Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Differentiation between the Vinegar Samples Based on Their Comprehensive Metabolite Profiles
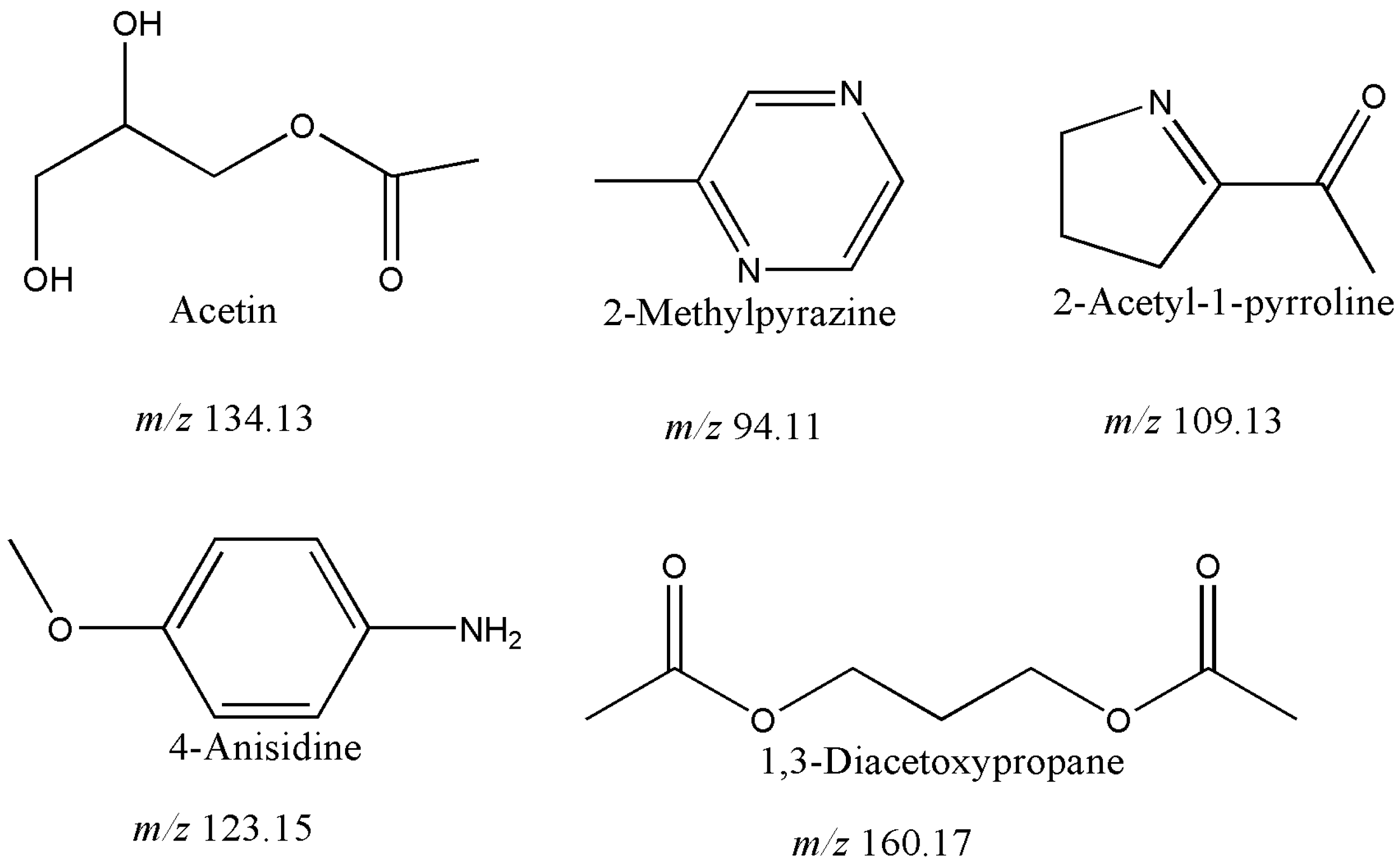
2.2. Five Volatile Metabolites Reported De Novo in Balsamic Vinegars
3. Materials and Methods
3.1. Chemicals
3.2. Vinegar Samples
3.3. Metabolite Profiling of Balsamic Vinegars by GC-MS
3.3.1. Methylchloroformate (MCF) Derivatization
3.3.2. Trimethyl Silyl (TMS) Derivatization
3.3.3. Analysis of Volatile Metabolites
3.3.4. Data Mining and Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography coupled to mass spectrometry |
| HCA | Hierarchical Cluster Analysis |
| MCF | Methylchloroformate |
| NMR | Nuclear magnetic resonance |
| RSD | Residual standard deviation |
| TVB | Traditional balsamic vinegars |
| TMS | Trimethylsilyl |
References
- Greco, E.; Cervellati, R.; Litterio, M.L. Antioxidant capacity and total reducing power of balsamic and traditional balsamic vinegar from Modena and Reggio Emilia by conventional chemical assays. Int. J. Food Sci. Technol. 2013, 48, 114–120. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Antioxidant properties of traditional balsamic vinegar and boiled must model systems. Eur. Food Res. Technol. 2008, 227, 835–843. [Google Scholar] [CrossRef]
- Johnston, C.S.; Quagliano, S.; White, S. Vinegar ingestion at mealtime reduced fasting blood glucose concentrations in healthy adults at risk for type 2 diabetes. J. Funct. Foods 2013, 5, 2007–2011. [Google Scholar] [CrossRef]
- Liatist, S.; Grammatikou, S.; Poulia, K.A.; Perrea, D.; Makrilakis, K.; Diakoumopoulou, E.; Katsilambros, N. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur. J. Clin. Nutr. 2010, 64, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Petsiou, E.I.; Mitrou, P.I.; Raptis, S.A.; Dimitriadis, G.D. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr. Rev. 2014, 72, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kimura, F.; Takashima, A.; Shimizu, Y.; Takebayashi, A.; Kita, N.; Zhang, G.; Murakami, T. Intake of vinegar beverage is associated with restoration of ovulatory function in women with polycystic ovary syndrome. Tohoku J. Exp. Med. 2013, 230, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R.; Rinaldini, S.; Incerti, A. Analytical method for authentication of traditional balsamic Vinegar of Modena. Talanta 2008, 75, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Falcone, P.M.; Chillo, S.; Giudici, P.; Del Nobile, M.A. Measuring rheological properties for applications in quality assessment of Traditional Balsamic Vinegar: Description and preliminary evaluation of a model. J. Food Eng. 2007, 80, 234–240. [Google Scholar] [CrossRef]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and microbiological aspects of traditional balsamic vinegar and their influence on quality and sensorial properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [PubMed]
