Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use
Abstract
:1. Introduction
2. Results
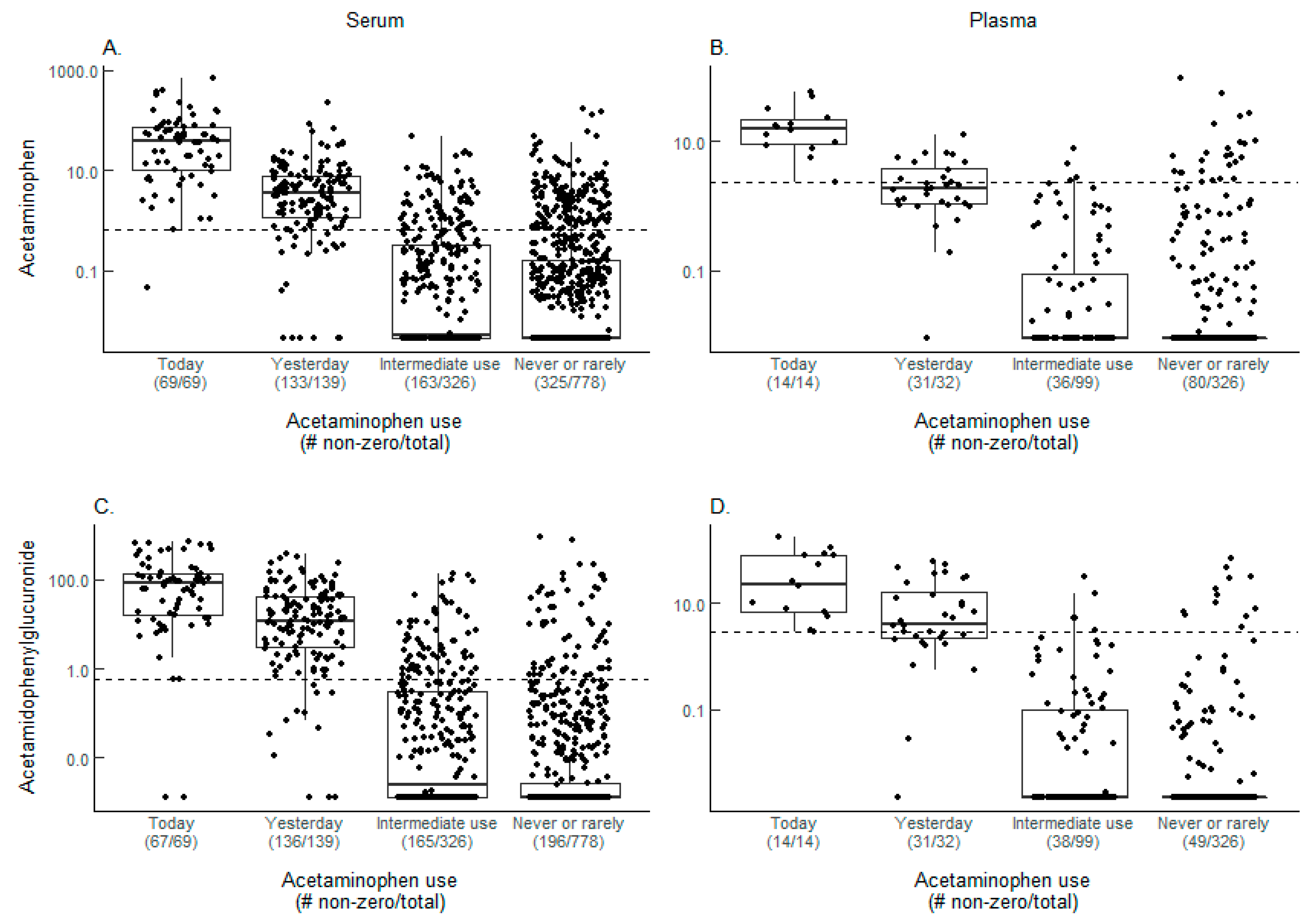
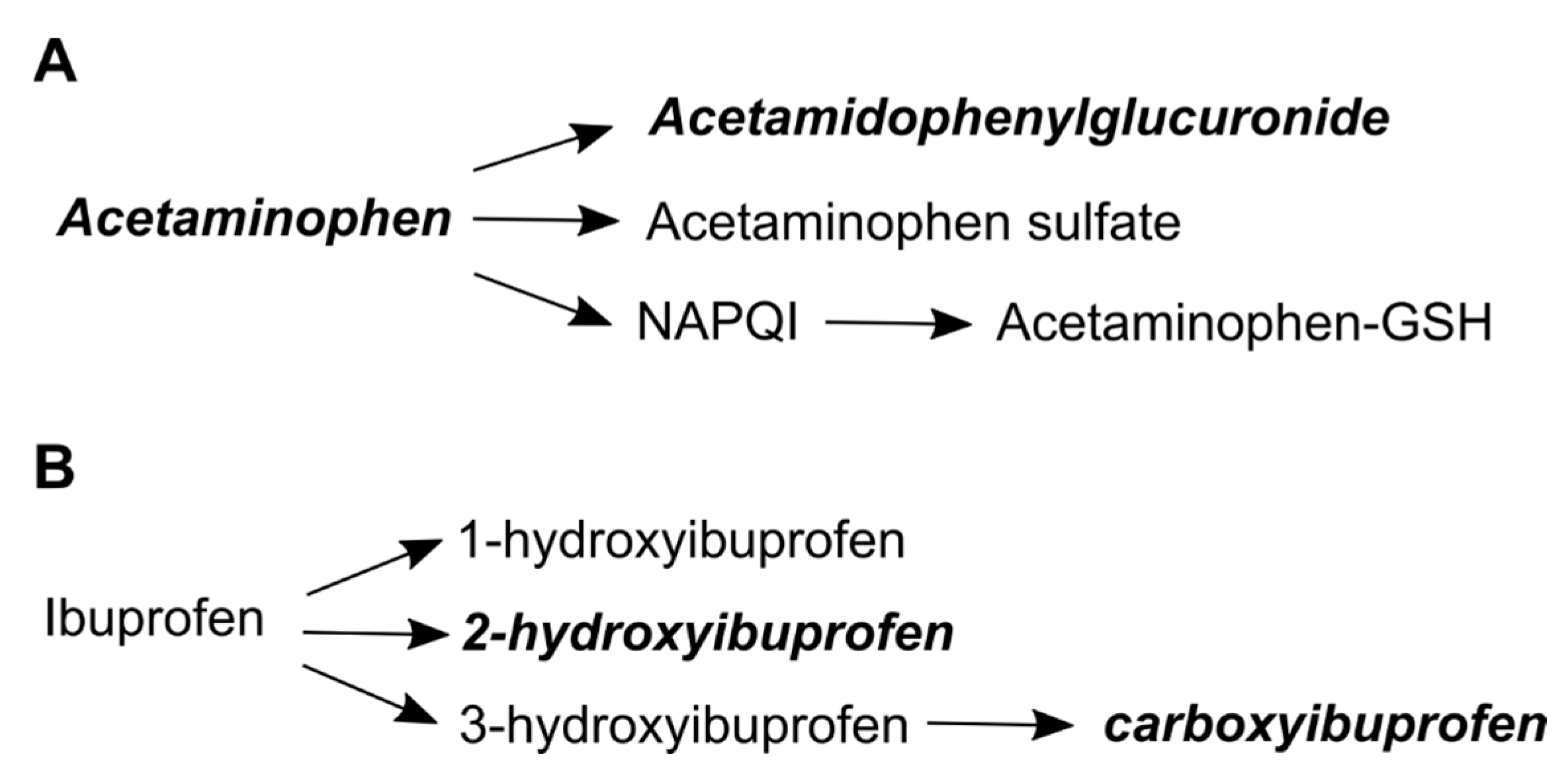
2.1. Acetaminophen Metabolites and Self-Reported Acetaminophen Use
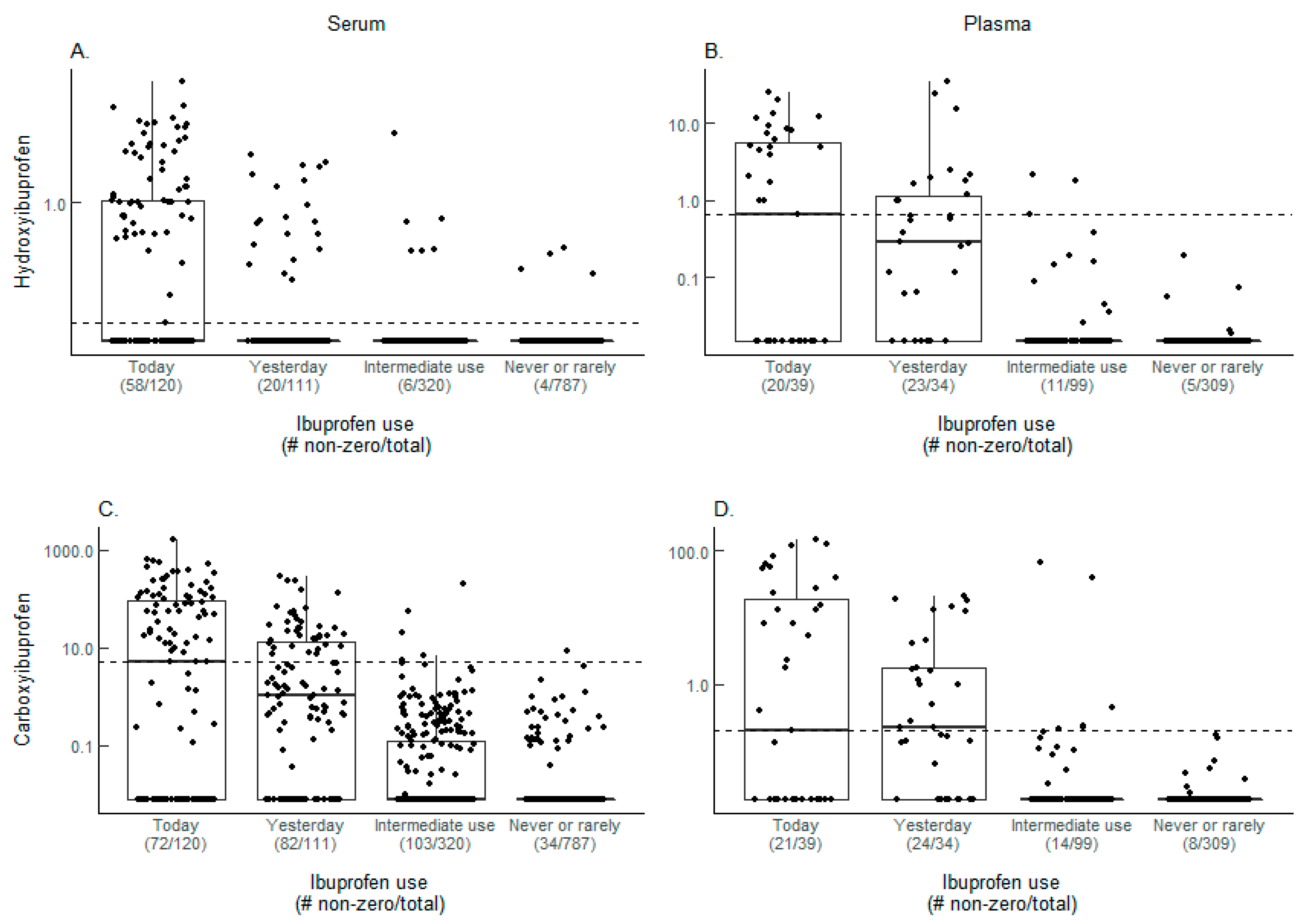
2.2. Ibuprofen Metabolites and Self-Reported Ibuprofen Use
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Questionnaire Data
4.3. Metabolomics Analysis
4.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Boudreau, D.M.; Freedman, A.N. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. Population. Pharmacoepidemiol. Drug Saf. 2014, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Paulose-Ram, R.; Hirsch, R.; Dillon, C.; Gu, Q. Frequent monthly use of selected non-prescription and prescription non-narcotic analgesics among u.S. Adults. Pharmacoepidemiol. Drug Saf. 2005, 14, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Beebe-Donk, J.; Namboodiri, K.K. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol. Rep. 2001, 8, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Din, F.V.; Theodoratou, E.; Farrington, S.M.; Tenesa, A.; Barnetson, R.A.; Cetnarskyj, R.; Stark, L.; Porteous, M.E.; Campbell, H.; Dunlop, M.G. Effect of aspirin and nsaids on risk and survival from colorectal cancer. Gut 2010, 59, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Maximo, I.X.; Brenner, A.J.; Beeram, M.; Hursting, S.D.; Price, R.S.; Tekmal, R.R.; Jolly, C.A.; deGraffenried, L.A. Nsaid use reduces breast cancer recurrence in overweight and obese women: Role of prostaglandin-aromatase interactions. Cancer Res. 2014, 74, 4446–4457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, L.; Wang, H.F.; Tan, C.C.; Meng, X.F.; Wang, C.; Tang, S.W.; Yu, J.T. Anti-inflammatory drugs and risk of alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimers Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Garcia Rodriguez, L.A.; Landolfi, R.; Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rodriguez, L.A.; Jick, H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994, 343, 769–772. [Google Scholar] [CrossRef]
