure 2). The positive ionization mass spectra demonstrated the presence of weak signals from the protonated ion [M+H]+ and dehexosylated ion [(M+H)–Hex]+ and strong signals of adduct ions such as [M+Na]+ and/or [M+K]+. Loganic acid (18), loganin (25), swertiamarin (33), gentiopicroside (38), and sweroside (40) are the most common examples of these types of iridoid glycosides found in gentians here and elsewhere [42], and they were identified using standards.
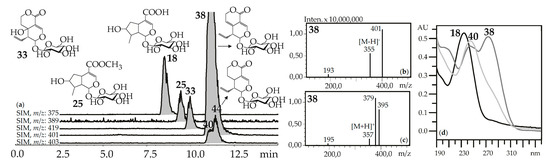
Figure 2.
(a) HPLC-ESI-MS chromatogram of G. septemfida root extract in SIM-mode {m/z 375 for loganic acid (18), m/z 389 for loganin (25), m/z 419 for swertiamarin (33), m/z 401 for gentiopicroside (38), m/z 403 for sweroside (40; isomeric 44)}. (b,c) Mass spectra of compound 38 in negative and positive ionization mode. (d) UV spectra of compounds 18, 38 and 40.
The rare, for the gentians, morroniside (23) was also compared with a standard and found in the G. septemfida herb only. In addition to the mentioned iridoid glycosides, similar mass spectrometric patterns were found for twenty-one compounds. There were compounds isomeric to swertiamarin (41), gentiopicroside (56), and sweroside (44) and eighteen compounds with less obvious structures. Compound 14 gave a deprotonated ion with m/z 389 and was tentatively identified as eustomoside, an iridoid glycoside first found in Eustoma russellianum [43] and later in G. septemfida herb [28]. Seven compounds (3, 5, 6, 10, 13, 17, 19) have the molecular weight 408 and these could be isomers of eustomorusside detected in the G. septemfida herb [28]. A tentative identification was made for compound 12, which gave a deprotonated ion with m/z 471 that is usual for eustoside, which was also discovered in the G. septemfida herb [28]. From the spectral data of compounds 7 (m/z 477 [M–H]–), 22, 24 (m/z 445 [M–H]–), 67 (m/z 683 [M–H]–), 96 (m/z 435 [M–H]–), and 86, 100 (m/z 561 [M–H]–), they were concluded to have an iridoid glycoside nature.
Iridoid glycosides with additional sugar fragments have molecular weights of 162 a.m.u. more than the parent compound. Also, in the MSn spectra, they gave extra signals of dehexosylated fragments [(M–H)–n×Hex]–. Loganic acid-6′-O-glucoside (15) found in G. cruciata roots gave a deprotonated ion with m/z 537 and, in the MS2 spectrum, signals with m/z 375 and 213 belonging to the fragments [(M–H)–Glc]– and [(M–H)–2×Glc]–, respectively. Similar spectral patterns were detected in the mass spectra of swertiamarin-6′-O-glucoside (26) and its isomers 8 and 9, gentiopicroside-6′-O-glucoside (27) and gentiopicroside-di-O-hexoside (20), and sweroside-6′-O-glucoside (28).
Iridoid glycosides with a 2,3-dihydroxybenzoyl fragment were characterized by specific UV absorptions at 235–238, 255–257 and 322–326 nm, and the mass spectra demonstrated the loss of a fragment with 136 a.m.u. belonging to an aromatic acid [44]. These types of iridoids are rare in plants, and they are distributed mainly in the Gentiana genus [42]. Eight iridoid glycosides were found including the known compounds 2′-O-(2,3-dihydroxybenzoyl)-loganic acid (algidiside I, 58), 6′-O-(2,3-dihydroxybenzoyl)-loganic acid (algidiside II, 69) [14], and 6′-O-(2,3-dihydroxybenzoyl)-sweroside (83) [44]. Other dihydroxybenzoyl ethers with unknown types of substitution were derivatives of loganic acid (77), loganin (91, 103), and gentiopicroside (74, 81).
Iridoid glycosides with 2,3-dihydroxybenzoyl and acetyl fragments have spectral properties close to the previous group with additional signals in mass spectra generated by elimination of acetyl moieties (42 a.m.u.). Tri-O-acetyl-O-2,3-dihydroxybenzoyl-swertiamarin (134) and two tri-O-acetyl-O-2,3-dihydroxybenzoyl-swerosides 111 and 135 were found in four gentians (G. asclepiadea, G. gelida, G. paradoxa, G. septemfida) and their most likely structures were deglucosylated gelidosides (found previously in G. robusta [45]) and deglucosylated trifloroside, described in Gentiana triflora subsp. japonica (Kusn.) Vorosch. [46].
Iridoid glycosides with 2,3-dihydroxybenzoyl and hexose fragments were characterized by the primary loss of a hexosyl fragment with m/z 162 followed by the expected elimination of a 2,3-dihydroxybenzoyl moiety (m/z 136). Two loganic acid-O-2,3-dihydroxybenzoyl ether-O-hexosides, 59 and 62, from G. asclepiadea roots and sweroside-O-2,3-dihydroxybenzoyl ether-O-hexoside, 72, from G. septemfida roots were detected. The latter compound’s structure could be tentatively identified as a deacetylated trifloroside compound. A similar structure was found in G. straminea roots and identified as 6′-O-{(2″-hydroxy-3″-glucosyloxy) benzoyl}-sweroside [47], also known as a gentiotrifloroside isolated from G. triflora [48]. Compounds 59 and 62 have no analogues in plants.
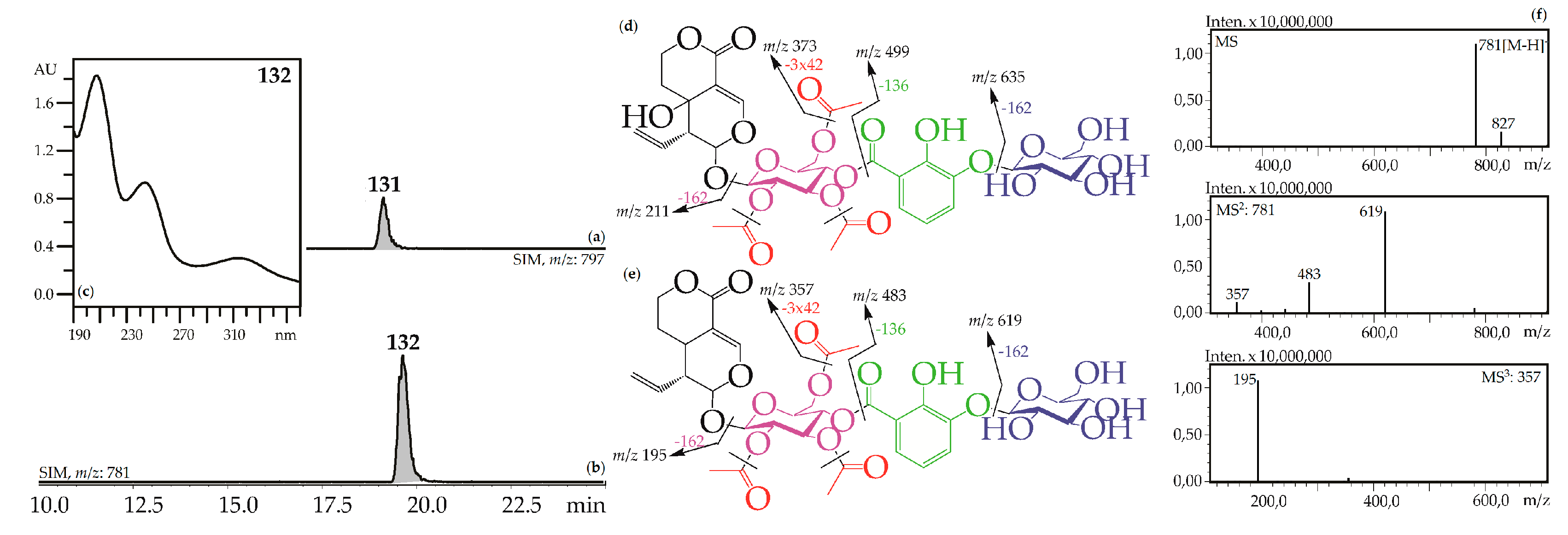
Iridoid glycosides with 2,3-dihydroxybenzoyl, acetyl and hexose fragments were members of the largest group of iridoids found in Caucasian gentians. Their mass spectra contain a sequence of signals caused by the serial elimination of hexose (or hexoses), a 2,3-dihydroxybenzoyl fragment, an acetyl group (or groups), and final loss of glucose and liberation of an iridoid aglycone (Figure 3). Twenty-one compounds gave an analogous fragmentation pattern. Among these were derivatives of loganic acid (82, 89, 130), swertiamarin (93, 102, 108, 113, 117, 120, 128, 131), sweroside (101, 110, 121, 126, 127, 129, 132) and tentatively eustomorusside (88) and eustomoside (116, 133). Comparison with standard compounds allowed identification of gelidoside (rindoside, 131) and trifloroside (132) which were 2′,3′,6′-tri-O-acetyl-4′-O-{(2″-hydroxy-3″-glucosyloxy) benzoyl}-swertiamarin [29] and 2′,3′,6′-tri-O-acetyl-4′-O-{(2″-hydroxy-3″-glucosyloxy) benzoyl}-sweroside, respectively [46]. When replacing swertiamarin in 131 or sweroside in 132 with loganic acid, the new iridoid glycosides 82, 89, and 130 would be obtained, but real examples of this are still unknown.
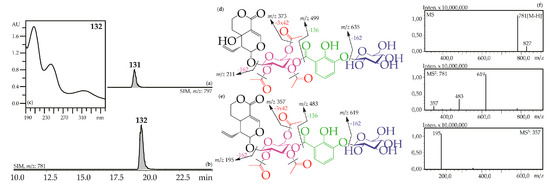
Figure 3.
(a,b) HPLC-ESI-MS chromatogram of G. gelida root extract in SIM-mode: {m/z 797 for gelidoside (rindoside, 131, a); m/z 781 for trifloroside (132, b)}. (c) UV spectrum of compounds 132. (d,e) Mass spectrometric fragmentation of compounds 131 (d) and 132 (e). (f) MSn spectra of compound 132.
Three monoacetylated (93, 102, 108) and two diacetylated (113, 117) analogues of gelidoside were found in G. gelida, G. cruciata, G. pneumonanthe and G. septemfida as well as two hexosylated derivatives of gelidoside (120, 128). Only one diacetylated compound, 3′,4′-di-O-acetyl-6′-O-{(2″-hydroxy-3″-O-glucopyranosyloxy)- benzoyloxy}-swertiamarin (gentistraminoside B), was found previously as a component of G. straminea roots [49]. Data about monoacetylated and hexosylated gelidosides is absent. Two compounds with one acetyl group (101, 110), two compounds with two acetyl groups (121, 132), and two hexosylated derivatives of trifloroside (126, 129) were discovered in gentians. The known data about similar compounds include two monoacetylated analogues of trifloroside – 3′-O-{(2″-hydroxy-3″-glucosyloxy) benzoyl}-6′-O-acetyl-sweroside from G. manshurica roots [50] and 4′-O-acetyl-6′-O-{(2″-hydroxy-3″-glucosyloxy)benzoyl}-sweroside (gentistraminoside A) from G. straminea roots [49], and two hexosylated triflorosides such as 4‴-O-glucosyl trifloroside from G. scabra roots [51] and 6′"-O-glucosyl trifloroside from G. linearis roots [52]. The unknown compound, which was found in G. gelida herb and gave a deprotonated molecular ion with m/z 831, was tentatively identified as tri-O-acetyl-O-2,3-dihydroxybenzoyl-O-hexosyl-eustomorusside, 88. Compounds 116 and 133 were two isomeric tri-O-acetyl-O-2,3-dihydroxybenzoyl-O-hexosyl-eustomosides for which only one known structure is available, 2′,3′,6′-tri-O-acetyl-4′-O-{(2″-hydroxy-3″-glucosyloxy) benzoyl}- eustomoside or gentomoside isolated from G. gelida [29].
Iridoid glycosides with a caffeoyl fragment have specific UV patterns with maxima at 325–330 nm, and their mass spectra gave the signals of decaffeoylated ions derived from the parent molecular ions after loss of a fragment with m/z 162 (caffeoyl). Two compounds were found and identified as O-caffeoyl-loganic acid (76) from G. gelida roots and O-caffeoyl-sweroside (115) from G. cruciata roots. The known iridoid glycoside caffeates are 2′-O-caffeoyl-loganic acid from G. loureirii herb [53] and 3′-O-caffeoyl-sweroside from Anthocephalus chinensis bark (Rubiaceae) [54].