- Lemmetti, F.; Falcone, P.M.; Giudici, P. Traditional balsamic vinegar: The role of the viscosity on the objective and perceived quality. Ind. Delle Bevande 2013, 42, 9–27. [Google Scholar]
- Caligiani, A.; Silva, G.; Palla, G. Traditional balsamic vinegar. J. Agric. Food Chem. 2007, 55, 7810–7815. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Tesfaye, W.; Soria-Diaz, M.E.; Jesus Torija, M.; Mateo, E.; Carmen Garcia-Parrilla, M.; Troncoso, A.M. Effect of wood on the phenolic profile and sensory properties of wine vinegars during ageing. J. Food Compos. Anal. 2010, 23, 175–184. [Google Scholar] [CrossRef]
- Chinnici, F.; Duran Guerrero, E.; Sonni, F.; Natali, N.; Natera Marin, R.; Riponi, C. Gas Chromatography-Mass Spectrometry (GC-MS) characterization of volatile compounds in quality vinegars with Protected European Geographical Indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Caligiani, A.; Palla, L.; Palla, G. HS-SPME/GC-MS and chemometrics for the classification of Balsamic Vinegars of Modena of different maturation and ageing. Food Chem. 2011, 124, 1678–1683. [Google Scholar] [CrossRef]
- Daglia, M.; Amoroso, A.; Rossi, D.; Mascherpa, D.; Maga, G. Identification and quantification of alpha-dicarbonyl compounds in balsamic and traditional balsamic vinegars and their cytotoxicity against human cells. J. Food Compos. Anal. 2013, 31, 67–74. [Google Scholar] [CrossRef]
- Giordano, L.; Calabrese, R.; Davoli, E.; Rotilio, D. Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry using isotope dilution. J. Chromatogr. A 2003, 1017, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, H.; Mattes, J.; Brockhoff, A.; Dunkel, A.; Meyerhof, W.; Hofmann, T. Sensomics analysis of taste compounds in balsamic vinegar and discovery of 5-acetoxymethyl-2-furaldehyde as a novel sweet taste modulator. J. Agric. Food Chem. 2012, 60, 9974–9990. [Google Scholar] [CrossRef] [PubMed]
- Marrufo-Curtido, A.; Cejudo-Bastante, M.J.; Duran-Guerrero, E.; Castro-Mejias, R.; Natera-Marin, R.; Chinnici, F.; Garcia-Barroso, C. Characterization and differentiation of high quality vinegars by stir bar sorptive extraction coupled to gas chromatography-mass spectrometry (SBSE-GC-MS). LWT Food Sci. Technol. 2012, 47, 332–341. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Miglietta, F. Extraction and identification by GC-MS of phenolic acids in traditional balsamic vinegar from Modena. J. Food Compos. Anal. 2006, 19, 49–54. [Google Scholar] [CrossRef]
- Ugliano, M.; Squillante, E.; Genovese, A.; Moio, L. Investigation on aroma compounds of Modena balsamic vinegars. In Flavour Research at the Dawn of the Twenty-First Century, Proceedings of the 10th Weurman Flavour Research Symposium, Beaune, France, 24–28 June 2002; pp. 733–736.