- Clark, R.; Borirakchanyavat, V.; Davidson, A.R.; Thompson, R.P.; Widdop, B.; Goulding, R.; Williams, R. Hepatic damage and death from overdose of paracetamol. Lancet 1973, 1, 66–70. [Google Scholar] [CrossRef]
- Boudreau, D.M.; Daling, J.R.; Malone, K.E.; Gardner, J.S.; Blough, D.K.; Heckbert, S.R. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am. J Epidemiol. 2004, 159, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Sato, Y.; Nagashima, K.; Takahashi, S.; Hata, A. Validity assessment of self-reported medication use by comparing to pharmacy insurance claims. BMJ Open 2015, 5, e009490. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Savitz, D.A.; Koch, G.; Strom, B.L.; Guess, H.A.; Hartzema, A. Recall accuracy for prescription medications: Self-report compared with database information. Am. J. Epidemiol. 1995, 142, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Pit, S.; Byles, J. Older australians’ medication use: Self-report by phone showed good agreement and accuracy compared with home visit. J. Clin. Epidemiol. 2010, 63, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H.; Dela Cruz, B.L.; Thompson, D.M.; Farmer, K.C.; Medina, P.J. Use of prescription and nonprescription medications and supplements by cancer patients during chemotherapy: Questionnaire validation. J. Oncol. Pharm. Pract. 2008, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.S.; Giovino, G.A.; Pechacek, T.F.; Mowery, P.D. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third national health and nutrition examination survey, 1988–1994. Am. J. Epidemiol. 2001, 153, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Bidny, S.; Gago, K.; Chung, P.; Albertyn, D.; Pasin, D. Simultaneous screening and quantification of basic, neutral and acidic drugs in blood using UPLC-QTOF-MS. J. Anal. Toxicol. 2016, 41, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.F.; King, A.D.; van den Anker, J.N.; Wilkins, D.G. Simultaneous quantification of acetaminophen and five acetaminophen metabolites in human plasma and urine by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: Method validation and application to a neonatal pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1007, 30–42. [Google Scholar]
