Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts
Abstract
:1. Introduction
2. Results
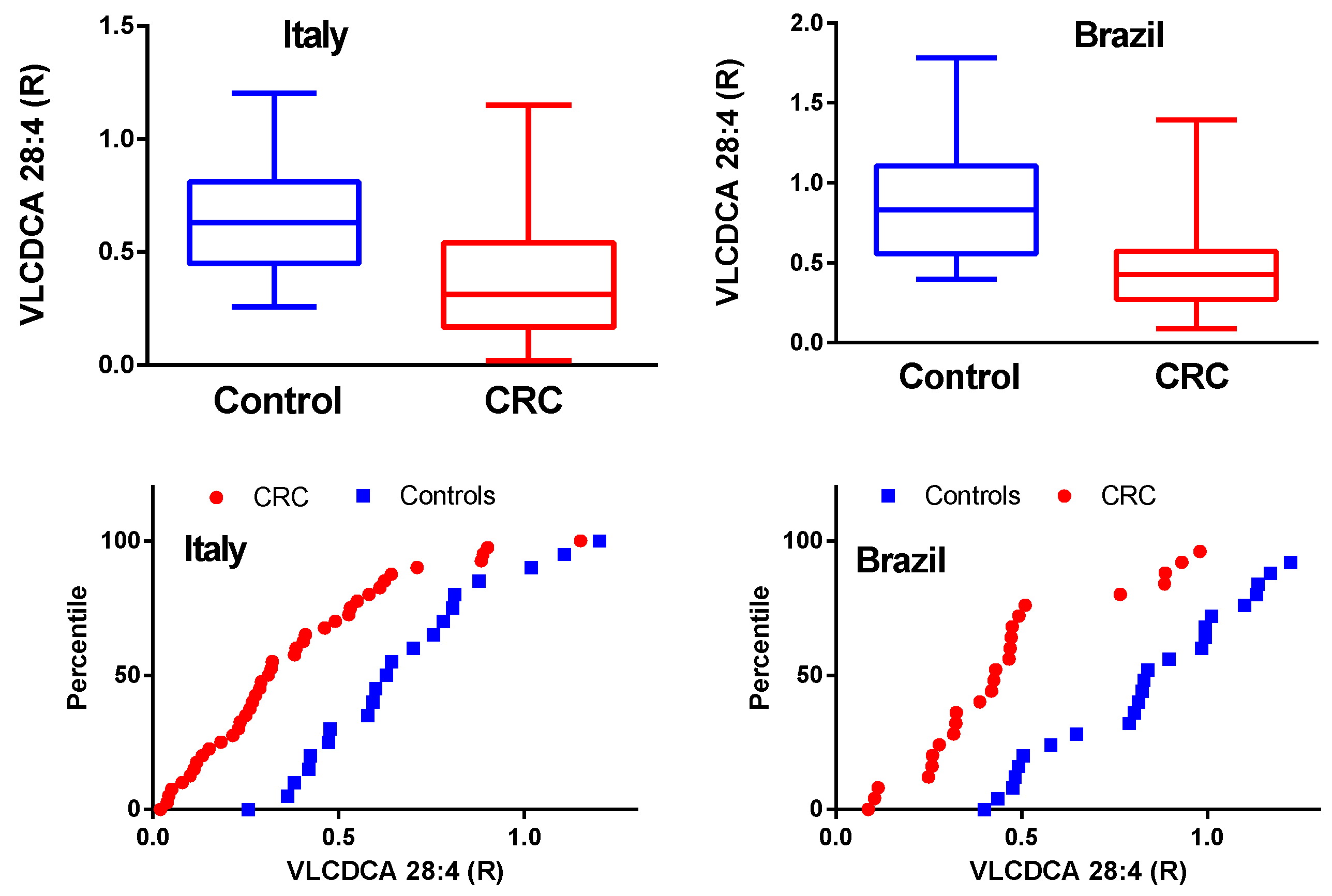
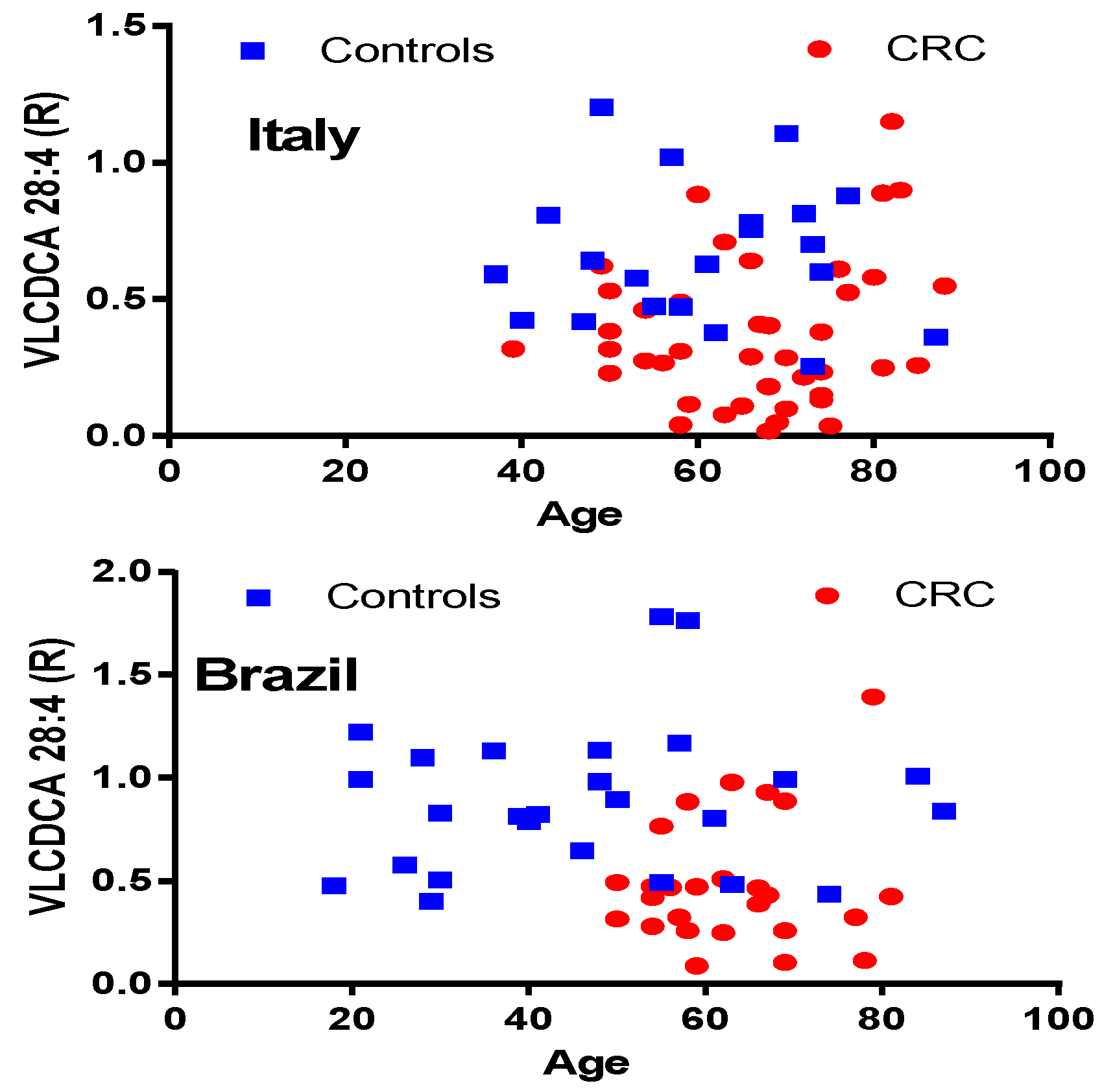
2.1. VLCDCA 28:4 Plasma Levels in Italian and Brazilian CRC Cohorts
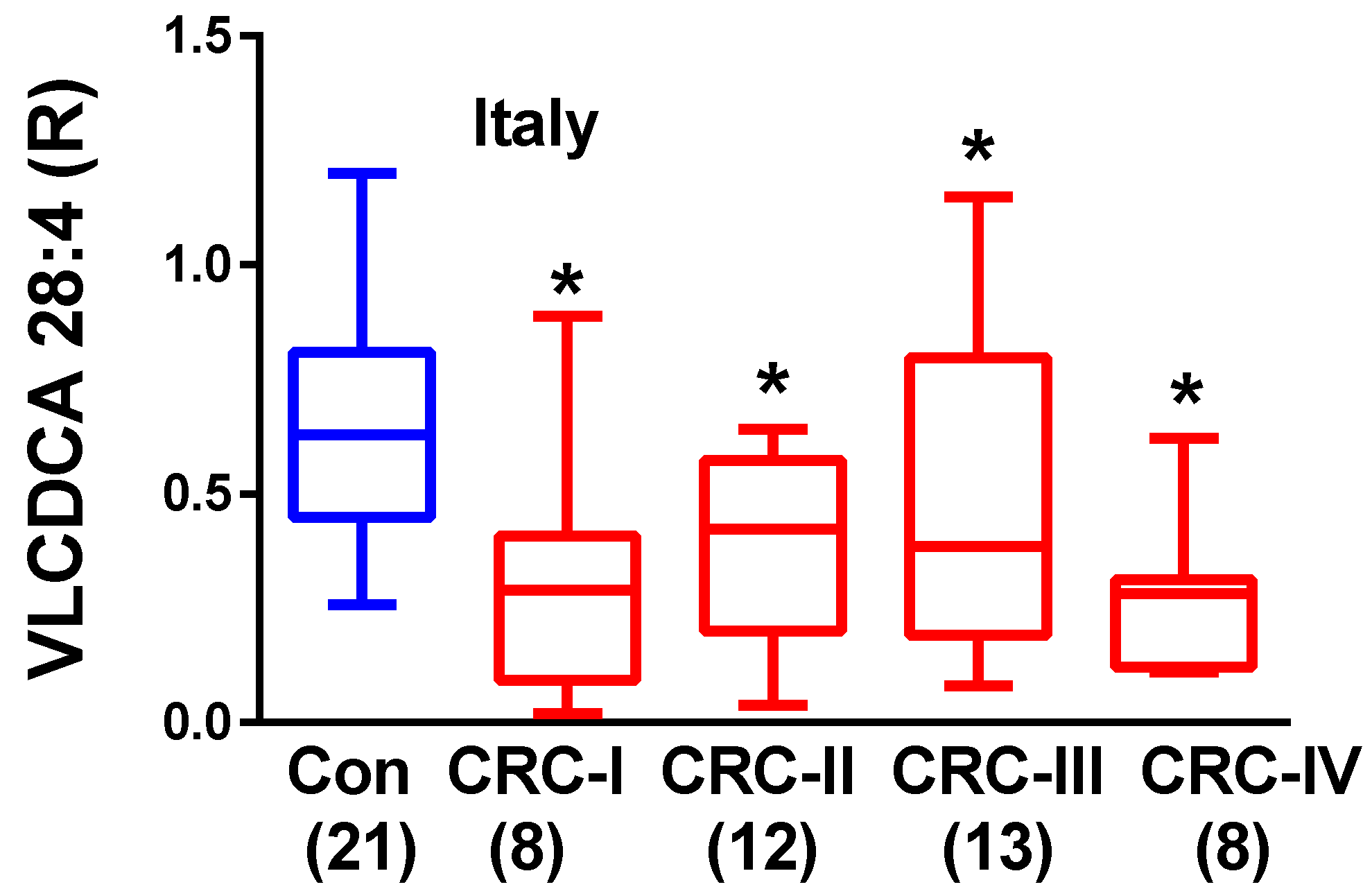
2.2. Plasma VLCDCA 28:4 in Stage I–IV CRC Patients
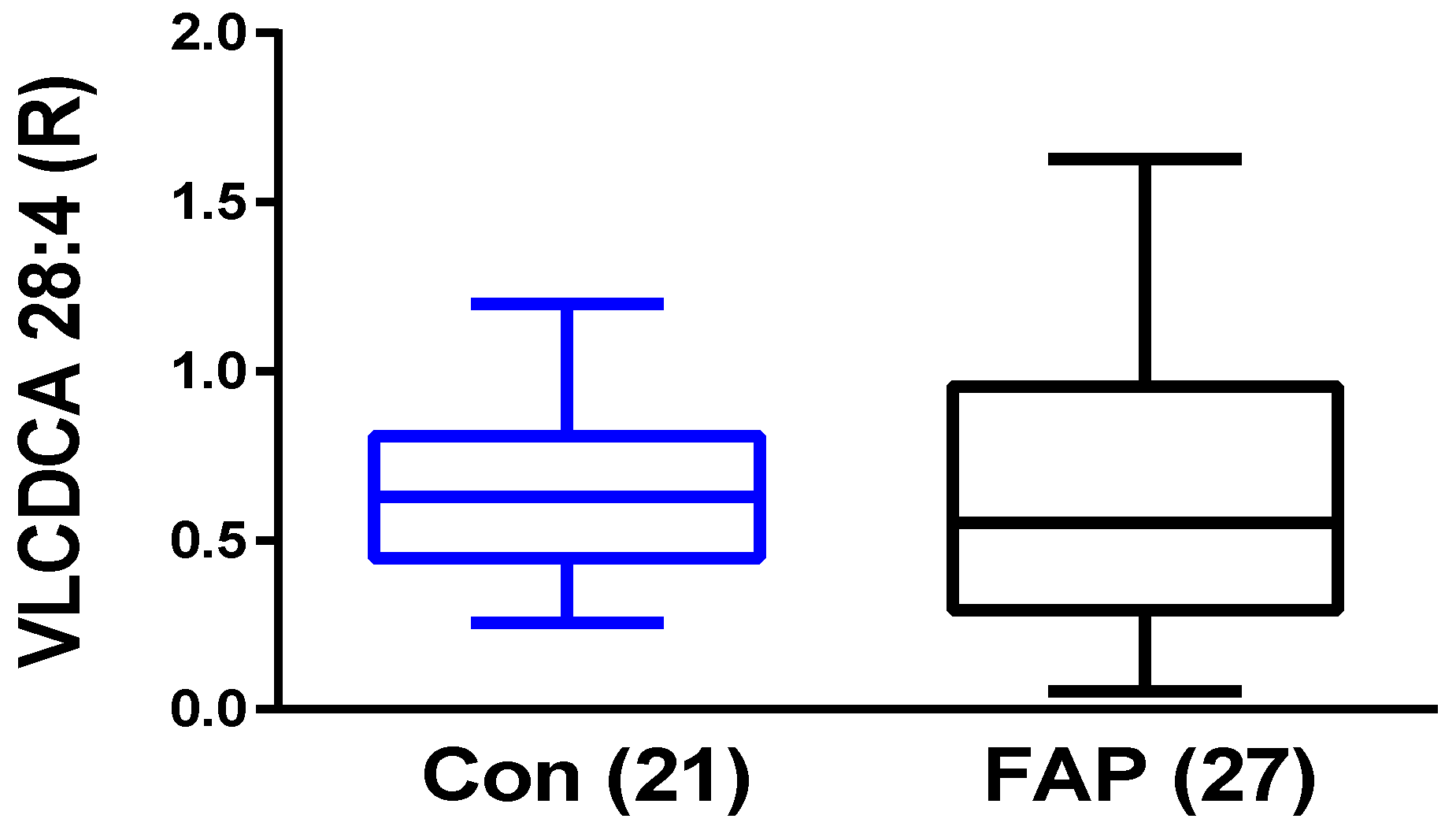
2.3. Plasma VLCDCA 28:4 in FAP Patients
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. VLCDCA 28:4 Analyses
4.3. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Tao, S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur. J. Cancer 2013, 49, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Kim, H.P.; Shin, J.Y.; Jeong, E.G.; Lee, W.C.; Lee, K.H.; Won, J.K.; Kim, T.Y.; Oh, D.Y.; Im, S.A.; et al. Targeted sequencing of cancer-related genes in colorectal cancer using next-generation sequencing. PLoS ONE 2013, 8, e64271. [Google Scholar] [CrossRef] [PubMed]
- Bedin, C.; Crotti, S.; Ragazzi, E.; Pucciarelli, S.; Agatea, L.; Tasciotti, E.; Ferrari, M.; Traldi, P.; Rizzolio, F.; Giordano, A.; et al. Alterations of the Plasma Peptidome Profiling in Colorectal Cancer Progression. J. Cell Physiol. 