- Caligiani, A.; Acquotti, D.; Palla, G.; Bocchi, V. Identification and quantification of the main organic components of vinegars by high resolution H-1 NMR spectroscopy. Anal. Chim. Acta 2007, 585, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Lambertini, P.; Manzini, D.; Marchetti, A.; Ulrici, A. Determination of carboxylic acids in vinegars and in aceto balsamico tradizionale di modena by HPLC and GC methods. J. Agric. Food Chem. 2002, 50, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Sanarico, D.; Motta, S.; Bertolini, L.; Antonelli, A. HPLC determination of organic acids in Traditional Balsamic Vinegar of Reggio Emilia. J. Liquid Chromatogr. Relat. Technol. 2003, 26, 2177–2187. [Google Scholar] [CrossRef]
- Cocchi, M.; Durante, C.; Grandi, M.; Lambertini, P.; Manzini, D.; Marchetti, A. Simultaneous determination of sugars and organic acids in aged vinegars and chemometric data analysis. Talanta 2006, 69, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Koulman, A.; Lane, G.A. Analytical methods from the perspective of method standardization. Top. Curr. Genet. 2007, 18, 11–52. [Google Scholar]
- Villas-Bôas, S.G.; Mas, S.; Åkesson, M.; Smedsgaard, J.; Nielsen, J. Mass spectrometry in metabolome analysis. Mass Spectrom. Rev. 2005, 24, 613–646. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.F.; Aggio, R.B.M.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Skogerson, K.; Runnebaum, R.; Wohlgemuth, G.; de Ropp, J.; Heymann, H.; Fiehn, O. Comparison of gas chromatography-coupled time-of-flight mass spectrometry and H-1 nuclear magnetic resonance spectroscopy metabolite identification in white wines from a sensory study investigating wine body. J. Agric. Food Chem. 2009, 57, 6899–6907. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Paglia, G.; Williams, J.P.; Menikarachchi, L.; Thompson, J.W.; Tyldesley-Worster, R.; Halldórsson, S.; Rolfsson, O.; Moseley, A.; Grant, D.; Langridge, J.; et al. Ion mobility derived collision cross sections to support metabolomics applications. Anal. Chem. 2014, 86, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Brown, M.; Baker, P.N.; Redman, C.W.G.; Kenny, L.C.; Kell, D.B. Metabolic profiling of serum using Ultra Performance Liquid Chromatography and the LTQ-Orbitrap mass spectrometry system. J. Chromagr. B 2008, 871, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Durán-Guerrero, E.; Riponi, C. Discrimination of some European vinegars with protected denomination of origin as a function of their amino acid and biogenic amine content. J. Sci. Food Agric. 2016, 96, 3762–3771. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Masino, F.; Chinnici, F.; Franchini, G.C.; Ulrici, A.; Antonelli, A. A study of the relationships among acidity, sugar and furanic compound concentrations in set of casks for Aceto Balsamico Tradizionale of Reggio Emilia by multivariate techniques. Food Chem. 2005, 92, 673–679. [Google Scholar] [CrossRef]
- Guerrero, E.D.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Caligiani, A.; Palla, G. Formation of glucose and fructose acetates during maturation and ageing of balsamic vinegars. Food Chem. 2009, 112, 51–56. [Google Scholar] [CrossRef]
- Chinnici, F.; Masino, F.; Antonelli, A. Determination of furanic compounds in traditional balsamic vinegars by ion-exclusion liquid chromatography and diode-array detection. J. Chromatogr. Sci. 2003, 41, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.C.R.; Itabaiana, I., Jr.; Flores, M.C.; Lourenço, A.C.; Leite, S.G.F.; Miranda, L.S.; Leal, I.C.R.; De Souza, R.O.M.A. Biocatalyzed acetins production under continuous-flow conditions: Valorization of glycerol derived from biodiesel industry. J. Flow Chem. 2013, 3, 41–45. [Google Scholar] [CrossRef]
