Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis
Abstract
1. Introduction
2. Results
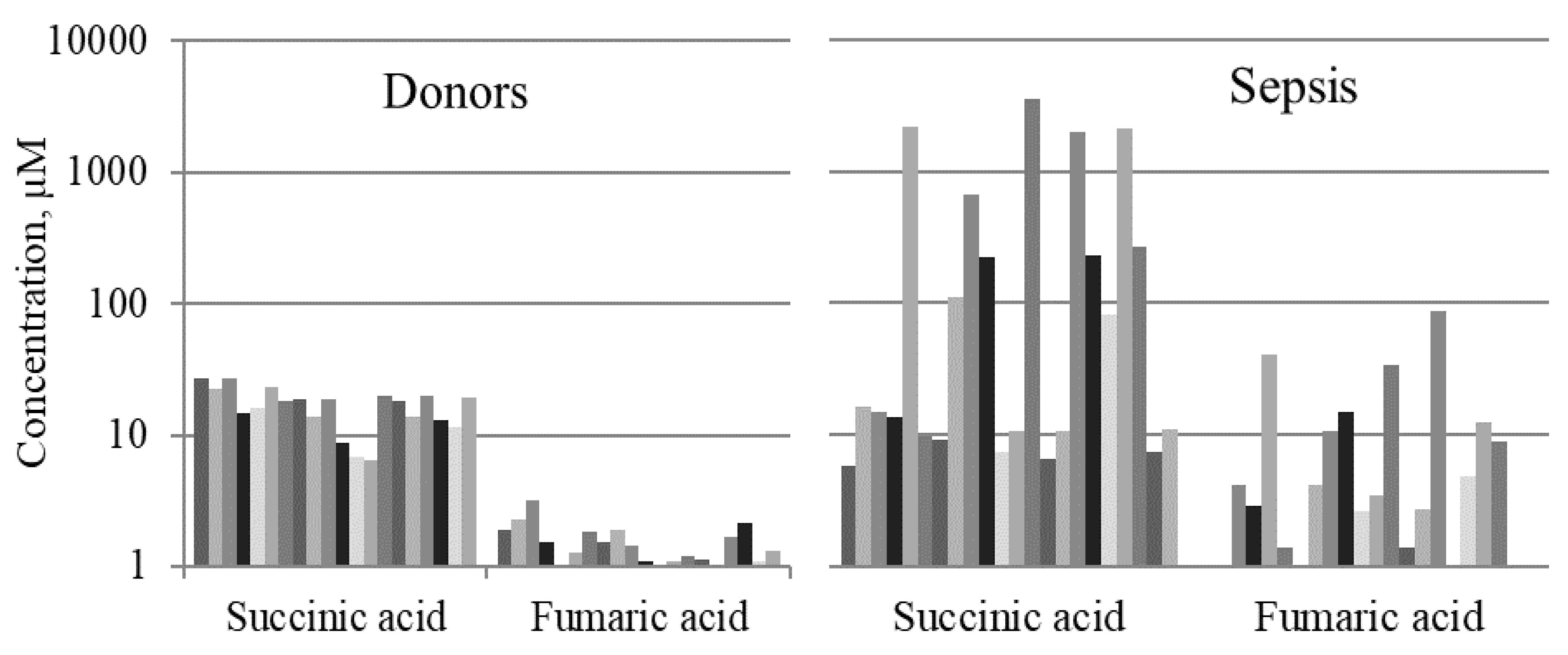
2.1. Mitochondrial Metabolites in Blood Serum of Septic Patients
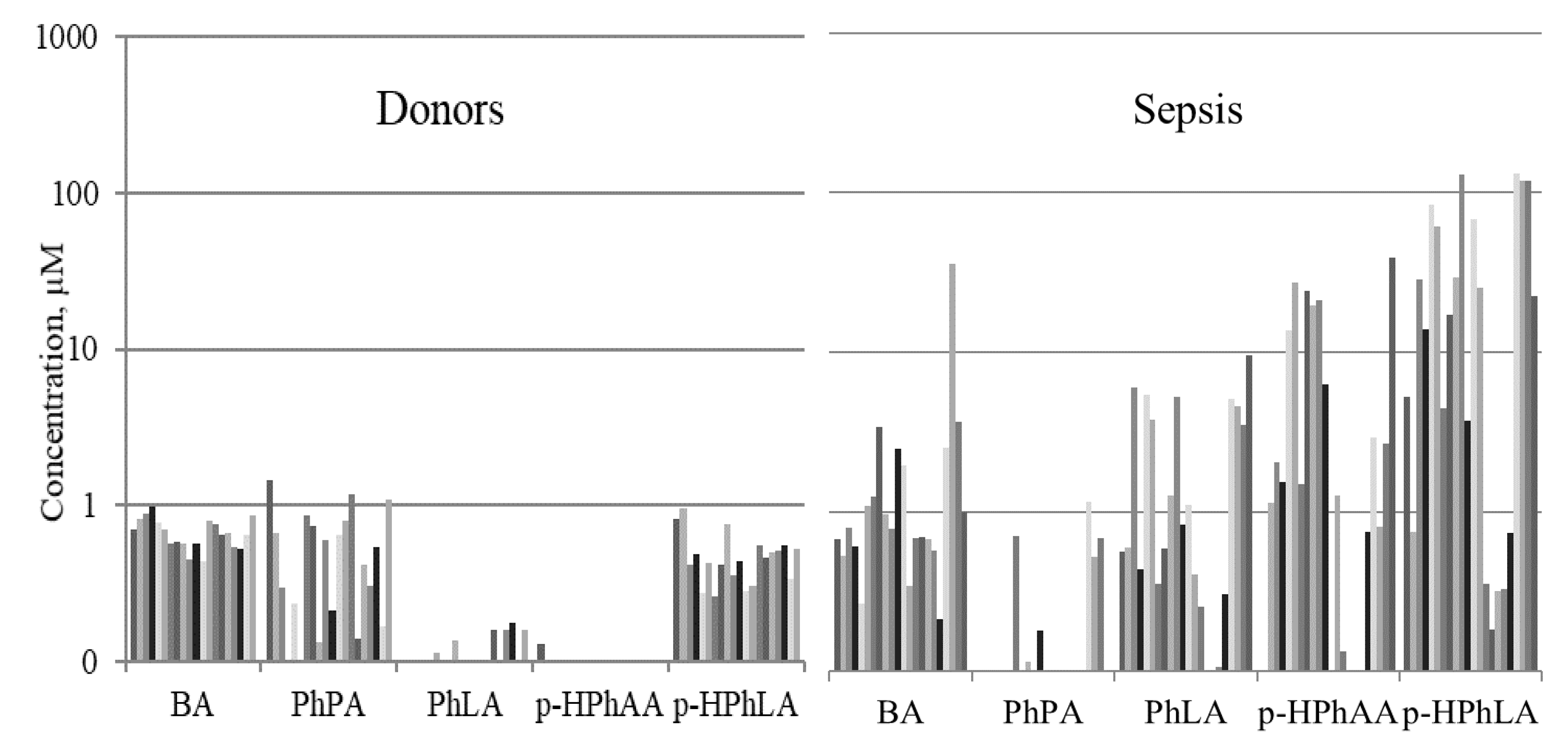
2.2. Aromatic Microbial Metabolites in the Blood Serum of Septic Patients
2.3. Features of the Results in the Late Sepsis Group
2.3.1. Microbial and Mitochondrial Metabolites in Groups II–III
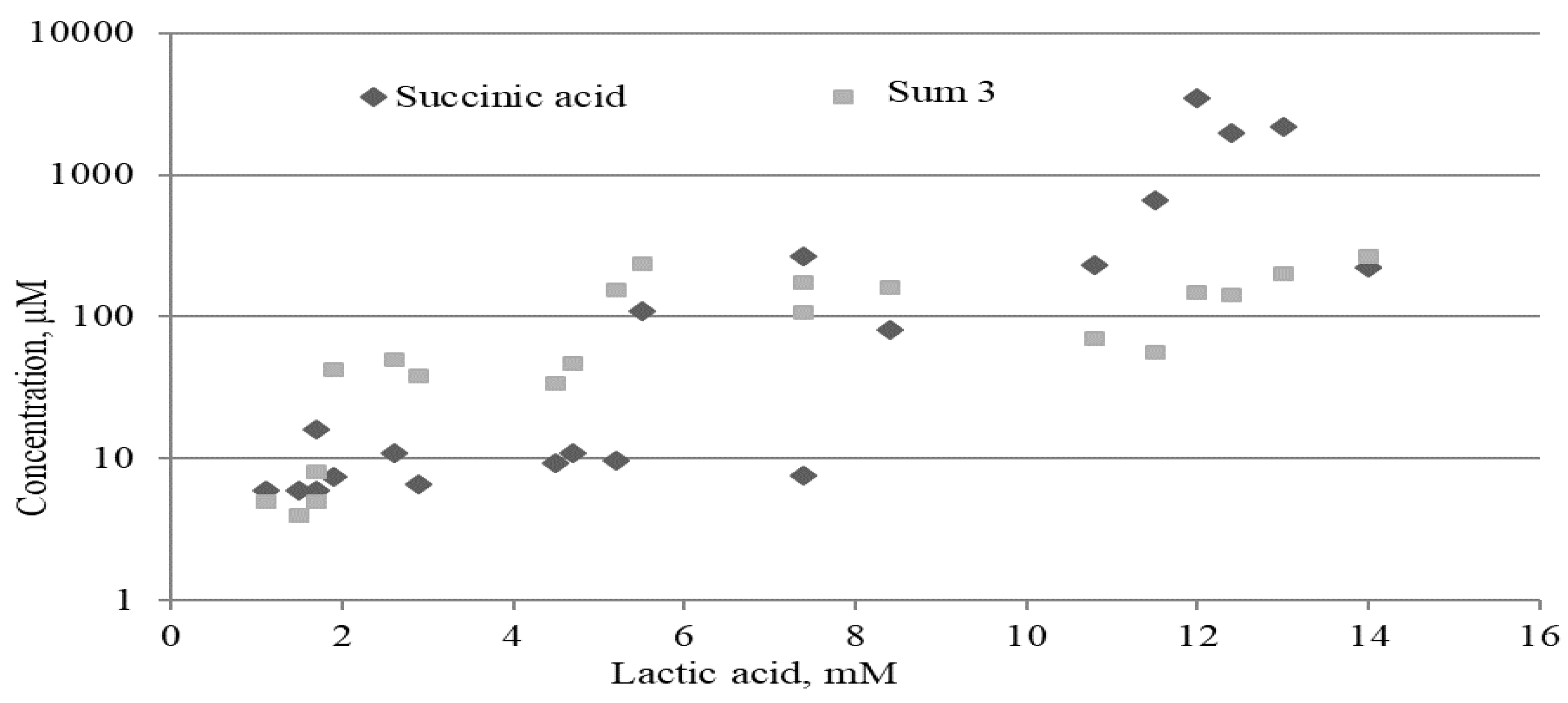
2.3.2. Lactate, Mitochondrial and Microbial Metabolites
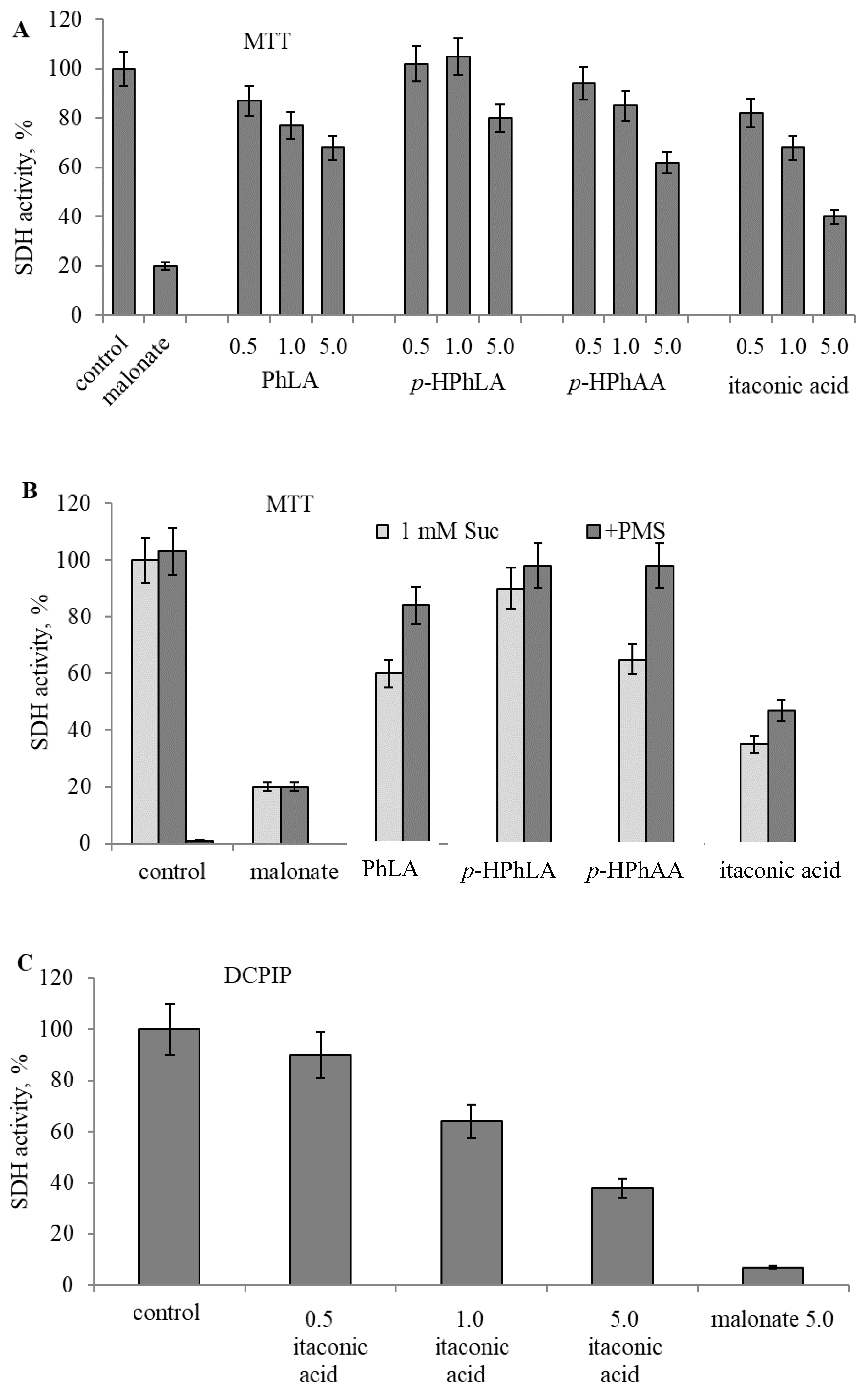
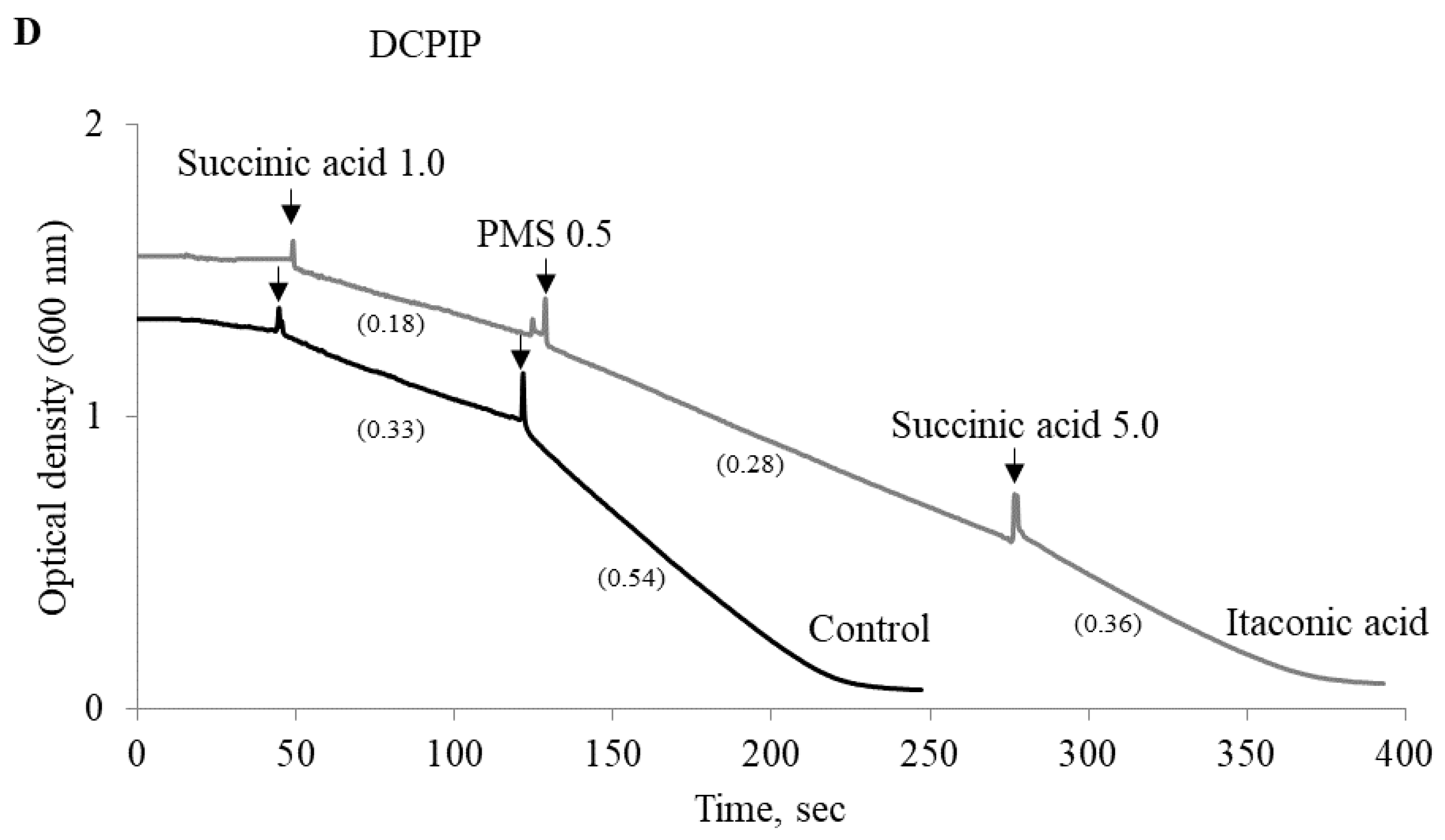
2.4. Experimental Studies on Mitochondria: Effect of Phenylcarboxylic and Itaconic Acids on the Activity of Succinate Dehydrogenase
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Patients and Samples
4.3. Serum Sample Preparation and GC–MS Analysis
4.4. Preparation of Rat Liver Mitochondria
4.5. Determination of Succinate Dehydrogenase Activity from Reduction of Methyl Thiazolyl Tetrazolium and Dichlorophenolindophenol
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | benzoic acid |
| DCPIP | dichlorophenolindophenol |
| ICU | intensive care unit |
| IQR | interquartile range |
| GC-MS | gas chromatography-mass spectrometry |
| LPS | lipopolysaccharides |
| MTT | methyl thiazolyl tetrazolium |
| PhAA | phenylacetic acid |
| PhCA | phenylcarboxylic acid |
| PhPA | phenylpropionic acid |
| PhLA | phenyllactic acid |
| p-HPhAA | 4-hydroxyphenylacetic acid |
| p-HPhLA | 4-hydroxyphenyllactic acid |
| PMS | phenazine methosulfate |
| ROS | reactive oxygen species |
| SDH | succinate dehydrogenase |
| SOFA score | sequential organ failure assessment score |
| Sum3 | sum of PhLA, p-HPhAA and p-HPhLA |
| TCA | tricarboxylic acid cycle |
References