One compound, amarogentin (105), was not among the mentioned types of gentian iridoid glycosides and was identified using a standard compound. Amarogentin is a common bitter component of G. lutea roots and was also found to be a trace compound in the roots of G. asclepiadea and G. pneumonanthe [27]. In our study it was detected only in G. gelida roots.
2.1.3. Phenolic Acid O-glycosides
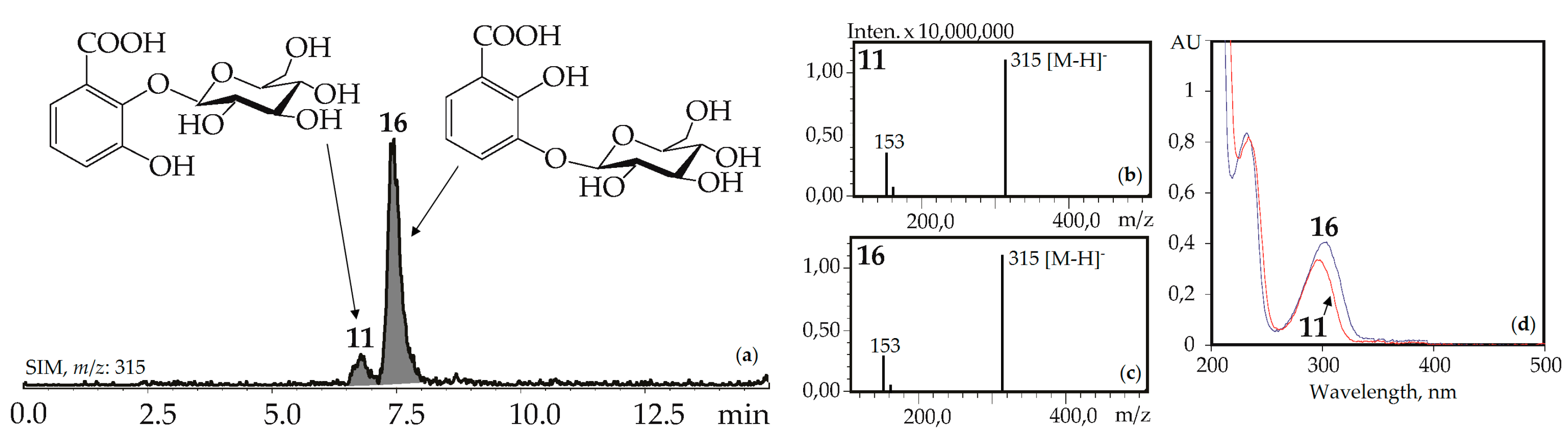
The UV patterns of two compounds, 11 and 16, were close (λmax 203, 235–240, 300–304 nm) and specific for the 2,3-dihydroxybenzoic acids with one substituted hydroxyl (Figure 4). Basic fragments in the negative mass spectra of 11 and 16 showed signals with m/z 315 [M–H]– and 153 [(M–H)–hexose]– that are characteristic for O-hexosides of 2,3-dihydroxybenzoic acid [55]. The most likely identification of the dominant 16 is 2,3-dihydroxybenzoic acid 3-O-glucoside, because its fragment can be seen in the structures of gelidoside (rindoside, 131) and trifloroside (132), suggesting a need of 16 for biosynthesis of selected iridoids in gentians. The trace component 11 is isomeric to 16 and may be discovered as 2,3-dihydroxybenzoic acid 2-O-glucoside. Both compounds were found in gentians for the first time.
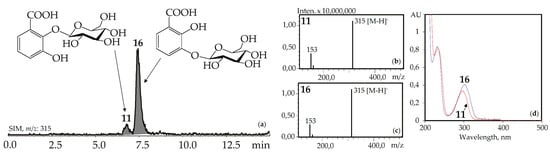
Figure 4.
(a) HPLC-ESI-MS chromatogram of G. gelida herb extract in SIM-mode (m/z 315). (b, c) Mass spectra (negative ionization) of compounds 11 (2,3-dihydroxybenzoic acid 2-O-glucoside) and 16 (2,3-dihydroxybenzoic acid 3-O-glucoside), respectively. (d) UV spectra of compounds 11 and 16.
2.1.4. Hydroxycinnamates
Hydroxycinnamates are rare gentian components. Only five caffeic acid derivatives were detected in two gentians including 1-O-caffeoyl-glucose (21) and 6-O-caffeoyl-glucose (45) from G. pneumonanthe herb and 2-O-caffeoyl-glucaric acid (51), 1,3-di-O-caffeoyl-glycerol (79), and 1,2-di-O-caffeoyl-glycerol (tentative, 90) from G. septemfida herb and none of these have been found in gentians. Previously known gentian hydroxycinnamates are ferulic acid in G. scabra roots, O-feruloyl-glucose from G. loureirii whole plant, and 3-O-caffeoyl-glucose from G. rigescens roots [42].
2.1.5. Xanthones
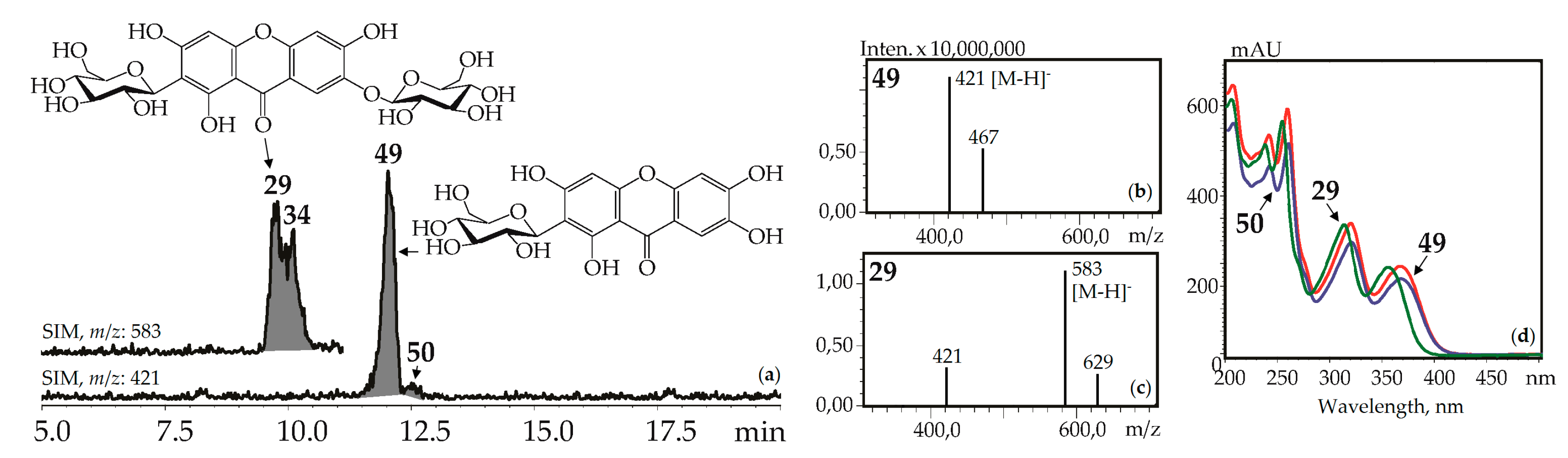
Xanthone-C-glycosides mangiferin (1,3,6,7-tetrahydroxyxanthone-2-C-glucoside, 49) and isomangiferin (1,3,6,7-tetrahydroxyxanthone-4-C-glucoside, 50) were identified using reference compounds, UV spectra (λmax 240, 257, 318, 365 nm), and mass spectrometric patterns (m/z 421 [M–H]–, 467 [(M–H)+HCOOH]–). These were in known sources such as the herbs of G. asclepiadea [15], G. cruciata [17], G. pneumonanthe [26], and for the first time in G. paradoxa herb (Figure 5).
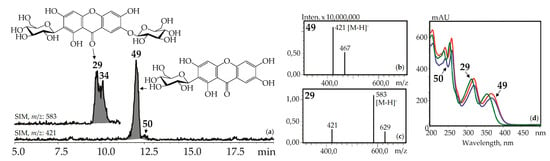
Figure 5.
(a) HPLC-ESI-MS chromatogram of G. asclepiadea herb extract in SIM-mode (m/z 421, 583). (b, c) Mass spectra (negative ionization) of compounds 49 (mangiferin) and 29 (mangiferin-7-O-glucoside), respectively. (d) UV spectra of compounds 29, 49, and 50.
Additionally, two isomeric to mangiferin compounds, 30 and 39, with lower retention times were found in G. cruciata and G. paradoxa. Mangiferin-7-O-glucoside (neomangiferin, 29) gave the signals of deprotonated ion [M–H]– with m/z 583 and a deglucosylated fragment with m/z 421 [(M–H)–glucose]–. It is a known component of G. asclepiadea herb [20] and was newly found in G. cruciata, G. paradoxa, and G. pneumonanthe. A compound isolated from G. asclepiadea herb, but with a longer retention time than 29, was tentatively identified as mangiferin-6-O-glucoside, 34, an isomer of 29 [20]. Gentisin (1,7-dihydroxy-3-methoxyxanthone, 137) and its 1-O-primveroside (gentioside, 109) were identified using reference compounds as a component of G. asclepiadea roots.
2.1.6. Flavonoids
Forty O-, C-, and O,C-glycosides and acylated compounds were identified as derivatives of luteolin, apigenin, chrysoeriol, and acacetin. Only one aglycone chrysoeriol (136) was detected in herbs of G. asclepiadea and G. pneumonanthe.
Among the fifteen luteolin glycosides found, seven compounds compared with reference compounds were luteolin-7-O-glucoside (98), luteolin-6-C-glucoside (isoorientin, 61), luteolin-8-C-glucoside (orientin, 80), isoorientin-7-O-glucoside (35), isoorientin-2″-O-glucoside (43), isoorientin-4″-O-glucoside (52), and isoorientin-6″-O-glucoside (54). Isoorientin is one of the most often described flavonoids of the Gentiana genus and it was found in all herb samples and in G. asclepiadea root. The same distribution was shown for the isoorientin-2″-O-glucoside, the known flavonoid of G. asclepiadea herb [15]. Compound 63 was isomeric to 35, 43, 52, and 54 and was determined as luteolin-C-hexoside-O-hexoside.
The UV spectrum of compound 97 was specific for the luteolin glycosides acylated by a fragment of caffeic acid (λmax 326 nm) [56] (Figure 6). The mass spectrometric data (m/z 609 [M–H]–; MS2 609: 447; MS3 447: 357, 327, 299) indicate that its possible structure is luteolin-C-hexoside-O-caffeate, especially since a similar compound (isoorientin-2″-O-caffeate) was already isolated from G. cruciata [17].