- Gazzi, L.; D’Ambra, R.; Di Cintio, R.; Rescalli, C.; Vetere, A. Cryogenic process for the Selective Removal of Acid Gases from Gas Mixtures by Means of a Solvent. U.S. Patent US4710211 A, 31 December 1985. [Google Scholar]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Prabavathi, N. Scaled quantum chemical calculations and FTIR, FT-Raman spectral analysis of 2-Methylpyrazine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Noel, S.; Lane, G.A.; Attwood, G.; Cookson, A. Extracellular metabolomics: A metabolic footprinting approach to assess fiber degradation in complex media. Anal. Biochem. 2006, 349, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Aggio, R.; Villas-Bôas, S.G.; Ruggiero, K. Metab: An R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 2011, 27, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | RT (min) | m/z | Avg RSD (%); n = 6 | Relative Abundance in Vinegar Samples | |||||
|---|---|---|---|---|---|---|---|---|---|
| D | M1 | L | T | M4 | P | ||||
| Amino acids (25): | |||||||||
| 2-aminobutyric acid | 11.40 | 116 | 10 | 0.08 ± 0.01 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.08 ± 0.01 | 0.10 ± 0.02 | 0.28 ± 0.05 |
| 4-aminobutyric acid (GABA) | 14.98 | 102 | 11 | 0.62 ± 0.09 | 0.69 ± 0.05 | 0.47 ± 0.09 | 0.35 ± 0.05 | 0.46 ± 0.12 | 0.41 ± 0.06 |
| 4-Hydroxyproline | 12.75 | 144 | 9 | ND | 0.08 ± 0.00 | 0.04 ± 0.00 | ND | 0.07 ± 0.01 | 0.12 ± 0.03 |
| Alanine | 11.15 | 102 | 6 | 2.77 ± 0.55 | 3.05 ± 0.16 | 2.08 ± 0.52 | 1.89 ± 0.61 | 2.81 ± 0.24 | 3.87 ± 0.59 |
| Asparagine | 16.70 | 70 | 11 | 0.17 ± 0.02 | 0.23 ± 0.04 | ND | ND | 0.16 ± 0.09 | 0.20 ± 0.04 |
| Aspartic acid | 16.45 | 160 | 11 | 0.51 ± 0.08 | 0.54 ± 0.03 | 0.42 ±0.09 | 0.82 ±0.03 | 0.72 ±0.12 | 0.60 ±0.08 |
| Beta-Alanine | 12.75 | 88 | 7 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.08 ± 0.09 | 0.06 ± 0.02 | 0.04 ± 0.00 |
| Glycine | 11.50 | 88 | 11 | 0.52 ± 0.12 | 0.49 ± 0.18 | 0.34 ± 0.04 | 0.42 ± 0.16 | 0.44 ± 0.06 | 0.98 ± 0.17 |
| Glutamic acid | 18.20 | 174 | 14 | 0.15 ± 0.05 | 0.14 ± 0.00 | 0.21 ± 0.06 | 0.44 ± 0.12 | 0.40 ± 0.08 | 0.57 ± 0.03 |
| Glutamine | 17.99 | 174 | 24 | ND | ND | 0.37 ± 0.00 | ND | ND | 2.41 ± 0.67 |
| Histidine | 26.90 | 139 | 12 | 0.07 ± 0.00 | 0.11 ± 0.01 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.02 |
| Isoleucine | 14.15 | 115 | 10 | 0.67 ± 0.24 | 0.83 ± 0.26 | 0.53 ± 0.11 | 0.36 ± 0.08 | 0.62 ± 0.09 | 0.39 ± 0.11 |
| Leucine | 14.10 | 144 | 6 | 0.40 ± 0.00 | 0.50 ± 0.07 | 0.48 ± 0.11 | 0.73 ± 0.17 | 0.40 ± 0.08 | 0.66 ± 0.12 |
| Lysine | 26.35 | 142 | 17 | 0.21 ± 0.05 | 0.38 ± 0.11 | 0.26 ± 0.02 | 0.34 ± 0.01 | 0.21 ± 0.03 | 0.21 ± 0.05 |
| Methionine | 18.10 | 147 | 14 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | ND |
| N-Acetylglutamic acid | 19.28 | 116 | 13 | 0.05 ± 0.01 | 0.08 ± 0.00 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.00 | 0.03 ± 0.00 |
| Ornithine | 24.85 | 128 | 19 | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.07 ± 0.00 | 0.19 ± 0.06 | 0.05 ± 0.00 | ND |
| Phenylalanine | 20.05 | 162 | 14 | 0.24 ± 0.04 | 0.37 ± 0.07 | 0.22 ± 0.00 | 0.34 ± 0.06 | 0.28 ± 0.02 | 0.46 ± 0.07 |
| Proline | 15.15 | 128 | 6 | 7.31 ± 1.31 | 9.26 ± 1.67 | 6.81 ± 0.66 | 7.55 ± 0.98 | 8.74 ± 1.11 | 12.35 ± 1.09 |