- Heitmeier, S.; Blaschke, G. Direct determination of paracetamol and its metabolites in urine and serum by capillary electrophoresis with ultraviolet and mass spectrometric detection. J. Chromatogr. B Biomed. Sci. Appl. 1999, 721, 93–108. [Google Scholar] [CrossRef]
- Huber, G.; Garg, U. Quantitation of ibuprofen in blood using gas chromatography-mass spectrometry (GC-MS). Methods Mol. Biol. 2010, 603, 289–296. [Google Scholar] [PubMed]
- Hlozek, T.; Bursova, M.; Cabala, R. Fast ibuprofen, ketoprofen and naproxen simultaneous determination in human serum for clinical toxicology by GC-FID. Clin. Biochem. 2014, 47, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Loo, R.L.; Chan, Q.; Brown, I.J.; Robertson, C.E.; Stamler, J.; Nicholson, J.K.; Holmes, E.; Elliott, P.; Group, I.R. A comparison of self-reported analgesic use and detection of urinary ibuprofen and acetaminophen metabolites by means of metabonomics: The intermap study. Am. J. Epidemiol. 2012, 175, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Loo, R.L.; Coen, M.; Ebbels, T.; Cloarec, O.; Maibaum, E.; Bictash, M.; Yap, I.; Elliott, P.; Stamler, J.; Nicholson, J.K.; et al. Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. Anal. Chem. 2009, 81, 5119–5129. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Aa, J.Y.; Wang, G.J.; Yan, B.; Zhang, Y.; Wang, X.W.; Zhao, C.Y.; Cao, B.; Shi, J.A.; Li, M.J.; et al. Differences in metabolite profile between blood plasma and serum. Anal. Biochem. 2010, 406, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Kastenmuller, G.; He, Y.; Belcredi, P.; Moller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Denery, J.R.; Nunes, A.A.K.; Dickerson, T.J. Characterization of differences between blood sample matrices in untargeted metabolomics. Anal. Chem. 2011, 83, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 1980, 10 (Suppl. 2), 291S–298S. [Google Scholar] [CrossRef]