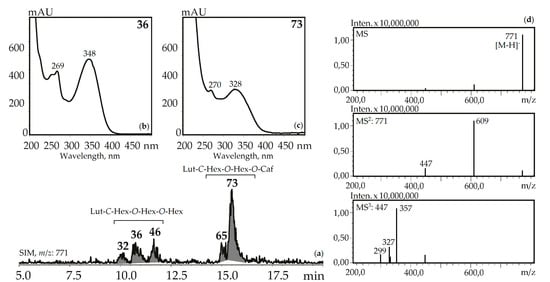
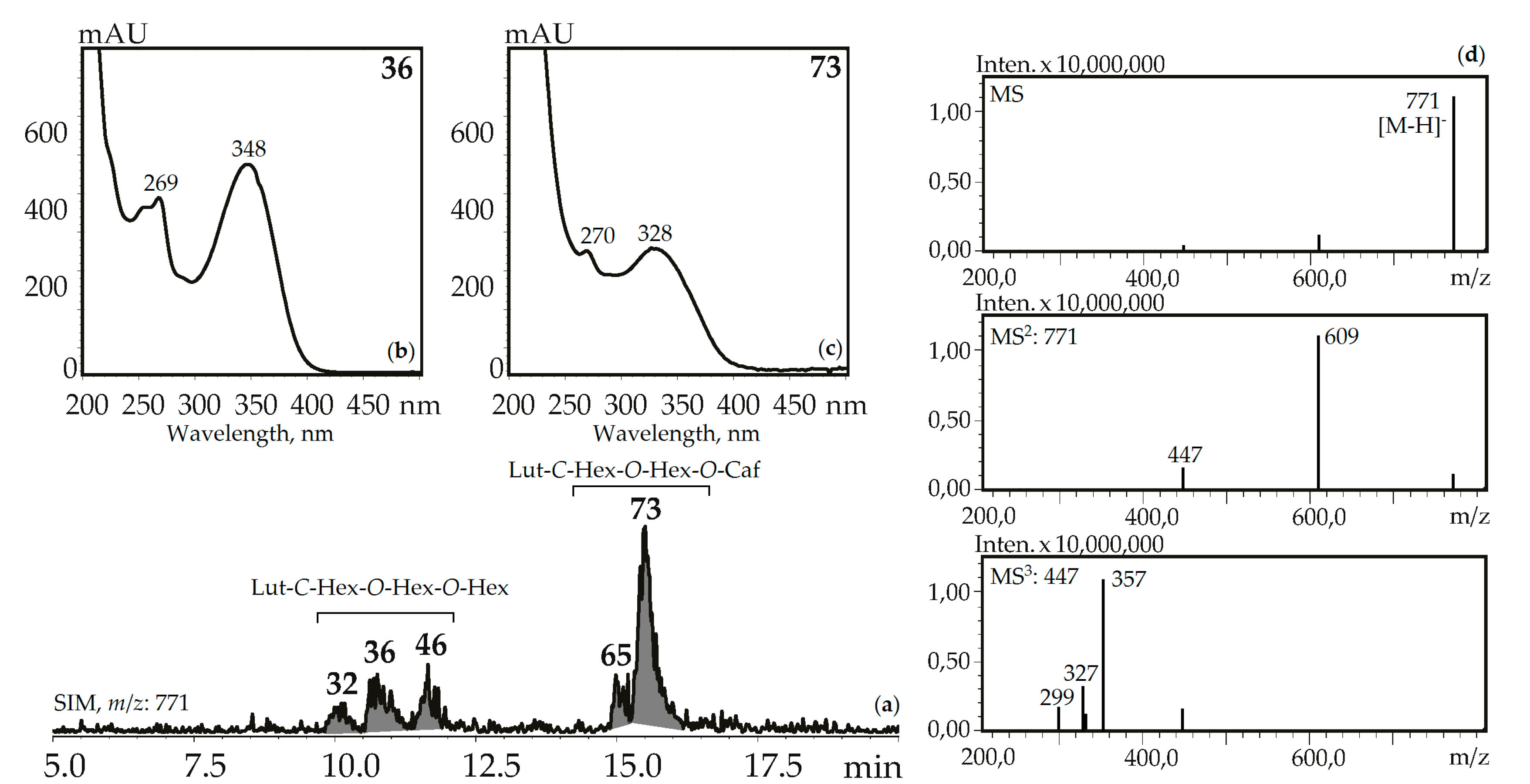
Figure 6.
(a) HPLC-ESI-MS chromatogram of G. gelida herb extract in SIM-mode (m/z 771). (b, c) UV spectra of compounds 36 and 73. (d) MSn spectra (negative ionization) of compound 36. Lut-C-Hex-O-Hex-O-Hex—zone of isomeric luteolin-C-hexoside-O-hexoside-O-hexosides, Lut-C-Hex-O-Hex-O-Caf—zone of isomeric luteolin-C-hexoside-O-hexoside-O-caffeates.
Three luteolin-C-hexoside-O-di-hexosides (32, 36 and 46) with the same UV pattern gave MSn spectra fragments with m/z 771, 609, and 447 that are specific for a deprotonated ion and its dehexosylated fragments followed by the further cleavage typical for C-glycosylflavones [57]. Possible examples are isoorientin-2″,4′-di-O-glucoside isolated from G. asclepiadea herb [23] and isoorientin-3″,6″-di-O-glucoside from G. pedicellata leaves [58]. Two compounds, 65 and 73, gave a similar MS pattern but the existence of a hypsochromic shift in band II of the UV spectra (λmax 348→328 nm) and increased retention times pointed to acylation of luteolin-C-hexoside-O-hexoside by a caffeic acid fragment [56]. Despite the relatively rare occurrence of luteolin-C-hexoside-O-hexoside-O-caffeates in plants, there are two known compounds isolated from the gentians, i.e., isoorientin-4′-O-glucoside-2″-O-caffeate from G. punctata leaves [59] and isoorientin-2″-O-(4″-O-glucosyl)-caffeate from G. marcailhouana leaves [60]. Compound 66 from G. asclepiadea, G. paradoxa, and G. pneumonanthe herbs was tentatively identified as luteolin-C-hexoside-O-hexoside-O-p-hydroxybenzoate. This was based on its deprotonated ion with m/z 729 and ions with m/z 609 and 447 that were caused by the loss of p-hydroxybenzoyl and hexosyl fragments, respectively. A flavone glycoside with a similar structure was isolated from G. asclepiadea and known as isoorientin-4′-O-(2″-O-p-hydroxybenzoyl)-glucoside [25].
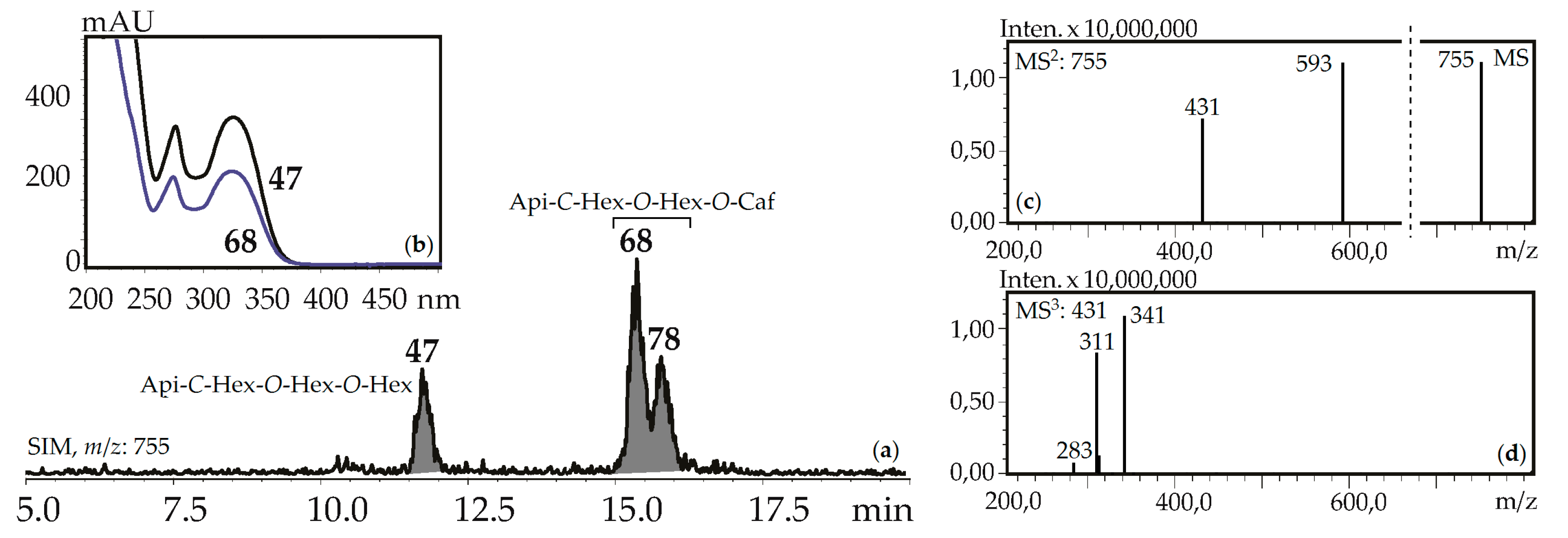
Fourteen apigenin glycosides were the components of gentian herbs and roots. Apigenin-7-O-glucoside (125), apigenin-6-C-glucoside (isovitexin, 71) and isovitexin-7-O-glucoside (saponarin, 42) were found in all gentian herb samples, and 42 and 71 were in the roots of G. asclepiadea, G. gelida, and G. paradoxa. Isovitexin is also a frequently detected flavonoid of the gentians [42]. Four C,O-glycosylflavones were identified by comparing with reference compounds like isovitexin-2″-O-glucoside (55), isovitexin-4′-O-glucoside (64), isovitexin-7,2″-di-O-glucoside (37), and isovitexin-2″,4″-di-O-glucoside (47). Compounds 57 and 107 are found in some gentians and have spectral data similar to 36 and 97, respectively, but 16 a.m.u. less, confirming their nature as apigenin-C-hexoside-O-hexoside-O-hexosides and apigenin-C-hexoside-O-caffeate. Five isomeric compounds (68, 78, 92, 114, 118) with molecular weights 756 were detected in all gentian herbs, and it was concluded that they are acylated C,O-glycosylflavones or apigenin-C-hexoside-O-hexoside-O-caffeates. Their spectral properties were similar to an apigenin-C-hexoside-O-hexoside-O-hexoside like isovitexin-7,2″-di-O-glucoside (37) or isovitexin-2″,4″-di-O-glucoside (47), but the long retention times (15.09–18.09 min) suggested the existence of an additional functional group with high lipophilicity (Figure 7). Because the molecular weight of the loss group was 162 a.m.u., it was identified as caffeoyl. To date, only one compound satisfies these criteria, the isovitexin-7-O-(6″-caffeoyl)glucoside isolated from Bryonia herbs [61]. The discovery of five isomeric compounds illustrates the necessity for additional study to find other new isomers.
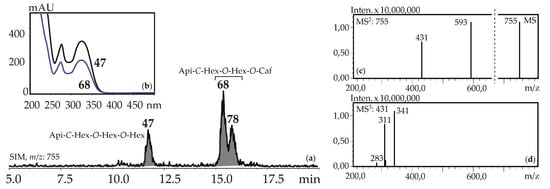
Figure 7.
(a) HPLC-ESI-MS chromatogram of G. gelida herb extract in SIM-mode (m/z 755). (b) UV spectra of compounds 47 and 68. (c) MS and MS2 spectra (negative ionization) of compound 68. Api-C-Hex-O-Hex-O-Hex—apigenin-C-hexoside-O-hexoside-O-hexoside (isovitexin-2″,4″-di-O- glucoside), Api-C-Hex-O-Hex-O-Caf—zone of isomeric apigenin-C-hexoside-O-hexoside-O-caffeates.
Five gentians showed the presence of chrysoeriol and its seven glycosides. The known compounds isoscoparin (85), isoscoparin-7-O-glucoside (48), and isoscoparin-2″-O-glucoside (60) were identified after comparing with reference compounds as well as chrysoeriol-C-hexoside-O-hexoside (53), chrysoeriol-C-hexoside-O-hexoside-O-caffeate (70), and two chrysoeriol-C-hexoside-O-caffeates 104 and 106. Compounds 48 and 85 were reported once in G. pneumonanthe herb [21]. Chrysoeriol and its glycosides are considered to be rare gentian components [42], and its caffeoyl esters are still unknown. Less expected metabolites of the Gentian genus are the acacetin derivatives found in our study in G. cruciata herb (87, 124) and G. gelida herb (122). There were acacetin-C-hexoside-O-hexoside-O-caffeate (87) and two acacetin-C-hexoside-O-caffeates (122, 124). Only the isocytisoside-7-O-glucoside (acacetin-6-C-glucoside-7-O-glucoside) isolated from G. pyrenaica herb [62] is a known acacetin glycoside of the gentians. There is not any information about caffeoylated glycosides of acacetin of plant origin.