| Putrescine | 21.80 | 115 | 15 | 0.04 ± 0.00 | ND | ND | 0.15 ± 0.00 | 0.14 ± 0.05 | 0.07 ± 0.01 |
| Pyroglutamic acid | 16.55 | 84 | 13 | ND | 0.66 ± 0.11 | ND | ND | ND | 1.85 ± 0.87 |
| Serine | 17.45 | 100 | 14 | 0.05 ± 0.00 | 0.07 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.08 ± 0.02 |
| Threonine | 15.75 | 115 | 8 | 0.27 ± 0.11 | 0.31 ± 0.04 | 0.18 ± 0.02 | 0.13 ± 0.05 | 0.22 ± 0.01 | 0.23 ± 0.03 |
| Tyrosine | 28.75 | 236 | 11 | 0.16 ± 0.00 | 0.24 ± 0.05 | 0.12 ± 0.00 | 0.16 ± 0.00 | 0.17 ± 0.04 | 0.41 ± 0.09 |
| Valine | 12.85 | 130 | 10 | 1.16 ± 0.28 | 1.40 ± 0.11 | 0.93 ± 0.08 | 1.12 ± 0.04 | 1.09 ± 0.03 | 1.82 ± 0.55 |
| Tripeptide (1): | |||||||||
| Glutathione | 19.00 | 142 | 8 | 0.03 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 | ND | 0.01 ± 0.00 | ND |
| Carboxylic acids (26): | |||||||||
| 3-hydroxybenzoic acid | 17.01 | 135 | 16 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.12 ± 0.02 |
| 4-hydroxycinnamic acid | 23.22 | 161 | 16 | ND | 0.03 ± 0.00 | 0.07 ± 0.01 | ND | ND | 0.02 ± 0.00 |
| 2-isopropylmalic acid | 12.87 | 145 | 13 | 0.04 ± 0.00 | 0.10 ± 0.03 | 0.07 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.10 ± 0.02 |
| 4-hydroxyphenylacetic acid | 18.89 | 121 | 23 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.14 ± 0.04 | ND | 0.26 ± 0.06 | 0.47 ± 0.09 |
| 2-oxoglutaric acid | 13.85 | 115 | 15 | 0.14 ± 0.01 | ND | 0.13 ± 0.00 | ND | ND | 0.32 ± 0.l2 |
| 2-oxovaleric acid | 7.17 | 71 | 24 | ND | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.01 |
| Benzoic acid | 9.70 | 105 | 8 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 |
| cis-Aconitic acid | 9.70 | 105 | 12 | 0.07 ± 0.02 | 0.03 ± 0.00 | 0.10 ± 0.01 | ND | 0.16 ± 0.03 | 1.76 ± 0.55 |
| Citric acid | 15.55 | 153 | 9 | 0.20 ± 0.05 | 0.45 ± 0.02 | 0.26 ± 0.03 | 0.15 ± 0.01 | 0.25 ± 0.06 | 1.94 ± 0.78 |
| Citraconic acid | 16.45 | 143 | 19 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.16 ± 0.06 |
| Citramalic acid | 10.20 | 127 | 10 | 0.19 ± 0.05 | 0.11 ± 0.02 | 0.14 ± 0.00 | 0.26 ± 0.06 | 0.21 ± 0.03 | 0.22 ± 0.05 |
| Fumaric acid | 10.75 | 117 | 13 | 0.94 ± 0.67 | 1.03 ± 0.09 | 0.61 ± 0.05 | 0.44 ± 0.02 | 0.98 ± 0.08 | 2.68 ± 0.55 |
| Glutaric acid | 9.25 | 113 | 13 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.05 ± 0.01 |
| Glyceric acid | 11.75 | 119 | 16 | ND | ND | ND | 0.05 ± 0.02 | 0.02 ± 0.00 | ND |
| Glyoxalic acid | 11.25 | 75 | 14 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | ND | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Isocitric acid | 21.05 | 129 | 14 | 0.07 ± 0.01 | 0.12 ± 0.03 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.10 ± 0.02 | 0.18 ± 0.03 |
| Itaconic acid | 10.22 | 127 | 19 | 0.05 ± 0.00 | 0.16 ± 0.04 | 0.10 ± 0.01 | 0.07 ± 0.03 | 0.09 ± 0.02 | 1.14 ± 0.06 |
| Lactic acid | 9.28 | 103 | 9 | 3.33 ± 0.85 | 1.65 ± 0.12 | 1.98 ± 0.50 | 2.22 ± 0.43 | 2.61 ± 0.65 | 4.33 ± 1.11 |
| Levulinic acid | 8.75 | 99 | 8 | 0.45 ± 0.04 | 0.68 ± 0.08 | 0.47 ± 0.05 | 0.04 ± 0.00 | 0.51 ± 013 | 0.33 ± 0.05 |
| Malic acid | 11.45 | 103 | 8 | 0.25 ± 0.07 | 0.41 ± 0.05 | 0.21 ± 0.11 | 0.59 ± 0.16 | 0.35 ± 0.54 | 0.78 ± 0.54 |
| Malonic acid | 7.50 | 101 | 17 | 0.48 ± 0.12 | 0.69 ± 0.24 | 0.46 ± 0.07 | 0.40 ± 0.03 | 0.63 ± 0.08 | 1.00 ± 0.04 |
| Oxaloacetic acid | 9.73 | 101 | 32 | 0.13 ± 0.03 | 0.18 ± 0.02 | 0.13 ± 0.04 | 0.10 ± 0.01 | 0.13 ± 0.03 | 0.18 ± 0.08 |