2016, 231, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Yang, Y.; Zhang, X.; Wang, W.; Liu, Y.; Zheng, G.; Du, L.; Wang, C. Development of a preoperative prediction nomogram for lymph node metastasis in colorectal cancer based on a novel Plasma miRNA signature and CT scans. EBioMedicine 2018, 37, 125–133. [Google Scholar] [CrossRef]
- Fernandes-Messias, M.C.; Mecatti, G.C.; Figueiredo-Angolini, C.F.; Eberlin, M.N.; Credidio, L.; Real-Martinez, C.A.; Rodrigues-Coy, C.S.; de Oliveira, C.P. Plasma Lipidomic Signature of Rectal Adenocarcinoma Reveals Potential Biomarkers. Front. Oncol. 2018, 7, 325. [Google Scholar] [CrossRef]
- Crotti, S.; Agnoletto, E.; Cancemi, G.; Di Marco, V.; Traldi, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Altered plasma levels of decanoic acid in colorectal cancer as a new diagnostic biomarker. Anal. Bioanal. Chem. 2016, 408, 6321–6328. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Jayasinghe, D.; Davies, G.F.; Ahiahonu, P.; Ma, H.; Goodenowe, D.B. Human Plasma-derived hydroxy long-chain fatty acids exhibit anti-inflammatory and anti-proliferative activity. J. Exp. Clin. Cancer Res. 2011, 30, 59. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Heath, D.; Yamazaki, Y.; Grimmalt, B.; Kavianpour, A.; Krenitsky, K.; Elshoni, H.; Takemasa, I.; Miyake, M.; Sekimoto, M.; et al. Reduction of novel circulating long-chain fatty acids in