- Butler, J.M.; Begg, E.J. Free drug metabolic clearance in elderly people. Clin. Pharmacokinet. 2008, 47, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Critchley, J.A.; Critchley, L.A.; Anderson, P.J.; Tomlinson, B. Differences in the single-oral-dose pharmacokinetics and urinary excretion of paracetamol and its conjugates between hong kong chinese and caucasian subjects. J. Clin. Pharm. Ther. 2005, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H.; Duan, S.X.; von Moltke, L.L.; Greenblatt, D.J.; Patten, C.J.; Miners, J.O.; Mackenzie, P.I. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: Identification of relevant acetaminophen udp-glucuronosyltransferase isoforms. J. Pharmacol. Exp. Ther. 2001, 299, 998–1006. [Google Scholar] [PubMed]
- Klaassen, C.D.; Cui, J.Y. Review: Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab. Dispos. 2015, 43, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [PubMed]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Jacobs, E.J.; Almon, M.L.; Chao, A.; McCullough, M.L.; Feigelson, H.S.; Thun, M.J. The american cancer society cancer prevention study ii nutrition cohort: Rationale, study design, and baseline characteristics. Cancer 2002, 94, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; American Joint Committee on Cancer; American Cancer Society. AJCC Cancer Staging Handbook: From the Ajcc Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; p. 718. [Google Scholar]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 35 (Suppl. 1), D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Overview of cotinine cutoff values for smoking status classification. Int. J. Environ. Res. Public Health 2016, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
| Drug Metabolite(s) Detected a | Serum | Plasma | ||||||
|---|---|---|---|---|---|---|---|---|