| p-Coumaric acid | 10.52 | 164 | 15 | 0.11 ± 0.01 | 0.16 ± 0.03 | 0.07 ± 0.00 | 0.12 ± 0.03 | 0.14 ± 0.05 | 0.37 ± 0.11 |
| Succinic acid | 9.10 | 115 | 6 | 3.80 ± 0.98 | 4.86 ± 0.87 | 3.53 ± 0.56 | 4.03 ± 0.76 | 4.51 ± 0.22 | 6.87 ± 0.44 |
| Syringic acid | 24.40 | 211 | 15 | 0.05 ± 0.00 | 0.01 ± 0.00 | ND | ND | 0.11 ± 0.03 | 0.08 ± 0.02 |
| Tartaric acid | 20.80 | 59 | 11 | 3.26 ± 0.78 | 2.22 ± 0.55 | 1.54 ± 0.34 | 4.54 ± 0.13 | 3.46 ± 0.24 | 3.89 ± 0.11 |
| Fatty acids (4): | |||||||||
| 3-Methyl-2-oxopentanoic acid | 7.69 | 57 | 14 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.00 |
| 4-Methyl-2-oxopentanoic acid | 7.79 | 85 | 10 | ND | 0.02 ± 0.00 | 0.01 ± 0.00 | ND | 0.04 ± 0.01 | 0.06 ± 0.01 |
| Stearic acid | 24.22 | 74 | 15 | 0.67 ± 0.22 | 0.89 ± 0.13 | 0.22 ± 0.05 | 0.17 ± 0.01 | 0.55 ± 0.12 | 0.46 ± 0.07 |
| 10,13-Dimethyltetradecanoic acid | 20.22 | 180 | 13 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | ND |
| Sugars (9): | |||||||||
| d-Galactose | 18.75 | 319 | 4 | 1.91 ± 0.76 | 1.79 ± 0.55 | 1.83 ± 0.56 | 2.02 ± 0.87 | 2.20 ± 0.26 | 2.94 ± 0.65 |
| d-Glucose | 19.13 | 319 | 2 | 1.82 ± 0.11 | 1.85 ± 0.45 | 1.89 ± 0.67 | 1.84 ± 0.28 | 2.09 ± 0.45 | 1.85 ± 0.16 |
| d-Fructose | 18.26 | 103 | 1 | 1.94 ± 0.65 | 1.83 ± 0.23 | 1.96 ± 0.41 | 2.01 ± 0.22 | 2.28 ± 0.09 | 1.91 ± 0.41 |
| d-Mannose | 18.72 | 319 | 8 | 1.92 ± 0.12 | 1.93 ± 0.34 | 1.73 ± 0.25 | 1.75 ± 0.16 | 1.85 ± 0.11 | 1.95 ± 0.45 |
| d-Ribose | 15.54 | 103 | 3 | 1.94 ± 0.56 | 1.83 ± 0.22 | 1.96 ± 0.55 | 2.01 ± 0.17 | 2.27 ± 0.21 | 1.91 ± 0.50 |
| d-Sorbose | 18.17 | 103 | 1 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.02 | 0.01 ± 0.00 |
| d-Xylose | 13.98 | 103 | 4 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 |
| Trehalose | 32.40 | 361 | 6 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Sucrose | 32.76 | 361 | 4 | ND | ND | ND | ND | 0.02 ± | ND |
| Sugar alcohols (4): | |||||||||
| d-mannitol | 18.91 | 319 | 5 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.27 ± 0.06 | 1.92 ± 0.65 | 0.15 ± 0.02 |
| Glycerol | 10.43 | 147 | 4 | 0.76 ± 0.03 | 0.80 ± 0.09 | 0.97 ± 0.06 | 1.19 ± 0.16 | 0.81 ± 0.04 | 0.72 ± 0.05 |
| Meso-erythritol | 13.61 | 217 | 6 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.03 ± 0.00 |
| Meso-inositol | 22.27 | 305 | 6 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.20 ± 0.05 | 0.08 ± 0.03 |
| Vitamin and derivative (2): | |||||||||
| Nicotinic acid | 10.82 | 137 | 14 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Nicotinamide | 6.04 | 56 | 20 | ND | 0.02 ± 0.00 | 0.01 ± 0.00 | ND | ND | 0.02 ± 0.00 |
| Others (2): | |||||||||
| 5-hydroxymethyl-2-furaldehyde | 14.25 | 168 | 3 | 0.02 ± 0.00 | ND | ND | 0.12 ± 0.03 | ND | ND |
| Phosphate | 11.72 | 299 | 12 | 0.15 ± 0.04 | 0.13 ± 0.02 | 0.15 ± 0.03 | 0.28 ± 0.06 | 0.50 ± 0.08 | 0.15 ± 0.02 |
| Metabolite | RT (min) | m/z | Ag RSD (%), n = 6 | Relative Abundance in Vinegar Samples | |||||
|---|---|---|---|---|---|---|---|---|---|
| D | M1 | L | T | M4 | P | ||||
| Volatile acids (14): | |||||||||
| 2-Ethylhexanoic acid | 9.18 | 88 | 6 | ND | ND | ND | 0.19 | ND | ND |
| 2-Methybutyric acid | 6.59 | 74 | 7 | 0.23 ± 0.04 | 0.16 ± 0.02 | 0.26 ± 0.05 | 0.29 ± 0.08 | 0.24 ± 0.02 | 0.23 ± 0.04 |
| 4-Methyl-2-pentenoic acid | 7.59 | 60 | 8 | 0.03 ± 0.00 | ND | 0.04 ± 0.01 | 0.05 ± 0.01 | ND | 0.03 ± 0.00 |
| Acetic acid | 4.43 | 43 | 4 | 2.26 ± 0.56 | 2.25 ± 0.76 | 2.20 ± 0.12 | 2.33 ± 0.09 | 2.20 ± 0.15 | 2.26 ± 0.23 |