2.1.7. Triterpene Glycosides
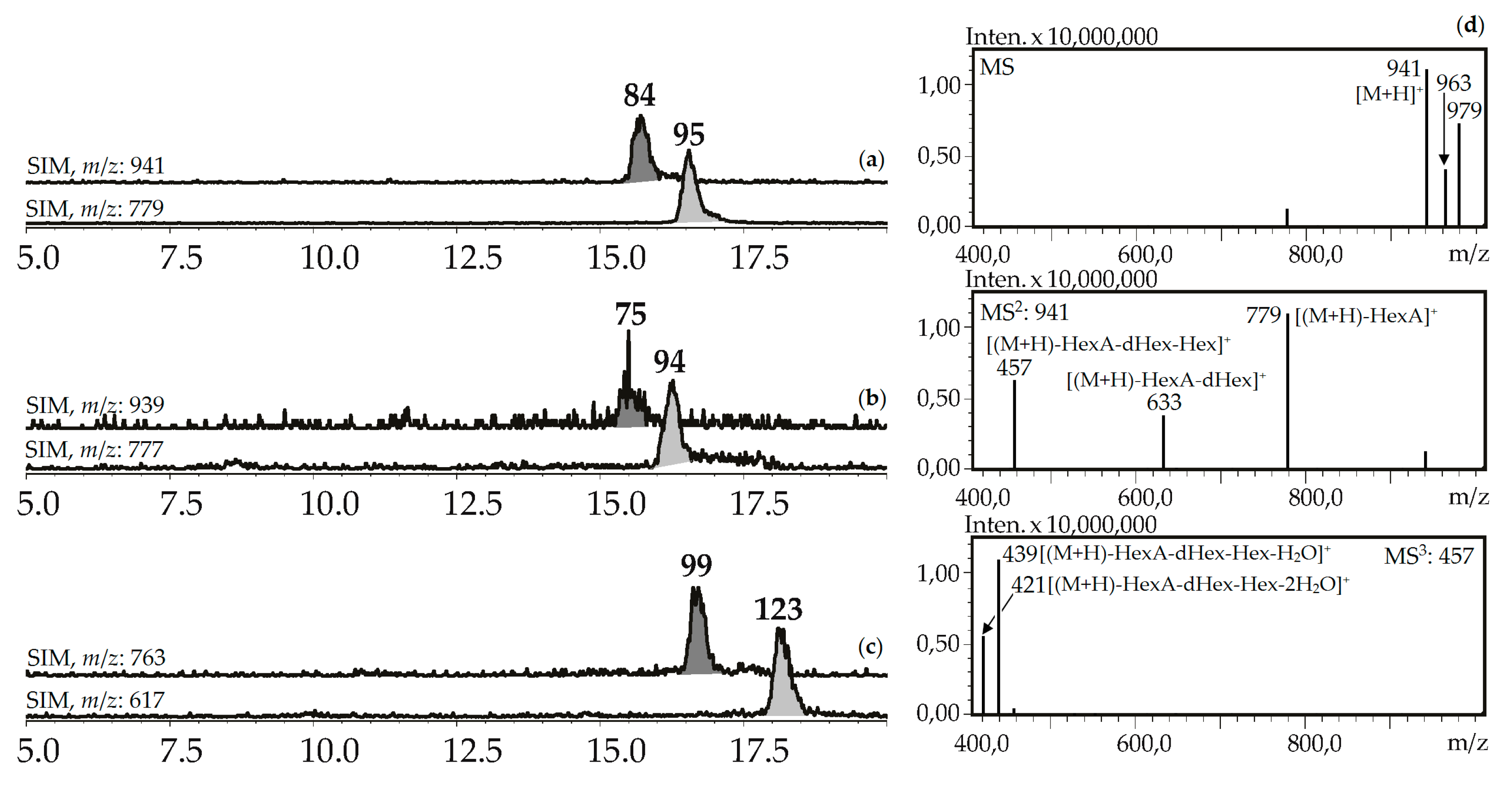
Seven compounds (75, 84, 94, 95, 99, 112, 123) were determined to be triterpenic glycosides based on their mass spectra in positive ionization mode. Their glycosidic nature was proved by the presence of a series of signals caused by elimination of carbohydrate fragments like hexose (162 a.m.u.), desoxyhexose (146 a.m.u.), and hexuronic acid (176 a.m.u.) (Figure 8). Aglycone fragments in MS3 spectra gave the signals of dehydrated ions characteristic of triterpenoids (triterpenes, sterols, ecdysteroids) [63,64]. Two compounds, 84 and 95, from the herb of G. asclepiadea, G. cruciata and G. paradoxa were determined to be oleanolic acid-O-hexuronide-O-desoxyhexoside-O-hexoside and oleanolic acid-O-hexuronide-O-desoxyhexoside, respectively. Compounds 75 and 94 have similar mass spectrometric patterns, but the atomic numbers of the basic fragments were 2 a.m.u. less that presumably caused by the absence of two hydrogens in an aglycone fragment. Dehydrooleanolic acid was tentatively concluded to be an aglycone of 75 and 94, and they may be described as dehydrooleanolic acid-O-hexuronide-O-desoxyhexoside-O-hexoside (75) and dehydrooleanolic acid-O-hexuronide-O-desoxyhexoside (94). Compound 123 and two isomers 99 and 112 were desoxyoleanolic acid derivatives coupled with fragments of O-hexuronic acid and O-hexuronyl-O-desoxyhexose, respectively. The known triterpenes of the Gentiana genus are mostly derivatives of oleanane, ursane, and dammarane in aglycone state [42]. Triterpene glycosides are rare components of gentians and compounds with the described structural features are still unknown.
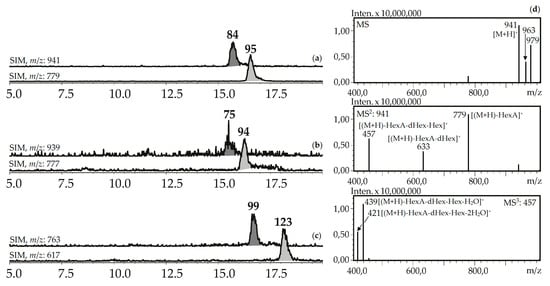
Figure 8.
(a,b,c) HPLC-ESI-MS chromatograms of G. asclepiades herb extract in SIM-mode (positive ionization; m/z 941, 779 (oleanolic acid glycosides; a), 939, 777 (dehydrooleanolic acid glycosides; b), 763, 617 (desoxyoleanolic acid glycosides; c)). (d) MSn spectra (positive ionization) of compound 84.
In general, the chemical profiles of the studied gentian species are similar: flavonoids and iridoids accumulate predominately in all types of herbs, and iridoids are the most diverse class of compounds in the roots. Of the 137 compounds detected, 71 compounds (52% of the total) are iridoids and 40 compounds are flavonoids (29% of the total). In total, these account for more than 80% of the total variety of compounds. In gentian herb samples, 44 (G. pneumonanthe) to 58 compounds (G. paradoxa) were detected and in roots 21 (G. pneumonanthe) to 32 compounds (G. septemfida). The largest number of compounds known for the species (12) was found in the herb of G. asclepiadea, which is considered to be the most studied of the species studied. Most of the compounds mentioned in this study were found in gentian species for the first time (Table 1). Four samples have never been studied before; these include the herb and roots of G. paradoxa, and the roots of G. gelida and G. septemfida.
The common components of all the studied species of both herb and roots were iridoid glycosides, including loganic acid (18), loganin (25), swertiamarin (33), gentiopicroside (38), and sweroside (40). These compounds are markers for gentian sections of Pneumonanthe and Aptera and the genus Gentiana, in general; therefore, their obligate occurrence is not a surprise. Compounds that were found in all herb samples (and rarely in the roots) are glycosylflavones saponarin (42), isoorientin-2″-O-glycoside (43), isoorientin (61), isovitexin (71), and apigenin-7-O-glucoside (125). Specific compounds identified mainly in the roots of the studied gentians include some minor iridoid glycosides (28, 27, 126, 129, etc.), but these findings are inconclusive and require additional research.
Is it possible to say that any components are characteristic for a particular type of gentian? The most suitable example is the detection of gentisin (137) and gentioside (109) in the roots of G. asclepiadea. This occasion is really rare, because for gentians of the Pneumonanthe section, the presence of xanthones (except mangiferin) is uncharacteristic. The possible reason for this phenomenon may be the least evolutionary advancement of G. asclepiadea, which makes it close to the species of the Coelanthe section, which contains such xanthon-containing species as G. lutea and G. punctata [42].
A notable fact is the single occurrence of some hydroxycinnamates such as caffeoyl-glucose 21 and 45 in G. pneumonanthe herb or caffeoyl-glycerins 79 and 90 in G. septemfida herb. Commonly, hydroxycinnamates of various structures accumulate in the green parts of plants in the form of esters with quinic acid, shikimic acid, or glucose [65]. For plants of the genus Gentiana, such a phenomenon is extremely rare [42].
The presence of flavonoids in the form of aglycones was detected only in two species (G. asclepiadea, G. paradoxa). This is typical for gentian sections of Pneumonanthe and Aptera produced flavonoids in the form of O-, C-, C, O-glycosides and most often derivatives of apigenin (or isovitexin) and/or luteolin (or isoorientin) [26,66]. The distribution of xanthone C-glycoside mangiferin (49) within the genus Gentiana is irregular, and its presence was previously observed only in some types of sections of Pneumonanthe, Frigida, Aptera, and Chondrophyllae [67]. Mangiferin is a useful therapeutic molecule with various bioactivities [68]. New plant sources are needed and G. paradoxa herb was shown to be a mangiferin source for the first time.
2.2. HPLC-DAD Quantification of Selected Compounds in Six Caucasian Gentians: Comparison of Herb and Roots
Continuing the metabolomic study of six Caucasian gentians, we determined the quantitative content of selected compounds by high performance liquid chromatography with diode array detection (HPLC-DAD) technique [12]. Six iridoids, nine flavones and mangiferin were chosen as quantitative markers of the herb samples, and roots were analysed using seven iridoids, isoorientin-2″-O-glucoside and gentioside (Tables S4, S5, Figure S2). Comparative analysis of quantitative data showed a strong variation of iridoid, flavonoid, and xanthone content in herbs and roots (Table 2). Gentiopicroside was the predominant iridoid glycoside in herb samples of G. asclepiadea, G. cruciata, and G. pneumonanthe, and swertiamarin had maximal content in other herbs of G. gelida, G. paradoxa, and G. septemfida. The loganic acid level in gentian herb was also high and sweroside was a trace compound. Gelidoside was quantified in two herbs, G. gelida and G. septemfida, and trifloroside content was determined in G. gelida, G. paradoxa and G. septemfida. The main iridoid of roots was gentiopicroside for all samples and its derivative gentiopicroside-6″-O-glucoside was quantified only in roots. The earlier information showed a high content of gentiopicroside in G. asclepiadea, G. cruciata, and G. pneumonanthe roots from Hungary (50–60 mg/g) [27], G. cruciata herb/roots from East Serbia (10.67/19.57 mg/g; as extract) [22], and G. pneumonanthe roots from Serbia (40.02–56.68 mg/g) [18]. In this regard, the herbs and roots of six Caucasian gentians are a good source of gentiopicroside.

Table 2.
Content of selected compounds in gentian herbs and roots a, mg/g of dry plant weight (±S.D.).
The following iridoids were present in much lower amounts: swertiamarin and sweroside in G. asclepiadea, G. cruciata, and G. pneumonanthe roots [27], swertiamarin and sweroside in G. cruciata herb/roots extracts [22], swertiamarin, and sweroside in G. pneumonanthe herb/roots [18]. By this data, we see a remarkable similarity between known information about iridoid content in gentian parts and the data obtained in the present study.
Flavonoids were quantifiable in all gentian herbs and G. asclepiadea roots. The basic flavonoids of herb samples were isoorientin and isoorientin-2″-O-glucoside. The content of isovitexin and its O-glycosides was less but appropriate for analysis. Isoscoparin was quantifiable in three herb samples such as G. septemfida, G. gelida, and G. pneumonanthe. Only isoorientin-2″-O-glucoside was found in G. asclepiadea roots. As previously shown, the isoorientin and isovitexin content in G. pneumonanthe herb from Serbia was 0.27–2.67 and 0.12–0.88 mg/g, respectively [18]. Isoorientin was mentioned as the dominant flavonoid of some Turkish gentian herbs such as G. asclepiadea (1.00–30.72 mg/g), G. cruciata (2.41–22.78 mg/g), G. gelida (2.64–35.16 mg/g), and G. septemfida (1.17–15.19 mg/g) [24]. By contrast to apigenin glycosides, luteolin derivatives were the principal flavonoids with high content in gentian herbs of Caucasus origin and this was also reported in early research of European and Turkish populations of various gentians.
Xanthone content in gentian herbs was formed by their mangiferin value. The herbs of G. gelida and G. septemfida were free of mangiferin and other species showed high mangiferin levels. The roots of G. asclepiadea accumulate xanthone-O-glycoside gentioside at a low level. Mangiferin content was also determined previously in G. pneumonanthe herb (0.44–5.81 mg/g) [18] and in G. asclepiadea flowers and stem extracts (0.26–1.48 mM) [37]. In view of mangiferin’s importance as a bioactive compound, the herbs of four gentian species could be concluded to be a rich source of this xanthone, especially G. asclepiadea herb.