- Arulkumaran, N.; Deutschman, C.S.; Pinsky, M.R.; Zuckerbraun, B.; Schumacker, P.T.; Gomez, H.; Gomez, A.; Murray, P.; Kellum, J.A.; ADQI XIV Workgroup. Mitochondrial function in sepsis. Shock 2016, 45, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Hüttemann, M. Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochim. Biophys. Acta 2014, 1842, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, G.; Duchen, M.R.; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Carrick, M.M.; Mains, C.W.; Rael, L.T.; Slone, D.; Brody, E.N. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Rep. 2015, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, Y.; Hiller, K. Biochemistry of proinflammatory macrophage activation. Cell. Mol. Life Sci. 2018, 75, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.; Griss, T.; et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.C.; O’Neill, L.A.J. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J.; Kraemer, L.; Jaeger, C.; Madry, H.; Link, A.; Lepper, P.M.; Hiller, K.; Schneider, J.G. Itaconic acid indicates cellular but not systemic immune system activation. Oncotarget 2018, 9, 32098–32107. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.V.; Bairamov, I.T.; Olenin, A.Y.; Shubina, V.; Teplova, V.V.; Fedotcheva, N.I. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Kazakov, R.E.; Kondrashova, M.N.; Beloborodova, N.V. Toxic effects of microbial phenolic acids on the functions of mitochondria. Toxycol. Lett. 2008, 180, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S. The inflammation induced by lipopolysaccharide can be mitigated by short chain fatty acid, butyrate, through up-regulation of IL-10 in septic shock. Scand. J. Immunol. 2017, 85, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P.; Darra, A.; Agrawal, A. Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion 2018, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.; Wilmer, M.J.; Reijnders, D.; Jansen, J.; Van den Broek, P.H.; Forkink, M.; Schepers, E.; Glorieux, G.; Vanholder, R.; Van den Heuvel, L.P.; et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim. Biophys. Acta 2013, 1832, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef]
- Zhang, H.; Du, M.; Yang, Q.; Zhu, M.J. Butyrate suppresses murine mast cell roliferation and cytokine production through inhibiting histone deacetylase. J. Nutr. Biochem. 2015, 27, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef]
- Stehle, H.W.; Leblebicioglu, B.; Walters, J.D. Short-chain carboxylic acids produced by gram-negative anaerobic bacteria can accelerate or delay polymorphonuclear leukocyte apoptosis in vitro. J. Periodontol. 2001, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Teplova, V.V.; Beloborodova, N.V. Participation of phenolic acids of microbial origin in mitochondrial dysfunction in sepsis. Biol. Membr. 2010, 27, 60–66. [Google Scholar]
- Fedotcheva, N.I.; Teplova, V.V.; Beloborodova, N.V. The effect of microbial metabolites on the functions of mitochondria in acidosis and deficiency of the substrates of oxidation. Biol. Membr. 2019, 36, 44–52. [Google Scholar] [CrossRef]
- Sarshor, Y.N.; Beloborodova, N.V.; Bedova, A.Y.; Osipov, A.A.; Chernevskaya, E.A.; Getsina, M.L. New criteria of bacterial load in critically ill patients. Shock 2013, 40 (Suppl. 1), 31. [Google Scholar]
- Beloborodova, N.V.; Khodakova, A.S.; Bairamov, I.T.; Olenin, A.Y. Microbial origin of phenylcarboxylic acids in the human body. Biochemistry 2009, 74, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Khodakova, A.; Beloborodova, N. Microbial metabolites in the blood of patients with sepsis. Crit. Care 2007, 11 (Suppl. 