| Benzeneacetic acid | 11.13 | 91 | 7 | 0.27 ± 0.12 | 0.06 ± 0.01 | 0.31 ± 0.09 | 0.74 ± 0.17 | 0.42 ± 0.12 | 0.52 ± 0.23 |
| Butyric acid | 4.46 | 60 | 7 | 0.58 ± 0.11 | 0.54 ± 0.16 | 0.40 ± 0.09 | 0.09 ± 0.02 | 0.05 ± 0.00 | 0.17 ± 0.04 |
| Carbolic acid | 8.56 | 94 | 9 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.10 ± 0.02 | ND | 0.03 ± 0.00 |
| Furoic acid | 9.75 | 112 | 8 | 0.41 ± 0.18 | 0.41 ± 0.11 | 0.38 ± 0.09 | 0.23 ± 0.04 | 0.13 ± 0.03 | 0.21 ± 0.07 |
| Hexanoic acid | 8.00 | 60 | 5 | 0.33 ± 0.11 | 0.27 ± 0.06 | 0.24 ± 0.03 | 0.17 ± 0.05 | 0.23 ± 0.08 | 0.44 ± 0.11 |
| Isobutyric acid | 5.44 | 73 | 6 | 1.04 ± 0.34 | 0.45 ± 0.11 | 1.04 ± 0.30 | 0.84 ± 0.13 | 0.02 ± 0.00 | 0.74 ± 0.21 |
| Isovaleric acid | 6.98 | 60 | 4 | 2.15 ± 0.67 | 1.99 ± 0.44 | 3.09 ± 0.81 | 5.31 ± 0.96 | 0.11 ± 0.01 | 3.46 ± 0.54 |
| Octanoic acid | 10.13 | 73 | 8 | 0.25 ± 0.04 | 0.34 ± 0.02 | 0.19 ± 0.01 | ND | 0.22 ± 0.03 | 0.18 ± 0.03 |
| Propionic acid | 5.28 | 74 | 4 | 0.84 ± 0.55 | 0.46 ± 0.10 | 0.54 ± 0.18 | 0.18 ± 0.05 | 0.25 ± 0.05 | 0.14 ± 0.04 |
| Valeric acid | 7.44 | 60 | 6 | 0.23 ± 0.04 | 0.08 ± 0.01 | 0.05 ± 0.00 | ND | ND | 0.37 ± 0.06 |
| Esters (12): | |||||||||
| 2-Carboxymethyl-3-n-hexylmaleic acid anhydride | 12.47 | 126 | 7 | 0.22 ± 0.05 | 0.21 ± 0.02 | 0.31 ± 0.05 | 0.12 ± 0.01 | 0.15 ± 0.03 | 0.31 ± 0.08 |
| (−)-Ethyl L-Lactate | 5.65 | 45 | 7 | 0.08 ± 0.02 | 0.39 ± 0.14 | 0.12 ± 0.05 | 0.10 ± 0.01 | 0.04 ± 0.00 | 0.12 ± 0.02 |
| 2-Methyl-1-butyl acetate | 5.37 | 70 | 6 | 0.10 ± 0.02 | 0.17 ± 0.03 | 0.07 ± 0.00 | 0.08 ± 0.02 | 0.01 ± 0.00 | 0.06 ± 0.01 |
| 1,3-Propylene diacetate | 8.76 | 43 | 9 | 0.17 ± 0.05 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.16 ± 0.05 | 0.03 ± 0.00 |
| Diethyl succinate | 9.59 | 129 | 5 | 0.41 ± 0.17 | 0.71 ± 0.26 | 0.87 ± 0.21 | 0.15 ± 0.05 | 0.03 ± 0.00 | 0.14 ± 0.03 |
| Ethylisovalerate | 4.91 | 40 | 9 | 0.01 ± 0.00 | ND | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Ethyl hydrogen succinate | 10.47 | 101 | 4 | 2.10 ± 0.23 | 1.89 ± 0.55 | 2.28 ± 0.38 | 2.32 ± 0.43 | 1.26 ± 0.17 | 1.17 ± 0.17 |
| Isoamyl acetate | 5.34 | 43 | 6 | 0.83 ± 0.34 | 1.65 ± 0.33 | 0.61 ± 0.09 | 0.09 ± 0.00 | 0.16 ± 0.03 | 0.42 ± 0.11 |
| Methyl 2-furoate | 4.98 | 95 | 8 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.01 | 0.01 ± 0.00 |
| Methylsuccinic anhydride | 9.52 | 42 | 4 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.01 | ND | 0.03 ± 0.00 | ND |
| Phenethyl acetate | 10.29 | 104 | 8 | 0.44 ± 0.17 | 0.44 ± 0.11 | 0.55 ± 0.08 | 0.24 ± 0.04 | 0.12 ± 0.02 | 0.21 ± 0.06 |
| p-Hydroxycinnamic acid, ethyl ester | 15.98 | 147 | 7 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.16 ± 0.05 | 0.06 ± 0.00 | 0.14 ± 0.03 | 0.05 ± 0.00 |
| Higher alcohols (9): | |||||||||
| 2,3-butanediol | 5.76 | 45 | 7 | 0.42 ± 0.08 | 0.41 ± 0.11 | 0.50 ± 0.18 | 0.43 ± 0.09 | 0.32 ± 0.07 | 0.39 ± 0.10 |
| 1,2,3-Benzenetriol | 13.40 | 126 | 5 | 0.15 ± 0.02 | 0.21 ± 0.04 | 0.73 ± 0.21 | 0.13 ± 0.06 | 0.39 ± 0.11 | 0.22 ± 0.06 |
| 1,4-Benzenediol | 12.34 | 81 | 8 | 0.19 ± 0.05 | 0.17 ± 0.03 | 0.10 ± 0.01 | 0.13 ± 0.02 | ND | 0.17 ± 0.11 |
| 2-Methyl-1-butanol | 4.05 | 57 | 3 | 1.40 ± 0.45 | 1.78 ± 0.25 | 1.45 ± 0.11 | 0.73 ± 0.08 | 0.16 ± 0.04 | 0.99 ± 0.17 |
| 2-Phenylethanol | 8.81 | 107 | 4 | 4.10 ± 1.08 | 5.55 ± 1.16 | 4.38 ± 0.98 | 2.56 ± 0.45 | 1.81 ± 0.76 | 2.39 ± 0.54 |
| Benzyl alcohol | 8.45 | 79 | 5 | 0.18 ± 0.06 | 0.22 ± 0.04 | 0.13 ± 0.06 | 0.14 ± 0.03 | 0.22 ± 0.06 | 0.11 ± 0.02 |