colorectal cancer patients is independent of tumor burden and correlates with age. BMC Gastroenterol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Ahiahonu, P.W.; Jayasinghe, D.; Heath, D.; Liu, J.; Lu, Y.; Jin, W.; Kavianpour, A.; Yamazaki, Y.; Khan, A.M.; et al. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the plasma of colorectal cancer patients: Implications for early screening and detection. BMC Med. 2010, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Tonita, J.; Alvi, R.; Lehotay, D.; Elshoni, H.; Myat, S.; McHattie, J.; Goodenowe, D.B. Low-Plasma GTA-446 anti-inflammatory fatty acid levels as a new risk factor for colon cancer. Int. J. Cancer 2013, 132, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Endogenous anti-inflammatory very-long-chain dicarboxylic acids: Potential chemopreventive lipids. Metabolites 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Wintjens, D.S.J.; Bogie, R.M.M.; van den Heuvel, T.R.A.; le Clercq, C.M.C.; Oostenbrug, L.E.; Romberg-Camps, M.J.L.; Straathof, J.W.; Stassen, L.P.S.; Masclee, A.A.M.; Jonkers, D.M.A.E.; et al. Incidence and Classification of Postcolonoscopy Colorectal Cancers in Inflammatory Bowel Disease: A Dutch Population-Based Cohort Study. Incidence and Classification of postcolonoscopy Colorectal Cancers in Inflammatory Bowel Disease: A Dutch Population-Based Cohort Study. J. Crohns. Colitis. 