4), 5. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Sarshor, Y.N.; Bedova, A.Y.; Chernevskaya, E.A.; Pautova, A.K. Involvement of aromatic metabolites in the pathogenesis of septic shock. Shock 2018, 50, 273–279. [Google Scholar] [CrossRef]
- Moroz, V.V.; Beloborodova, N.V.; Osipov, A.A.; Vlasenko, A.V.; Bedova, A.Y.; Pautova, A.K. Phenylcarboxylic acids in the assessment of the severity of patient condition and the efficiency of intensive treatment in critical care medicine. Gen. Reanimatol. 2016, 12, 37–48. [Google Scholar] [CrossRef]
- Jenner, A.M.; Rafter, J.; Halliwell, B. Human fecal water content of phenylcarboxylics: The extent of colonic exposure to phenylcarboxylic compounds. Free Radic. Biol. Med. 2005, 38, 763–772. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Olenin, A.Y.; Pautova, A.K. Metabolomic findings in sepsis as a damage of host-microbial metabolism integration. J. Crit. Care 2018, 43, 246–255. [Google Scholar] [CrossRef]
- Renwick, A.G. Gut bacteria and the metabolism of aromatic amino acids. In Microbial Metabolism in the Digestive Tract; Hill, M.J., Ed.; Academic Press: New York, NY, USA, 1986; pp. 108–119. [Google Scholar]
- Pautova, A.K.; Bedova, A.Y.; Sarshor, Y.N.; Beloborodova, N.V. Determination of aromatic microbial metabolites in blood serum by gas chromatography–mass spectrometry. J. Anal. Chem. 2018, 73, 160–166. [Google Scholar] [CrossRef]
- Forni, L.G.; McKinnon, W.; Lord, G.A.; Treacher, D.F.; Peron, J.M.; Hilton, P.J. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit. Care 2005, 9, R591–R595. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauer, M.; Dawkins, S.; Costa, A.S.H.; Lee, R.; Young, T.; Pell, V.R.; Choudhury, R.P.; Banning, A.P.; Kharbanda, R.K.; Oxford Acute Myocardial Infarction (OxAMI) Study; et al. Metabolomic profiling in acute ST-segment-elevation myocardial infarction identifies succinate as an early marker of human ischemia-reperfusion injury. J. Am. Heart Assoc. 2018, 7, e007546. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.C.; Deen, P.M.; Robben, J.H. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Front. Endocrinol. 2012, 3, 22. [Google Scholar] [CrossRef]
- Weinberg, J.M.; Venkatachalam, M.A.; Roeser, N.F.; Saikumar, P.; Dong, Z.; Senter, R.A.; Nissim, I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am. J. Physiol. Ren. Physiol. 2000, 279, F927–F943. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.R.; Kruglov, E.A.; Thompson, M.; Leite, M.F.; Dranoff, J.A.; Nathanson, M.H. Succinate is a paracrine signal for liver damage. J. Hepatol. 2007, 47, 262–269. [Google Scholar] [CrossRef]
- Kushnir, M.M.; Komaromy-Hiller, G.; Shushan, B.; Urry, F.M.; Roberts, W.L. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin. Chem. 2001, 47, 1993–2002. [Google Scholar]
- He, W.; Miao, F.J.; Lin, D.C.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef]
- Gibala, M.J.; MacLean, D.A.; Graham, T.E.; Saltin, B. Anaplerotic processes in human skeletal muscle during brief dynamic exercise. J. Physiol. 1997, 502, 703–713. [Google Scholar] [CrossRef]
- Tiao, G.; Fagan, J.M.; Samuels, N.; James, J.H.; Hudson, K.; Lieberman, M.; Fischer, J.E.; Hasselgren, P.O. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J. Clin. Investig. 1994, 94, 2255–2264. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Microbial metabolites of ingested caffeic acid are absorbed by the monocarboxylic acid transporter (MCT) in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Jeger, V.; Djafarzadeh, S.; Jakob, S.M.; Takala, J. Mitochondrial function in sepsis. Eur. J. Clin. Investig. 2013, 43, 532–542. [Google Scholar] [CrossRef]
