Population-Level Analysis to Determine Parameters That Drive Variation in the Plasma Metabolite Profiles
Abstract
1. Introduction
2. Results
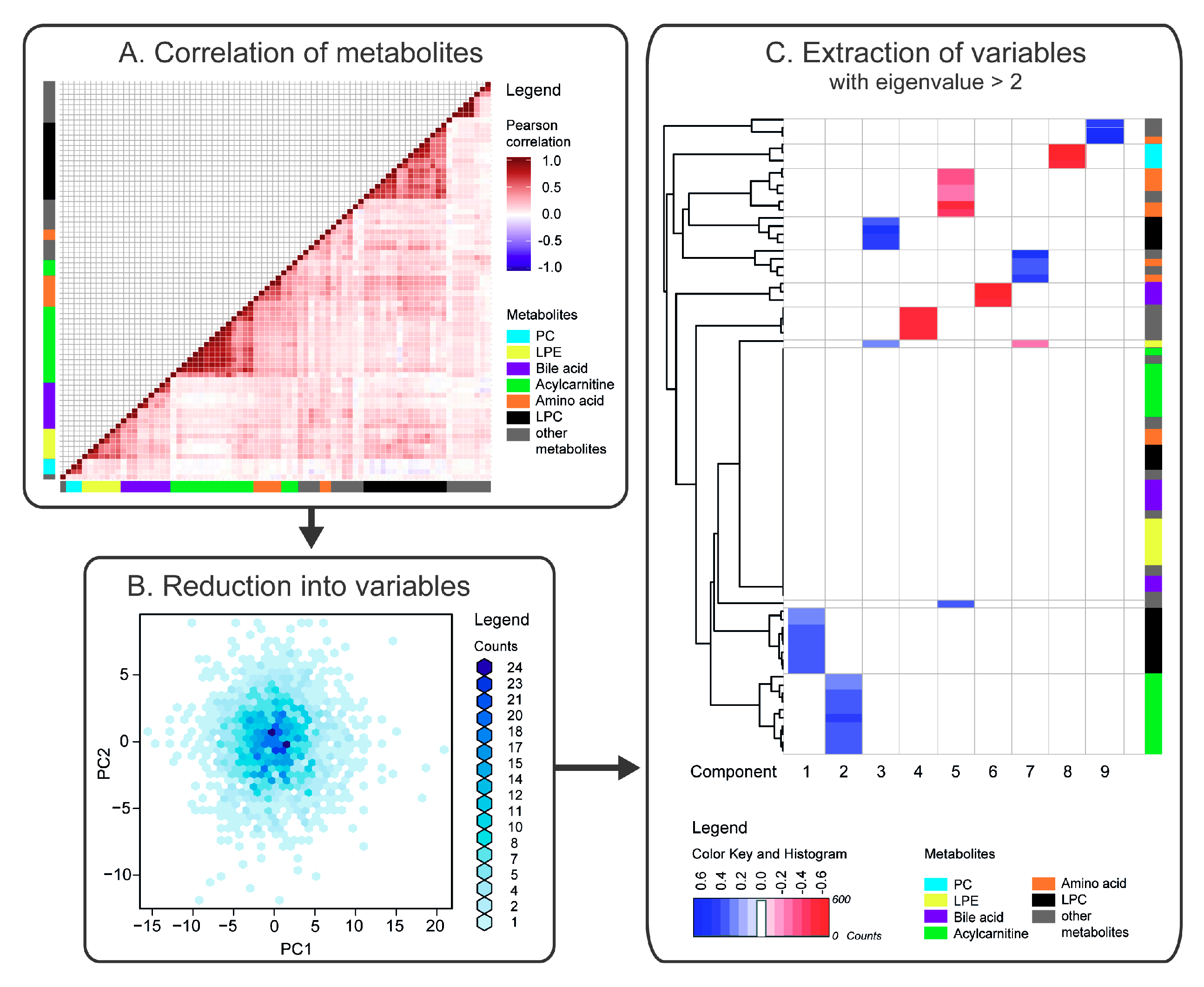
2.1. Correlation of Metabolites
2.2. Reduction of Metabolites into Uncorrelated Variables
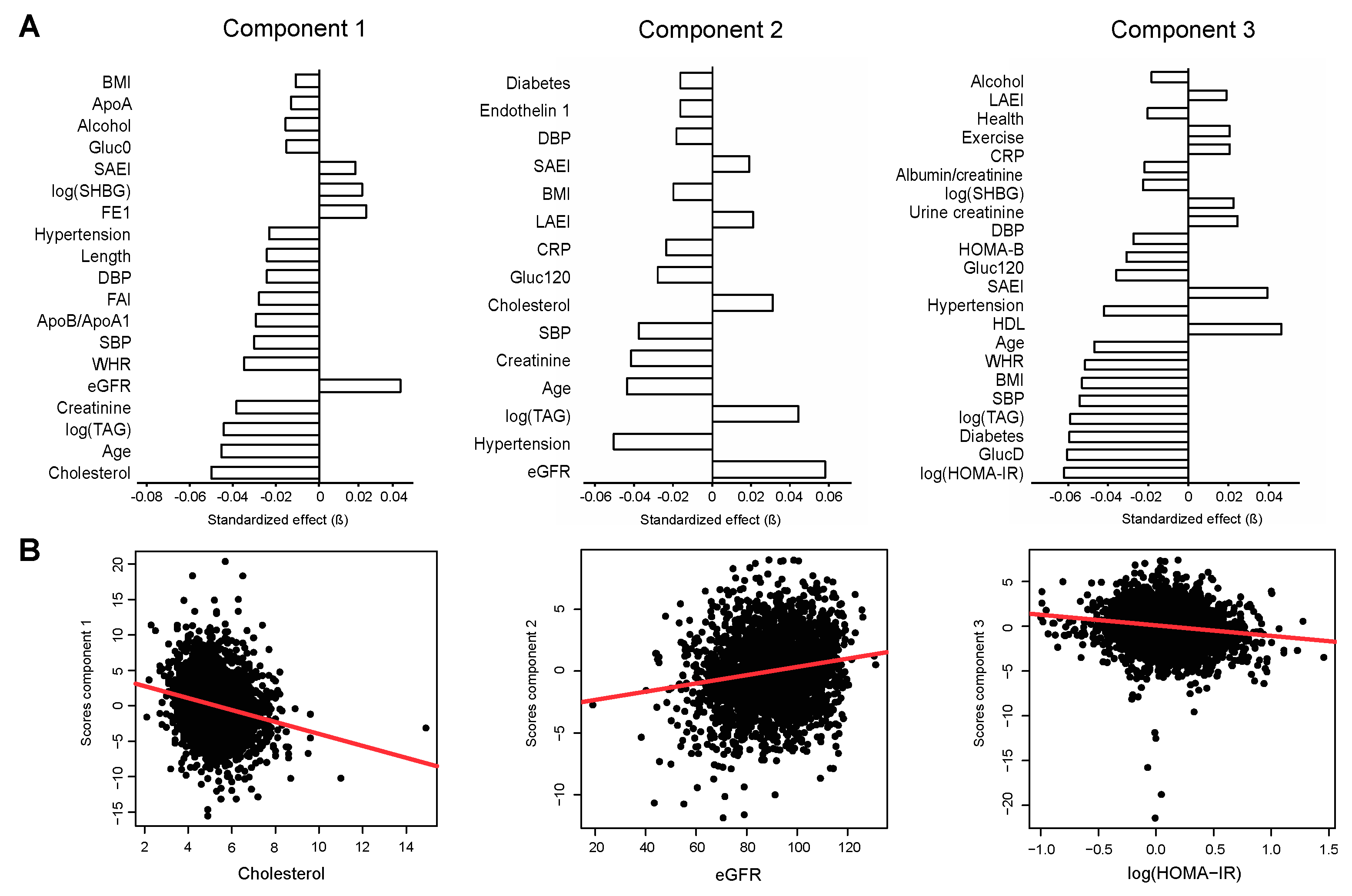
2.3. Association of Metabolite Factors with Phenotypic Parameters
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Clinical and Anthropometric Assays
4.3. Metabolite Profiling
4.4. Statistical Analysis and Data Visualisation
4.5. Compliance with Ethical Standards
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mamas, M.; Dunn, W.B.; Neyses, L.; Goodacre, R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Arch. Toxicol. 2011, 85, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Hu, Y.; Li, P.; Wan, J.B. Current state of the art of mass spectrometry-based metabolomics studies-a review focusing on wide coverage, high throughput and easy identification. RSC Adv. 2015, 5, 78728–78737. [Google Scholar] [CrossRef]
- Kordalewska, M.; Markuszewski, M.J. Metabolomics in cardiovascular diseases. J. Pharm. Biomed. Anal. 2015, 113, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engström, G.; Östling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.E.; Kinross, J.; Nicholson, J.K. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology 2014, 146, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine deficiency and the development of diabetes. Diabetes 2015, 64, 3010–3016. [Google Scholar] [CrossRef] [PubMed]
- McKillop, A.M.; Flatt, P.R. Emerging applications of metabolomic and genomic profiling in diabetic clinical medicine. Diabetes Care 2011, 34, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K. Hepatic clearance of plasma low density lipoproteins. Semin. Liver Dis. 1992, 12, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.A.; Cianflone, K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler. Thromb. Vasc. Biol. 1993, 13, 629–636. [Google Scholar] [CrossRef]