| Self-Reported Drug Use | Self-Reported Drug Use | |||||||
| Today | Yesterday | Intermediate b | Never/Rare | Today | Yesterday | Intermediate b | Never/Rare | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Acetaminophen | 69 | 139 | 326 | 778 | 14 | 32 | 99 | 326 |
| Acetaminophen + Acetamidophenylglucuronide | 67 (97.1) | 115 (82.7) | 41 (12.6) | 49 (6.3) | 14 (100.0) | 11 (34.4) | 1 (1.0) | 9 (2.8) |
| Acetaminophen only | 1 (1.4) | 3 (2.2) | 25 (7.7) | 94 (12.1) | 0 (0) | 0 (0) | 3 (3.0) | 16 (4.9) |
| Acetamidophenylglucuronide only | 0 (0) | 12 (8.6) | 25 (7.7) | 14 (1.8) | 0 (0) | 8 (25.0) | 4 (4.0) | 3 (0.9) |
| None | 1 (1.4) | 9 (6.5) | 235 (72.1) | 621 (79.8) | 0 (0) | 13 (40.6) | 91 (91.9) | 298 (91.4) |
| Ibuprofen | 120 | 111 | 320 | 787 | 39 | 34 | 99 | 309 |
| Carboxyibuprofen + Hydroxyibuprofen | 57 (47.5) | 17 (15.3) | 3 (0.9) | 0 (0) | 20 (51.3) | 10 (29.4) | 2 (2.0) | 0 (0) |
| Hydroxyibuprofen Only | 1 (0.8) | 3 (2.7) | 3 (0.9) | 4 (0.5) | 0 (0) | 1 (2.9) | 0 (0) | 0 (0) |
| Carboxyibuprofen Only | 5 (4.2) | 20 (18.0) | 2 (0.6) | 1 (0.1) | 0 (0) | 8 (23.5) | 4 (4.0) | 0 (0) |
| None | 57 (47.5) | 71 (64.0) | 312 (97.5) | 782 (99.4) | 19 (48.7) | 15 (44.1) | 93 (93.9) | 309 (100.0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, K.K.; Carter, B.D.; Gapstur, S.M.; Stevens, V.L. Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use. Metabolites 2018, 8, 55. https://doi.org/10.3390/metabo8040055
Dennis KK, Carter BD, Gapstur SM, Stevens VL. Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use. Metabolites. 2018; 8(4):55. https://doi.org/10.3390/metabo8040055
Chicago/Turabian StyleDennis, Kristine K., Brian D. Carter, Susan M. Gapstur, and Victoria L. Stevens. 2018. "Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use" Metabolites 8, no. 4: 55. https://doi.org/10.3390/metabo8040055
APA StyleDennis, K. K., Carter, B. D., Gapstur, S. M., & Stevens, V. L. (2018). Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use. Metabolites, 8(4), 55. https://doi.org/10.3390/metabo8040055