- Brealey, D.; Karyampudi, S.; Jacques, T.S.; Novelli, M.; Stidwill, R.; Taylor, V.; Smolenski, R.T.; Singer, M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R491–R497. [Google Scholar] [CrossRef] [PubMed]
- Eyenga, P.; Roussel, D.; Morel, J.; Rey, B.; Romestaing, C.; Teulier, L.; Sheu, S.S.; Goudable, J.; Négrier, C.; Viale, J.P. Early septic shock induces loss of oxidative phosphorylation yield plasticity in liver mitochondria. J. Physiol. Biochem. 2014, 70, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Bakker, J. Blood lactate monitoring in critically ill patients: A systematic health technology assessment. Crit. Care Med. 2009, 37, 2827–2839. [Google Scholar] [PubMed]
- Cheng, H.H.; Chen, F.C.; Change, M.W.; Kung, C.T.; Cheng, C.Y.; Tsai, T.C.; Hsiao, S.Y.; Su, C.M. Difference between elderly and non-elderly patients in using serum lactate level to predict mortality caused by sepsis in the emergency department. Medicine 2018, 97, e0209. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Moore, H.B.; Moore, E.E.; Reisz, J.A.; Wither, M.J.; Ghasasbyan, A.; Chandler, J.; Silliman, C.C.; Hansen, K.C.; Banerjee, A. Plasma succinate is a predictor of mortality in critically injured patients. J. Trauma Acute Care Surg. 2017, 83, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Andrienko, T.N.; Pasdois, P.; Pereira, G.C.; Ovens, M.J.; Halestrap, A.P. The role of succinate and ROS in reperfusion injury—A critical appraisal. J. Mol. Cell. Cardiol. 2017, 110, 1–14. [Google Scholar] [CrossRef]