- Lehmann, R.; Franken, H.; Dammeier, S.; Rosenbaum, L.; Kantartzis, K.; Peter, A.; Zell, A.; Adam, P.; Li, J.; Xu, G.; et al. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care 2013, 36, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S. Predictors of New-Onset Kidney Disease in a Community-Based Population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Nkuipou-Kenfack, E.; Duranton, F.; Gayrard, N.; Argiles, A.; Lundin, U.; Weinberger, K.M.; Dakna, M.; Delles, C.; Mullen, W.; Husi, H.; et al. Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS ONE 2014, 9, e96955. [Google Scholar] [CrossRef] [PubMed]
- Goek, O.N.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Prehn, C.; Römisch-Margl, W.; Wang-Sattler, R.; Illig, T.; Suhre, K.; et al. Serum metabolite concentrations and decreased GFR in the general population. Am. J. Kidney Dis. 2012, 60, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Holt, S.; Guebre-Egziabher, F.; Nakamura, K.; Vianey-Saban, C.; Hadj-Aïssa, A.; Hoppel, C.L.; Kopple, J.D. Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. J. Ren. Nutr. 2006, 16, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sanchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transpl. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body Mass Index and Risk for End-Stage Renal Disease. Ann. Intern. Med. 2006, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P. Estimating the Glomerular Filtration Rate in Obese Adult Patients for Drug Dosing. Adv. Chronic Kidney Dis. 2010, 17, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Koning, S.H.; Gansevoort, R.T.; Mukamal, K.J.; Rimm, E.B.; Bakker, S.J.L.; Joosten, M.M. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int. 2015, 87, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Klein, R.; Klein, B.E.K. The association among smoking, heavy drinking, and chronic kidney disease. Am. J. Epidemiol. 2006, 164, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Traversy, G.; Chaput, J.P. Alcohol Consumption and Obesity: An Update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Fong, K.; Larive, B.; Beck, G.J.; Chertow, G.M.; Levin, N.W.; Kliger, A.S.; Plummer, N.S.; Meyer, T.W. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney Int. 2017, 91, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Al-Majdoub, M.; Ali, A.; Storm, P.; Rosengren, A.H.; Spégel, P.; Groop, L.; Spégel, P. Metabolite profiling of LADA challenges the view of a metabolically distinct subtype. Diabetes 2017, 66, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Häring, H.U.; De Angelis, M.H.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kobayashi, T.; Kamata, K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. AJP Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.A.; Gullberg, B.; Råstam, L.; Lindblad, U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: A cross-sectional study. BMC Endocr. Disord. 2009, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.A.; Krøll, L.; Bennet, L.; Gullberg, B.; Rastam, L.; Lindblad, U. Leisure time and occupational physical activity in relation to obesity and insulin resistance: A population-based study from the Skaraborg Project in Sweden. Metabolism 2012, 61, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, U.; Ek, J.; Eckner, J.; Larsson, C.A.; Shan, G.; Råstam, L. Prevalence, awareness, treatment, and control of hypertension: Rule of thirds in the Skaraborg project. Scand. J. Prim. Health Care 2012, 30, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Ekman, I.; Friberg, F.; Daka, B.; Lindblad, U.; Larsson, C.A. The association between self-rated health and impaired glucose tolerance in Swedish adults: A cross-sectional study. Scand. J. Prim. Health Care 2013, 31, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Løchen, M.L.; Rasmussen, K. The Tromsø study: Physical fitness, self reported physical activity, and their relationship to other coronary risk factors. J. Epidemiol. Community Health 1992, 46, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Available online: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf (accessed on 14 November 2018).