| Isoamyl alcohol | 5.32 | 70 | 3 | 2.70 ± 0.76 | 5.67 ± 0.76 | 2.02 ± 0.32 | 1.01 ± 0.32 | 1.02 ± 0.06 | 2.26 ± 0.43 |
| Methionol | 7.79 | 106 | 9 | 0.04 ± 0.00 | 0.18 ± 0.04 | 0.02 ± 0.00 | ND | ND | ND |
| Tyrosol | 13.43 | 107 | 3 | 0.73 ± 0.16 | 0.43 ± 0.08 | 0.48 ± 0.09 | 0.53 ± 0.08 | 0.05 ± 0.00 | 0.26 ± 0.07 |
| Aldehydes and ketones (6): | |||||||||
| 1,3-Diacetoxypropane * | 7.75 | 61 | 8 | 0.41 ± 0.06 | 0.16 ± 0.03 | 0.19 ± 0.04 | 0.06 ± 0.00 | 0.51 ± 0.12 | 0.07 ± 0.00 |
| 4,4-Diethyl-3-methylene-2-oxetanone | 15.09 | 14 | 25 | 0.08 ± 0.00 | 0.04 ± 0.00 | 0.10 ± 0.02 | 0.04 ± 0.00 | 0.15 ± 0.04 | 0.08 ± 0.01 |
| 3-Methyl-1,2-cyclopentanedione | 8.21 | 112 | 13 | 0.02 ± 0.00 | ND | 0.02 ± 0.00 | 0.05 ± 0.00 | ND | 0.02 ± 0.00 |
| 5-Hydrxoymethylfurfural | 11.34 | 97 | 10 | 0.46 ± 0.12 | 0.34 ± 0.04 | 0.43 ± 0.08 | 0.23 ± 0.04 | 4.15 ± 0.78 | 0.41 ± 0.23 |
| 5-Methyl furfural | 7.41 | 110 | 5 | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.24 ± 0.08 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.14 ± 0.03 |
| Acetoin | 7.17 | 43 | 0.26 ± 0.06 | 0.14 ± 0.04 | 0.15 ± 0.02 | 0.22 ± 0.06 | 0.36 ± 0.10 | 0.16 ± 0.02 | |
| Butyrolactone | 4.65 | 42 | 6 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Others (6): | |||||||||
| 2-Acetyl-1-pyrroline * | 8.87 | 66 | 12 | ND | ND | ND | 0.01 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.00 |
| 4-Anisidine * | 8.37 | 45 | 4 | ND | ND | ND | ND | 0.21 ± 0.05 | 0.18 ± 0.04 |
| 2-Methylpyrazine * | 4.96 | 94 | 2 | ND | ND | ND | 0.08 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.00 |
| Acetin * | 10.00 | 43 | 8 | ND | ND | 0.03 ± 0.00 | 0.12 ± 0.03 | 0.22 ± 0.06 | ND |
| Coumaran | 10.97 | 120 | 4 | 0.89 | 0.64 ± 0.11 | 0.81 ± 0.16 | 0.68 ± 0.09 | 0.45 ± 0.05 | 0.90 ± 0.02 |
| N-acetyl tyramine | 13.85 | 107 | 10 | 0.18 ± 0.04 | 0.18 ± 0.07 | 0.13 ± 0.05 | 0.16 ± 0.02 | 0.03 ± 0.00 | 0.09 ± 0.02 |
| Brand Name | Type of Vinegar | Code | Origin | Bottling Site |
|---|---|---|---|---|
| MazzettiTM | L’originale balsamic vinegar of Modena, four leaves | M4 | Modena, Italy | Pertini 440-41032, Cavezzo |
| MazzettiTM | L’originale aceto balsamic vinegar of Modena IGP, one leaf | M1 | Modena, Italy | Pertini 440-41032, Cavezzo |
| LupiTM | Aceo balsamic vinegar of Modena IGP | L | Modena, Italy | Montanara 22/24 41051 |
| Pam’sTM | Balsamic vinegar of Modena | P | Modena, Italy | n°CSQA 216311 |
| DelmaineTM | Balsamic vinegar of Modena IGP | D | Modena, Italy | n°CSQA 216311 |
| TastemakerTM | Aged balsamic vinegar of Modena, premium | T | New Zealand | Merton Road, Fernside |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinu, F.R.; De Carvalho-Silva, S.; Trovatti Uetanabaro, A.P.; Villas-Boas, S.G. Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry. Metabolites 2016, 6, 22. https://doi.org/10.3390/metabo6030022
Pinu FR, De Carvalho-Silva S, Trovatti Uetanabaro AP, Villas-Boas SG. Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry. Metabolites. 2016; 6(3):22. https://doi.org/10.3390/metabo6030022
Chicago/Turabian StylePinu, Farhana R., Samuel De Carvalho-Silva, Ana Paula Trovatti Uetanabaro, and Silas G. Villas-Boas. 2016. "Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry" Metabolites 6, no. 3: 22. https://doi.org/10.3390/metabo6030022
APA StylePinu, F. R., De Carvalho-Silva, S., Trovatti Uetanabaro, A. P., & Villas-Boas, S. G. (2016). Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry. Metabolites, 6(3), 22. https://doi.org/10.3390/metabo6030022