- Levy, R.J. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock 2007, 28, 24–28. [Google Scholar] [CrossRef]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Litvinova, E.G.; Kamzolova, S.V.; Morgunov, I.G.; Amerkhanov, Z.G. Mitochondrial metabolites in tissues as indicators of metabolic alterations during hibernation. CryoLetters 2010, 31, 392–400. [Google Scholar] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Litvinova, E.G.; Zakharchenko, M.V.; Khunderyakova, N.V.; Fadeev, R.S.; Teplova, V.V.; Kondrashova, M.N.; Fedotcheva, T.A.; Beloborodova, N.V. Substrate-specific reduction of tetrazolium salts by isolated mitochondria, tissues, and leukocytes. Biochemistry 2017, 82, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Kondrashova, M.N.; Litvinova, E.G.; Zakharchenko, M.V.; Khunderyakova, N.V.; Beloborodova, N.V. Modulation of the activity of succinate dehydrogenase by acetylation with chemicals, drugs, and microbial metabolites. Biophysics 2018, 63, 743–750. [Google Scholar] [CrossRef]

| Metabolite | Group I (Early Stage of Sepsis) | Group II (Late Stage of Sepsis) | Group III (Healthy Control) | p I, II | p II, III | p I, III |
|---|---|---|---|---|---|---|
| Aromatic Microbial Metabolites | ||||||
| PhLA | 1.1 (0.8–1.8) | 0.8 (0.4–2.2) | <LOD * | ns | <0.0001 | <0.0001 |
| p-HPhAA | 2.6 (1.0–8.6) | 1.1 (0.3–5.8) | <LOD * | ns | <0.0001 | <0.0001 |
| p-HPhLA | 2.7 (1.3–6.0) | 13.9 (1.1–28.9) | 0.7 (0.6–1.0) | <0.05 | <0.0001 | <0.0001 |
| Sum3 | 7.4 (3.2–22.8) | 15.9 (3.4–59.4) | 0.8 (0.6–1.1) | ns | <0.0001 | <0.0001 |
| Mitochondrial Metabolites | ||||||
| Succinic Acid | 4.9 (4.0–13.7) | 11.0 (7.4–169.8) | 22.0 (15.5–26.2) | <0.0001 | ns | <0.0001 |
| Fumaric Acid | 1.0 (0.9–2.7) | 2.7 (0.9–6.3) | 1.8 (1.3–2.4) | 0.002 | ns | 0.007 |
| Itaconic Acid | 1.2 (0.5–2.3) | <LOD * | <LOD * | - | - | - |
| α-Ketoglutaric Acid | 77% ** | 45% ** | 5% ** | - | - | - |