By comparing results of the quantitative profile of herbs and roots of six Caucasian gentians, it can be concluded that the herbs are a good source of iridoids, flavonoids, and xanthones, and the roots can concentrate mainly iridoids.
2.3. Bioactivity of Gentian Extracts as a Function of Phenolic Compounds Content
Antioxidant activity is one of the basic bioactivity properties of plant extracts due to the presence of the various groups of antioxidants. The gentian extracts are no exceptions, and they were previously found to be good antioxidant sources [13,22,39]. In the present work, we studied the antioxidant properties of herb and root extracts of six Caucasian gentians by three methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, superoxide anion-radical scavenging assay, and lipid peroxidation inhibition assay.
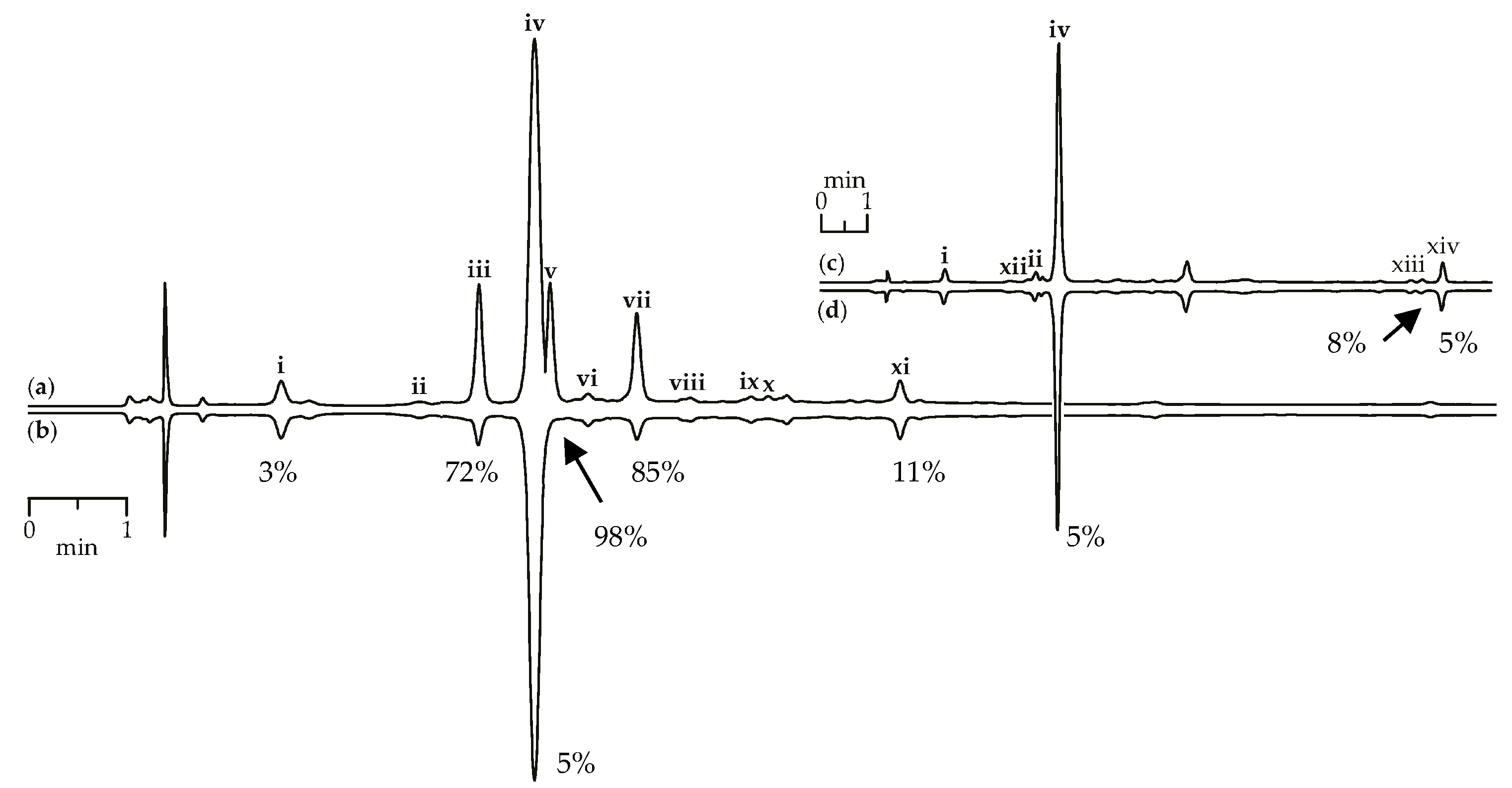
Scavenging activity against DPPH radicals of gentian herb extracts were medium to high and varied from 147.57 mg trolox/g for G. paradoxa to 580.71 mg trolox/g for G. asclepiadea (Table 3). Extracts of gentian roots were characterized by mostly low activity, with activity values <10 mg trolox/g. Using the HPLC-DAD assay coupled with pre-chromatographic reaction with DPPH radicals, the most active compounds were characterized [69,70]. Figure 9a,b show chromatograms of G. asclepiadea herb extract before and after reaction with DPPH radical. The peak areas of the most active scavengers isoorientin-2″-O-glucoside (zone iii), mangiferin (zone v), and isoorientin (zone vii) were reduced by 72–98% compared with the initial value, but iridoid glycosides such as loganic acid (zone i) and gentiopicroside (zone iv) gave week peak reduction (3–5%). The chromatograms of root extracts appeared relatively unchanged after reaction with DPPH radicals, demonstrating a low reduction of all peaks (Figure 9c,d). Analysing the scavenging activity of the selected compounds, we found that iridoid glycosides such as loganic acid and gentiopicroside have low activity (<10 mg trolox/g). Additional phenolic groups such as 2,3-dihydroxybenzoyl in iridoid structures of gelidoside (rindoside) and trifloroside can increase the radical scavenging potential, but not much (20.82–25.14 mg trolox/g). Isoorientin (2523.27 mg trolox/g) and mangiferin (3824.20 mg trolox/g) were the most active compounds found in gentian extracts.

Table 3.
Parameters of antioxidant activity and α-amylase/α-glycosidase inhibitory potential of gentian extracts and pure compounds a,b.
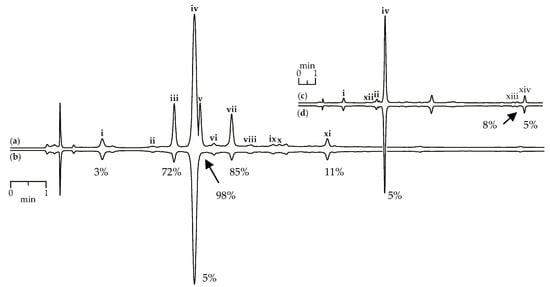
Figure 9.
HPLC-DAD chromatograms (210 nm) of G. asclepiadea herb extract before (a) and after (b) pre-chromatographic reaction with DPPH radicals and G. gelida roots extract before (c) and after (d) pre-chromatographic reaction with DPPH radicals. Zone of compounds numbered as follows: i—loganic acid; ii—swertiamarin; iii—isoorientin-2″-O-glucoside; iv—gentiopicroside; v—mangiferin; vi—isovitexin-2″-O-glucoside; vii—isoorientin; viii—saponarin; ix—isovitexin; x—luteolin-7-O-glucoside; xi—apigenin-7-O-glucoside; xii—gentiopicroside-6″-O-glucoside; xiii—gelidoside (rindoside); xiv—trifloroside. The numbers demonstrate the percentage of peak area reduction after pre-chromatographic reaction with DPPH radicals.
The scavenging activity of gentian herb extracts against superoxide anion-radicals was medium to high with values of potential from 52.18 mg quercetin/g for G. paradoxa to 235.54 mg quercetin/g for G. septemfida, and root extracts had low activity (<10 mg quercetin/g). The lipid peroxidation inhibition values of gentian herb extracts were also high with activities ranging from 63.23–242.08 mg caffeic acid/g. The root extracts had low activity from <10 mg caffeic acid/g to 17.68 mg caffeic acid/g (G. asclepiadea roots). The main reason for the high activity of the herb extracts was the high phenolic content (flavonoids, xanthones), which showed the maximum intensity of antioxidant protection.
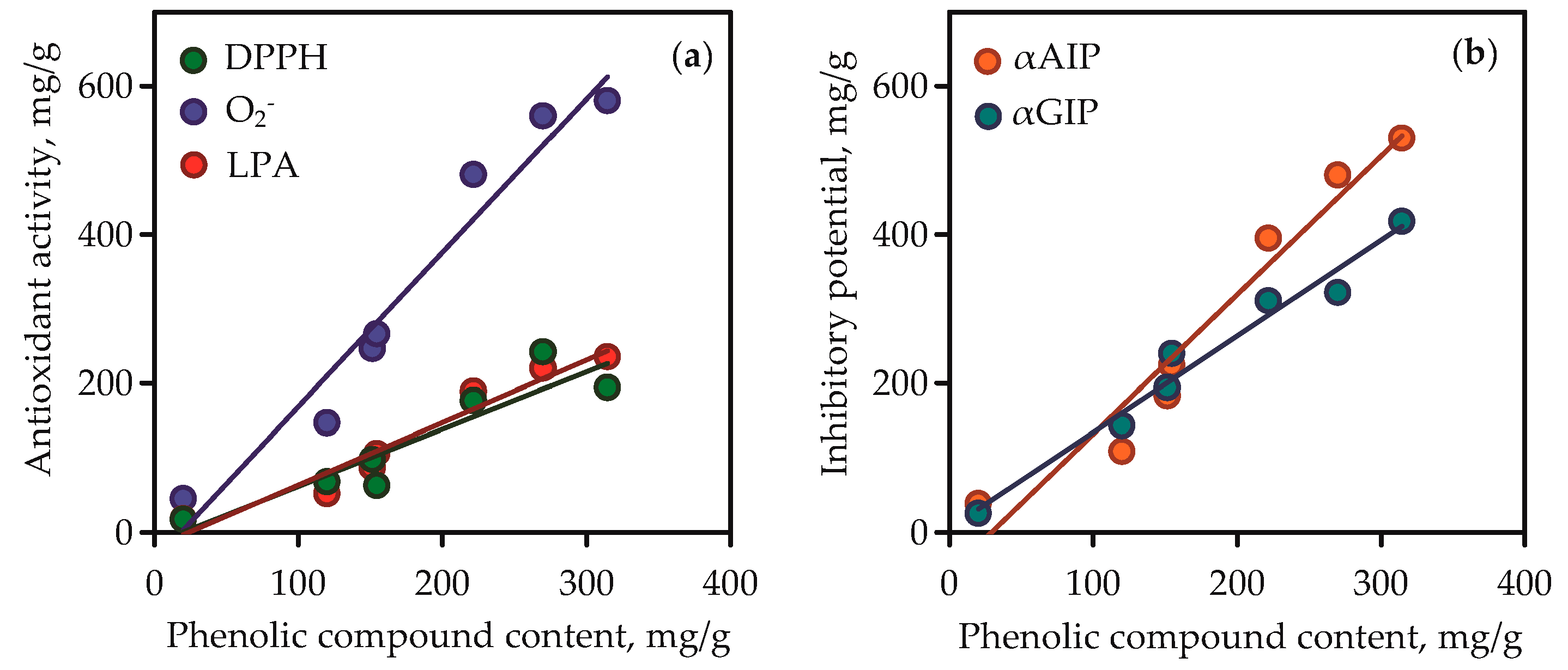
Isoorientin and mangiferin demonstrated superoxide anion-radical scavenging activity at 863.15 and 927.07 mg quercetin/g, respectively, and in the lipid peroxidation inhibition assay the parameters of activity for the same compounds were 486.56 and 522.14 mg caffeic acid/g, respectively. It was thus evident that the high content of phenolic compounds such as flavonoids and xanthones in gentian extracts gave them high antioxidant activity. This was clearly confirmed by the results of regression analysis between phenolic compound content in gentian extracts (Table S6) and their antioxidant activity values (Figure 10a). Good linearity was shown for three equations that gave high r (correlation coefficient) values from 0.9232 to 0.9752. The fact of the positive direct relationships between phenolic content and antioxidant activity of plant extracts was postulated elsewhere [71], but the compliance of this rule in case of Caucasian gentians was observed for the first time.