2018, 12, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Quach, D.T.; Hiyama, T.; Nguyen, T.A.; Ly, H.Q.; Tanaka, S. Asia-Pacific Colorectal Screening score: A useful tool to stratify risk for colorectal advanced neoplasms in Vietnamese patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2018, 33, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Veettil, S.K.; Jinatongthai, P.; Nathisuwan, S.; Teerawattanapong, N.; Ching, S.M.; Lim, K.G.; Saokaew, S.; Phisalprapa, P.; Reid, C.M.; Chaiyakunapruk, N. Efficacy and safety of chemopreventive agents on colorectal cancer incidence and mortality: Systematic review and network meta-analysis. Clin. Epidemiol. 2018, 10, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Tomić, T.; Domínguez-López, S.; Barrios-Rodríguez, R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: A systematic review and meta-analysis. Cancer Epidemiol. 2018, 58, 52–62. [Google Scholar] [CrossRef]
- Witold, K.; Anna, K.; Maciej, T.; Jakub, J. Adenomas—Genetic factors in colorectal cancer prevention. Rep. Pract. Oncol. Radiother. 2018, 23, 75–83. [Google Scholar] [CrossRef]
- Alidoust, M.; Hamzehzadeh, L.; Rivandi, M.; Pasdar, A. Polymorphisms in non-coding RNAs and risk of colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 132, 100–110. [Google Scholar] [CrossRef]
- Pierce, B.L.; Kraft, P.; Zhang, C. Mendelian randomization studies of cancer risk: A literature review. Curr. Epidemiol. Rep. 2018, 5, 184–196. [Google Scholar] [CrossRef]
- Rex, D.K.; Ahnen, D.J.; Baron, J.A.; Batts, K.P.; Burke, C.A.; Burt, R.W.; Goldblum, J.R.; Guillem, J.G.; Kahi, C.J.; Kalady, M.F.; et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am. J. Gastroenterol. 2012, 107, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Yang, H.; Jiang, B.; Wang, Z.; Ji, J.; Su, X. Whole exome sequencing reveals intertumor heterogeneity and distinct genetic origins of sporadic synchronous colorectal cancer. Int. J. Cancer 2018, 142, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Wood, P.L. Non-targeted lipidomics utilizing constant infusion high resolution ESI mass spectrometry. Lipidomics 2017, 125, 13–19. [Google Scholar]
| Group | Age (Yr. ± SD) | Age Range | N | Number of Females |
|---|---|---|---|---|
| Italy—Controls | 60.3 ± 13.4 | 37–87 | 21 | 11 |
| Italy—Colorectal cancers (CRC) | 66.4 ± 11.6 | 39–88 | 41 | 23 |
| Italy—Familial adenomatous polyposis (FAP) | 45.0 ± 14.1 | 21–59 | 27 | 23 |
| Brazil—Controls | 46.7 ± 19.1 | 18–87 | 26 | 14 |