| Group I (Early Stage of Sepsis) | Group II (Late Stage of Sepsis) | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | SOFA | Succinic Acid | Fumaric Acid | Parameter | SOFA | Succinic Acid | Fumaric Acid |
| SOFA | - | 0.38 | ns | SOFA | - | 0.61 | 0.55 |
| Succinic Acid | 0.38 | - | ns | Succinic acid | 0.61 | - | 0.74 |
| Fumaric Acid | ns | ns | - | Fumaric acid | 0.55 | 0.74 | - |
| PhLA | 0.45 | 0.39 | −0.29 | PhLA | 0.69 | 0.45 | 0.62 |
| p-HPhAA | 0.59 | 0.40 | ns | p-HPhAA | 0.57 | ns | 0.39 |
| p-HPhLA | 0.39 | 0.39 | ns | p-HPhLA | 0.84 | 0.54 | 0.68 |
| Sum3 | 0.52 | 0.45 | ns | Sum3 | 0.83 | 0.54 | 0.68 |
| Acid Derivatives | Retention Time, min | m/z Value | Range of Concentrations, µM | Linear Equation | R2 |
|---|---|---|---|---|---|
| Succinic | 10.67 | 247 and 172 | 0.5–70 | y = 435 × x | 0.9920 |
| Fumaric | 11.22 | 245 and 217 | 0.5–70 | y = 31 × x | 0.9988 |
| α-Ketoglutaric | 13.15 | 157 and 173 | * | * | * |
| Itaconic | 11.10 | 215 and 259 | 0.5–15 | y = 481 × x | 0.9886 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beloborodova, N.; Pautova, A.; Sergeev, A.; Fedotcheva, N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites 2019, 9, 196. https://doi.org/10.3390/metabo9100196
Beloborodova N, Pautova A, Sergeev A, Fedotcheva N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites. 2019; 9(10):196. https://doi.org/10.3390/metabo9100196
Chicago/Turabian StyleBeloborodova, Natalia, Alisa Pautova, Aleksandr Sergeev, and Nadezhda Fedotcheva. 2019. "Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis" Metabolites 9, no. 10: 196. https://doi.org/10.3390/metabo9100196
APA StyleBeloborodova, N., Pautova, A., Sergeev, A., & Fedotcheva, N. (2019). Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites, 9(10), 196. https://doi.org/10.3390/metabo9100196