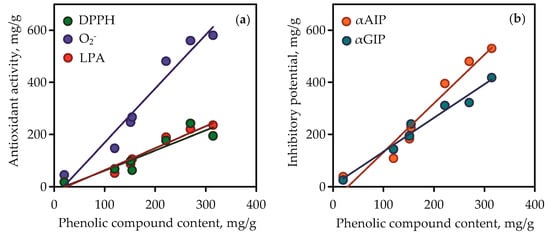
Figure 10.
Correlation graphs between phenolic compound content in gentian extracts (mg/g) and their bioactivity. (a) DPPH—2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (as mg of trolox per gram of dry extract weight; correlation equation y = 2.07·x − 38.48, r = 0.9752); O2-—superoxide anion-radical scavenging activity (as mg of quercetin per gram of dry extract weight; correlation equation y = 0.84·x − 20.21, r = 0.9736); LPA—lipid peroxidation inhibition activity (as mg of caffeic acid per g of dry extract weight; correlation equation y = 0.76·x − 14.74, r = 0.9232). (b) αAIP—α-amylase inhibitory potential (as mg of acarbose per g of dry extract weight; correlation equation y = 1.86·x − 54.73, r = 0.9739); αGIP—α-glycosidase inhibitory potential (as mg of acarbose per gram of dry extract weight; correlation equation y = 1.29·x + 5.08, r = 0.9849).
The calculated IC50 values in the DPPH assay for the Serbian species were 426.67–1000 μg/mL for G. asclepiadea root fractions and 181–614 μg/mL for G. asclepiadea herb fractions (ascorbic acid as a reference IC50 5.23 μg/mL) [39], for G. cruciata herb and roots extracts – 1263 and 2603 μg/mL, respectively (ascorbic acid as a reference IC50 6.05 μg/mL) [22]. The fractions of the G. septemfida herb of Turkey origin showed a scavenging effect at a dose of 1000 μg/mL with values of 15.01–80.17% [19]. The superoxide scavenging potential of extracts from the herb and roots of G. cruciata (Serbia) was 135 and >1000 μg/mL (gallic acid as a reference IC50 360 μg/mL) [22]. The root fractions of G. asclepiadea showed inhibitory activity toward lipid peroxidation with IC50 values of 40–183 μg/mL (butylated hydroxytoluene as a reference IC50 1.00 μg/mL) [22], and the extracts of the herb and roots of G. cruciata were active with IC50 792 and 894 μg/mL, respectively (butylated hydroxytoluene as a reference IC50 1.00 μg/mL) [22]. All this points out that despite differences in plant origin (Serbia, Turkey, Caucasus), it should be possible to postulate that the high antioxidant activity of gentian herbs makes them a valuable source of antioxidant phytophenolics.
The inhibitory potential of gentian extracts against α-amylase and α-glycosidase as basic digestion enzymes was studied using known spectrophotometric microplate assays [57]. All herb extracts showed high activity in the range 108.85–530.11 mg acarbose/g for α-amylase inhibition and 144.77–418.80 mg acarbose/g for α-glycosidase inhibition.
The root extracts demonstrated low activity (<10–38.87 mg acarbose/g) for α-amylase inhibition and <10–25.64 mg acarbose/g for α-glycosidase inhibition. The reasons for the varied activity of gentian extracts are in the various chemical profiles and quantitative composition of the extracts. The values of inhibitory potential of pure compounds were low for iridoid glycosides (<10 mg acarbose/g), medium for isovitexin, and high for isoorientin and mangiferin. The latter two compounds were the most active inhibitors, whereas isoorientin was the most potent inhibitor of α-amylase (1242.03 mg acarbose/g in α-amylase inhibition vs. 811.10 mg acarbose/g in α-glycosidase inhibition), and mangiferin was the most potent inhibitor of α-glycosidase (296.14 mg acarbose/g in α-amylase inhibition vs. 1562.84 mg acarbose/g in α-glycosidase inhibition). It is reasonable to expect that the high phenolic content resulted in the high inhibitory activity of gentian extracts against α-amylase and α-glycosidase. Regression equation data showed good linearity for correlation graphs between phenolic compound content in gentian extracts and α-amylase/α-glycosidase inhibitory activity (Figure 10b). In view of all this, there is a need to characterise gentian herbs as potent plant sources of anti-α-amylase and anti-α-glycosidase phenolics.
3. Conclusions
The data obtained are in compliance with known facts about high antioxidant and antidiabetic activity of flavones with luteolin skeleton [40,71,72] as well as the xanthone mangiferin [40,68]. Both of these have two phenolic ortho-hydroxyl groups. Although Caucasian gentians contain a great number of various phytochemicals, the most abundant active compounds were flavones and mangiferin which resulted in the high efficiency of derived plant extracts as antioxidants and digestive enzymes inhibitors. To our knowledge, this is the first paper combining detailed metabolite profiling by the HPLC-DAD-ESI-QQQ-MS technique and HPLC-DAD quantification of the main compounds with an antioxidant, anti-α-amylase, and anti-α-glycosidase study of the six gentian species (herb, roots) widely distributed in the Caucasus and used as phytopharmaceuticals.
4. Materials and Methods
4.1. Plant Materials and Chemicals
The information about samples of gentian herb and roots is listed in Table 4. The species were authenticated by authors Alexey S. Prokopyev (Siberian Botanic Garden, Tomsk State University, Tomsk Russia) and Javanshir I. Isaev (Azerbaijan Medical University, Baku, Azerbaijan). Plant material was dried and powdered before analysis.

Table 4.
Detailed information of gentiana samples.
The chemicals were purchased from ChemFaces (Wuhan, Hubei, PRC) — chrysoeriol (Cat. No. CFN98785, ≥98%), isoscoparin (Cat. No. CFN90965, ≥98%), morroniside (Cat. No. CFN98161, ≥98%), neomangiferin (Cat. No. CFN98122, ≥98%), sweroside (Cat. No. CFN99455, ≥98%); Extrasynthese (Lyon, France) — amarogentin (Cat. No. 0218S, ≥98%), apigenin-7-O-glucoside (Cat. No. 1004 S, ≥99%), orientin (Cat. No. 1054 S, ≥99%); Sigma-Aldrich (St. Louis, MO, USA) — acarbose (Cat. No. A8980, ≥95%), acetonitrile for HPLC (Cat. No 34851, ≥99.9%), α-amylase from Aspergillus oryzae (Cat. No. 10065, ~30 U/mg), caffeic acid (Cat. No. C0625, ≥98%), 2,2-diphenyl-1-picrylhydrazyl (Cat. No. D9132), formic acid (Cat. No. F0507, ≥95%), gentiopicroside (Cat. No. SMB00416, ≥98%), α-glucosidase from Saccharomyces cerevisiae (Cat. No. G5003, ≥10 units/mg), isomangiferin (Cat. No. PHL83514, ≥98%), isoorientin (Cat. No. 02187, ≥98%), isovitexin (Cat. No. 17804, ≥98%), lithium perchlorate (Cat. No. 431567, ≥99%), loganic acid (Cat. No. SMB00231, ≥95%), loganin (Cat. No. 36483, ≥97%), luteolin-7-O-glucoside (Cat. No. 49968, ≥98%), mangiferin (Cat. No. 06279, ≥98%), methanol (Cat. No. 322415, ≥99.8%), perchloric acid 70% (Cat. No. 311421, ≥99%), quercetin (Cat. No. Q4951, ≥95%), saponarin (Cat. No. PHL89784, ≥98%), swertiamarin (Cat. No. 90957, ≥95%), trolox (Cat. No. 238813, ≥97%). Some reference substances were isolated previously as loganic acid-6′-O-glucoside, algidiside I, algidiside II, gentiopicroside-6′-O-glucoside, sweroside-6′-O-glucoside, 6′-O-(2,3-dihydroxybenzoyl)-sweroside, gelidoside (rindoside), and trifloroside from Gentiana algida [14], swertiamarin-6′-O-glucoside from Gentianella azurea [73], 1-O-caffeoyl-glucose from Spiraea salicifolia [74], 6-O-caffeoyl-glucose from Filipendula ulmaria [75], 2-O-caffeoyl-glucaric acid from Leonurus deminutus [76], 1,3-di-O-caffeoyl-glycerin from Bupleurum longifolium [77], gentisin and gentioside from Anagallidium dichotomum [78], isoorientin-7-O-glucoside, isoorientin-4′-O-glucoside, and isovitexin-4′-O-glucoside from Gentiana decumbens [12], isoorientin-2″-O-glucoside from Silene nutans [79], isoorientin-6″-O-glucoside and isovitexin-2″-O-glucoside from Gastrolychnis tristis [80], isovitexin-7,2″-di-O-glucoside and isovitexin-2″,4″-di-O-glucoside from Melandrium divaricatum [81], isoscoparin-2″-O-glucoside and isoscoparin-7-O-glucoside from Silene aprica [82].
4.2. Total Extract Preparation
To prepare the total extract of gentian herb and roots the powdered sample (100 g) was triple extracted in a conical glass flask (2 L) with 60% methanol (2 L) with stirring and sonication for 60 min at 40 °C with ultrasound power of 100 W and the frequency 35 kHz. The final extracts were filtered through a cellulose filter, combined, evaporated in vacuo until dryness, and stored at 4 °C until further chemical composition analysis and bioactivity assays. The yields of total extracts of gentian herbs and roots listed in Table 4.
4.3. High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-DAD-ESI-QQQ-MS)
Reversed-phase high-performance liquid chromatography with diode array detection and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-DAD-ESI-QQQ-MS) procedure was used for phenolic compounds profiling. Experiments were performed on an LCMS 8050 liquid chromatograph coupled with diode-array-detector and triple-quadrupole electrospray ionization detector (Shimadzu, Columbia, MD, USA), using a GLC Mastro C18 column (150 × 2.1 mm, Ø 3 μm; Shimadzu, Kyoto, Japan), column temperature was 35 °C. Eluent A was 0.5% formic acid in water and eluent B was 0.5% formic acid in acetonitrile. The injection volume was 1 μL, and elution flow was 100 μL/min. Gradient program: 0.0–6.0 min 5–20% B, 6.0–12.0 min 20–40% B, 12.0–16.0 min 40–55% B, 16.0–21.0 min 55–60% B, 21.0–30.0 min 60–100% B, 30.0–35.0 min 100–5% B. The DAD acquisitions were performed in the range of 200–600 nm. MS detection was performed in negative ESI mode using the parameters as follows: temperature levels of ESI interface, desolvation line and heat block were 300 °C, 250 °C and 400 °C, respectively. The flow levels of nebulizing gas (N2), heating gas (air) and collision-induced dissociation gas (Ar) were 3 L/min, 10 L/min and 0.3 mL/min, respectively. The MS and MS/MS spectra were both recorded in negative (−3 kV source voltage) and positive mode (+3 kV source voltage) by scanning in the range of m/z 100–1900 at the collision energy of 10–45 eV. The system was operated under LabSolutions workstation software with the internal LC-MS library.
4.4. HPLC-DAD Quantification
Quantification of iridoid glycosides, flavonoids, and xanthones was realized in mc-HPLC-DAD experiments using microcolumn HPLC apparatus. Experiments were performed on an microcolumn chromatograph Econova MiLiChrom A-02 (Novosibirsk, Russia), using a ProntoSIL-120-5-C18 AQ column (1 × 50 mm, ∅ 1 μm; Metrohm AG; Herisau, Switzerland), column temperature was 30 °C. Eluent A was 0.2 М LiClO4 in 0.01 M HClO4 and eluent B was 0.01 M HClO4 in acetonitrile. The injection volume was 1 μL, and elution flow was 150 μL/min. Gradient program: 0.0–10.0 min 12–35% B, 10.0–15.0 min 35–70% B, 15.0–20.0 min 70–12% B. The chromatograms were recorded at 210 nm.
To prepare the stock solutions of reference compounds, 18 mg loganic acid, swertiamarin, gelidoside, gentiopicroside, gentiopicroside-6″-O-glucoside, sweroside, trifloroside, isovitexin, isovitexin-2″-O-glucoside, saponarin, apigenin-7-O-glucoside, isoorientin, isoorientin-2″-O-glucoside, isoorientin-6″-O-glucoside, luteolin-7-O-glucoside, isoscoparin, mangiferin, and gentioside were accurately weighed and individually dissolved in methanol in volumetric flask (20 mL). The external standard calibration curve was generated using six data points, covering the concentration 1.75, 14.06, 56.25, 225.00, 450, and 900.00 µg/mL. The calibration curves were created by plotting the peak area vs. the concentration levels. All the analyses were carried out in triplicate and the data were expressed as mean value ± standard deviation (SD).