- Chorell, E.; Moritz, T.; Branth, S.; Antti, H.; Svensson, M.B. Predictive metabolomics evaluation of nutrition-modulated metabolic stress responses in human blood serum during the early recovery phase of strenuous physical exercise. J. Proteome Res. 2009, 8, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albert, F.; Llorach, R.; Garcia-Aloy, M.; Ziyatdinov, A.; Andres-Lacueva, C.; Perera, A. Intensity drift removal in LC/MS metabolomics by common variance compensation. Bioinformatics 2014, 30, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Hrydziuszko, O.; Viant, M.R. Missing values in mass spectrometry based metabolomics: An undervalued step in the data processing pipeline. Metabolomics 2012, 8, 161–174. [Google Scholar] [CrossRef]
- Troyanskaya, O.; Cantor, M.; Sherlock, G.; Brown, P.; Hastie, T.; Tibshirani, R.; Botstein, D.; Altman, R.B. Missing value estimation methods for DNA microarrays. Bioinformatics 2001, 17, 520–525. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean/Median/ Frequency | SD/IQR a |
|---|---|---|
| N (men/woman) | 1246/1257 | - |
| Age (years) | 47.8 | 11.8 |
| Waist/hip-ratio (WHR) | 0.9 | 0.1 |
| Length (cm) | 172.2 | 9.5 |
| Body mass index (BMI; kg m−2) | 26.9 | 4.6 |
| Fasting plasma glucose (Gluc0; mmol L−1) | 5.4 | 1.1 |
| 120 min OGTT plasma glucose (Gluc120; mmol L−1) | 5.6 | 2.2 |
| Homeostasis model assessment of insulin resistance (HOMA-IR) | 1.2 | 0.8–1.9 |
| Homeostasis model assessment of beta-cell function (HOMA-B) | 59.8 | 42.6–84.2 |
| Type 2 diabetes (T2D) (yes/no; %) | 141/2359; 5.6% | - |
| Small artery elasticity index (SAEI; mL mmHg−1) | 7.4 | 3.5 |
| Large artery elasticity index (LAEI; mL mmHg−1) | 16.4 | 5 |
| Pulse (min−1) | 63.7 | 8.4 |
| Systolic blood pressure (SBP; mm Hg) | 121.8 | 16.9 |
| Diastolic blood pressure (DBP; mm HG) | 70.3 | 10.1 |
| Hypertension (yes/no; %) | 363/2140; 14.5% | - |
| Apolipoprotein A1 (ApoA1; g L−1) | 1.7 | 0.3 |
| ApoB/ApoA1 | 0.6 | 0.2 |
| Triacylglycerides (TAG; g L−1) | 1.1 | 0.8-1.6 |
| High density lipoprotein (HDL; mmol L−1) | 1.3 | 0.3 |
| Cholesterol (mmol L−1) | 5.3 | 1.1 |
| Creatinine (mol L−1) | 78.8 | 13.9 |
| Albumin/creatinine-ratio | 0.3 | 0.2–0.5 |
| Urine creatinine (mol L−1) | 12.2 | 5.9 |
| Estimated glomerular filtration rate (eGFR; mL min−1 1.73 m−2) | 89.9 | 14.4 |
| Testosterone (nmol L−1) | 22.7 | 2.4–46.0 |
| Estradiol (nmol L−1) | 4.4 | 2.9–6.9 |
| Sex hormone binding globulin (SHBG; nmol L−1) | 37.5 | 26.7–52.2 |
| Health (1/2/3/4/5) b | 434/1380/581/76/7 | - |
| Exercise (1/2/3/4) c | 171/1432/748/73 | - |
| Smoking (yes/no; %) | 454/2040; 18.2% | - |
| Alcohol (g/week) | 25.2 | 6.3-59.7 |
| C-reactive protein (CRP; mg L−1) | 1.3 | 0.7–2.7 |
| Endothelin 1 (pg mL−1) | 2.4 | 1.3 |
| Cortisol (nmol L−1) | 2 | 2.0–3.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Majdoub, M.; Herzog, K.; Daka, B.; Magnusson, M.; Råstam, L.; Lindblad, U.; Spégel, P. Population-Level Analysis to Determine Parameters That Drive Variation in the Plasma Metabolite Profiles. Metabolites 2018, 8, 78. https://doi.org/10.3390/metabo8040078
Al-Majdoub M, Herzog K, Daka B, Magnusson M, Råstam L, Lindblad U, Spégel P. Population-Level Analysis to Determine Parameters That Drive Variation in the Plasma Metabolite Profiles. Metabolites. 2018; 8(4):78. https://doi.org/10.3390/metabo8040078
Chicago/Turabian StyleAl-Majdoub, Mahmoud, Katharina Herzog, Bledar Daka, Martin Magnusson, Lennart Råstam, Ulf Lindblad, and Peter Spégel. 2018. "Population-Level Analysis to Determine Parameters That Drive Variation in the Plasma Metabolite Profiles" Metabolites 8, no. 4: 78. https://doi.org/10.3390/metabo8040078
APA StyleAl-Majdoub, M., Herzog, K., Daka, B., Magnusson, M., Råstam, L., Lindblad, U., & Spégel, P. (2018). Population-Level Analysis to Determine Parameters That Drive Variation in the Plasma Metabolite Profiles. Metabolites, 8(4), 78. https://doi.org/10.3390/metabo8040078