| Brazil—CRC | 63.0 ± 8.8 | 50–81 | 26 | 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, P.L.; Donohue, M.M.; Cebak, J.E.; Beckmann, T.G.; Messias, M.C.F.; Credidio, L.; Coy, C.S.R.; Carvalho, P.d.O.; Crotti, S.; D’Aronco, S.; et al. Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts. Metabolites 2018, 8, 91. https://doi.org/10.3390/metabo8040091
Wood PL, Donohue MM, Cebak JE, Beckmann TG, Messias MCF, Credidio L, Coy CSR, Carvalho PdO, Crotti S, D’Aronco S, et al. Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts. Metabolites. 2018; 8(4):91. https://doi.org/10.3390/metabo8040091
Chicago/Turabian StyleWood, Paul L., Michelle M. Donohue, John E. Cebak, Taylor G. Beckmann, Márcia Cristina Fernandes Messias, Laura Credidio, Cláudio Saddy Rodrigues Coy, Patrícia de Oliveira Carvalho, Sara Crotti, Sara D’Aronco, and et al. 2018. "Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts" Metabolites 8, no. 4: 91. https://doi.org/10.3390/metabo8040091
APA StyleWood, P. L., Donohue, M. M., Cebak, J. E., Beckmann, T. G., Messias, M. C. F., Credidio, L., Coy, C. S. R., Carvalho, P. d. O., Crotti, S., D’Aronco, S., Urso, E. D. L., & Agostini, M. (2018). Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts. Metabolites, 8(4), 91. https://doi.org/10.3390/metabo8040091