For preparation of sample solution, an accurately weighted powdered plant (100 mg) was placed in an Eppendorf tube, 2 mL of 60% methanol was added. Then the sample was extracted twice in an ultrasonic bath for 30 min at 40 °C and centrifuged (3000× g, 15 min). Combined supernatants were transferred to volumetric flask (5 mL) and the final volume was reduced to 5 mL. The resultant extract was filtered through a 0.22-μm PTFE syringe filter before injection into the HPLC system for analysis.
4.5. Validation Analysis
The linearity of HPLC-DAD quantification method was studied by injecting six concentrations (1.75–900.00 µg/mL) of the 18 reference standards (loganic acid, swertiamarin, gelidoside, gentiopicroside, gentiopicroside-6″-O-glucoside, sweroside, trifloroside, isovitexin, isovitexin-2″-O-glucoside, saponarin, apigenin-7-O-glucoside, isoorientin, isoorientin-2″-O-glucoside, isoorientin-6″-O-glucoside, luteolin-7-O-glucoside, isoscoparin, mangiferin, gentioside). Results from each analysis were averaged and subjected to regression analysis. Limits of detection (LOD) and quantification (LOQ) were determined using the following equations: LOD = (3.3 × SYX)/a; LOQ = (10 × SYX)/a, where SYX is a standard deviation of the response (Y intercept) and a is a slope of calibration curve. Intra- and inter-day variations, which are presented in terms of percent relative standard deviation (%RSD) of the analyte’s peak area and variability assessed the precision of the HPLC-DAD quantification. For the intra-day variability test, the mixture solution containing 18 reference standards was analysed for five replicates within one day (56.25 µg/mL), while inter-day assay was analysed using the same concentration for intra-day precision on four different days (interval of 1 day). The repeatability test of the sample was performed on 7-fold experiments of the mixture solution contain 18 reference standards (225 µg/mL). The stability test was performed with one sample solution, which was stored at room temperature and analysed at regular intervals (0, 2, 4, 8, 12, 24 and 48 h.). For analysis of recovery data, the appropriate amounts of the powdered sample of 18 reference standards were weighted and spiked with a known amount of reference compound and then analysed five times.
4.6. Antioxidant Activity Analysis
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was performed as described earlier [83]. The trolox was used as a positive control (IC50 11.62 ± 0.23 µg/mL), and water was used as a negative control. The IC50 value was found as the effective concentration at which DPPH radicals were scavenged by 50%. The value of antioxidant activity (ADPPH) against DPPH radicals was found as a ratio of trolox IC50 to sample IC50 [ADPPH = (IC50Trolox/ IC50Sample) × 1000] and expressed as mg of trolox in 1 g of sample. Values are expressed as mean obtained from five independent experiments.
The known assay [84] was used to determine superoxide anion radical scavenging activity. Quercetin was used as a positive control (IC50 21.74 ± 0.42 µg/mL), and water was used as a negative control. The IC50 value was found as the effective concentration at which superoxide anion radicals were scavenged by 50%. The value of antioxidant activity (AO2•) against superoxide anion radicals was found as a ratio of quercetin IC50 to sample IC50 [AO2• = (IC50Quercetin/ IC50Sample) × 1000] and expressed as mg of quercetin in 1 g of sample. Values are expressed as mean obtained from five independent experiments.
The previously described method [85] was used to investigate the lipid peroxidation inhibition potency. Caffeic acid was used as a positive control (IC50 58.96 ± 1.14 µg/mL), and water was used as a negative control. The IC50 value was found as the effective sample concentration gave 50% reduction of optical density of initial solution. The value of lipid peroxidation inhibition (I) was found as a ratio of caffeic acid IC50 to sample IC50 [I = (IC50Caffeic acid/ IC50Sample) × 1000] and expressed as mg of caffeic acid in 1 g of sample. Values are expressed as mean obtained from five independent experiments.
4.7. Digestive Enzymes Inhibiting Potential
The α-amylase inhibiting potential and α-glucosidase inhibiting potential were performed using spectrophotometric assays [86,87]. The α-amylase from Aspergillus oryzae (3 U/mL) and α-glucosidase from Saccharomyces cerevisae (0.5 U/mL) were used as substrates. Acarbose was used as a positive control (IC50 311.14 ± 7.79 µg/mL for α-amylase inhibiting potential; IC50 1282.64 ± 38.46 µg/mL for α-glucosidase inhibiting potential), and water was used as a negative control. The IC50 value was found as the effective sample concentration gave 50% inhibition of digestive enzyme. The values of α-amylase/α-glucosidase inhibiting potential (P) was found as a ratio of acarbose IC50 to sample IC50 [I = (IC50Acarbose/ IC50Sample) × 1000] and expressed as mg of acarbose in 1 g of sample. Values are expressed as mean obtained from six independent experiments.
4.8. Statistical and Multivariative Analysis
Statistical analyses were performed using a one-way analysis of variance (ANOVA), and the significance of the mean difference was determined by Duncan’s multiple range test. Differences at p < 0.05 were considered statistically significant. The results are presented as mean values ± SD (standard deviations) of the three replicates. Advanced Grapher 2.2 (Alentum Software Inc., Ramat-Gan, Israel) was used to perform linear regression analysis and to generate graphs.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/9/11/271/s1, Table S1: Ethnopharmacological use of Gentiana species by the various Caucasus people, Table S2: Known compounds found in Gentiana species mentioned in present study (literature data), Table S3: Retention times and mass spectrometric data of compounds 1–137 found in herb and roots of six Caucasian Gentiana species, Table S4: Regression equations, correlation coefficients, standard deviation, limits of detection, limits of quantification and linear ranges for 18 compounds, Table S5: Intra- and inter-day precision, repeatability, stability and recovery for 18 compounds, Table S6: Content of selected phenolic compounds in dry extracts of gentian herbs and roots, Figure S1: High-Performance Liquid Chromatography with Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection chromatogram in base peak chromatogram mode of six Gentiana herb and root extracts, Figure S2: Structures of reference compounds, Figure S3: High-Performance Liquid Chromatography with Diode Array Detection chromatograms of gentian herb and roots extracts at 210 nm.
Author Contributions
Conceptualization, D.N.O., N.K.C. and A.I.G.; methodology, D.N.O. and C.V.; validation, N.I.K., N.K.C., and A.I.G.; formal analysis, A.I.G. and J.I.I.; investigation, D.N.O., N.I.K., A.I.G., and A.S.P.; resources, N.I.K., N.K.C., A.I.G., and A.S.P.; data curation, J.I.I. and T.N.K.; writing—original draft preparation, D.N.O. and N.I.K.; writing—review and editing, C.V.; visualization, D.N.O.; supervision, N.K.C.; project administration, N.I.K.; funding acquisition, D.N.O., N.I.K., and N.K.C.
Funding
This research was funded by Ministry of Education and Science of Russia, project number AAAA-A17-117011810037-0, and the Science Development Foundation under the President of the Republic of Azerbaijan, project number EIF-2013-9(15)-46/01/3-M-13.
Acknowledgments
The authors acknowledge the Buryat Research Resource Centre for the technical support in chromatographic and mass-spectrometric research.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- The Plant List. Available online: http://www.theplantlist.org/1.1/browse/A/Gentianaceae/Gentiana/ (accessed on 24 September 2019).
- Sirotuk, E.A. The Gentians of the West Caucasus: The aspects of biology and conservation. Ph.D. Thesis, Kuban State Agrarian University, Krasnodar, Russia, 22 March 2007. [Google Scholar]
- Mamedov, N.; Mehdiyeva, N.P.; Craker, L.E. Medicinal plants used in traditional medicine of the Caucasus and North America. J. Med. Act. Plants 2015, 4, 42–66. [Google Scholar] [CrossRef]
- Ibadullayeva, S.J.; Mamedova, S.E.; Sultanova, Z.R.; Movsumova, N.V.; Jafarli, I.A. Medicinal plants of Azerbaijan flora used in the treatment of certain diseases. Afr. J. Pharm. Pharmacol. 2010, 4, 545–548. [Google Scholar]
- Karyagin, I.I. Flora of Azerbaijan; Academy of Science: Baku, Azerbaijan, 1957; Volume 7, pp. 87–99. [Google Scholar]
- Bussmann, R.W.; Paniagua, Z.N.Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Hart, R.E. Medicinal and food plants of Svaneti and Lechkhumi, Sakartvelo (Republic of Georgia), Caucasus. Med. Aromat. Plants 2016, 5, 5. [Google Scholar] [CrossRef]
- Damirov, I.A.; Prilipko, L.I.; Shukurov, D.Z.; Kerimov, U.B. Medical Plants of Azerbaijan; Baku: Baku, Azerbaijan, 1983; pp. 310–319. [Google Scholar]
- Bussmann, R.W.; Paniagua Zambrana, N.Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Khutsishvili, M.; Batsatsashvili, K.; Hart, R.E. A comparative ethnobotany of Khevsureti, Samtskhe-Javakheti, Tusheti, Svaneti, and Racha-Lechkhumi, Republic of Georgia (Sakartvelo), Caucasus. J. Ethnobiol. Ethnomed. 2016, 12, 43. [Google Scholar] [CrossRef]
- Gagiidze, R.I.; Kutateladze, S.I. Flora of Georgia; Metzniereba: Tbilisi, Georgia, 1985; Volume 10, pp. 123–140. [Google Scholar]
- Sokolov, P.D. (Ed.) Plant Resources of USSR; Nauka: Leningrad, Russia, 1990; Volume 5, pp. 50–58. [Google Scholar]
- Rollov, A.K.H. Wild-Growing Plants of Caucasus: Distribution, Properties and Uses; Nauka: Tyfliz, Georgia, 1908; pp. 598–599. [Google Scholar]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Tankhaeva, L.M. Iridoids and flavonoids of four Siberian gentians: Chemical profile and gastric stimulatory effect. Molecules 2015, 20, 19172–19188. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Koryakina, L.P.; Vladimirov, L.N. Bitter gentian teas: Nutritional and phytochemical profiles, polysaccharide characterisation and bioactivity. Molecules 2015, 20, 20014–20030. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. Algidisides I and II, new iridoids glycosides from Gentiana algida. Chem. Nat. Compd. 2016, 52, 550–554. [Google Scholar] [CrossRef]
- Goetz, M.; Hostettmann, K.; Jacot-Guillarmod, A. A new C-glycosylflavone from Gentiana asclepiadea. Phytochemistry 1976, 15, 2014. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Van, D.T.; Asenov, I.V. Chemical composition of the roots of Gentiana asclepiadea. Chem. Nat. Compd. 1991, 27, 369–370. [Google Scholar] [CrossRef]
- Goetz, M.; Hostettmann, K.; Jacot-Guillarmod, A. C-glucosides flavoniques et xanthoniques de Gentiana cruciata. Phytochemistry 1976, 15, 2015. [Google Scholar] [CrossRef]
- Popović, Z.; Krstić-Milošević, D.; Stefanović, M.; Matić, R.; Vidaković, V.; Bojović, S. Chemical and morphological inter- and intrapopulation variability in natural populations of Gentiana pneumonanthe L. Chem. Biodivers. 2019, 16, e1800509. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Yagci Tuzun, C.; Toker, G.; Orhan, I.E. An in vitro perspective to cholinesterase inhibitory and antioxidant activity of five Gentiana species and Gentianella caucasea. Int. J. Food Sci. Nutr. 2012, 63, 802–812. [Google Scholar] [CrossRef]
- Goetz, M.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana, XXII. Identification de nouveaux O-glucosides de la mangiférine dans Gentiana asclepiadea L. Helv. Chim. Acta 1977, 60, 2104–2106. [Google Scholar] [CrossRef]
- Burret, F.; Chulia, A.J.; Debelmas, A.M.; Hosteltmann, K. Presence of isoscoparine, isoscoparine-7-O-glucoside and saponarine in Gentiana pneumonanthe L. Planta Med. 1978, 34, 176–179. [Google Scholar] [CrossRef]
- Mihailović, V.; Mišić, D.; Matić, S.; Mihailović, M.; Stanić, S.; Vrvić, M.M.; Katanić, J.; Mladenović, M.; Stanković, N.; Boroja, T.; et al. Comparative phytochemical analysis of Gentiana cruciata L. Roots and aerial parts, and their biological activities. Ind. Crops Prod. 2015, 73, 49–62. [Google Scholar] [CrossRef]
- Goetz, M.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana, XX. Identification de nouveaux di-O-glucosides de C-glucosylflavones dans Gentiana asclepiadea L. Helv. Chim. Acta 1977, 60, 1322–1324. [Google Scholar] [CrossRef]
- Toker, G.; Edis, M.; Yeşilada, E. Quantitative analysis of isoorientin in several Turkish Gentiana species by high performance liquid chromatography. Fabad J. Pharm. Sci. 2011, 36, 149–154. [Google Scholar]
- Goetz, M.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana, XXIV. Nouveaux C-glycosides flavoniques dans les feuilles de Gentiana asclepiadea L. Helv. Chim. Acta 1978, 61, 1373–1375. [Google Scholar] [CrossRef]
- Hostettmann-Kaldas, M.; Hostettmann, K.; Sticher, O. Xanthones, flavones and secoiridoids of american Gentiana species. Phytochemistry 1981, 20, 443–446. [Google Scholar] [CrossRef]
- Szucs, Z.; Dános, B.; Nyiredy, S.Z. Comparative analysis of the underground parts of Gentiana species by HPLC with diode-array and mass spectrometric detection. Chromatographia 2002, 56, S19–S23. [Google Scholar] [CrossRef]
- Çalis, İ.; Ersöz, T.; Chulla, A.J.; Rüedi, P. Septemfidoside: A new bis-iridoid diglucoside from Gentiana septemfida. J. Nat. Prod. 1992, 55, 385–388. [Google Scholar] [CrossRef]
- Calis, I.; Ruegger, H.; Chun, Z.; Sticher, O. Secoiridoid glucosides isolated from Gentiana gelida. Planta Med. 1990, 56, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Mpondo Mpondo, E.; Chulia, A.J. 6′-O-β-D-Glucosyl gentiopicroside: A new secoiridoid from Gentiana asclepiadea. Planta Med. 1988, 54, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Hayta, S.; Akgun, I.H.; Ganzera, M.; Bedir, E.; Gurel, A. Shoot proliferation and HPLC-determination of iridoid glycosides in clones of Gentiana cruciata L. Plant Cell Tissue Organ Cult. 2011, 107, 175–180. [Google Scholar] [CrossRef]
- Chulia, A.J.; Mpondo, E.M.; Nardin, R. Pneumonanthoside, premier glycoside de l’oxo-3α-ionol chez Gentiana pneumonanthe. J. Nat. Prod. 1987, 50, 248–250. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Spassov, S.L. A naphthodipyranodione from Gentiana asclepiadea. Phytochemistry 1992, 31, 1067–1068. [Google Scholar] [CrossRef]
- Hudecová, A.; Hašplová, K.; Miadoková, E.; Gregáň, F.; Dušinská, M. Cytotoxic and genotoxic effect of methanolic flower extract from Gentiana asclepiadea on COS 1 cells. Neuro Endocrinol. Lett. 2010, 31, 21–25. [Google Scholar]
- Mihailović, V.; Vuković, N.; Nićiforović, N.; Mašković, P.; Stanković, M.S. Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J. Med. Plants Res. 2011, 5, 1164–1174. [Google Scholar]
- Hudecová, A.; Hašplová, K.; Miadoková, E.; Gregáň, F.; Dušinská, M. Gentiana asclepiadea protects human cells against oxidation DNA lesions. Cell Biochem. Funct. 2012, 30, 101–107. [Google Scholar] [CrossRef]
- Hudecová, A.; Kusznierewicz, B.; Hašplová, K.; Gálová, E.; Dušinská, M. Gentiana asclepiadea exerts antioxidant activity and enhances DNA repair of hydrogen peroxide- and silver nanoparticles-induced DNA damage. Food Chem. Toxicol. 2012, 50, 3352–3359. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Solujić, S.; Matić, S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, O.; Ličina, B.; Vasić, S.; Radojević, I.; Čomić, L. Bioactive extracts of Gentiana asclepiadea: Antioxidant, antimicrobial, and antibiofilm activity. Bot. Serbica 2018, 42, 223–229. [Google Scholar] [CrossRef]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Massias, M.; Carbonnier, J.; Mohlo, D. Implications chimiotaxonomiques de la répartition des substances osidiques dans le genre Gentiana L. Bull. Mus. Nat. Hist. Nat. Sci. Phys. Chim. 1977, 13, 41–54. [Google Scholar]
- Pan, Y.; Zhao, Y.-L.; Zhang, J.; Li, W.-Y.; Wang, Y.-Z. Phytochemistry and pharmacological activities of the genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef]
- Uesato, S.; Hashimoto, T.; Inouye, H. Three new secoiridoid glucosides from Eustoma russellianum. Phytochemistry 1979, 18, 1981–1986. [Google Scholar] [CrossRef]
- Tan, R.X.; Wolfender, J.-L.; Ma, W.G.; Zhang, L.X.; Hostettmann, K. Secoiridoids and antifungal aromatic acids from Gentiana algida. Phytochemistry 1996, 41, 111–116. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Wu, L.; Wang, Z. Authentication of Gentiana straminea Maxim. and its substitutes based on chemical profiling of iridoids using liquid chromatography with mass spectrometry. Biomed. Chromatogr. 2016, 30, 2061–2066. [Google Scholar] [CrossRef]
- Inouye, H.; Ueda, S.; Nakamura, Y.; Inoue, K.; Hayano, T.; Matsumura, H. Über die monoterpenglucoside und verwandte naturstoffe-XXIV. Triflorosid, ein neues secoiridoidglucosid aus Gentiana triflora var. japonica. Tetrahedron 1974, 30, 571–577. [Google Scholar] [CrossRef]
- Chen, G.; Wei, S.-H.; Yu, C.-Y. Secoiridoids from the roots of Gentiana straminea. Biochem. Syst. Ecol. 2009, 37, 766–771. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Wong, K.-L.; Chan, Y.-M.; But, P.P.-H.; Shaw, P.-C. Isolation of iridoid and secoiridoid glycosides and comparative study on Radix gentianae and related adulterants by HPLC analysis. Phytochemistry 2005, 66, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, M.; Zhang, Y.-J.; Yang, C.-R. New acylated secoiridoid glucosides from Gentiana straminea (Gentianaceae). Helv. Chim. Acta 2009, 92, 321–327. [Google Scholar] [CrossRef]
- Liu, Q.; Chou, G.-X.; Wang, Z.-T. New iridoid and secoiridoid glucosides from the roots of Gentiana manshurica. Helv. Chim. Acta 2012, 95, 1094–1101. [Google Scholar] [CrossRef]
- Kim, J.A.; Son, N.S.; Son, J.K.; Jahng, Y.; Chang, H.W.; Jang, T.S.; Na, M.; Lee, S.H. Two new secoiridoid glycosides from the rhizomes of Gentiana scabra Bunge. Arch. Pharm. Res. 2009, 32, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Marston, A.; Gauthier, R.; Hostettmann, K. Iridoids and secoiridoids from Gentiana linearis. Phytochemistry 1997, 44, 633–637. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Liu, M.; Xie, H.; Jiang, Y.; Wei, X. Iridoids from Gentiana loureirii. Phytochemistry 2009, 70, 746–750. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wei, H.; Nagao, S.; Mahmud, T.; Hori, K.; Kobayashi, M.; Uji, T.; Shibuya, H. Indonesian Medicinal Plants. XIV. Characterization of 3′-O-caffeoylsweroside, a new secoiridoid glucoside, and kelampayosides A and B, two new phenolic apioglucosides, from the bark of Anthocephalus chinensis (Rubiaceae). Chem. Pharm. Bull. 1996, 44, 1162–1167. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Cardellina, J.H.; Boyd, M.R. A benzoic acid glycoside from Geniostoma antherotrichum. Phytochemistry 1996, 41, 1205–1207. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New flavonoids and turkesterone-2-O-cinnamate from leaves of Rhaponticum uniflorum. Chem. Nat. Compd. 2019, 55, 256–264. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Chulia, A.J.; Mariotte, A.M. Nouvelles C-glucosylflavones chez Gentiana pedicellata. J. Nat. Prod. 1985, 48, 480–483. [Google Scholar] [CrossRef]
- Luong, M.D.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana XXI.; Les cinnamoyl-C-glucosylflavones et leurs O-glucosides dans Gentiana punctata L. Helv. Chim. Acta 1977, 60, 2099–2103. [Google Scholar] [CrossRef]
- Luong, M.D.; Fombasso, P.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana. XXV. Etude des composés flavoniques et xanthoniques dans les feuilles de Gentiana × marcailhouana RY. Nouveaux cinnamoyl-C-glucosides flavoniques. Helv. Chim. Acta 1980, 63, 244–249. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Cisowski, W. Flavone C-glycosides from Bryonia alba and B. dioica. Phytochemistry 1995, 39, 727–729. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K.; Jacot-Guillarmod, A. Contribution à la phytochimie du genre Gentiana, XIX. Identification de nouveaux C-glucosides flavoniques dans Gentiana pyrenaica L. Helv. Chim. Acta 1976, 59, 2596–2600. [Google Scholar] [CrossRef]
- Rhourri-Frih, B.; Chaimbault, P.; Claude, B.; André, P.; Lafosse, M. Analysis of pentacyclic triterpenes by LC-MS. A comparative study between APCI and APPI. J. Mass Spectrom. 2009, 44, 71–80. [Google Scholar] [CrossRef]
- Olennikov, D.N. Makisterone C-20,22-acetonide from Rhaponticum uniflorum. Chem. Nat. Compd. 2018, 54, 930–933. [Google Scholar] [CrossRef]
- Gil, M.; Wianowska, D. Chlorogenic acids—Their properties, occurrence and analysis. Ann. Univ. Mariae Curie Skłodowska 2017, 72, 61–104. [Google Scholar] [CrossRef]
- Hostettmann, K.; Wagner, H. Xanthone glycosides. Phytochemistry 1977, 16, 821–829. [Google Scholar] [CrossRef]
- Struwe, L.; Albert, V.A. (Eds.) Gentianaceae: Systematics and Natural History; Cambridge University Press: Cambridge, UK, 2002; pp. 602–604. [Google Scholar]
- Jyotshna; Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. Biofactors 2016, 42, 504–514. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic acids and flavonoids of fringed sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS profile, HPLC-DAD quantification, in vitro digestion stability, and antioxidant capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vennos, C. Chemical composition, antioxidant and anticholinesterase activities of Gentianella azurea from Russian Federation. Nat. Prod. Commun. 2017, 12, 55–56. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Spireasalicin, a new acylated glycoside of quercetin from Spiraea salicicfolia. Chem. Nat. Compd. 2017, 53, 1038–1044. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kruglova, M.Y. A new quercetin glycoside and other phenolic compounds from the genus Filipendula. Chem. Nat. Compd. 2013, 49, 610–616. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. Caffeoylglucaric acids and other phenylpropanoids of the Siberian Leonurus species. Chem. Nat. Compd. 2016, 52, 780–782. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Partilkhaev, V.V. Flavonoids and phenylpropanoids from several species of Bupleurum growing in Buryatia. Chem. Nat. Compd. 2012, 48, 972–976. [Google Scholar] [CrossRef]
- Olennikov, D.N. Chemical investigation of Anagallidium dichotomum and anticholinesterase activity of its constituents. Chem. Nat. Compd. 2013, 49, 977–979. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ecdysteroids, flavonoids, and phenylpropanoids from Silene nutans. Chem. Nat. Compd. 2019, 55, 127–130. [Google Scholar] [CrossRef]
- Olennikov, D.N. Phytoecdysteroids and flavonoids from Gastrolychnis tristis. Chem. Nat. Compd. 2018, 54, 204–206. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New C,O-glycosylflavones from Melandrium divaricatum. Chem. Nat. Compd. 2019, 55, 890–895. [Google Scholar]
- Olennikov, D.N.; Chirikova, N.K. C-Glycosyl flavones from two Eastern Siberian species of Silene. Chem. Nat. Compd. 2019, 55, 642–647. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules 2017, 22, 16. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Gornostai, T.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017, 18, 2579. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Agrimoniin, an active ellagitannin from Comarum palustre herb with anti-α-glucosidase and antidiabetic potential in streptozotocin-induced diabetic rats. Molecules 2017, 22, 73. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).